Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes
Abstract
1. Introduction
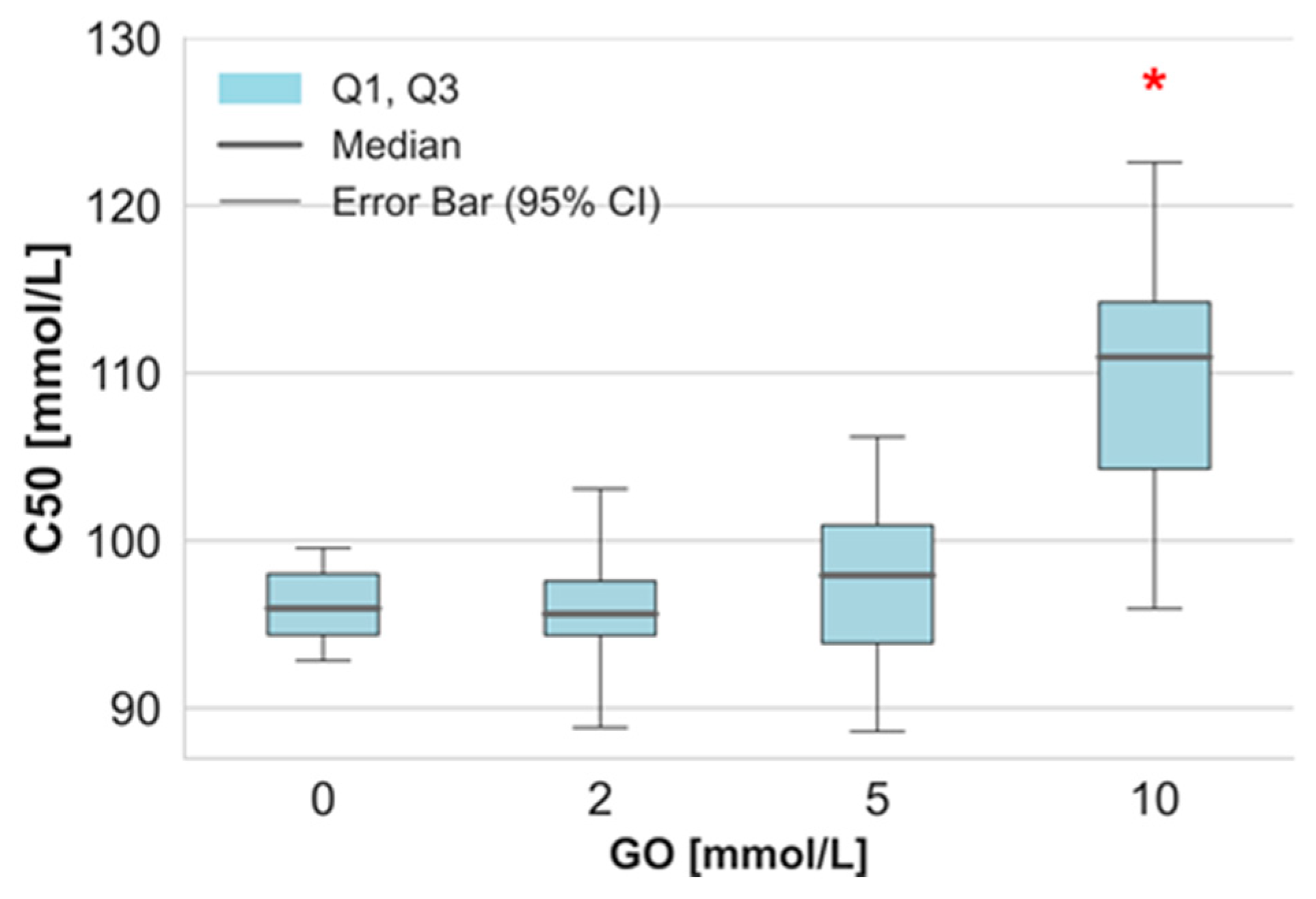
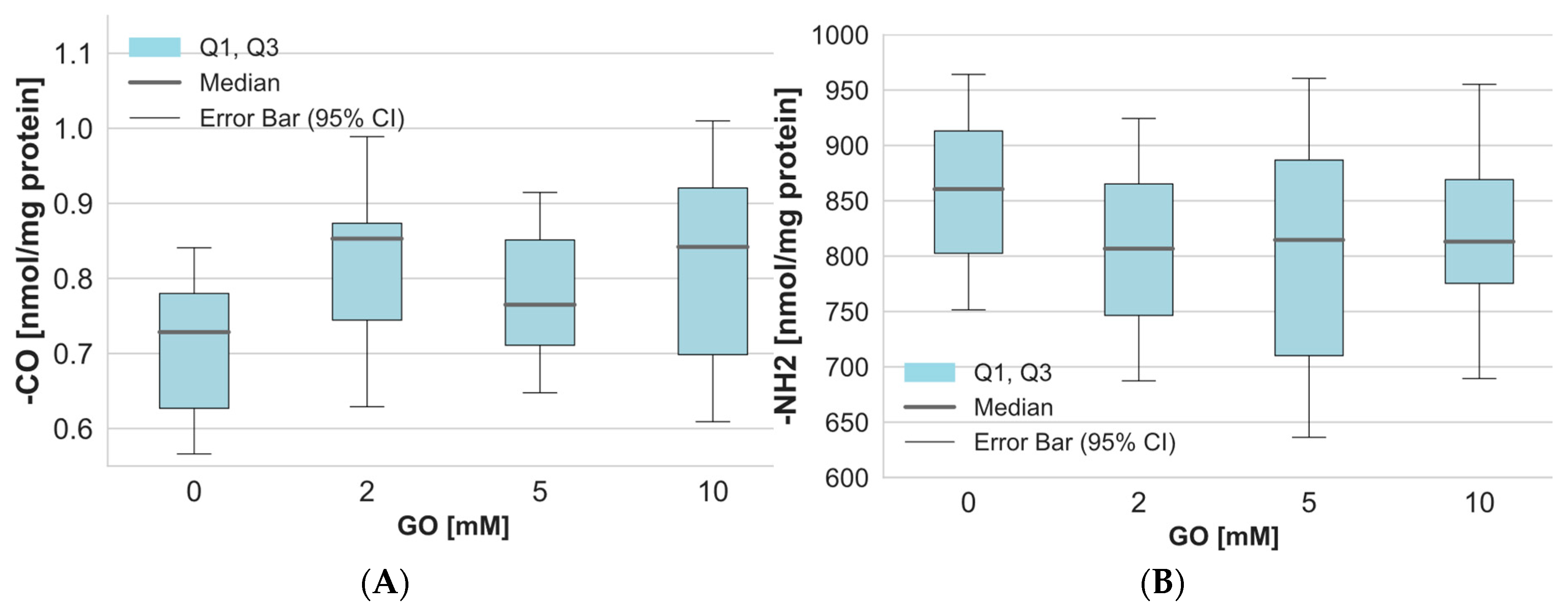
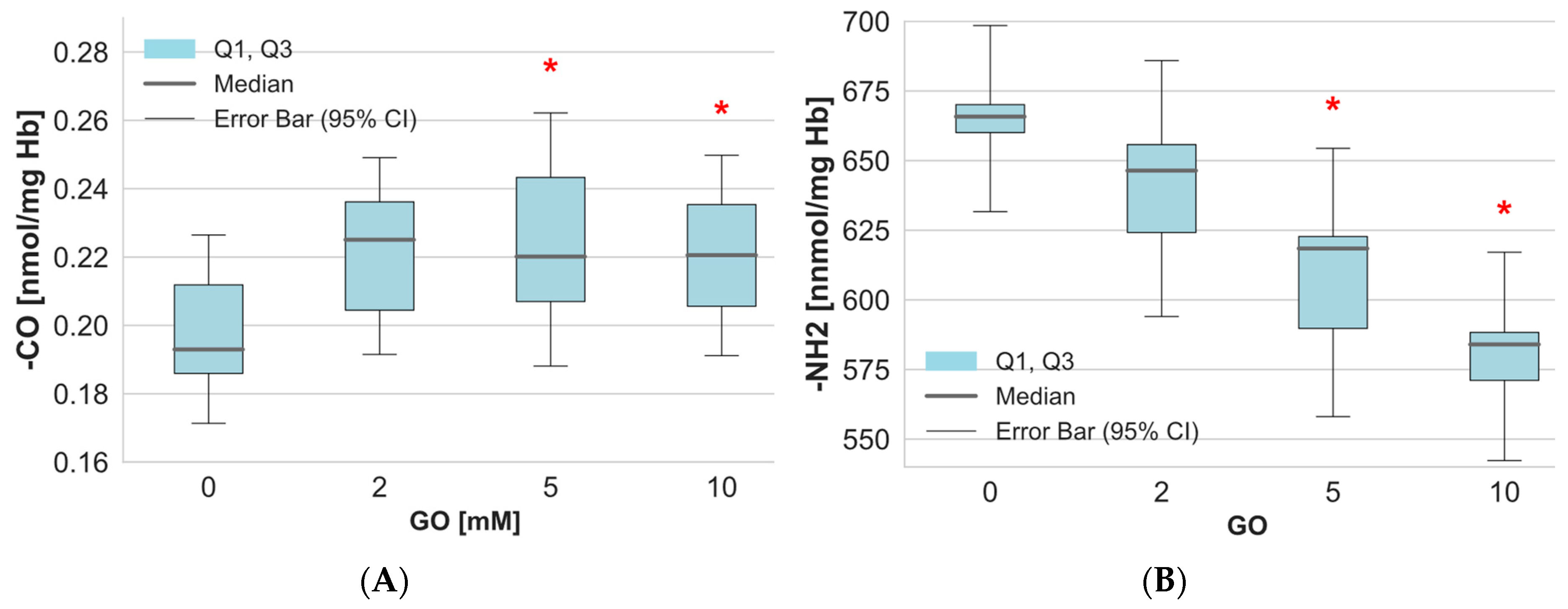
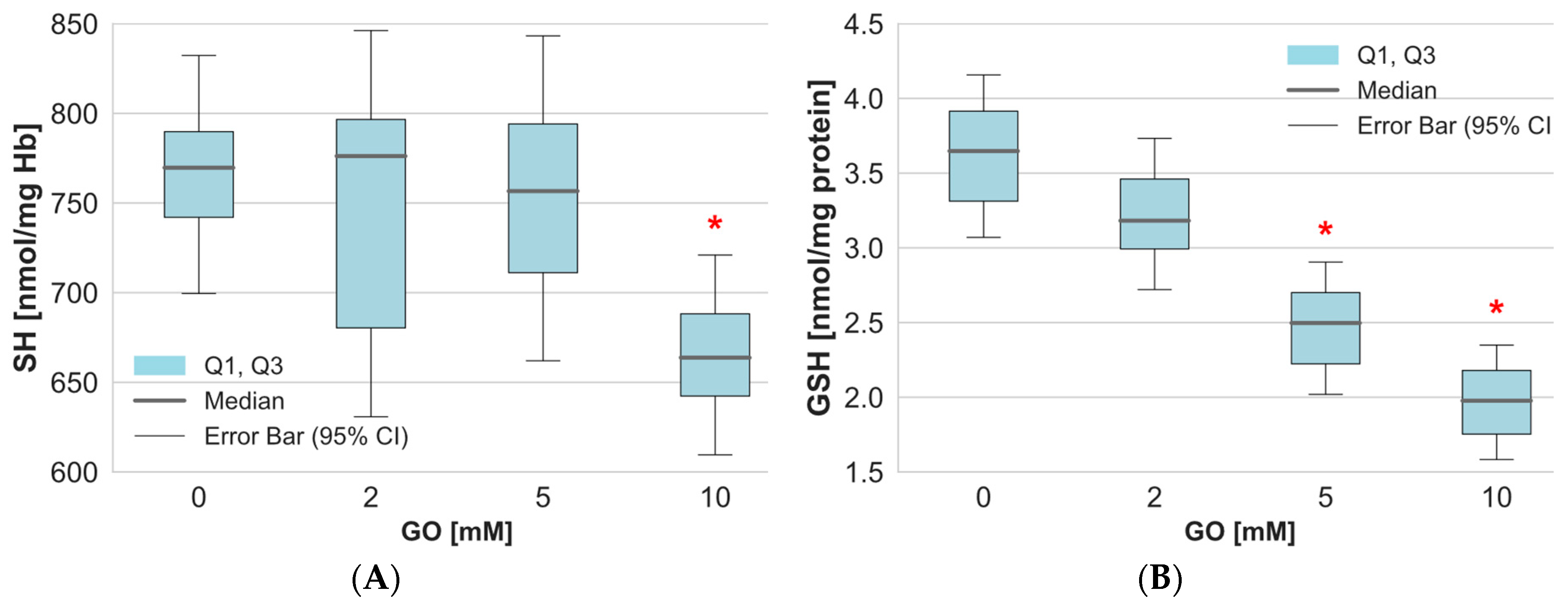
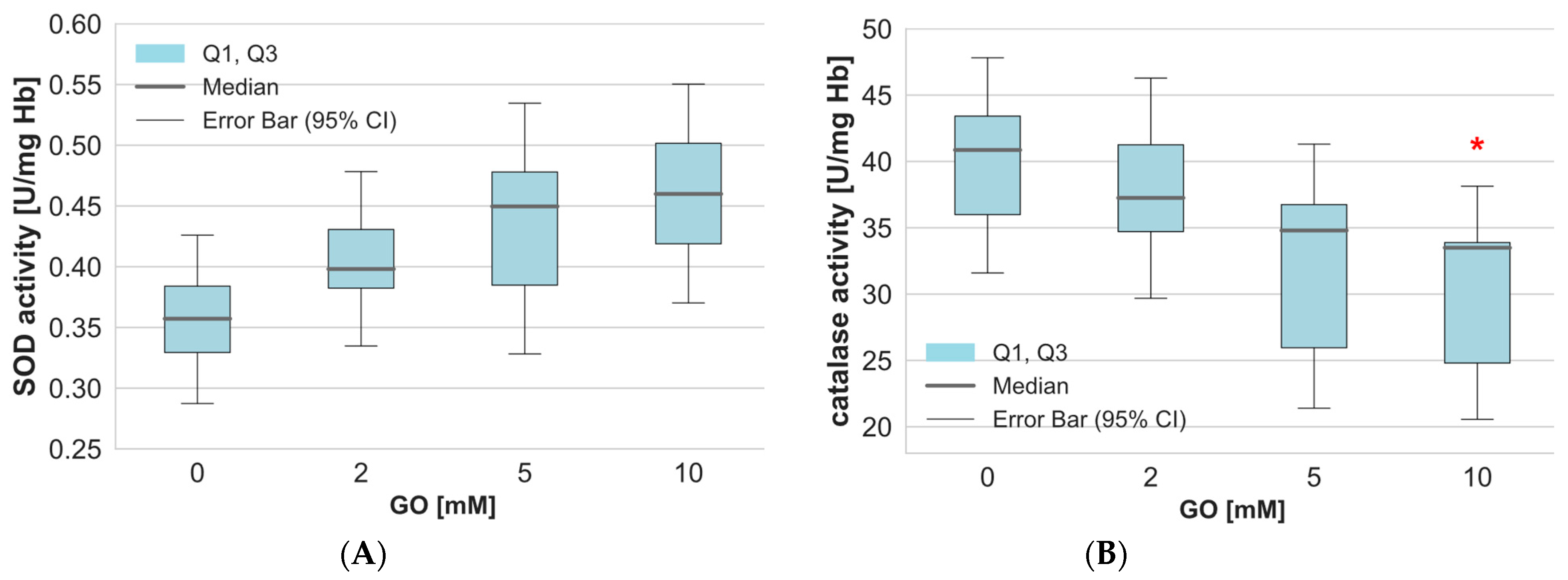
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Measurement of Osmotic Fragility
4.4. Measurement of Catalase (CAT) Activity
4.5. Measurement of Superoxide Dismutase (SOD) Activity
4.6. Determination of Thiobarbituric Acid-Reactive Substances (TBARS)
4.7. Determination of Total Non-Enzymatic Antioxidant Capacity (NEAC)
4.8. Determination of Free Thiol Group Content
4.9. Determination of Free-Amino Group Content
4.10. Measurement of Carbonyl Group Content
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end-products |
| CAT | Catalase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GO | Glyoxal |
| GSH | Glutathione |
| NEAC | Non-enzymatic antioxidant capacity |
| RBCs | Erythrocytes |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
References
- Xie, M.-Z.; Guo, C.; Dong, J.-Q.; Zhang, J.; Sun, K.-T.; Lu, G.-J.; Wang, L.; Bo, D.-Y.; Jiao, L.-Y.; Zhao, G.-A. Glyoxal damages human aortic endothelial cells by perturbing the glutathione, mitochondrial membrane potential, and mitogen-activated protein kinase pathways. BMC Cardiovasc. Disord. 2021, 21, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, C.; Ou, J.; Liu, F.; Ou, S.; Zheng, J. Glyoxal in Foods: Formation, Metabolism, Health Hazards, and Its Control Strategies. J. Agric. Food Chem. 2024, 72, 2434–2450. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015, 458, 221–226. [Google Scholar] [CrossRef]
