Wisent Somatic Cells Resist Reprogramming by the PiggyBac Transposon System: A Case Study Highlighting Methodological and Conservation Hurdles
Abstract
1. Introduction
2. Results
2.1. Reprogramming Factors Delivery to Wisent Somatic Cells
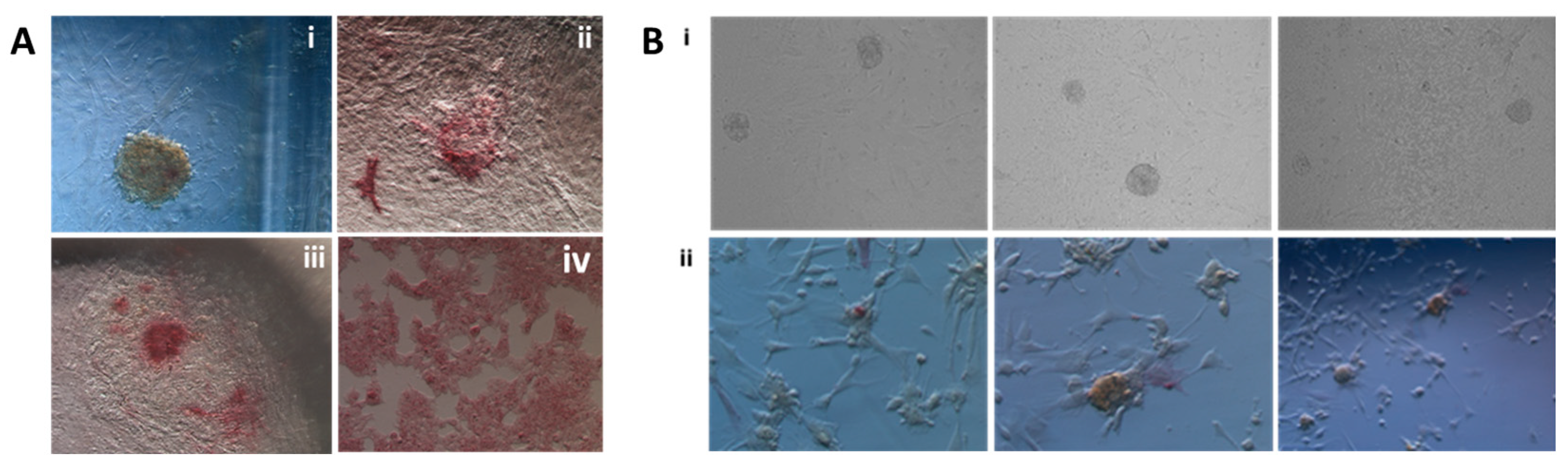
2.2. iPSC Derivation in Established Conditions
2.3. Determination of Permissible Culture Conditions for iPSCs Derivation
3. Discussion
4. Materials and Methods
4.1. Cell Source
4.1.1. Wisent Adult Fibroblasts (Bison bonasus Adult Fibroblasts—BAF)
4.1.2. Wisent Adult Ovarian Granulosa Cells (Bison bonasus Adult Ovarian Granulosa Cells—BOGCs) and Ovarian Fibroblasts (Bison bonasus Adult Ovarian Fibroblasts—BOFs)
4.2. Cells and Culture Conditions
4.3. Lipofection
4.4. Alkaline Phosphatase (AP) Staining
4.5. Immunostaining
4.6. PCR
4.7. Culture Conditions for iPSCs Derivation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6TFs | Six transcription factors |
| AA | Ascorbic acid |
| AP | Alkaline phosphatase |
| BAFs | Bison bonasus adult fibroblasts |
| BOFs | Bison bonasus Ovarian Fibroblasts |
| BOGCs | Bison bonasus Ovarian Granulosa Cells |
| COCs | Cumulus-oocyte complexes |
| ESCs | Embryonic stem cells |
| FVBS | Fetal bovine serum |
| iPSCs | Induced pluripotent stem cells |
| MEF | Mouse embryonic fibroblasts |
| VPA | Valproic acid |
References
- Saragusty, J.; Ajmone-Marsan, P.; Sampino, S.; Modlinski, J.A. Reproductive Biotechnology and Critically Endangered Species: Merging in Vitro Gametogenesis with Inner Cell Mass Transfer. Theriogenology 2020, 155, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.M.; Appeltant, R.; Burdon, T.; Bao, Q.; Bargaje, R.; Bodnar, A.; Chambers, S.; Comizzoli, P.; Cook, L.; Endo, Y.; et al. Advancing Stem Cell Technologies for Conservation of Wildlife Biodiversity. Development 2024, 151, dev203116. [Google Scholar] [CrossRef]
- Krasińska, M.; Krasiński, Z.A.; Perzanowski, K.; Olech, W. European Bison Bison bonasus (Linnaeus, 1758). In Ecology, Evolution and Behaviour of Wild Cattle; Melletti, M., Burton, J., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 115–173. ISBN 978-1-139-56809-8. [Google Scholar]
- Duszewska, A.M.; Baraniewicz-Kołek, M.; Wojdan, J.; Barłowska, K.; Bielecki, W.; Gręda, P.; Niżański, W.; Olech, W. Establishment of a Wisent (Bison bonasus) Germplasm Bank. Animals 2022, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.; Prochowska, S.; Duszewska, A.M.; Van Soom, A.; Olech, W.; Niżański, W. The Influence of Percoll® Density Gradient Centrifugation before Cryopreservation on the Quality of Frozen Wisent (Bison bonasus) Epididymal Spermatozoa. BMC Vet. Res. 2022, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Ogorevc, J.; Orehek, S.; Dovč, P. Cellular Reprogramming in Farm Animals: An Overview of iPSC Generation in the Mammalian Farm Animal Species. J. Anim. Sci. Biotechnol. 2016, 7, 10. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, J.; Salman, S.; Tang, Y. Induced Pluripotent Stem Cells from Farm Animals. J. Anim. Sci. 2020, 98, skaa343. [Google Scholar] [CrossRef]
