Overexpression of DWF1 Enhances Low-Nitrogen Stress Tolerance in Potato Plants
Abstract
1. Introduction
2. Results
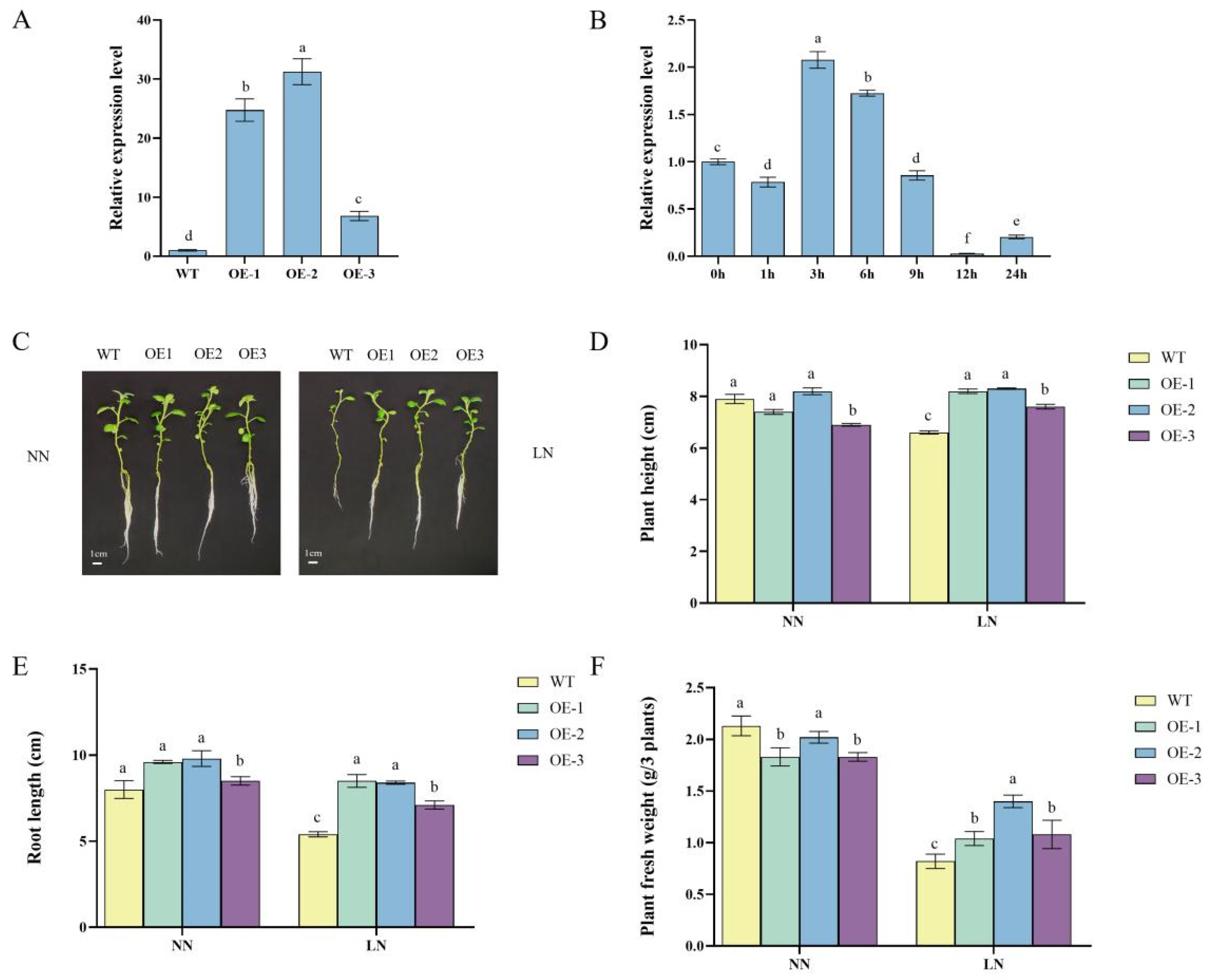
2.1. StDWF1 Expression and Phenotypic Characterization of Transgenic Lines
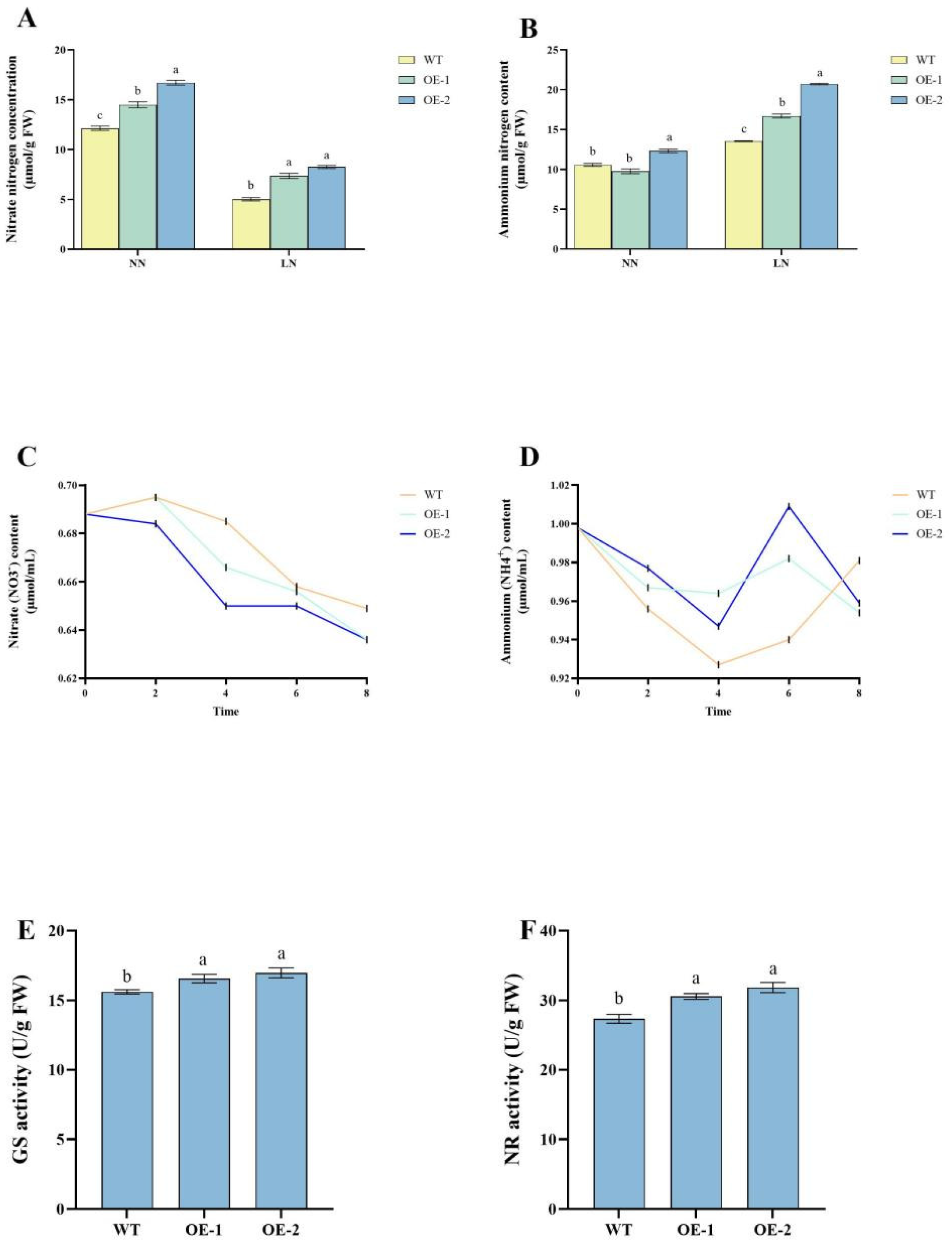
2.2. Enhanced Nitrogen Accumulation and Metabolic Efficiency in stDWF1-Overexpressing Lines
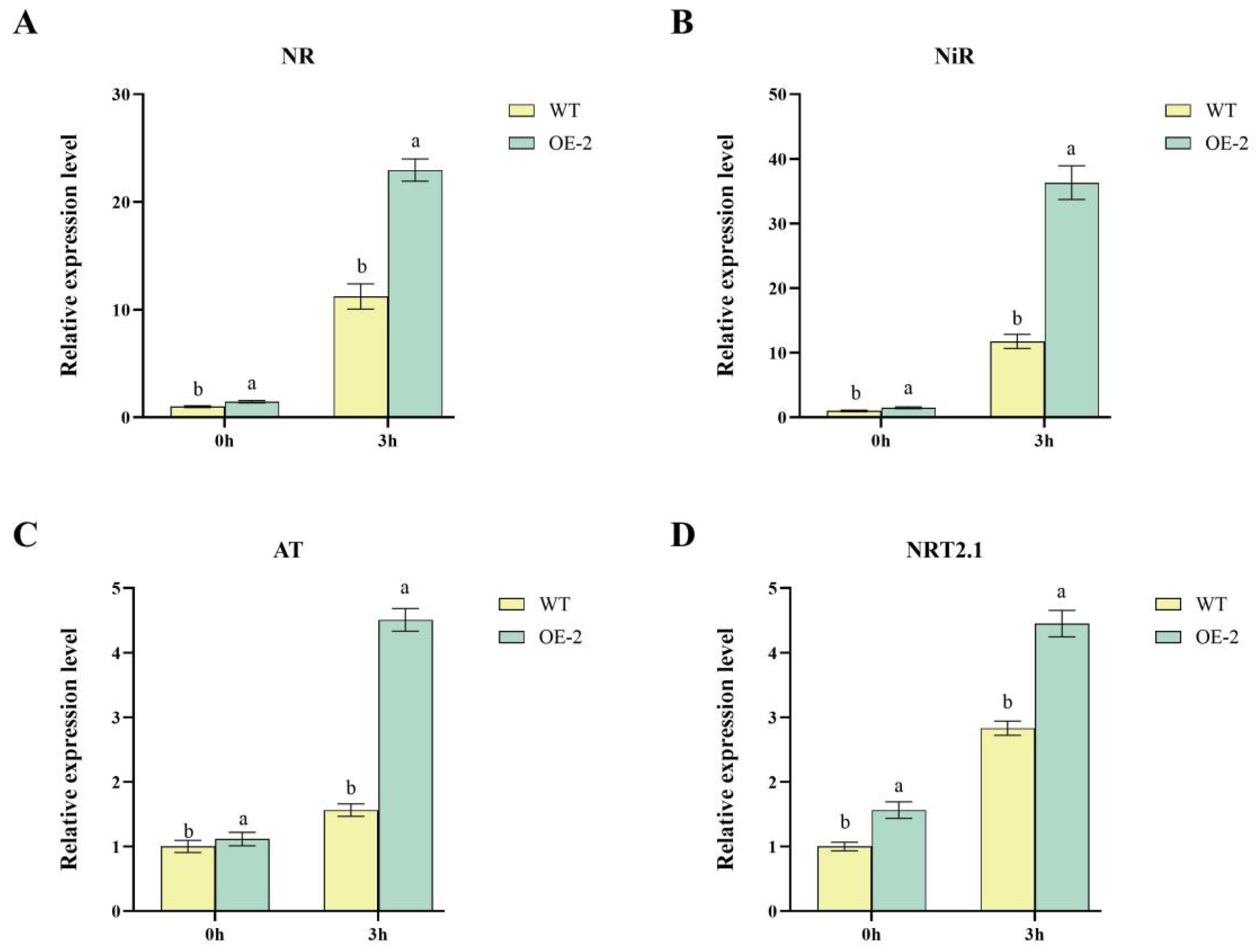
2.3. Upregulation of Nitrogen Metabolism-Related Genes in Transgenic Lines
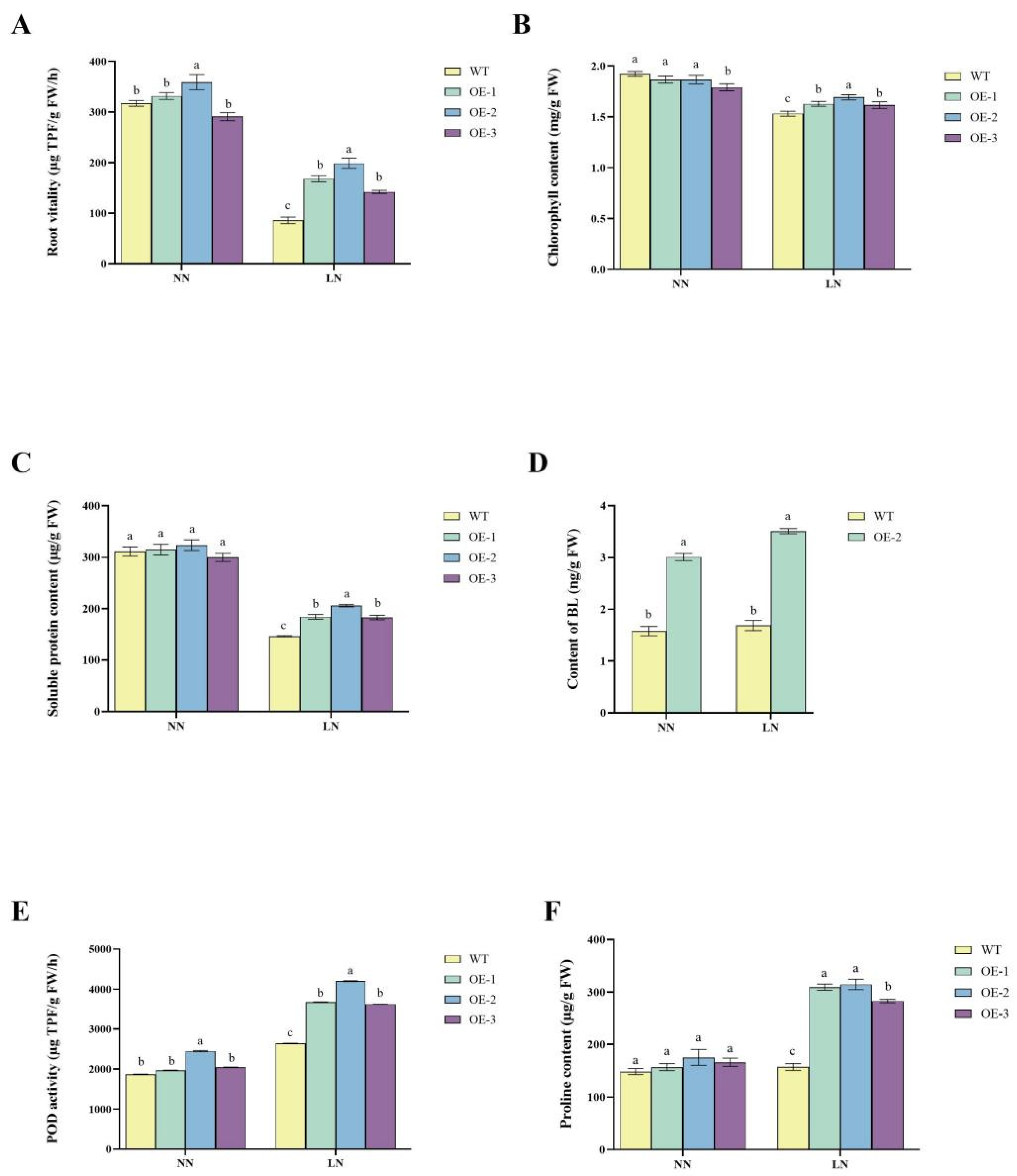
2.4. Improved Nitrogen-Use Efficiency and Stress Tolerance in Transgenic Lines
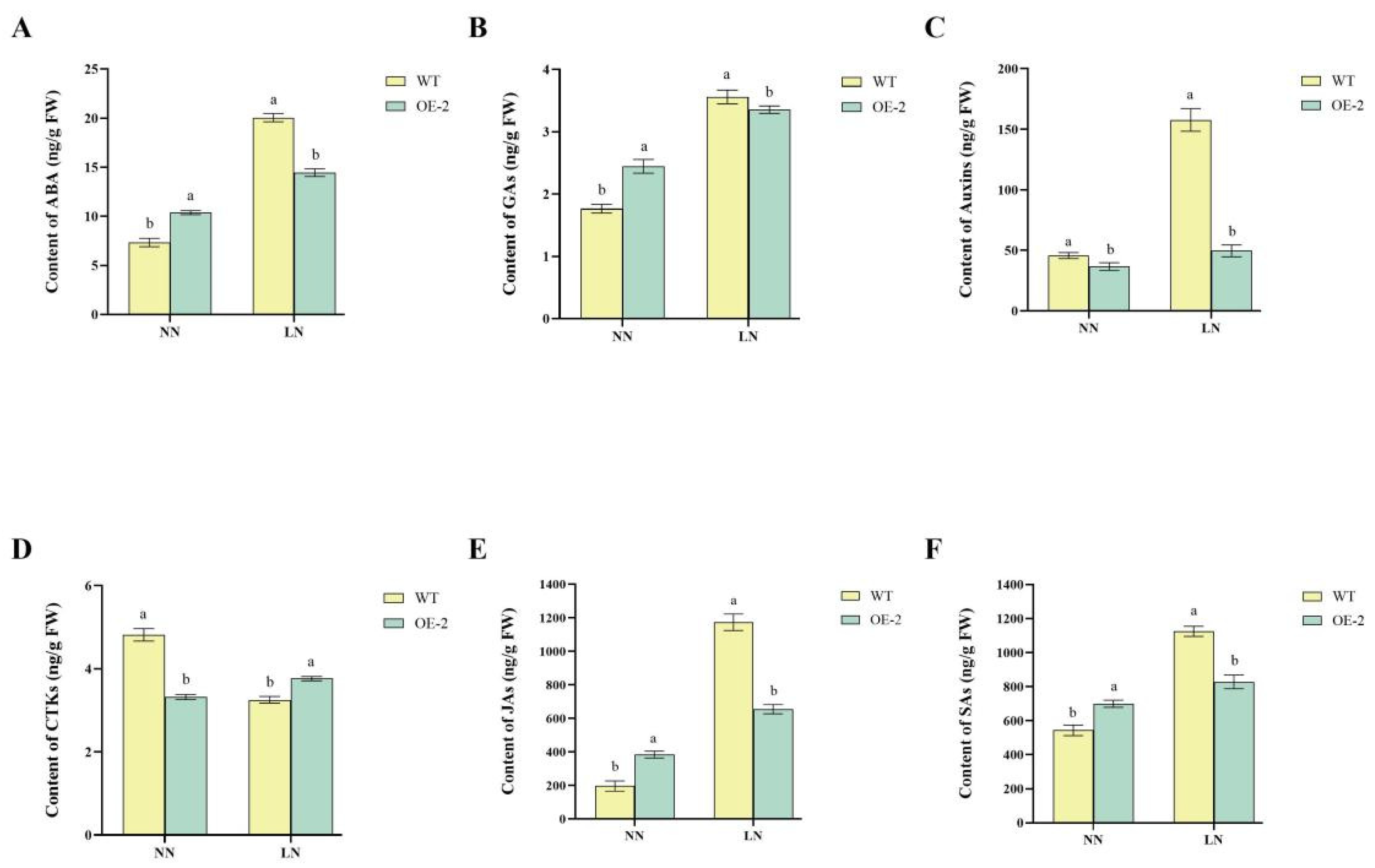
2.5. Regulation of Hormonal Pathways
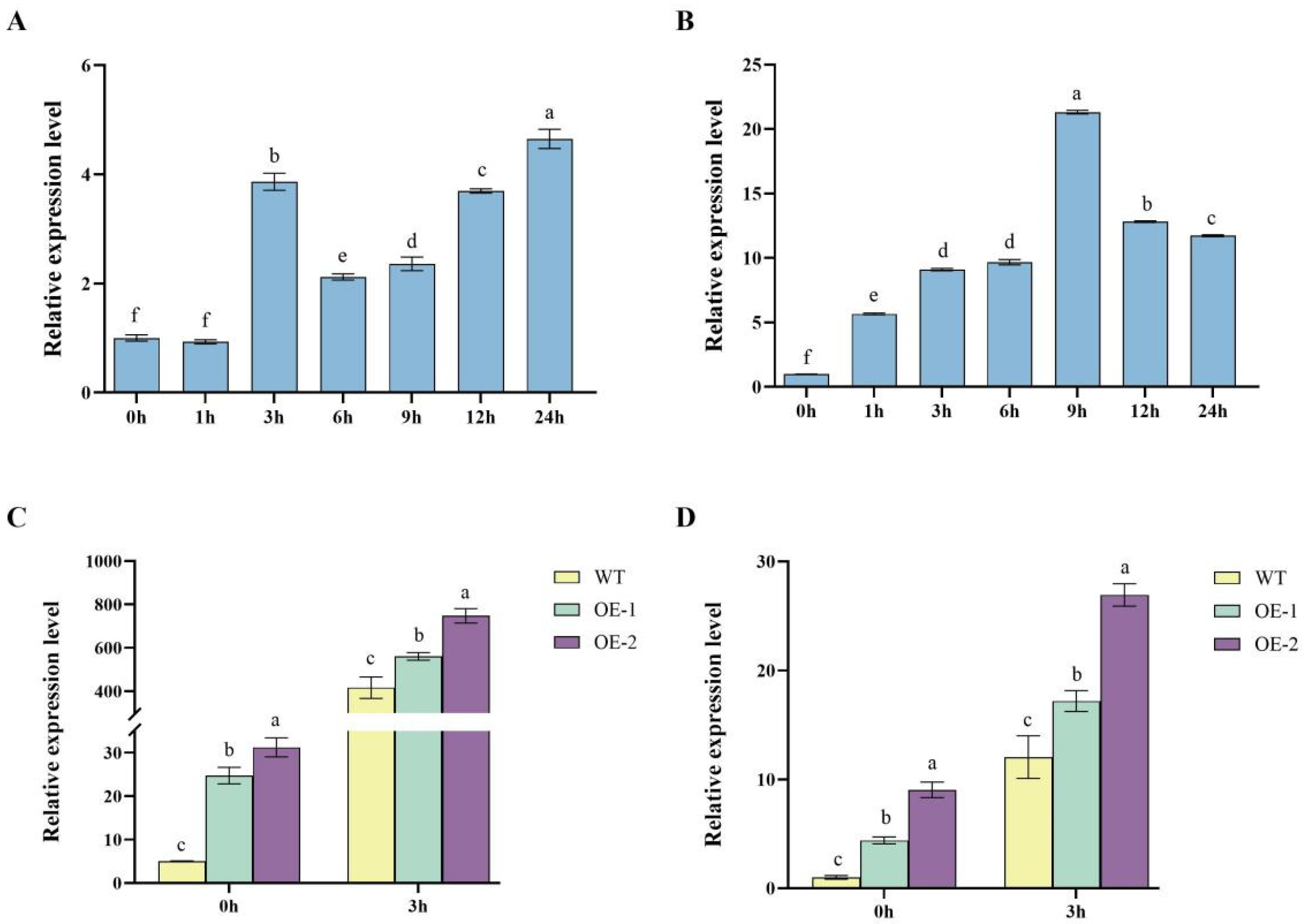
2.6. Dynamic Regulation of StDWF1, StGRP1 Expression
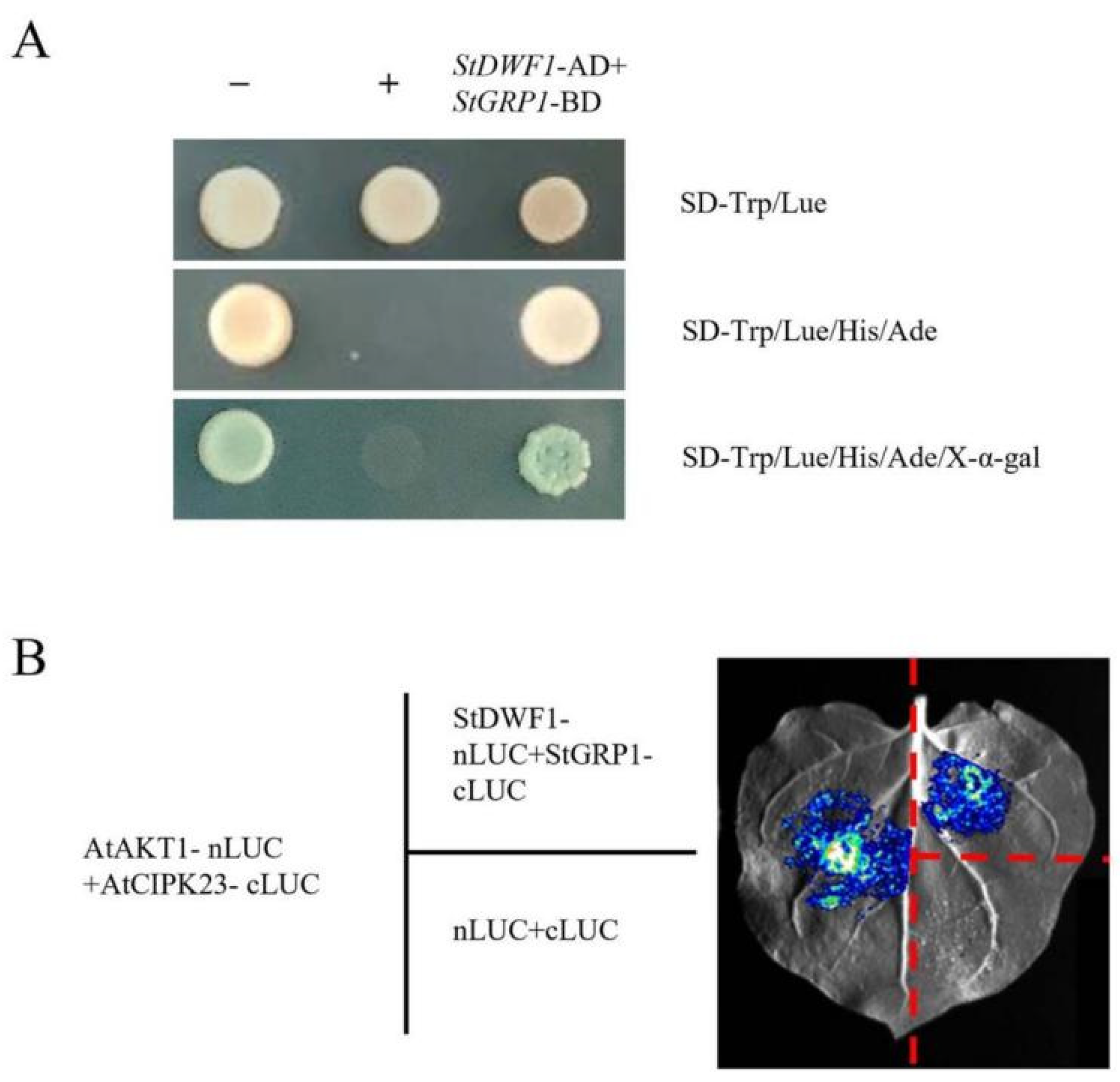
2.7. Validation of StDWF1 and StGRP Interactions Using Yeast Two-Hybrid and BiLC Assays
3. Discussion
4. Materials and Methods
4.1. Generation of StDWF1 Overexpression Transgenic Potato Lines
4.2. Hydroponic Cultivation of Seedlings and Phenotypic Observation
4.3. Nitrogen Depletion Experiment
4.4. Enzyme Activity Assay
4.5. Determination of Plant Hormones and Physiological Indicators
4.6. LUC Assay
4.7. Yeast Two-Hybrid Assay
4.8. RNA Extraction and qPCR Identification
4.9. Statistical Analysis and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khare, N.; Khare, P.; Singh, S. Molecular and Physiological Concepts: Macronutrients in Crop Plant Growth and Development. In Agricultural Crop Improvement; CRC Press: Boca Raton, FL, USA, 2025; pp. 148–164. [Google Scholar]