- Murata-Kamiya, N.; Kamiya, H.; Kaji, H.; Kasai, H. Glyoxal, a major product of DNA oxidation, induces mutations at G:C sites on a shuttle vector plasmid replicated in mammalian cells. Nucleic Acids Res. 1997, 25, 1897–1902. [Google Scholar] [CrossRef]
- Wetzels, S.; Wouters, K.; Schalkwijk, C.G.; Vanmierlo, T.; Hendriks, J.J.A. Methylglyoxal-Derived Advanced Glycation Endproducts in Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 421. [Google Scholar] [CrossRef]
- Liu, G.D.; Xu, C.; Feng, L.E.; Wang, F. The augmentation of O-GlcNAcylation reduces glyoxal-induced cell injury by attenuating oxidative stress in human retinal microvascular endothelial cells. Int. J. Mol. Med. 2015, 36, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Valtink, M.; Frenzel, A.; Goetze, D.; Knels, L.; Morawietz, H.; Funk, R.H.W. Stress responses of human retinal pigment epithelial cells to glyoxal. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, J.; Xie, Y.; Mei, X.; Xu, C.; Liu, J.; Cao, X. Investigating the molecular mechanisms of glyoxal-induced cytotoxicity in human embryonic kidney cells: Insights from network toxicology and cell biology experiments. Environ. Toxicol. 2022, 37, 2269–2280. [Google Scholar] [CrossRef]