- Ben-Nun, I.F.; Montague, S.C.; Houck, M.L.; Tran, H.T.; Garitaonandia, I.; Leonardo, T.R.; Wang, Y.-C.; Charter, S.J.; Laurent, L.C.; Ryder, O.A.; et al. Induced Pluripotent Stem Cells from Highly Endangered Species. Nat. Methods 2011, 8, 829–831. [Google Scholar] [CrossRef]
- Ben-Nun, I.F.; Montague, S.C.; Houck, M.L.; Ryder, O.; Loring, J.F. Generation of Induced Pluripotent Stem Cells from Mammalian Endangered Species. In Cell Reprogramming; Verma, P.J., Sumer, H., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2015; Volume 1330, pp. 101–109. ISBN 978-1-4939-2847-7. [Google Scholar]
- Verma, R.; Holland, M.K.; Temple-Smith, P.; Verma, P.J. Inducing Pluripotency in Somatic Cells from the Snow Leopard (Panthera uncia), an Endangered Felid. Theriogenology 2012, 77, 220–228.e2. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zou, G.; An, J.; Li, Y.; Lin, D.; Wang, D.; Li, Y.; Chen, J.; Feng, T.; et al. Generation and Characterization of Giant Panda Induced Pluripotent Stem Cells. Sci. Adv. 2024, 10, eadn7724. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Han, J.; Ding, F.; Cao, S.; Lim, S.S.; Dai, Y.; Zhang, R.; Zhang, Y.; Lim, B.; Li, N. Generation of Induced Pluripotent Stem Cells from Bovine Embryonic Fibroblast Cells. Cell Res. 2011, 21, 1509–1512. [Google Scholar] [CrossRef]
- Talluri, T.R.; Kumar, D.; Glage, S.; Garrels, W.; Ivics, Z.; Debowski, K.; Behr, R.; Niemann, H.; Kues, W.A. Derivation and Characterization of Bovine Induced Pluripotent Stem Cells by Transposon-Mediated Reprogramming. Cell. Reprogram. 2015, 17, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Tsukiyama, T.; Kimura, K.; Matsuyama, S.; Minami, N.; Yamada, M.; Imai, H. Generation of Naïve Bovine Induced Pluripotent Stem Cells Using PiggyBac Transposition of Doxycycline-Inducible Transcription Factors. PLoS ONE 2015, 10, e0135403. [Google Scholar] [CrossRef]
- Talluri, T.R.; Kumar, D.; Glage, S.; Garrels, W.; Ivics, Z.; Debowski, K.; Behr, R.; Kues, W.A. Non-Viral Reprogramming of Fibroblasts into Induced Pluripotent Stem Cells by Sleeping Beauty and piggyBac Transposons. Biochem. Biophys. Res. Commun. 2014, 450, 581–587. [Google Scholar] [CrossRef]
- Debowski, K.; Warthemann, R.; Lentes, J.; Salinas-Riester, G.; Dressel, R.; Langenstroth, D.; Gromoll, J.; Sasaki, E.; Behr, R. Non-Viral Generation of Marmoset Monkey iPS Cells by a Six-Factor-in-One-Vector Approach. PLoS ONE 2015, 10, e0118424. [Google Scholar] [CrossRef]
- Rodriguez-Polo, I.; Stauske, M.; Becker, A.; Bartels, I.; Dressel, R.; Behr, R. Baboon Induced Pluripotent Stem Cell Generation by piggyBac Transposition of Reprogramming Factors. Primate Biol. 2019, 6, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.T.; Silva, R.L.O.; Cantanhêde, L.F.; Ferreira-Silva, J.C.; Nascimento, P.S.; Benko-Iseppon, A.M.; Oliveira, M.A.L. Evolutionary-Driven C-MYC Gene Expression in Mammalian Fibroblasts. Sci. Rep. 2020, 10, 11056. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like Factor 4 (KLF4): What We Currently Know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; Melton, D.A. Induction of Pluripotent Stem Cells from Primary Human Fibroblasts with Only Oct4 and Sox2. Nat. Biotechnol. 2008, 26, 1269–1275. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, X.; Yu, D.; Li, T.; Cui, J.; Wang, G.; Hu, J.-F.; Li, W. Histone Deacetylase Inhibitor Valproic Acid Promotes the Induction of Pluripotency in Mouse Fibroblasts by Suppressing Reprogramming-Induced Senescence Stress. Exp. Cell Res. 2015, 337, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-L.; Brena, R.M.; Kolle, G.; Grimmond, S.M.; Berman, B.P.; Laird, P.W.; Pera, M.F.; Wolvetang, E.J. Vitamin C Promotes Widespread Yet Specific DNA Demethylation of the Epigenome in Human Embryonic Stem Cells. Stem Cells 2010, 28, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Wang, T.; Qin, B.; Yang, J.; Qin, D.; Cai, J.; Li, W.; Weng, Z.; Chen, J.; Ni, S.; et al. Vitamin C Enhances the Generation of Mouse and Human Induced Pluripotent Stem Cells. Cell Stem Cell 2010, 6, 71–79. [Google Scholar] [CrossRef]
- Rodin, S.; Antonsson, L.; Niaudet, C.; Simonson, O.E.; Salmela, E.; Hansson, E.M.; Domogatskaya, A.; Xiao, Z.; Damdimopoulou, P.; Sheikhi, M.; et al. Clonal Culturing of Human Embryonic Stem Cells on Laminin-521/E-Cadherin Matrix in Defined and Xeno-Free Environment. Nat. Commun. 2014, 5, 3195. [Google Scholar] [CrossRef]
- Mesquita, F.C.P.; Leite, E.S.; Morrissey, J.; Freitas, C.; Coelho-Sampaio, T.; Hochman-Mendez, C. Polymerized Laminin-521: A Feasible Substrate for Expanding Induced Pluripotent Stem Cells at a Low Protein Concentration. Cells 2022, 11, 3955. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.; Kei, T.G.; Reddy, S.E.; Das, M.; Abratte, C.; Cheong, S.H.; Selvaraj, V. Induced Pluripotent Stem Cell Generation from Bovine Somatic Cells Indicates Unmet Needs for Pluripotency Sustenance. Anim. Sci. J. 2019, 90, 1149–1160. [Google Scholar] [CrossRef]
- Pillai, V.V.; Koganti, P.P.; Kei, T.G.; Gurung, S.; Butler, W.R.; Selvaraj, V. Efficient Induction and Sustenance of Pluripotent Stem Cells from Bovine Somatic Cells. Biol. Open 2021, 10, bio058756. [Google Scholar] [CrossRef]
- Su, Y.; Wang, L.; Fan, Z.; Liu, Y.; Zhu, J.; Kaback, D.; Oudiz, J.; Patrick, T.; Yee, S.P.; Tian, X.C.; et al. Establishment of Bovine-Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2021, 22, 10489. [Google Scholar] [CrossRef]
- Kumar, D.; Talluri, T.R.; Anand, T.; Kues, W.A. Transposon-Based Reprogramming to Induced Pluripotency. Histol. Histopathol. 2015, 30, 1397–1409. [Google Scholar] [CrossRef]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. piggyBac Transposition Reprograms Fibroblasts to Induced Pluripotent Stem Cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takahashi, K.; Okita, K.; Ichisaka, T.; Yamanaka, S. Hypoxia Enhances the Generation of Induced Pluripotent Stem Cells. Cell Stem Cell 2009, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Zhou, W.; Xing, Y.; Sperber, H.; Ferreccio, A.; Agoston, Z.; Kuppusamy, K.T.; Moon, R.T.; Ruohola-Baker, H. Hypoxia-Inducible Factors Have Distinct and Stage-Specific Roles during Reprogramming of Human Cells to Pluripotency. Cell Stem Cell 2014, 14, 592–605. [Google Scholar] [CrossRef]
