Assessment of Plasma and Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease and Other Dementias: A Center-Based Study
Abstract
1. Introduction
2. Results
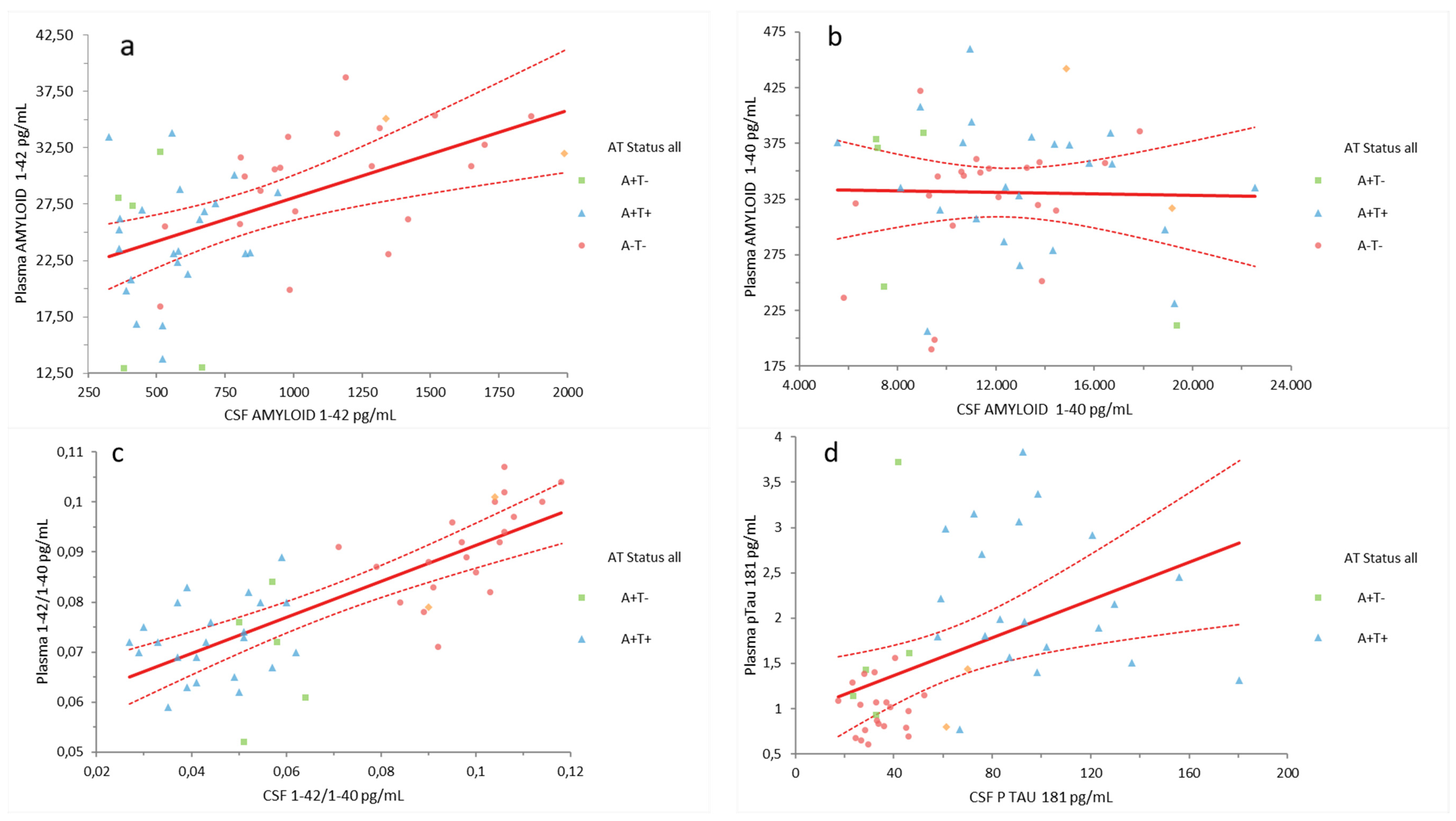
2.1. Plasma Aβ42, Aβ40, Aβ42/Aβ40, and pTau181 Correlation with CSF Biomarkers
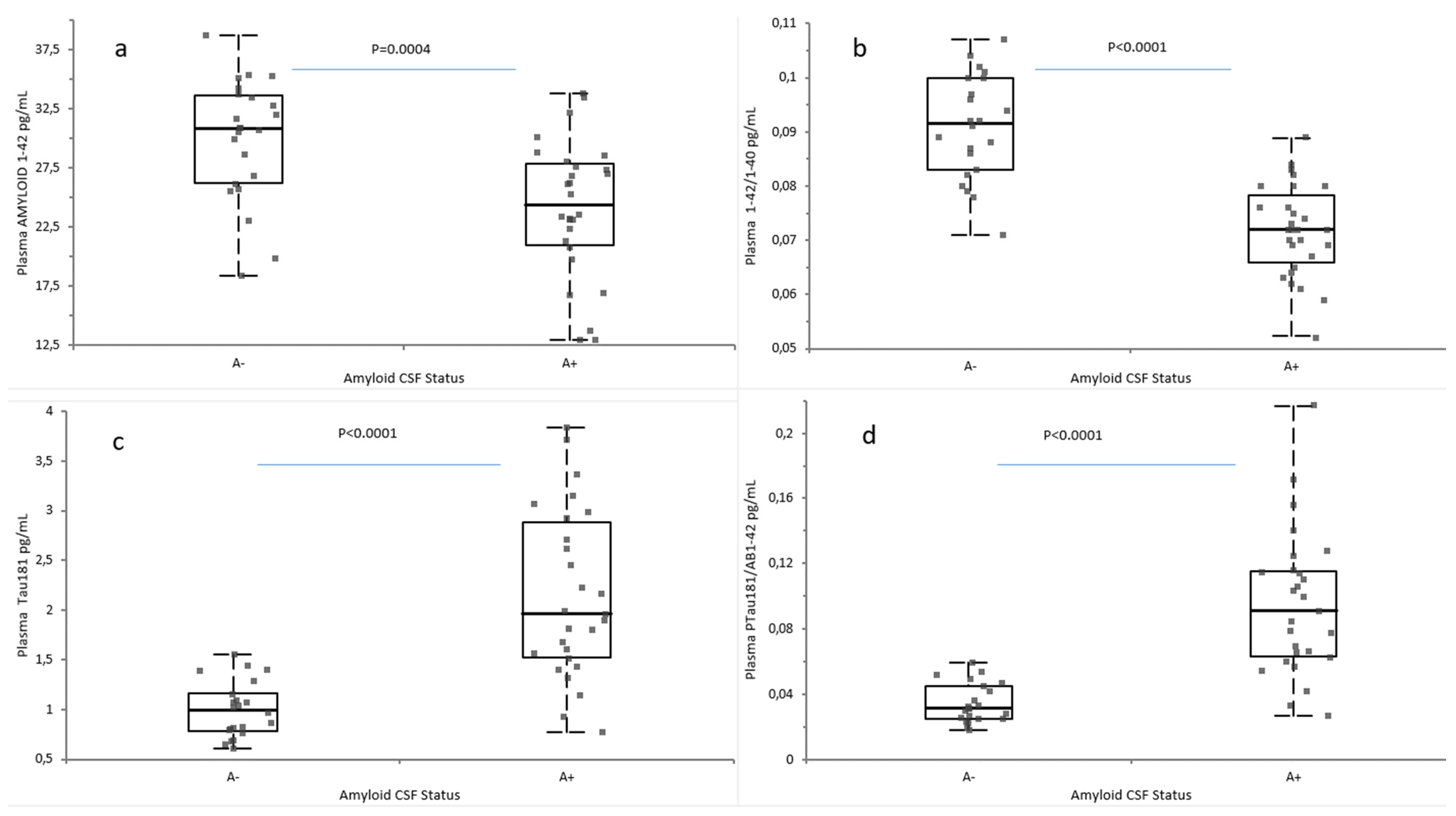
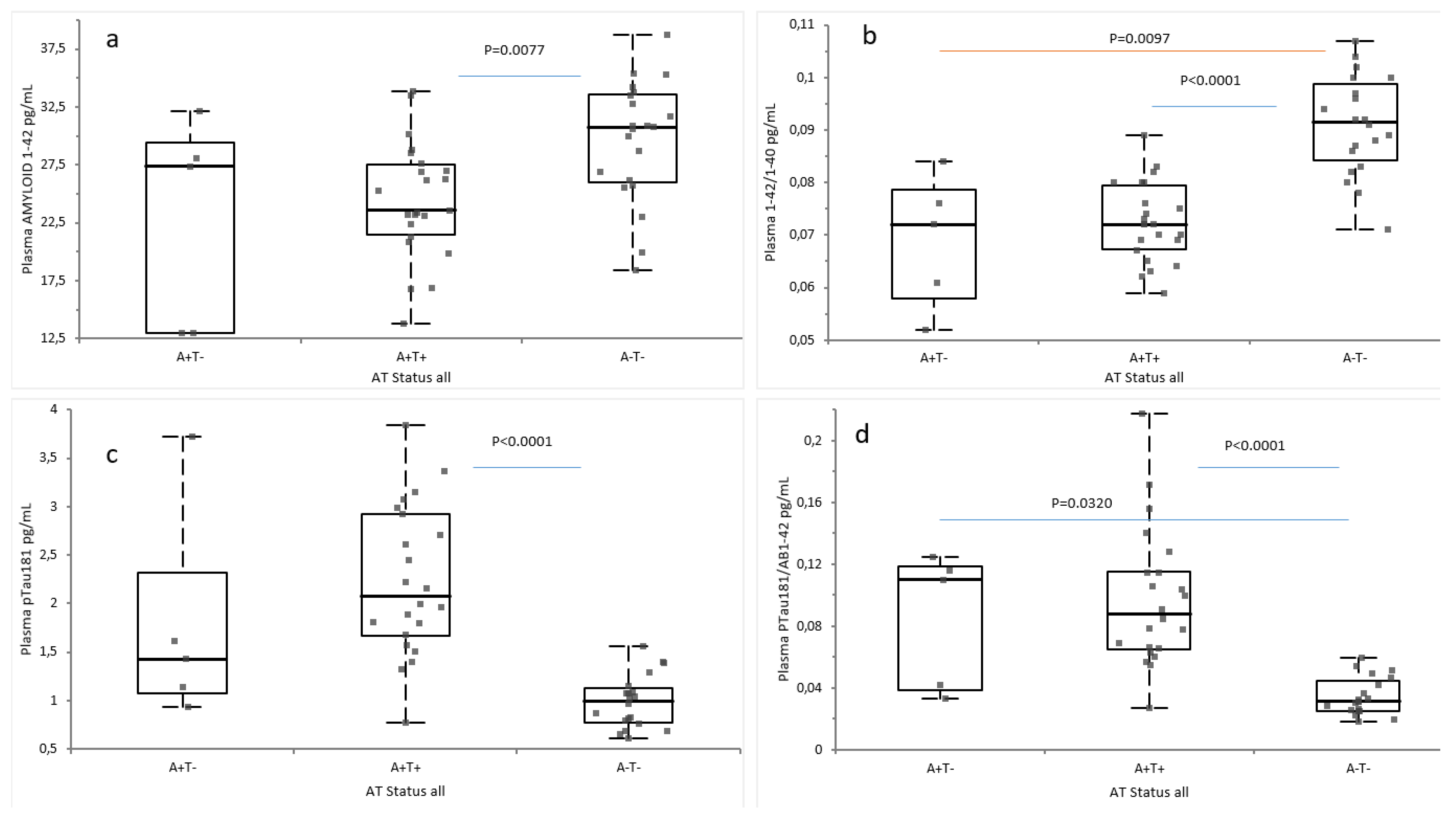
2.2. Plasma Biomarkers According to Amyloid and AT Classification
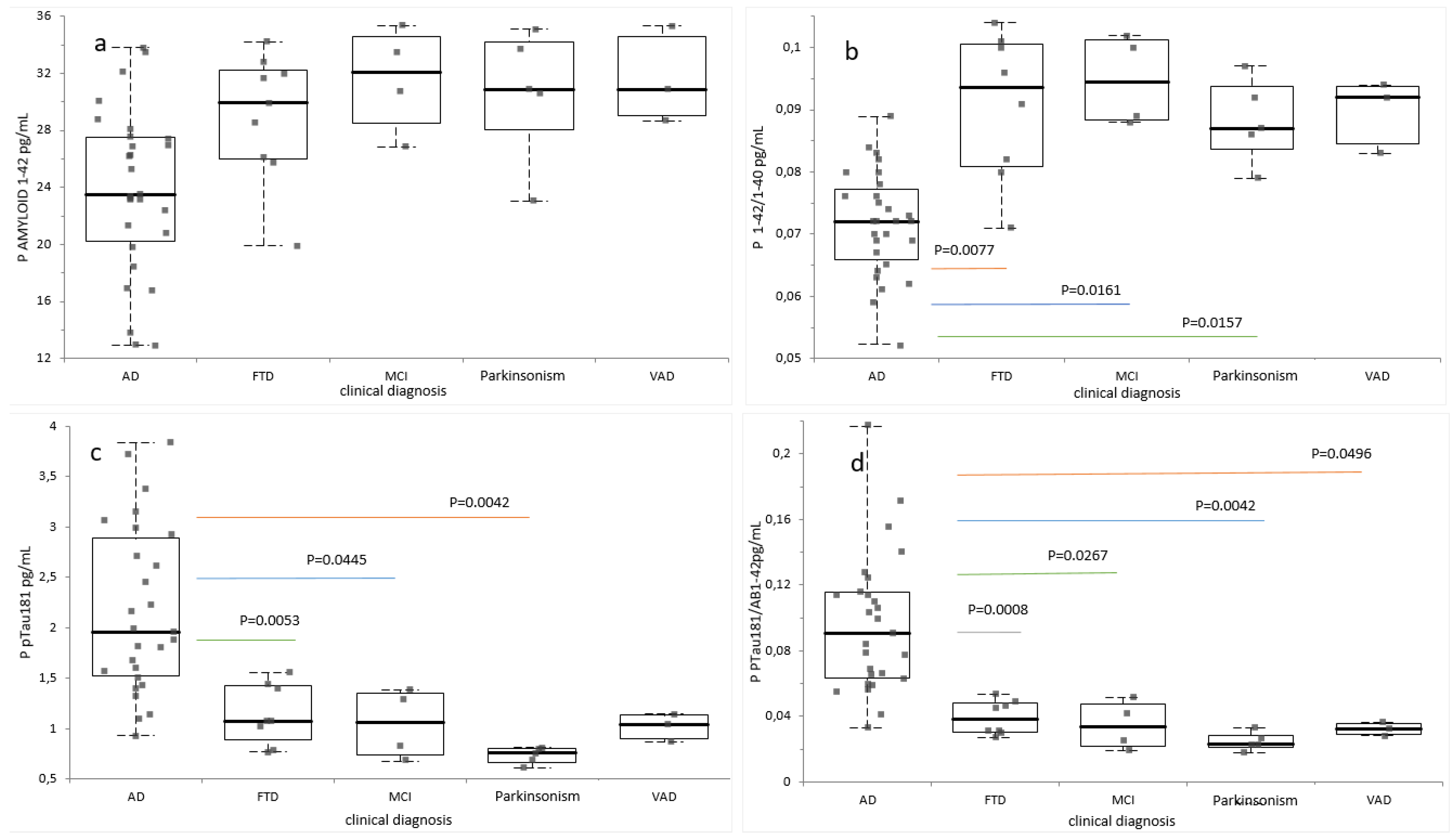
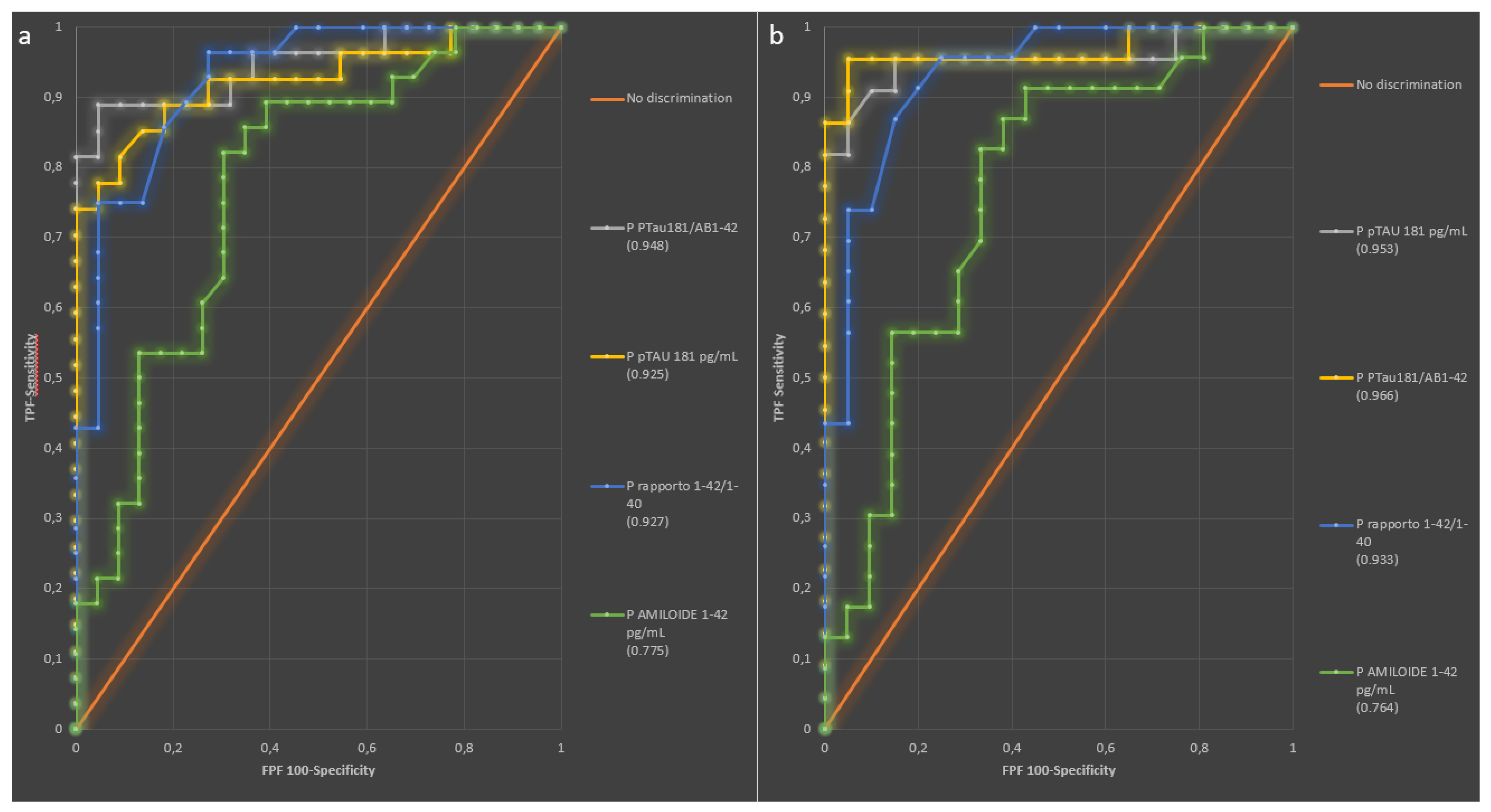
2.3. Diagnostic Performance of Plasma Biomarkers
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. CSF and Plasma Sample Collection
4.3. Measurement of CSF and Plasma Biomarkers
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Aβ | Amyloid β |
| AD | Alzheimer’s Disease |
| AUC | Area Under the Curve |
| CLEIA | ChemiLuminescent Enzyme ImmunoAssay |
| CI | Confidence Interval |
| CSF | Cerebrospinal Fluid |