- Knutson, S.D.; Sanford, A.A.; Swenson, C.S.; Korn, M.M.; Manuel, B.A.; Heemstra, J.M. Thermoreversible Control of Nucleic Acid Structure and Function with Glyoxal Caging. J. Am. Chem. Soc. 2020, 142, 17766–17781. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem. Pharmacol. 2004, 68, 1433–1442. [Google Scholar] [CrossRef]
- Nomi, Y.; Aizawa, H.; Kurata, T.; Shindo, K.; van Nguyen, C. Glutathione reacts with glyoxal at the N-terminal. Biosci. Biotechnol. Biochem. 2009, 73, 2408–2411. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, K.; Irrgang, F.; Raju, R.; Harman, D.G.; Moran, C.; Srikanth, V.; Münch, G. Determination of glyoxal and methylglyoxal in serum by UHPLC coupled with fluorescence detection. Anal. Biochem. 2019, 573, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Odani, H.; Shinzato, T.; Matsumoto, Y.; Usami, J.; Maeda, K. Increase in three alpha,beta-dicarbonyl compound levels in human uremic plasma: Specific in vivo determination of intermediates in advanced Maillard reaction. Biochem. Biophys. Res. Commun. 1999, 256, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef]
- Han, Y.; Randell, E.; Vasdev, S.; Gill, V.; Gadag, V.; Newhook, L.A.; Grant, M.; Hagerty, D. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol. Cell. Biochem. 2007, 305, 123–131. [Google Scholar] [CrossRef]
- Sejersen, H.; Rattan, S.I.S. Dicarbonyl-induced accelerated aging in vitro in human skin fibroblasts. Biogerontology 2009, 10, 203–211. [Google Scholar] [CrossRef]
- Iwata, H.; Ukeda, H.; Maruyama, T.; Fujino, T.; Sawamura, M. Effect of carbonyl compounds on red blood cells deformability. Biochem. Biophys. Res. Commun. 2004, 321, 700–706. [Google Scholar] [CrossRef]
