Ion-Channel Proteins in the Prepubertal Bitch Reproductive System: The Immunolocalization of ASIC2, ASIC4, and PIEZO2
Abstract
1. Introduction
2. Results
2.1. Ion Channels’ Evolutionary Conservation
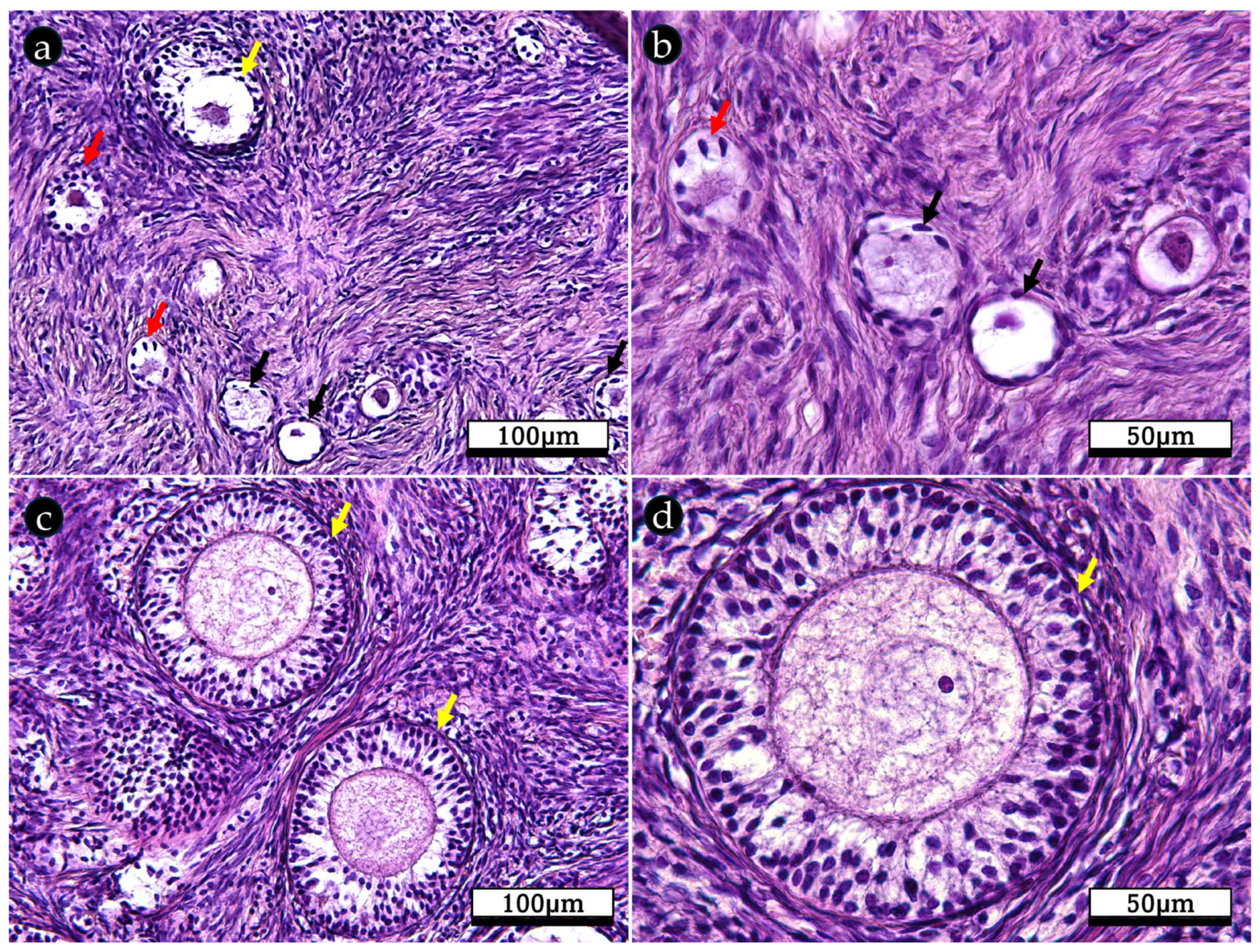
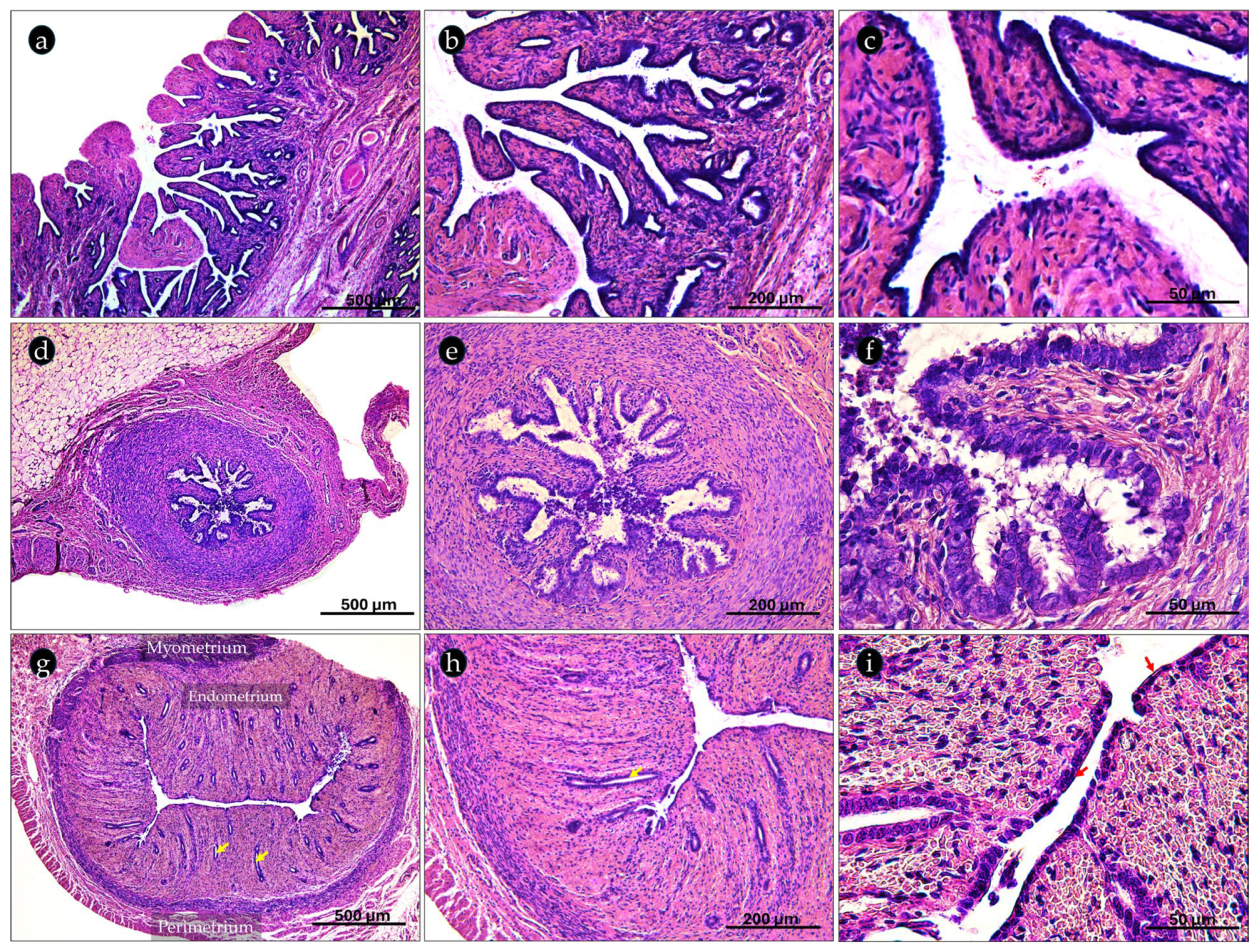
2.2. Histological Organization of the Canine Prepubertal Genital Tracts
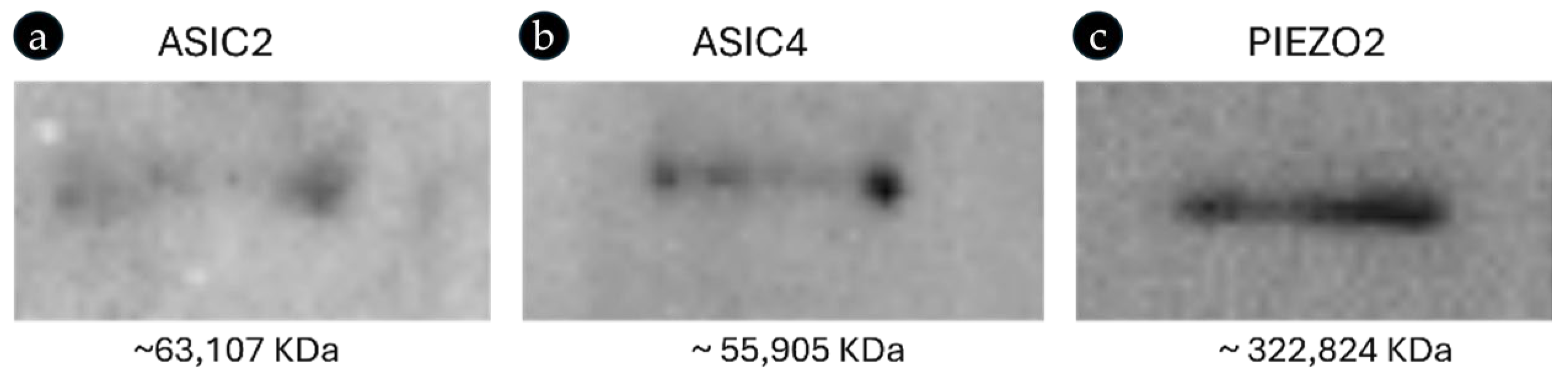
2.3. Ion-Channel Expression and Primary Antibody Specificity in Canine Sample
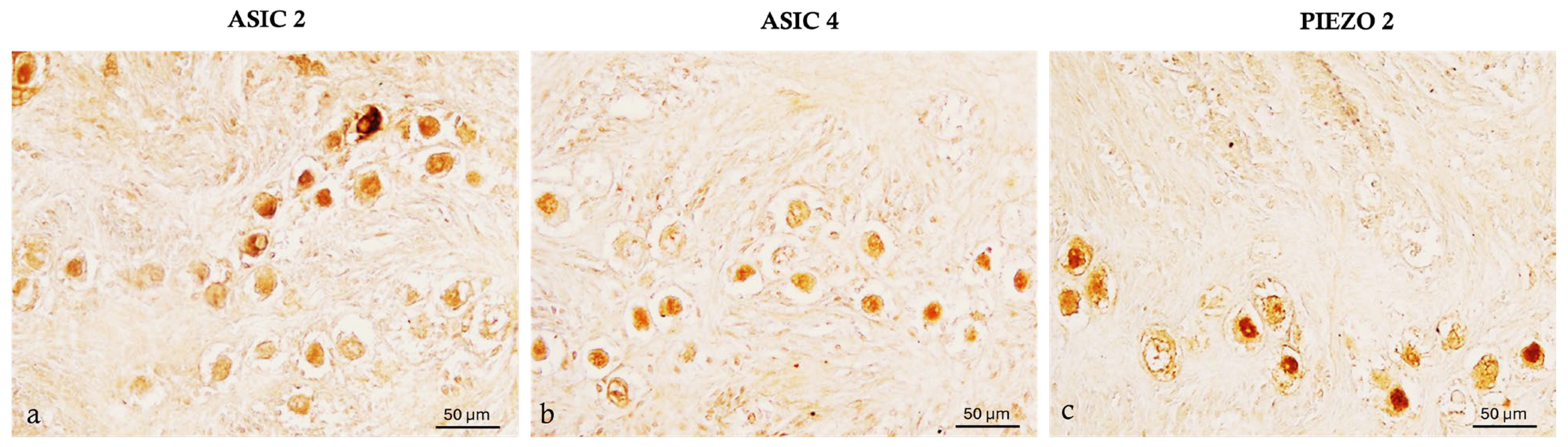
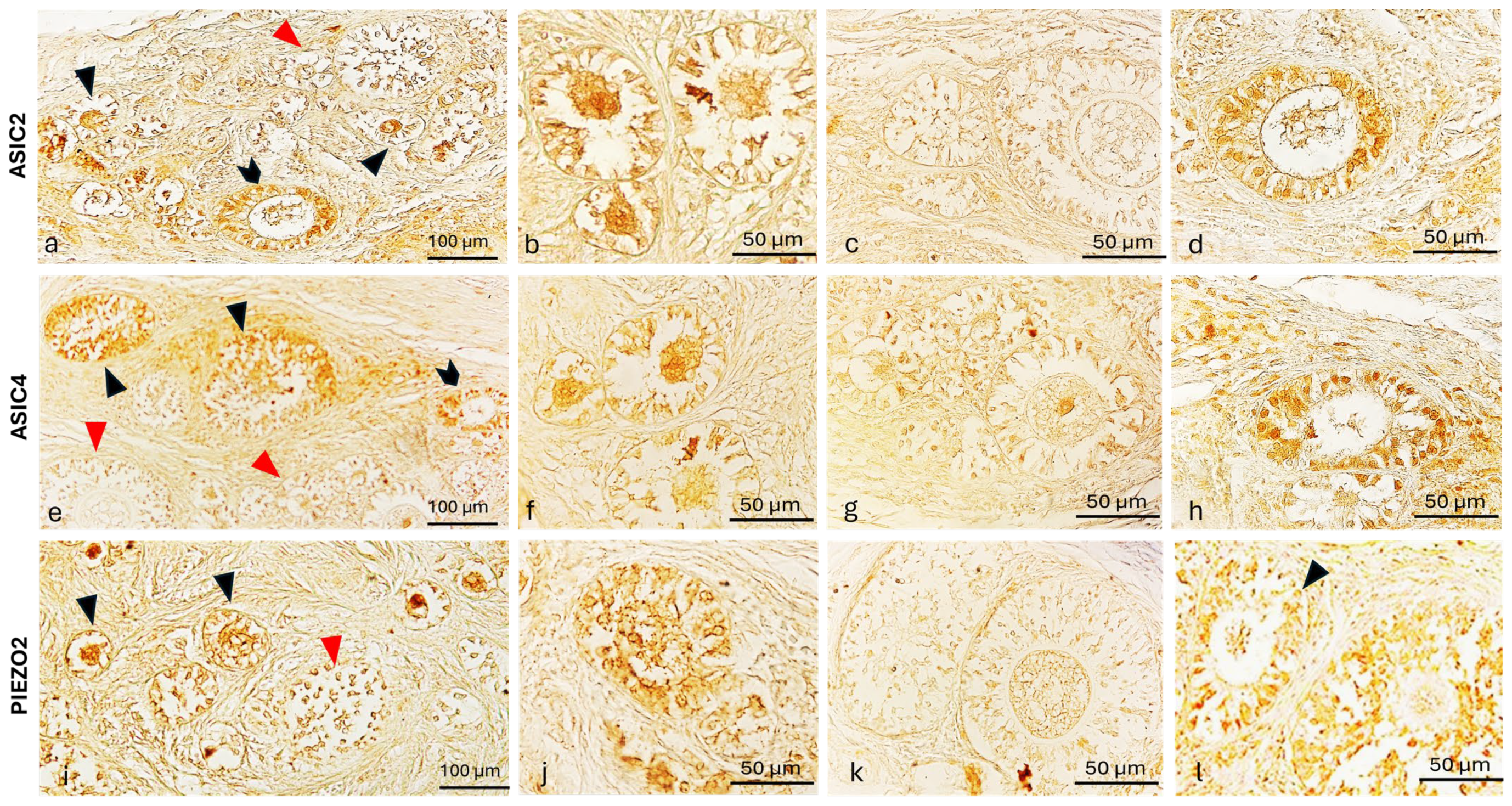
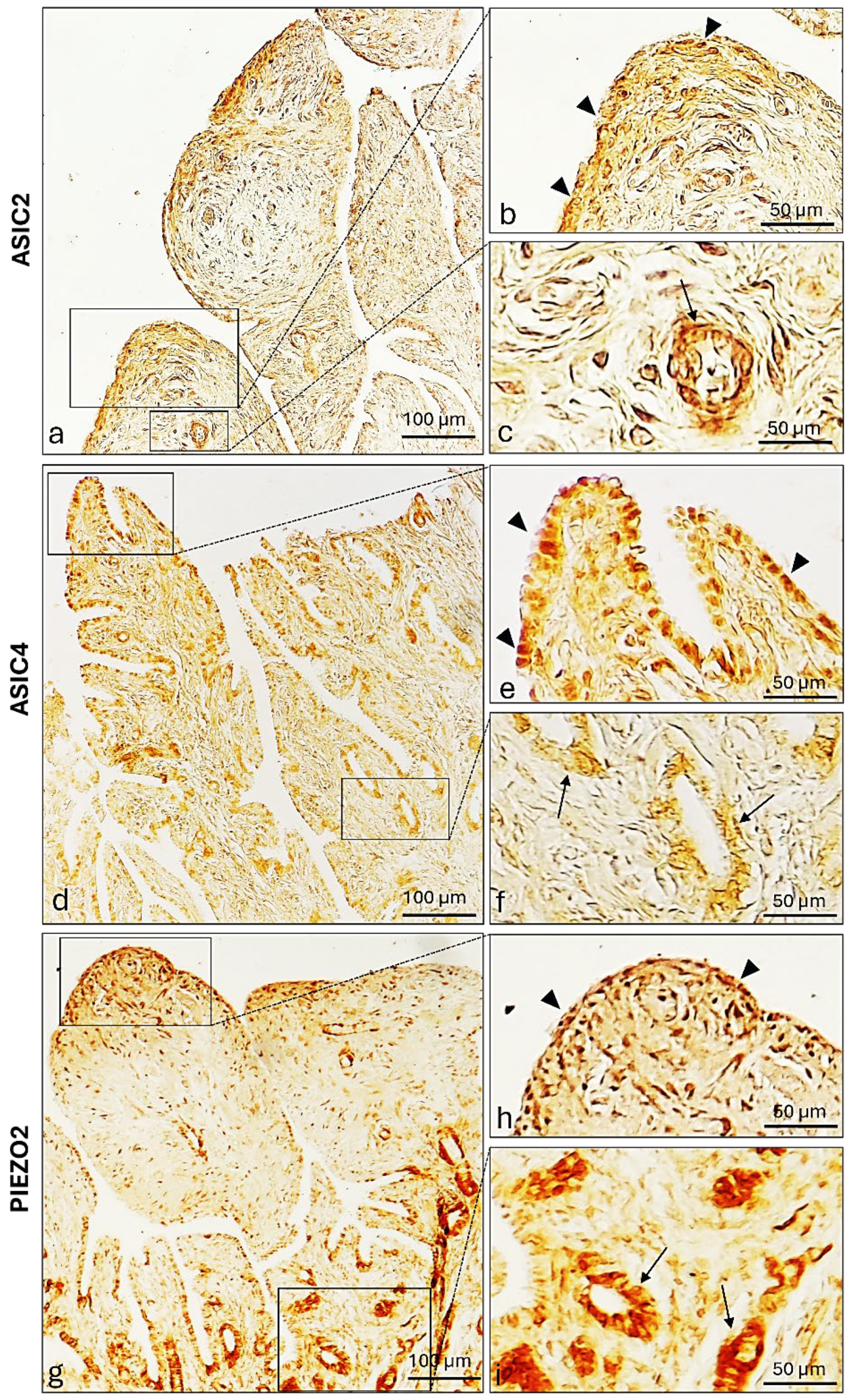
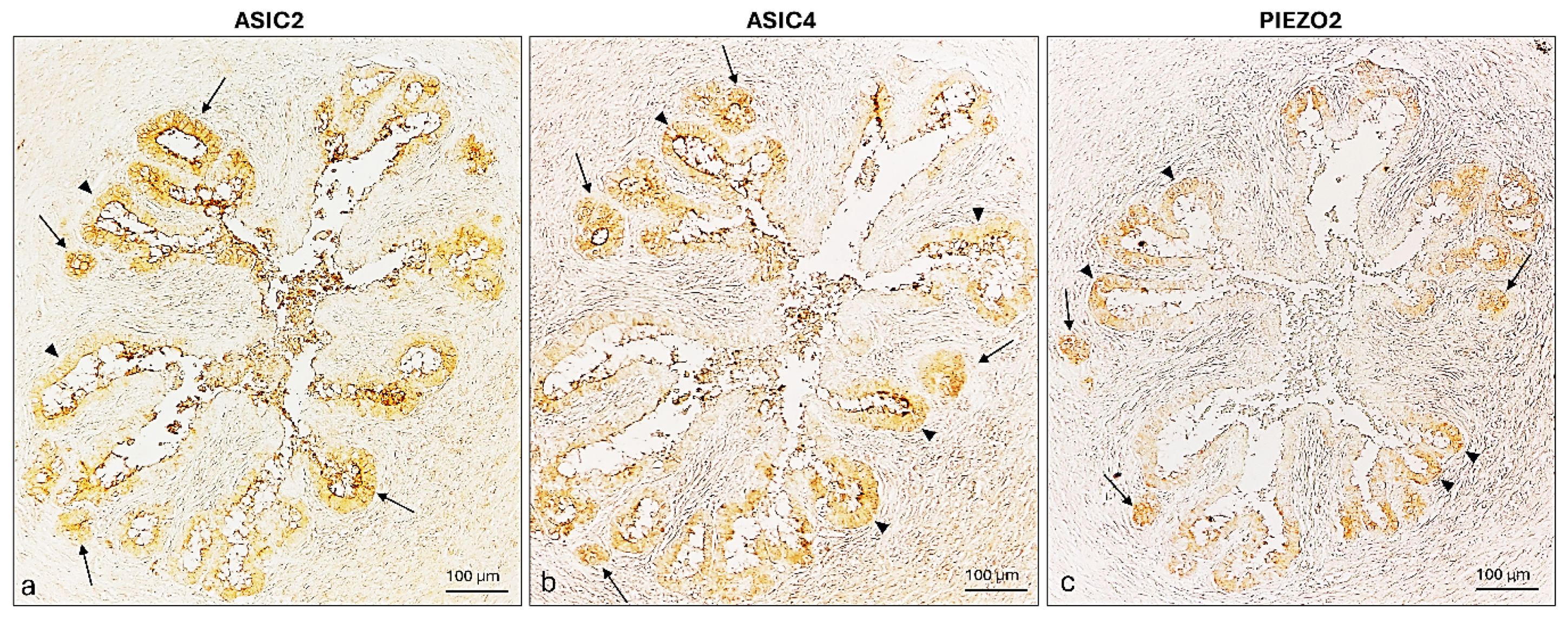
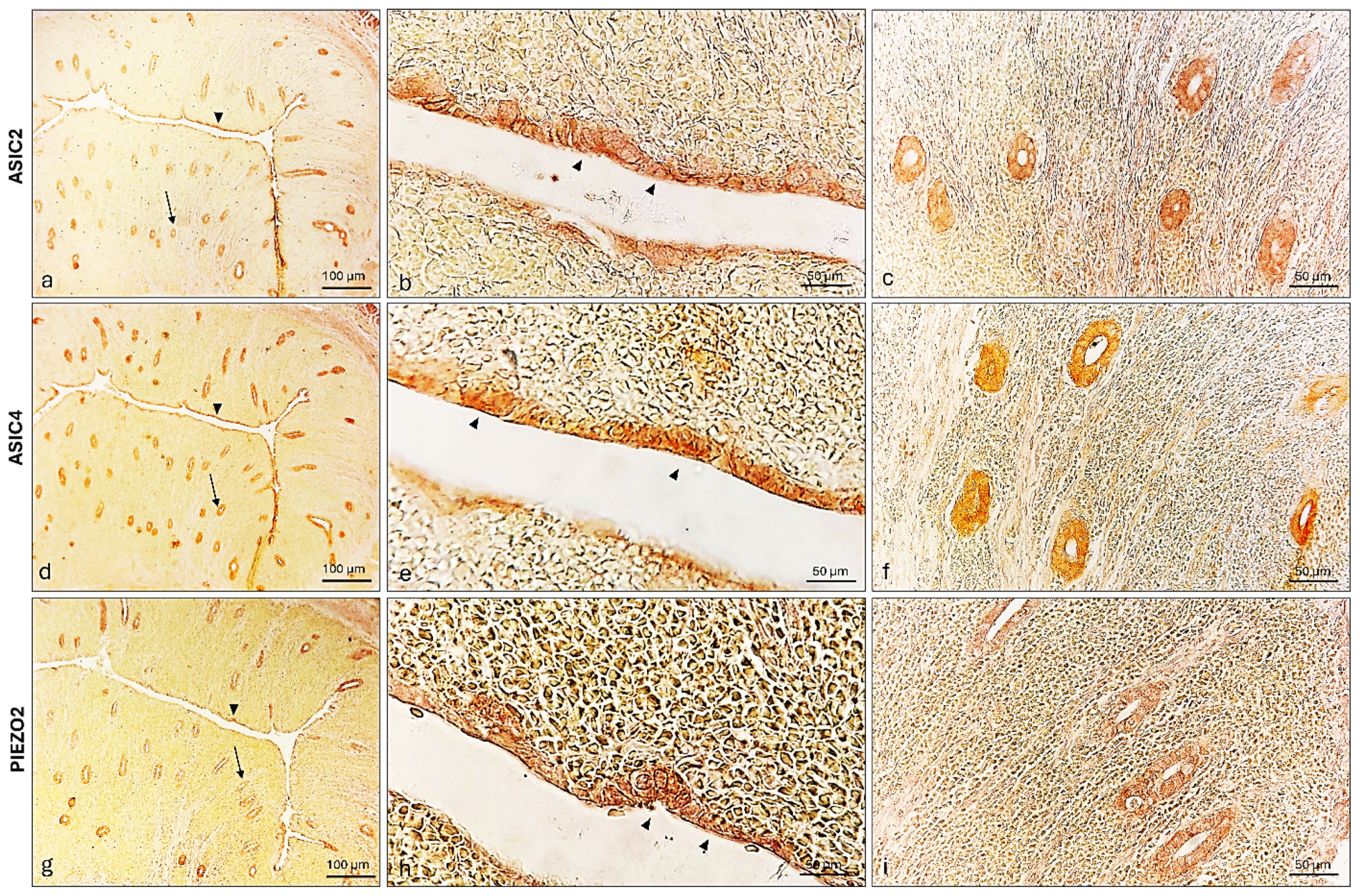
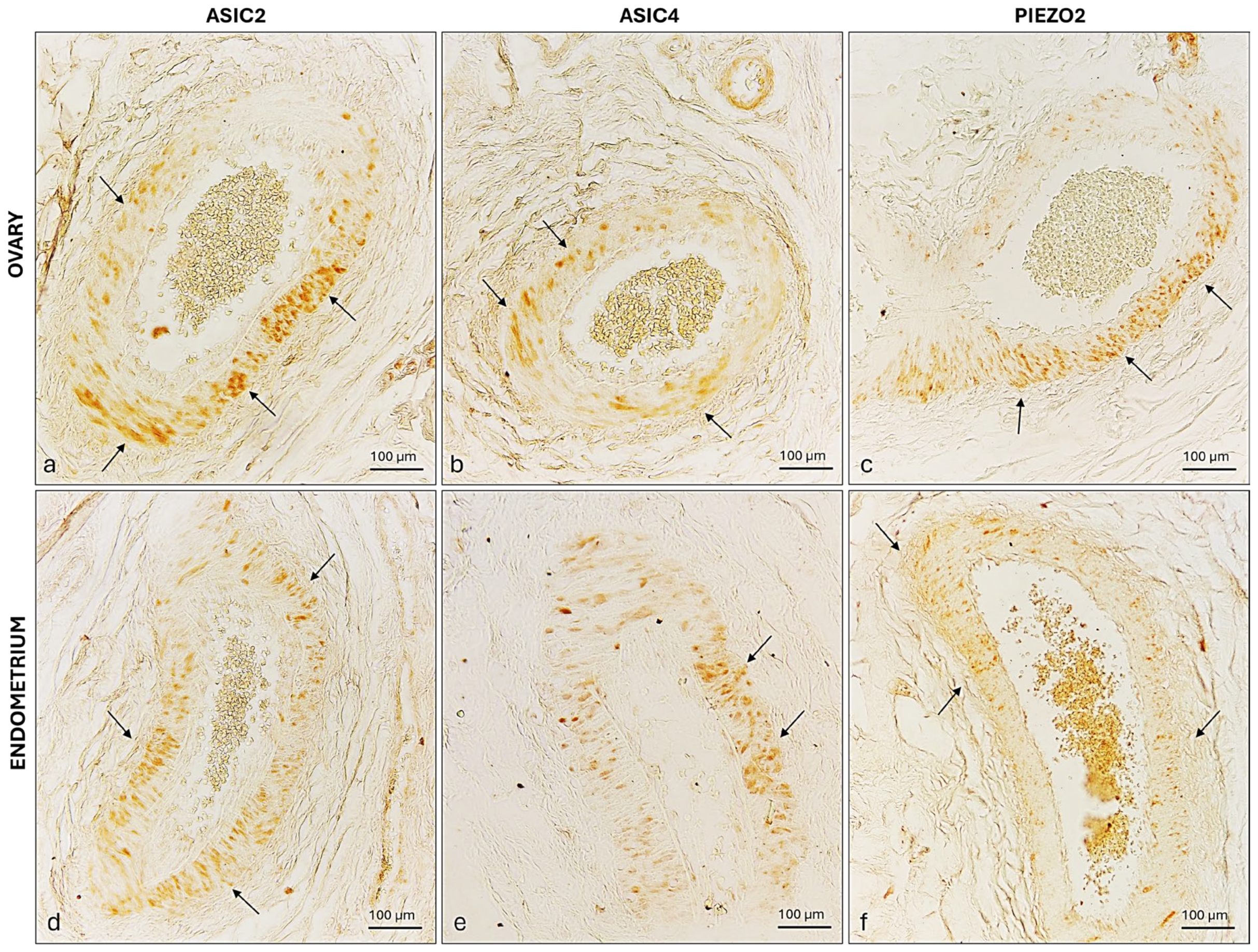
2.4. ASIC2, ASIC4 and PIEZO2 Localization in the Bitches’ Genital Tracts
3. Discussion
4. Materials and Methods
4.1. Evolutionary Conservation of Ion Channels Across Vertebrates: From High Sequence Homology to Potential Critical Function
4.2. Sampling and Characterization of Prepubertal Canine Reproductive Tissues
4.3. Histology
4.4. Ion-Channel Expression and Primary Antibody Specificity in Canine Sample
4.4.1. Alignment of Primary Antibodies Immunogen Sequences with the Respective Canine Proteins
4.4.2. Western Blot Analyses
4.5. Immunohistochemical and Immunofluorescent Detection of ASIC2, ASIC4, and PIEZO2 in Reproductive Tissues of Prepubertal Bitches
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Concannon, P.W. Reproductive Cycles of the Domestic Bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Partin, A.M.; Burghardt, G.M.; Springer, C.M.; Albright, J.D. A Descriptive Methodology for Studying the Ontogeny of Object Play and Breed Differences in Dogs (Canis lupus familiaris). Animals 2023, 13, 1371. [Google Scholar] [CrossRef] [PubMed]
- Gobello, C. Prepubertal and Pubertal Canine Reproductive Studies: Conflicting Aspects. Reprod. Domest. Anim. 2014, 49, e70–e73. [Google Scholar] [CrossRef] [PubMed]
- Mo, A.; Dang, Y.; Wang, J.; Liu, C.; Yuan, Y.C.; Yang, H. Sex Differences, Growth, Reproduction and Zinc Ion Homeostasis of Zebrafish after Chronic Dietary l-Selenomethionine Exposure. Chemosphere 2020, 259, 127455. [Google Scholar] [CrossRef]
- Benko, F.; Urminská, D.; Ďuračka, M.; Tvrdá, E. Signaling Roleplay between Ion Channels during Mammalian Sperm Capacitation. Biomedicines 2023, 11, 2519. [Google Scholar] [CrossRef]
- Delgado-Bermúdez, A.; Yeste, M.; Bonet, S.; Pinart, E. Physiological Role of Potassium Channels in Mammalian Germ Cell Differentiation, Maturation, and Capacitation. Andrology 2025, 13, 184–201. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, G.-S.; Ji, Y.-K.; Choi, K.-C.; Jeung, E.-B. Differential Expression of Uterine Calcium Transporter 1 and Plasma Membrane Ca2+ ATPase 1b during Rat Estrous Cycle. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E234–E241. [Google Scholar] [CrossRef]