| EDTA | EthyleneDiamineTetraacetic Acid |
| IQR | Interquartile Range |
| KD | Kidney Disease |
| MCI | Mild Cognitive Impairment |
| NIA-AA | National Institute on Aging and Alzheimer’s Association |
| pTau | Hyperphosphorilated tau |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Gamst, A.; Holtzmn, D.M.; Jaqust, W.J.; Petersen, R.C.; Synder, P.J.; Carrillo, M.C.; Thies, B.; Phelps, C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W.J.; Landau, S.M.; Koeppe, R.A.; Reiman, E.M.; Chen, K.; Mathis, C.A.; Price, J.C.; Foster, N.L.; Wang, A.Y. The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimers Dement. 2015, 11, 757–771. [Google Scholar] [CrossRef]
- Llorens, F.; Schmitz, M.; Karch, A.; Cramm, M.; Lange, P.; Gherib, K.; Varges, D.; Schmidt, C.; Zerr, I.; Stoeck, K. Comparative analysis of cerebrospinal fluid biomarkers in the differential diagnosis of neurodegenerative dementia. Alzheimers Dement. 2016, 12, 577–589. [Google Scholar] [CrossRef]
- Thijssen, E.H.; Verberk, I.M.W.; Kindermans, J.; Abramian, A.; Vanbrabant, J.; Ball, A.J.; Pijnenburg, Y.; Lemstra, A.W.; van der Flier, W.M.; Stoops, E.; et al. Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia. Alzheimers Dement. 2022, 14, e12285. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef]
- McFeely, A.; O’Connor, A.; Kennelly, S.P. Use of biomarkers in the diagnosis of Alzheimer’s disease in adults with intellectual disability. Lancet Healthy Longev. 2024, 5, 100639. [Google Scholar] [CrossRef]
- Marquez, F.; Yassa, M.A. Neuroimaging Biomarkers for Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 21. [Google Scholar] [CrossRef]
- Kehoe, E.G.; McNulty, J.P.; Mullins, P.G.; Bokde, A.L. Advances in MRI biomarkers for the diagnosis of Alzheimer’s disease. Biomark. Med. 2014, 8, 1151–1169. [Google Scholar] [CrossRef]