- Vanholder, R.; van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef]
- Sharma, G.S.; Bhattacharya, R.; Krishna, S.; Alomar, S.Y.; Alkhuriji, A.F.; Warepam, M.; Kumari, K.; Rahaman, H.; Singh, L.R. Structural and Functional Characterization of Covalently Modified Proteins Formed By a Glycating Agent, Glyoxal. ACS Omega 2021, 6, 20887–20894. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Defects in antioxidant defence enhance glyoxal toxicity in the yeast Saccharomyces cerevisiae. Ukr. Biokhim. Zh. (1999) 2013, 85, 50–60. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Bakker, W.; Sang, Y.; Rietjens, I.M.C.M. Absorption and intracellular accumulation of food-borne dicarbonyl precursors of advanced glycation end-product in a Caco-2 human cell transwell model. Food Chem. 2024, 452, 139532. [Google Scholar] [CrossRef] [PubMed]
- Gurney, T.O.; Oliver, P.J.; Sliman, S.M.; Yenigalla, A.; Eubank, T.D.; Nassal, D.M.; Miao, J.; Zhao, J.; Hund, T.J.; Parinandi, N.L. Cardiovascular Signaling in Health and Disease: Hyperglycemic Oxoaldehyde (Glyoxal)-Induced Vascular Endothelial Cell Damage Through Oxidative Stress Is Protected by Thiol Iron Chelator, Dimercaptosuccinic Acid–Role of Iron in Diabetic Vascular Endothelial Dysfunction; Springer: Cham, Switzerland, 2022; ISBN 9783031083082. [Google Scholar]