- Lee, G.-S.; Jeung, E.-B. Uterine TRPV6 Expression during the Estrous Cycle and Pregnancy in a Mouse Model. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E132–E138. [Google Scholar] [CrossRef]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper Channel Mediates Progesterone-Induced Ca2+ Influx in Human Sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Morales-Lázaro, S.L.; González-Ramírez, R.; Rosenbaum, T. Molecular Interplay Between the Sigma-1 Receptor, Steroids, and Ion Channels. Front. Pharmacol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Díaz, L.; Ceja-Ochoa, I.; Restrepo-Angulo, I.; Larrea, F.; Avila-Chávez, E.; García-Becerra, R.; Borja-Cacho, E.; Barrera, D.; Ahumada, E.; Gariglio, P.; et al. Estrogens and Human Papilloma Virus Oncogenes Regulate Human Ether-à-Go-Go-1 Potassium Channel Expression. Cancer Res. 2009, 69, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Irnaten, M.; Blanchard-Gutton, N.; Harvey, B.J. Rapid Effects of 17β-Estradiol on Epithelial TRPV6 Ca2+ Channel in Human T84 Colonic Cells. Cell Calcium 2008, 44, 441–452. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, F.; Alzamora, R.; Betts, V.; LaPaix, F.; Carter, D.; Irnaten, M.; Harvey, B.J. Female Gender-Specific Inhibition of KCNQ1 Channels and Chloride Secretion by 17β-Estradiol in Rat Distal Colonic Crypts. J. Biol. Chem. 2007, 282, 24563–24573. [Google Scholar] [CrossRef] [PubMed]
- Alzamora, R.; O’Mahony, F.; Bustos, V.; Rapetti-Mauss, R.; Urbach, V.; Cid, L.P.; Sepúlveda, F.V.; Harvey, B.J. Sexual Dimorphism and Oestrogen Regulation of KCNE3 Expression Modulates the Functional Properties of KCNQ1 K+ Channels. J. Physiol. 2011, 589, 5091–5107. [Google Scholar] [CrossRef]
- Ghavideldarestani, M.; Butler, A.E.; Shirian, S.; Atkin, S.L. Expression and Localization of Transient Receptor Potential Channels in the Bovine Uterus Epithelium throughout the Estrous Cycle. Mol. Biol. Rep. 2019, 46, 4077–4084. [Google Scholar] [CrossRef]
- Ghavideldarestani, M.; Atkin, S.L.; Leese, H.J.; Sturmey, R.G. Expression and Function of Transient Receptor Potential Channels in the Female Bovine Reproductive Tract. Theriogenology 2016, 86, 551–561. [Google Scholar] [CrossRef]
- Teilmann, S.C.; Byskov, A.G.; Pedersen, P.A.; Wheatley, D.N.; Pazour, G.J.; Christensen, S.T. Localization of Transient Receptor Potential Ion Channels in Primary and Motile Cilia of the Female Murine Reproductive Organs. Mol. Reprod. Dev. 2005, 71, 444–452. [Google Scholar] [CrossRef]
- Boscardin, E.; Alijevic, O.; Hummler, E.; Frateschi, S.; Kellenberger, S. The Function and Regulation of Acid-Sensing Ion Channels (ASICs) and the Epithelial Na+ Channel (ENaC): IUPHAR Review 19. Br. J. Pharmacol. 2016, 173, 2671–2701. [Google Scholar] [CrossRef]
- Gründer, S.; Pusch, M. Biophysical Properties of Acid-Sensing Ion Channels (ASICs). Neuropharmacology 2015, 94, 9–18. [Google Scholar] [CrossRef]
- Montalbano, G.; Levanti, M.; Mhalhel, K.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Germanà, A. Acid-Sensing Ion Channels in Zebrafish. Animals 2021, 11, 2471. [Google Scholar] [CrossRef]
- Cheng, Y.-R.; Jiang, B.-Y.; Chen, C.-C. Acid-Sensing Ion Channels: Dual Function Proteins for Chemo-Sensing and Mechano-Sensing. J. Biomed. Sci. 2018, 25, 46. [Google Scholar] [CrossRef]
- Hanukoglu, I. ASIC and ENaC Type Sodium Channels: Conformational States and the Structures of the Ion Selectivity Filters. FEBS J. 2017, 284, 525–545. [Google Scholar] [CrossRef]