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: A review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Xu, M.; Cheng, Z.; Yang, L.-T. Effects of nitrogen deficiency on the photosynthesis, chlorophyll a fluorescence, antioxidant system, and sulfur compounds in Oryza sativa. Int. J. Mol. Sci. 2024, 25, 10409. [Google Scholar] [CrossRef]
- Pandey, S.N. Biomolecular functions of micronutrients toward abiotic stress tolerance in plants. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2018; pp. 153–170. [Google Scholar]
- Ohyama, T. Nitrogen as a major essential element of plants. Nitrogen Assim. Plants 2010, 37, 1–17. [Google Scholar]
- Liu, M.; Xu, X.; Wanek, W.; Sun, J.; Bardgett, R.D.; Tian, Y.; Cui, X.; Jiang, L.; Ma, Z.; Kuzyakov, Y. Nitrogen availability in soil controls uptake of different nitrogen forms by plants. New Phytol. 2024, 245, 1450–1467. [Google Scholar] [CrossRef]
- Wani, B.A.; Rashid, S.; Rashid, K.; Javid, H.; Magray, J.A.; ul Qadir, R.; Islam, T. Nitrogen Deficiency in Plants. In Advances in Plant Nitrogen Metabolism; CRC Press: Boca Raton, FL, USA, 2022; pp. 28–37. [Google Scholar]
- Barhoumi, Z. Effects of Nitrogen Deficiency on Photosynthesis and Chlorophyll a Fluorescence Attributes at Two Contrasting Legume Forages. Russ. J. Plant Physiol. 2024, 71, 1. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Sakadevan, K.; Nguyen, M.-L. Livestock production and its impact on nutrient pollution and greenhouse gas emissions. Adv. Agron. 2017, 141, 147–184. [Google Scholar]