- Banerjee, S. Glyoxal-induced modification enhances stability of hemoglobin and lowers iron-mediated oxidation reactions of the heme protein: An in vitro study. Int. J. Biol. Macromol. 2018, 107, 494–501. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Carbonyl Stress in Red Blood Cells and Hemoglobin. Antioxidants 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef]
- Pieniazek, A.; Gwozdzinski, K. Changes in the conformational state of hemoglobin in hemodialysed patients with chronic renal failure. Oxid. Med. Cell. Longev. 2015, 2015, 783073. [Google Scholar] [CrossRef]
- Pieniazek, A.; Gwozdzinski, K. Carbamylation and oxidation of proteins lead to apoptotic death of lymphocytes. Chem. Biol. Interact. 2017, 270, 24–32. [Google Scholar] [CrossRef]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Konovalova, G.G.; Tikhaze, A.K.; Shumaev, K.B.; Belova Kumskova, E.M.; Grechnikova, M.A.; Viigimaa, M. Aldehyde inhibition of antioxidant enzymes in the blood of diabetic patients. J. Diabetes 2016, 8, 398–404. [Google Scholar] [CrossRef]
- Jabeen, R.; Saleemuddin, M.; Petersen, J.; Mohammad, A. Inactivation and modification of superoxide dismutase by glyoxal: Prevention by antibodies. Biochimie 2007, 89, 311–318. [Google Scholar] [CrossRef]
- Khan, M.A.; Younus, H. Superoxide Dismutase Glycation: A Contributor to Disease and Target for Prevention. Catalysts 2025, 15, 247. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Drabkin, D.L. Spectrophotometric studies. J. Biol. Chem. 1946, 164, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Tanaka, K.; Iwakiri, Y.; Tokuhiro, S.; Fukushima, S.; Takeuchi, Y. Protective effects of some neutral amino acids against hypotonic hemolysis. Biol. Pharm. Bull. 1995, 18, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972, 247, 6960–6962. [Google Scholar] [CrossRef]