- Nakamura, N.; Shi, X.; Darabi, R.; Li, Y. Hypoxia in Cell Reprogramming and the Epigenetic Regulations. Front. Cell Dev. Biol. 2021, 9, 609984. [Google Scholar] [CrossRef] [PubMed]
- Canizo, J.R.; Vazquez Echegaray, C.; Klisch, D.; Aller, J.F.; Paz, D.A.; Alberio, R.H.; Alberio, R.; Guberman, A.S. Exogenous Human OKSM Factors Maintain Pluripotency Gene Expression of Bovine and Porcine iPS-like Cells Obtained with STEMCCA Delivery System. BMC Res. Notes 2018, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Botigelli, R.C.; Pieri, N.C.G.; Bessi, B.W.; Machado, L.S.; Bridi, A.; De Souza, A.F.; Recchia, K.; Neto, P.F.; Ross, P.J.; Bressan, F.F.; et al. Acquisition and Maintenance of Pluripotency Are Influenced by Fibroblast Growth Factor, Leukemia Inhibitory Factor, and 2i in Bovine-Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2022, 10, 938709. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Jiang, Y.; An, X.-L.; Yu, H.; Cai, N.-N.; Zhai, Y.-H.; Li, Q.; Cheng, H.; Zhang, S.; Tang, B.; Li, Z.-Y.; et al. Transcriptome Profile of Bovine iPSCs Derived from Sertoli Cells. Theriogenology 2020, 146, 120–132. [Google Scholar] [CrossRef]
- Bar-Nur, O.; Russ, H.A.; Efrat, S.; Benvenisty, N. Epigenetic Memory and Preferential Lineage-Specific Differentiation in Induced Pluripotent Stem Cells Derived from Human Pancreatic Islet Beta Cells. Cell Stem Cell 2011, 9, 17–23. [Google Scholar] [CrossRef]
- Rouhani, F.J.; Zou, X.; Danecek, P.; Badja, C.; Amarante, T.D.; Koh, G.; Wu, Q.; Memari, Y.; Durbin, R.; Martincorena, I.; et al. Substantial Somatic Genomic Variation and Selection for BCOR Mutations in Human Induced Pluripotent Stem Cells. Nat. Genet. 2022, 54, 1406–1416. [Google Scholar] [CrossRef]
- Olech, W.; Wojciechowska, M.; Kloch, M.; Perlińska-Teresiak, M.; Nowak-Życzyńska, Z. Genetic Diversity of Wisent Bison bonasus Based on STR Loci Analyzed in a Large Set of Samples. Diversity 2023, 15, 399. [Google Scholar] [CrossRef]
- Chen, C.; Gao, Y.; Liu, W.; Gao, S. Epigenetic Regulation of Cell Fate Transition: Learning from Early Embryo Development and Somatic Cell Reprogramming. Biol. Reprod. 2022, 107, 183–195. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, D.D.; You, J.S.; Jones, P.A. DNA Methylation and Cellular Reprogramming. Trends Cell Biol. 2010, 20, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Liu, J.; Qi, J.; Wei, B.; Yang, J.; Liang, H.; Chen, Y.; Chen, J.; Wu, Y.; et al. H3K9 Methylation Is a Barrier during Somatic Cell Reprogramming into iPSCs. Nat. Genet. 2013, 45, 34–42. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Wu, J.; Vilarino, M.; Okamura, D.; Soto, D.A.; Zhong, C.; Sakurai, M.; Sampaio, R.V.; Suzuki, K.; Izpisua Belmonte, J.C.; et al. Efficient Derivation of Stable Primed Pluripotent Embryonic Stem Cells from Bovine Blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2090–2095. [Google Scholar] [CrossRef]