- McArthur, J.W.; McCord, G.C. Fertilizing growth: Agricultural inputs and their effects in economic development. J. Dev. Econ. 2017, 127, 133–152. [Google Scholar] [CrossRef]
- Krouk, G. Hormones and nitrate: A two-way connection. Plant Mol. Biol. 2016, 91, 599–606. [Google Scholar] [CrossRef]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, I.; Yang, X. Insights on phytohormonal crosstalk in plant response to nitrogen stress: A focus on plant root growth and development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, L.; Han, P.; Gu, C.; Li, Y.; Liao, X.; Qin, L. Metabolic profiles reveal changes in the leaves and roots of rapeseed (Brassica napus L.) seedlings under nitrogen deficiency. Int. J. Mol. Sci. 2022, 23, 5784. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Cao, X.; Zhang, M.; Wei, X.; Zhang, J.; Wan, X. Plant nitrogen availability and crosstalk with phytohormones signallings and their biotechnology breeding application in crops. Plant Biotechnol. J. 2023, 21, 1320–1342. [Google Scholar] [CrossRef]
- Jia, Z.; von Wirén, N. Signaling pathways underlying nitrogen-dependent changes in root system architecture: From model to crop species. J. Exp. Bot. 2020, 71, 4393–4404. [Google Scholar] [CrossRef] [PubMed]