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2017, 42, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Qiu, Z.; Woo, S.-H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a Mechanically Activated Ion Channel, Is Required for Vascular Development in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Lewis, A.H.; Grandl, J. Physics of Mechanotransduction by Piezo Ion Channels. J. Gen. Physiol. 2022, 154, e202113044. [Google Scholar] [CrossRef]
- Parpaite, T.; Coste, B. Piezo Channels. Curr. Biol. 2017, 27, R250–R252. [Google Scholar] [CrossRef]
- Aragona, M.; Mhalhel, K.; Cometa, M.; Franco, G.A.; Montalbano, G.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Abbate, F.; Vega, J.A.; et al. Piezo 1 and Piezo 2 in the Chemosensory Organs of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 7404. [Google Scholar] [CrossRef]
- Aragona, M.; Mhalhel, K.; Pansera, L.; Montalbano, G.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Abbate, F.; Vega, J.A.; Germanà, A. Localization of Piezo 1 and Piezo 2 in Lateral Line System and Inner Ear of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 9204. [Google Scholar] [CrossRef]
- Butenas, A.L.E.; Rollins, K.S.; Parr, S.K.; Hammond, S.T.; Ade, C.J.; Hageman, K.S.; Musch, T.I.; Copp, S.W. Novel Mechanosensory Role for Acid Sensing Ion Channel Subtype 1a in Evoking the Exercise Pressor Reflex in Rats with Heart Failure. J. Physiol. 2022, 600, 2105–2125. [Google Scholar] [CrossRef]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 Analogue (Dooku1) Which Antagonizes Yoda1-Evoked Activation of Piezo1 and Aortic Relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef] [PubMed]
- Akanji, O.; Weinzierl, N.; Schubert, R.; Schilling, L. Acid Sensing Ion Channels in Rat Cerebral Arteries: Probing the Expression Pattern and Vasomotor Activity. Life Sci. 2019, 227, 193–200. [Google Scholar] [CrossRef]
- Arishe, O.O.; McKenzie, J.; Dela Justina, V.; Dos Anjos Moraes, R.; Webb, R.C.; Priviero, F. Piezo1 Channels Mediate Vasorelaxation of Uterine Arteries from Pseudopregnant Rats. Front. Physiol. 2023, 14, 1140989. [Google Scholar] [CrossRef] [PubMed]
- Alcaino, C.; Farrugia, G.; Beyder, A. Mechanosensitive Piezo Channels in the Gastrointestinal Tract. Curr. Top. Membr. 2017, 79, 219–244. [Google Scholar] [CrossRef]
- Stewart, T.A.; Davis, F.M. Formation and Function of Mammalian Epithelia: Roles for Mechanosensitive PIEZO1 Ion Channels. Front. Cell Dev. Biol. 2019, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Ninomiya, T.; Kawamata, T.; Tatsumi, H. Expression of ASIC2 in Ciliated Cells and Stereociliated Cells. Cell Tissue Res. 2008, 333, 217–224. [Google Scholar] [CrossRef]
- Aspinall, V. Reproductive System of the Dog and Cat—Part 3. Reproductive Physiology of the Bitch. Vet. Nurs. J. 2011, 26, 153–157. [Google Scholar] [CrossRef]
- Linde-Forsberg, C. Biology of Reproduction and Modern Reproductive Technology. In The Genetics of the Dog; CABI Publishing: Wallingford, UK, 2001; pp. 401–429. ISBN 978-0-85199-078-1. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Songsasen, N. Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms. Animals 2021, 11, 653. [Google Scholar] [CrossRef]
- Carvacho, I.; Piesche, M.; Maier, T.J.; Machaca, K. Ion Channel Function During Oocyte Maturation and Fertilization. Front. Cell Dev. Biol. 2018, 6, 63. [Google Scholar] [CrossRef]