- Soto, D.A.; Navarro, M.; Zheng, C.; Halstead, M.M.; Zhou, C.; Guiltinan, C.; Wu, J.; Ross, P.J. Simplification of Culture Conditions and Feeder-Free Expansion of Bovine Embryonic Stem Cells. Sci. Rep. 2021, 11, 11045. [Google Scholar] [CrossRef]
- Loi, P.; Modlinski, J.A.; Ptak, G. Interspecies Somatic Cell Nuclear Transfer: A Salvage Tool Seeking First Aid. Theriogenology 2011, 76, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Madeja, Z.E.; Hryniewicz, K.; Orsztynowicz, M.; Pawlak, P.; Perkowska, A. WNT/β-Catenin Signaling Affects Cell Lineage and Pluripotency-Specific Gene Expression in Bovine Blastocysts: Prospects for Bovine Embryonic Stem Cell Derivation. Stem Cells Dev. 2015, 24, 2437–2454. [Google Scholar] [CrossRef]
- Eckert, J. mRNA Expression of Leukaemia Inhibitory Factor (LIF) and Its Receptor Subunits Glycoprotein 130 and LIF-Receptor-Beta in Bovine Embryos Derived in Vitro or in Vivo. Mol. Hum. Reprod. 1998, 4, 957–965. [Google Scholar] [CrossRef]


| Gene/Target | Forward Primer | Reverse Primer | Product Size (bp) | Annealing Temperature | Reference |
|---|---|---|---|---|---|
| POU5F1 (OCT4) | GTTCTCTTTGGAAAGGTGTTC | ACACTCGGACCACGTCTTTC | 313 | 56 °C | [15] |
| NANOG | AAACAACTGGCCGAGGAATA | AGGAGTGGTTGCTCCAAGAC | 194 | 56 °C | [49] |
| SOX2 | CACAACTCGGAGATCAGCAA | CATGAGCGTCTTGGTTTTCC | 162 | 56 °C | [49] |
| c-MYC | TGGACGCTAGATTTCCTTCG | GCTGCTGCTGGTGGTAGAAG | 155 | 56 °C | [49] |
| KLF4 | GCCCCTAGAGGCCCACTT | CACAACATCCCAGTCACAG | 433 | 56 °C | [15] |
| polyA | GTTTCCTCGGTGGTGTTTCCTGGGCTATGC | TGGAGTTCTGTTGTGGGTATGCTGGTGTAA | 252 | 56 °C | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętek, M.M.; Bihorac, A.; Wenta-Muchalska, E.; Duszewska, A.M.; Olech, W.; Sampino, S.; Bernat, A. Wisent Somatic Cells Resist Reprogramming by the PiggyBac Transposon System: A Case Study Highlighting Methodological and Conservation Hurdles. Int. J. Mol. Sci. 2025, 26, 4327. https://doi.org/10.3390/ijms26094327
Ziętek MM, Bihorac A, Wenta-Muchalska E, Duszewska AM, Olech W, Sampino S, Bernat A. Wisent Somatic Cells Resist Reprogramming by the PiggyBac Transposon System: A Case Study Highlighting Methodological and Conservation Hurdles. International Journal of Molecular Sciences. 2025; 26(9):4327. https://doi.org/10.3390/ijms26094327
Chicago/Turabian StyleZiętek, Marta Marlena, Ajna Bihorac, Elżbieta Wenta-Muchalska, Anna Maria Duszewska, Wanda Olech, Silvestre Sampino, and Agnieszka Bernat. 2025. "Wisent Somatic Cells Resist Reprogramming by the PiggyBac Transposon System: A Case Study Highlighting Methodological and Conservation Hurdles" International Journal of Molecular Sciences 26, no. 9: 4327. https://doi.org/10.3390/ijms26094327
APA StyleZiętek, M. M., Bihorac, A., Wenta-Muchalska, E., Duszewska, A. M., Olech, W., Sampino, S., & Bernat, A. (2025). Wisent Somatic Cells Resist Reprogramming by the PiggyBac Transposon System: A Case Study Highlighting Methodological and Conservation Hurdles. International Journal of Molecular Sciences, 26(9), 4327. https://doi.org/10.3390/ijms26094327