- Mussig, C.; Shin, G.-H.; Altmann, T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.; Meyer, R.C.; Altmann, T.; von Wirén, N. Natural variation of BSK3 tunes brassinosteroid signaling to regulate root foraging under low nitrogen. Nat. Commun. 2019, 10, 2378. [Google Scholar] [CrossRef]
- Yang, W.; Wan, G.-F.; Zhou, J.-Q.; Song, G.-C.; Zhao, J.; Huang, F.-L.; Meng, S. The Effects of Brassinosteroids on Nitrogen Utilization in Rice. Agronomy 2024, 14, 604. [Google Scholar] [CrossRef]
- Yadav, R.K.; Analin, B.; Panda, M.K.; Ranjan, A.; Singh, A.P. Brassinosteroids-regulated nitrogen metabolism fine-tunes growth physiology and low nitrogen response in tomato. Environ. Exp. Bot. 2023, 216, 105528. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Gao, N.; Liu, M.; Zhang, C.; Luo, J.; Sun, Y.; Feng, Y. Nitrate transporters and mechanisms of nitrate signal transduction in Arabidopsis and rice. Physiol. Plant. 2024, 176, e14486. [Google Scholar] [CrossRef]
- Deveshwar, P.; Prusty, A.; Sharma, S.; Tyagi, A.K. Phytohormone-mediated molecular mechanisms involving multiple genes and QTL govern grain number in rice. Front. Genet. 2020, 11, 586462. [Google Scholar] [CrossRef]
- Chu, W.; Chang, S.; Lin, J.; Zhang, C.; Li, J.; Liu, X.; Liu, Z.; Liu, D.; Yang, Q.; Zhao, D.; et al. Methyltransferase TaSAMT1 mediates wheat freezing tolerance by integrating brassinosteroid and salicylic acid signaling. Plant Cell 2024, 36, 2607–2628. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Deng, M.; Zou, X.; Li, L.; Zhu, Y.; Wang, X. Cloning and expression analysis of StDWF1 in Solanum tuberosum. Acta Agric. Zhejiangensis 2018, 30, 909–917. [Google Scholar]