- Bronson, F.H. Mammalian Reproduction: An Ecological Perspective. Biol. Reprod. 1985, 32, 1–26. [Google Scholar] [CrossRef]
- Jegla, T.J.; Zmasek, C.M.; Batalov, S.; Nayak, S.K. Evolution of the Human Ion Channel Set. Comb. Chem. High Throughput Screen 2009, 12, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Pascual-García, A.; Abia, D.; Méndez, R.; Nido, G.S.; Bastolla, U. Quantifying the Evolutionary Divergence of Protein Structures: The Role of Function Change and Function Conservation. Proteins Struct. Funct. Bioinform. 2010, 78, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Meinel, T. Function and Homology of Proteins Similar in Sequence: Phylogenetic Profiling. In Handbook of Research on Systems Biology Applications in Medicine; IGI Global: Hershey, PA, USA, 2009; Volume 1, pp. 143–166. ISBN 978-1-60566-076-9. [Google Scholar]
- Steinhauer, N.; Boos, A.; Günzel-Apel, A.-R. Morphological Changes and Proliferative Activity in the Oviductal Epithelium during Hormonally Defined Stages of the Oestrous Cycle in the Bitch. Reprod. Domest. Anim. 2004, 39, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Groppetti, D.; Aralla, M.; Bronzo, V.; Bosi, G.; Pecile, A.; Arrighi, S. Periovulatory Time in the Bitch: What’s New to Know?: Comparison between Ovarian Histology and Clinical Features. Anim. Reprod. Sci. 2015, 152, 108–116. [Google Scholar] [CrossRef]
- Schäfer-Somi, S.; Deichsel, K.; Beceriklisoy, H.; Korkmaz, D.; Walter, I.; Aslan, S. Morphological, Histological and Molecular Investigations on Canine Uterine Tissue after Ovariectomy. Theriogenology 2017, 102, 80–86. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Masserdotti, C. Reproductive System. In Canine Feline Cytology, 3rd ed.; Raskin, R.E., Meyer, D.J., Eds.; W.B. Saunders: St. Louis, MO, USA, 2015; pp. 313–352. ISBN 978-1-4557-4083-3. [Google Scholar] [CrossRef]
- Eppig, J.J. Oocyte Control of Ovarian Follicular Development and Function in Mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef]
- Fortune, J.E.; Cushman, R.A.; Wahl, C.M.; Kito, S. The Primordial to Primary Follicle Transition. Mol. Cell. Endocrinol. 2000, 163, 53–60. [Google Scholar] [CrossRef]
- Gougeon, A. Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef]
- Mhalhel, K.; Arena, R.; Rizzo, M.; Piccione, G.; Aragona, M.; Levanti, M.; Aragona, F.; Arfuso, F. Potential Implications of Acid-Sensing Ion Channels ASIC2 and ASIC4 in Gonadal Differentiation of Dicentrarchus Labrax Subjected to Water Temperature Increase during Gonadal Development. Animals 2024, 14, 1024. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.-Z.; Yu, Y.; Wang, J.-J. Inhibitory ASIC2-Mediated Calcineurin/NFAT against Colorectal Cancer by Triterpenoids Extracted from Rhus Chinensis Mill. J. Ethnopharmacol. 2019, 235, 255–267. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Song, J.-W.; Li, W.; Liu, X.; Cao, L.; Wan, L.-M.; Tan, Y.-X.; Ji, S.-P.; Liang, Y.-M.; Gong, F. The Acid-Sensing Ion Channel, ASIC2, Promotes Invasion and Metastasis of Colorectal Cancer under Acidosis by Activating the Calcineurin/NFAT1 Axis. J. Exp. Clin. Cancer Res. 2017, 36, 130. [Google Scholar] [CrossRef] [PubMed]