- Rice-Evans, C. Techniques in Free Radical Research; Elsevier: Amsterdam, The Netherlands, 1991; ISBN 0444813144. [Google Scholar]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Egwim, I.O.; Gruber, H.J. Spectrophotometric measurement of mercaptans with 4,4′-dithiodipyridine. Anal. Biochem. 2001, 288, 188–194. [Google Scholar] [CrossRef]
- Crowell, E.A.; Ough, C.S.; Bakalinsky, A. Determination of Alpha Amino Nitrogen in Musts and Wines by TNBS Method. Am. J. Enol. Vitic. 1985, 36, 175–177. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopera, M.; Adamkiewicz, M.; Pieniazek, A. Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes. Int. J. Mol. Sci. 2025, 26, 4328. https://doi.org/10.3390/ijms26094328
Kopera M, Adamkiewicz M, Pieniazek A. Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes. International Journal of Molecular Sciences. 2025; 26(9):4328. https://doi.org/10.3390/ijms26094328
Chicago/Turabian StyleKopera, Michal, Malgorzata Adamkiewicz, and Anna Pieniazek. 2025. "Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes" International Journal of Molecular Sciences 26, no. 9: 4328. https://doi.org/10.3390/ijms26094328
APA StyleKopera, M., Adamkiewicz, M., & Pieniazek, A. (2025). Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes. International Journal of Molecular Sciences, 26(9), 4328. https://doi.org/10.3390/ijms26094328