- Choe, S.; Dilkes, B.P.; Gregory, B.D.; Ross, A.S.; Yuan, H.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tanaka, A.; Yoshida, S.; et al. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999, 119, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zheng, J.; Xu, Z.; Zhang, X.; Zhang, K.; Wang, G. Functional analysis of ZmDWF1, a maize homolog of the Arabidopsis brassinosteroids biosynthetic DWF1/DIM gene. Plant Sci. 2004, 167, 743–751. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Aziz, T.; Maqsood, M.A.; Kanwal, S.; Hussain, S.; Ahmad, H.; Sabir, M. Fertilizers and environment: Issues and challenges. In Crop Production and Global Environmental Issues; Springer: Cham, Switzerland, 2015; pp. 575–598. [Google Scholar]
- Xuan, Y.H.; Duan, F.Y.; Je, B.I.; Kim, C.M.; Li, T.Y.; Liu, J.M.; Park, S.J.; Cho, J.H.; Kim, T.H.; von Wiren, N. Related to ABI3/VP1-Like 1 (RAVL1) regulates brassinosteroid-mediated activation of AMT1; 2 in rice (Oryza sativa). J. Exp. Bot. 2017, 68, 727–737. [Google Scholar]
- Anwar, A.; Li, Y.; He, C.; Yu, X. 24-Epibrassinolide promotes NO3− and NH4+ ion flux rate and NRT1 gene expression in cucumber under suboptimal root zone temperature. BMC Plant Biol. 2019, 19, 225. [Google Scholar]