- Tosti, E. Calcium Ion Currents Mediating Oocyte Maturation Events. Reprod. Biol. Endocrinol. 2006, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Wilhite, A.; Chokshi, S.; Singleton, M.H.; Scalici, J.; Lee, K. Abstract A032: The Role of ASIC2 and Calcium Influx in Epithelial Ovarian Cancer Pathogenesis. Cancer Res. 2024, 84, A032. [Google Scholar] [CrossRef]
- Nader, N.; Kulkarni, R.P.; Dib, M.; Machaca, K. How to Make a Good Egg!: The Need for Remodeling of Oocyte Ca2+ Signaling to Mediate the Egg-to-Embryo Transition. Cell Calcium 2013, 53, 41–54. [Google Scholar] [CrossRef]
- Kishimoto, T. Entry into Mitosis: A Solution to the Decades-Long Enigma of MPF. Chromosoma 2015, 124, 417–428. [Google Scholar] [CrossRef]
- Miao, Y.-L.; Stein, P.; Jefferson, W.N.; Padilla-Banks, E.; Williams, C.J. Calcium Influx-Mediated Signaling Is Required for Complete Mouse Egg Activation. Proc. Natl. Acad. Sci. USA 2012, 109, 4169–4174. [Google Scholar] [CrossRef]
- Wakai, T.; Mehregan, A.; Fissore, R.A. Ca2+ Signaling and Homeostasis in Mammalian Oocytes and Eggs. Cold Spring Harb. Perspect. Biol. 2019, 11, a035162. [Google Scholar] [CrossRef]
- Stricker, S.A. Comparative Biology of Calcium Signaling during Fertilization and Egg Activation in Animals. Dev. Biol. 1999, 211, 157–176. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Stefanov, V.E.; Iwasaki, T.; Sato, K.-I.; Fukami, Y. Calcium Signaling and Meiotic Exit at Fertilization in Xenopus Egg. Int. J. Mol. Sci. 2014, 15, 18659–18676. [Google Scholar] [CrossRef]
- Webb, S.E.; Miller, A.L. Ca2+ Signaling during Activation and Fertilization in the Eggs of Teleost Fish. Cell Calcium 2013, 53, 24–31. [Google Scholar] [CrossRef]
- Bai, X.; Bouffard, J.; Lord, A.; Brugman, K.; Sternberg, P.W.; Cram, E.J.; Golden, A. Caenorhabditis Elegans PIEZO Channel Coordinates Multiple Reproductive Tissues to Govern Ovulation. eLife 2020, 9, e53603. [Google Scholar] [CrossRef] [PubMed]
- Douguet, D.; Honoré, E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels. Cell 2019, 179, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Zhao, X.; Li, J.; Chen, Y. Piezo Channels in the Urinary System. Exp. Mol. Med. 2022, 54, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical Stretch Triggers Rapid Epithelial Cell Division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef]
- Mim, M.S.; Kumar, N.; Levis, M.; Unger, M.F.; Miranda, G.; Gazzo, D.; Robinett, T.; Zartman, J.J. Piezo Regulates Epithelial Topology and Promotes Precision in Organ Size Control. Cell Rep. 2024, 43, 114398. [Google Scholar] [CrossRef]
- Piddini, E. Epithelial Homeostasis: A Piezo of the Puzzle. Curr. Biol. 2017, 27, R232–R234. [Google Scholar] [CrossRef]
- Enders, A.C.; Given, R.L.; Schlafke, S. Differentiation and Migration of Endoderm in the Rat and Mouse at Implantation. Anat. Rec. 1978, 190, 65–77. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Hennes, A.; Held, K.; Boretto, M.; De Clercq, K.; Van den Eynde, C.; Vanhie, A.; Van Ranst, N.; Benoit, M.; Luyten, C.; Peeraer, K.; et al. Functional Expression of the Mechanosensitive PIEZO1 Channel in Primary Endometrial Epithelial Cells and Endometrial Organoids. Sci. Rep. 2019, 9, 1779. [Google Scholar] [CrossRef]
- Mahapatra, C.; Kumar, R. Biophysical Mechanisms of Vaginal Smooth Muscle Contraction: The Role of the Membrane Potential and Ion Channels. Pathophysiology 2024, 31, 225–243. [Google Scholar] [CrossRef]
- Suarez, S.S.; Wolfner, M.F. Cilia Take the Egg on a Magic Carpet Ride. Proc. Natl. Acad. Sci. USA 2021, 118, e2108887118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Hennig, G.W.; Zheng, H.; Yan, W. Oviductal Motile Cilia Are Essential for Oocyte Pickup but Dispensable for Sperm and Embryo Transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2102940118. [Google Scholar] [CrossRef] [PubMed]
- Arishe, O.O.; Ebeigbe, A.B.; Webb, R.C. Mechanotransduction and Uterine Blood Flow in Preeclampsia: The Role of Mechanosensing Piezo 1 Ion Channels. Am. J. Hypertens. 2020, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.; Shi, J.; Endesh, N.; Drinkhill, M.J.; Webster, P.J.; Lotteau, S.J.; Bailey, M.A.; Yuldasheva, N.Y.; Ludlow, M.J.; Cubbon, R.M.; et al. Piezo1 Channels Sense Whole Body Physical Activity to Reset Cardiovascular Homeostasis and Enhance Performance. Nat. Commun. 2017, 8, 350. [Google Scholar] [CrossRef]
- Barnett, S.D.; Asif, H.; Buxton, I.L.O. Novel Identification and Modulation of the Mechanosensitive Piezo1 Channel in Human Myometrium. J. Physiol. 2023, 601, 1675–1690. [Google Scholar] [CrossRef]
- Zhang, Y.; Hermanson, M.E.; Eddinger, T.J. Tonic and Phasic Smooth Muscle Contraction Is Not Regulated by the PKCα—CPI-17 Pathway in Swine Stomach Antrum and Fundus. PLoS ONE 2013, 8, e74608. [Google Scholar] [CrossRef]
- Brading, A.F. Spontaneous Activity of Lower Urinary Tract Smooth Muscles: Correlation between Ion Channels and Tissue Function. J. Physiol. 2006, 570, 13–22. [Google Scholar] [CrossRef]
- Amberg, G.C.; Koh, S.D.; Imaizumi, Y.; Ohya, S.; Sanders, K.M. A-Type Potassium Currents in Smooth Muscle. Am. J. Physiol. Cell Physiol. 2003, 284, C583–C595. [Google Scholar] [CrossRef]
- Jackson, W.F. Chapter Three—Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. In Advances in Pharmacology; Khalil, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 78, pp. 89–144. ISBN 1054-3589. [Google Scholar]
- Pablo, J.L.; DeCaen, P.G.; Clapham, D.E. Progress in Ciliary Ion Channel Physiology. J. Gen. Physiol. 2017, 149, 37–47. [Google Scholar] [CrossRef]
- Enuka, Y.; Hanukoglu, I.; Edelheit, O.; Vaknine, H.; Hanukoglu, A. Epithelial Sodium Channels (ENaC) Are Uniformly Distributed on Motile Cilia in the Oviduct and the Respiratory Airways. Histochem. Cell Biol. 2012, 137, 339–353. [Google Scholar] [CrossRef]
- Chapleau, M.W.; Lu, Y.; Abboud, F.M. Chapter 19—Mechanosensitive Ion Channels in Blood Pressure-Sensing Baroreceptor Neurons. In Current Topics in Membranes; Hamill, O.P., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 59, pp. 541–567. ISBN 1063-5823. [Google Scholar]
- López-Ramírez, O.; González-Garrido, A. The Role of Acid Sensing Ion Channels in the Cardiovascular Function. Front. Physiol. 2023, 14, 1194948. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Sabharwal, R.; Snitsarev, V.; Morgan, D.; Rahmouni, K.; Drummond, H.A.; Whiteis, C.A.; Costa, V.; Price, M.; et al. The Ion Channel ASIC2 Is Required for Baroreceptor and Autonomic Control of the Circulation. Neuron 2009, 64, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Briglia, M.; Aragona, M.; Porcino, C.; Abbate, F.; Guerrera, M.C.; Laurà, R.; Krichen, Y.; Guerbej, H.; Germanà, A.; et al. Nothobranchius as a Model for Anorexia of Aging Research: An Evolutionary, Anatomical, Histological, Immunohistochemical, and Molecular Study. Ann. Anat. Anat. Anz. 2023, 250, 152116. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Montalbano, G.; Giurdanella, G.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Germanà, A.; Levanti, M. Histological and Immunohistochemical Study of Gilthead Seabream Tongue from the Early Stage of Development: TRPV4 Potential Roles. Ann. Anat. 2022, 244, 151985. [Google Scholar] [CrossRef]
- DeTora, M.; McCarthy, R.J. Ovariohysterectomy versus Ovariectomy for Elective Sterilization of Female Dogs and Cats: Is Removal of the Uterus Necessary? J. Am. Vet. Med. Assoc. 2011, 239, 1409–1412. [Google Scholar] [CrossRef]
- Romagnoli, S.; Krekeler, N.; de Cramer, K.; Kutzler, M.; McCarthy, R.; Schaefer-Somi, S. WSAVA Guidelines for the Control of Reproduction in Dogs and Cats. J. Small Anim. Pract. 2024, 65, 424–559. [Google Scholar] [CrossRef]
- Germanà, A.; Guerrera, M.C.; Laurà, R.; Levanti, M.; Aragona, M.; Mhalhel, K.; Germanà, G.; Montalbano, G.; Abbate, F. Expression and Localization of BDNF/TrkB System in the Zebrafish Inner Ear. Int. J. Mol. Sci. 2020, 21, 5787. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; Calapai, F.; et al. Localization of BDNF and Calretinin in Olfactory Epithelium and Taste Buds of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 4696. [Google Scholar] [CrossRef]
- Mhalhel, K.; Kadmi, Y.; Ben Chira, A.; Levanti, M.; Pansera, L.; Cometa, M.; Sicari, M.; Germanà, A.; Aragona, M.; Montalbano, G. Urtica dioica Extract Abrogates Chlorpyrifos-Induced Toxicity in Zebrafish Larvae. Int. J. Mol. Sci. 2024, 25, 6631. [Google Scholar] [CrossRef]



| Taxa | Max Score | Total Score | Query Cover | E-Value | Per. Ident | Acc. Len | Accession | ||
|---|---|---|---|---|---|---|---|---|---|
| Proteins | ASIC2 | Canis lupus familiaris | - | - | - | - | - | - | XP_038533280.1 |
| Homo sapiens | 790 | 790 | 95% | 0.0 | 78.15% | 563 | NP_899233.1 | ||
| Mus musculus | 1051 | 1051 | 100% | 0.0 | 98.83% | 512 | NP_001029185.1 | ||
| Anolis carolinensis | 918 | 918 | 100% | 0.0 | 87.18% | 521 | XP_016850081.1 | ||
| Gallus gallus | 775 | 775 | 96% | 0.0 | 76.80% | 507 | XP_040548011.1 | ||
| Danio rerio | 768 | 768 | 100% | 0.0 | 71.67% | 533 | XP_068072559.1 | ||
| ASIC4 | Canis lupus familiaris | - | - | - | - | - | - | XP_038303989.1 | |
| Homo sapiens | 1026 | 1026 | 100% | 0.0 | 93.51% | 520 | NP_878267.3 | ||
| Mus musculus | 1084 | 1084 | 100% | 0.0 | 97.40% | 539 | NP_898843.1 | ||
| Anolis carolinensis | 756 | 756 | 100% | 0.0 | 70.61% | 538 | XP_008110128.2 | ||
| Gallus gallus | 777 | 777 | 100% | 0.0 | 73.11% | 538 | XP_015145601.3 | ||
| Danio rerio | 692 | 692 | 100% | 0.0 | 62.82% | 539 | NP_999952.1 | ||
| PIEZO2 | Canis lupus familiaris | - | - | - | - | - | - | XP_038528610.1 | |
| Homo sapiens | 4942 | 4942 | 100% | 0.0 | 90.24% | 2752 | AFC88283.1 | ||
| Mus musculus | 4953 | 4953 | 100% | 0.0 | 90.11% | 2822 | ADN28065.1 | ||
| Anolis carolinensis | 4093 | 4093 | 100% | 0.0 | 74.84% | 2818 | XP_062836599.1 | ||
| Gallus gallus | 4427 | 4427 | 99% | 0.0 | 79.72% | 2853 | XP_040520788.1 | ||