- Xing, J.; Wang, Y.; Yao, Q.; Zhang, Y.; Zhang, M.; Li, Z. Brassinosteroids modulate nitrogen physiological response and promote nitrogen uptake in maize (Zea mays L.). Crop J. 2022, 10, 166–176. [Google Scholar] [CrossRef]
- Nan, Y.Y.; Lü, T.T.; Cao, M.X.; Liu, H.Z.; Chen, Y.; Chen, Q. Cloning and expression analysis of potato nitrate transporter gene. Acta Bot. Boreali-Occident. Sin. 2019, 39, 588–594. [Google Scholar]
- Comas, L.H.; Bauerle, T.; Eissenstat, D. Biological and environmental factors controlling root dynamics and function: Effects of root ageing and soil moisture. Aust. J. Grape Wine Res. 2010, 16, 131–137. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Du, M.; Zhao, Y.; Chen, L. An Integrated Analysis of the Rice Transcriptome and Metabolome Reveals Root Growth Regulation Mechanisms in Response to Nitrogen Availability. Int. J. Mol. Sci. 2019, 20, 5893–5907. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as natural bioactive compounds existing in food by-products: A critical review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Sun, H.; Wang, W.; Liu, L.; Zhang, Y. Effects of drought on soluble protein content and protective enzyme system in cotton leaves. Front. Agric. China 2010, 4, 56–62. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zhou, L.; Pan, G.; Li, Z.; Cheng, F. Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. Biochem. 2016, 109, 248–261. [Google Scholar] [CrossRef]