| Danio rerio | 2045 | 3380 | 93% | 0.0 | 62.89% | 2972 | XP_021323952.1 |
| Taxa | Query ID | Max Score | Total Score | Query Cover | E-Value | Per. Ident | Acc. Len | Accession | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibodies | ASIC2 | Homo sapiens | - | - | - | - | - | - | - | - |
| Canis lupus familiaris | XP_038533280.1 | 67.4 | 67.4 | 6% | 4 × 10−20 | 100.00% | 32 | Query_1685301 | ||
| ASIC4 | Homo sapiens | - | - | - | - | - | - | - | - | |
| Canis lupus familiaris | XP_038303990.1 | 129 | 129 | 11% | 6 × 10−42 | 98.33% | 60 | Query_481487 | ||
| PIEZO2 | Homo sapiens | - | - | - | - | - | - | - | - | |
| Canis lupus familiaris | XP_048968903.1 | 85.5 | 85.5 | 1% | 2 × 10−25 | 92.00% | 50 | Query_1375639 |
| Primary Antibodies | ||||
|---|---|---|---|---|
| ASIC2 | Supplier: Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |||
| Catalog number | Source | Dilution | Antibody ID | |
| OSR00098W | rabbit | 1:100 | AB_2220076 | |
| ASIC4 | Supplier: Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |||
| Catalog number | Source | Dilution | Antibody ID | |
| OSR00101W | rabbit | 1:100 | AB_2222665 | |
| PIEZO2 | Supplier: Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |||
| Catalog number | Source | Dilution | Antibody ID | |
| PA5-72976 | rabbit | 1:100 | AB_2718830 | |
| Secondary Antibodies | ||||
| Anti-rabbit IgG-peroxidase conjugate | Supplier: Santa Cruz Biotechnology, Inc., Dallas, TX, USA | |||
| Catalog number | Source | Dilution | Antibody ID | |
| sc-2357 | mouse | 1:100 | AB_628497 | |
| Anti-rabbit IgG (H + L) Alexa Fluor 594 | Supplier: Molecular Probes, Invitrogen, Waltham, MA, USA | |||
| Catalog number | Source | Dilution | Antibody ID | |
| A32754 | Donkey | 1:300 | AB_2762827 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhalhel, K.; Cavallaro, M.; Pansera, L.; Franco, G.A.; Montalbano, G.; Laurà, R.; Abbate, F.; Germanà, A.; Levanti, M.; Aragona, M. Ion-Channel Proteins in the Prepubertal Bitch Reproductive System: The Immunolocalization of ASIC2, ASIC4, and PIEZO2. Int. J. Mol. Sci. 2025, 26, 4388. https://doi.org/10.3390/ijms26094388
Mhalhel K, Cavallaro M, Pansera L, Franco GA, Montalbano G, Laurà R, Abbate F, Germanà A, Levanti M, Aragona M. Ion-Channel Proteins in the Prepubertal Bitch Reproductive System: The Immunolocalization of ASIC2, ASIC4, and PIEZO2. International Journal of Molecular Sciences. 2025; 26(9):4388. https://doi.org/10.3390/ijms26094388
Chicago/Turabian StyleMhalhel, Kamel, Mauro Cavallaro, Lidia Pansera, Gianluca Antonio Franco, Giuseppe Montalbano, Rosaria Laurà, Francesco Abbate, Antonino Germanà, Maria Levanti, and Marialuisa Aragona. 2025. "Ion-Channel Proteins in the Prepubertal Bitch Reproductive System: The Immunolocalization of ASIC2, ASIC4, and PIEZO2" International Journal of Molecular Sciences 26, no. 9: 4388. https://doi.org/10.3390/ijms26094388
APA StyleMhalhel, K., Cavallaro, M., Pansera, L., Franco, G. A., Montalbano, G., Laurà, R., Abbate, F., Germanà, A., Levanti, M., & Aragona, M. (2025). Ion-Channel Proteins in the Prepubertal Bitch Reproductive System: The Immunolocalization of ASIC2, ASIC4, and PIEZO2. International Journal of Molecular Sciences, 26(9), 4388. https://doi.org/10.3390/ijms26094388