- Monson, R.K.; Trowbridge, A.M.; Lindroth, R.L.; Lerdau, M.T. Coordinated resource allocation to plant growth–defense tradeoffs. New Phytol. 2022, 233, 1051–1066. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Chen, X.; Wang, X. Effects of Nitrogen Deficiency Stress on Physiological Characteristics of Festuca arundinacea at Seedling Stage. Chin. J. Grassl. 2022, 44, 9–15. [Google Scholar]
- Wang, L.; Wang, Z.; Xu, Y.; Joo, S.H.; Kim, S.K.; Xue, Z.; Xu, Z.; Wang, Z.; Chong, K. OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J. 2009, 57, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Nahirñak, V.; Rivarola, M.; Almasia, N.I.; Barón, M.P.B.; Hopp, H.E.; Vile, D.; Paniego, N.; Rovere, C.V. Snakin-1 affects reactive oxygen species and ascorbic acid levels and hormone balance in potato. PLoS ONE 2019, 14, e0214165. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khan, M.O.; Ullah, R.; Ahmad, F.; Raza, G. Agrobacterium-mediated transformation for the development of transgenic crops; present and future prospects. Mol. Biotechnol. 2024, 66, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative Study on Several Determination Methods of Chlorophyll Content in Plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Grintzalis, K.; Georgiou, C.D.; Schneider, Y.J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal. Biochem. 2015, 480, 28–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Gao, X. A New Method for Accurate Determination of Peroxidase Activity Based on Fluorescence Decrease of Guaiacol. Chin. J. Anal. Chem. 2015, 43, 1040–1046. [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for Determination of Proline in Plants. Methods Mol. Biol. 2015, 639, 317–331. [Google Scholar]
- Luo, H.; Zhang, H.; Han, H.; Hu, Y.; Zhang, Y.; Zhang, W. Effects of water storage in deeper soil layers on growth, yield, and water productivity of cotton (Gossypium hirsutum L.) in arid areas of northwestern China. Irrig. Drain 2014, 63, 59–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Z.; Lu, Y.; Ren, B.; Zeng, F.; Yang, S.; Lu, L.; Li, L. Overexpression of DWF1 Enhances Low-Nitrogen Stress Tolerance in Potato Plants. Int. J. Mol. Sci. 2025, 26, 4374. https://doi.org/10.3390/ijms26094374
Li Z, Li Z, Lu Y, Ren B, Zeng F, Yang S, Lu L, Li L. Overexpression of DWF1 Enhances Low-Nitrogen Stress Tolerance in Potato Plants. International Journal of Molecular Sciences. 2025; 26(9):4374. https://doi.org/10.3390/ijms26094374
Chicago/Turabian StyleLi, Zi, Zhuangyan Li, Yifei Lu, Bi Ren, Fuchun Zeng, Shimin Yang, Liming Lu, and Liqin Li. 2025. "Overexpression of DWF1 Enhances Low-Nitrogen Stress Tolerance in Potato Plants" International Journal of Molecular Sciences 26, no. 9: 4374. https://doi.org/10.3390/ijms26094374
APA StyleLi, Z., Li, Z., Lu, Y., Ren, B., Zeng, F., Yang, S., Lu, L., & Li, L. (2025). Overexpression of DWF1 Enhances Low-Nitrogen Stress Tolerance in Potato Plants. International Journal of Molecular Sciences, 26(9), 4374. https://doi.org/10.3390/ijms26094374