- Ovod, V.; Ramsey, K.N.; Mawuenyega, K.G.; Bollinger, J.G.; Hicks, T.; Schneider, T.; Sullivan, M.; Paumier, K.; Holtzman, D.M.; Morris, J.C.; et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s Dement. 2017, 13, 841–849. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.; Xiong, C.; et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.-X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018, 554, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Bellomo, G.; Bayoumy, S.; Megaro, A.; Toja, A.; Nardi, G.; Gaetani, L.; Blujdea, E.R.; Paoletti, F.P.; Wojdaƚa, A.L.; Chiasserini, D.; et al. Fully automated measurement of plasma Aβ42/40 and p-tau181: Analytical robustness and concordance with cerebrospinal fluid profile along the Alzheimer’s disease continuum in two independent cohorts. Alzheimers Dement. 2024, 20, 2453–2468. [Google Scholar] [CrossRef]
- Figdore, D.J.; Wiste, H.J.; Bornhorst, J.A.; Bateman, R.J.; Li, Y.; Graff-Radford, J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Jack, C.R., Jr.; et al. Performance of the Lumipulse plasma Aβ42/40 and pTau181 immunoassays in the detection of amyloid pathology. Alzheimers Dement 2024, 16, e12545. [Google Scholar] [CrossRef]
- Martínez-Dubarbie, F.; Guerra-Ruiz, A.; López-García, S.; Lage, C.; Fernández-Matarrubia, M.; Infante, J.; Pozueta-Cantudo, A.; García-Martínez, M.; Corrales-Pardo, A.; Bravo, M.; et al. Accuracy of plasma Aβ40, Aβ42, and p-tau181 to detect CSF Alzheimer’s pathological changes in cognitively unimpaired subjects using the Lumipulse automated platform. Alzheimers Res. Ther. 2023, 15, 163. [Google Scholar] [CrossRef]
- Silva-Spínola, A.; Leitão, M.J.; Nadal, A.; Le Bastard, N.; Santana, I.; Baldeiras, I. Exploring the potential of fully automated LUMIPULSE G plasma assays for detecting Alzheimer’s disease pathology. Alzheimer’s Res. Ther. 2024, 16, 51. [Google Scholar] [CrossRef]
- Pais, M.V.; Forlenza, O.V.; Diniz, B.S. Plasma Biomarkers of Alzheimer’s Disease: A Review of Available Assays, Recent Developments, and Implications for Clinical Practice. J. Alzheimers Dis. Rep. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.T.; Wu, Z.; Mielke, M.M.; Murray, A.M.; Ryan, J. Plasma Biomarkers of Alzheimer’s Disease and Neurodegeneration According to Sociodemographic Characteristics and Chronic Health Conditions. J. Prev. Alzheimers Dis. 2024, 11, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Arranz, J.; Zhu, N.; Rubio-Guerra, S.; Rodríguez-Baz, Í.; Ferrer, R.; Carmona-Iragui, M.; Barroeta, I.; Illán-Gala, I.; Santos-Santos, M.; Fortea, J.; et al. Diagnostic performance of plasma pTau(217), pTau(181), Abeta(1-42) and Abeta(1-40) in the LUMIPULSE automated platform for the detection of Alzheimer disease. Alzheimers Res. Ther. 2024, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.N.; Young, C.B.; Benitez, J.R.; Swarovski, M.S.; Feinstein, I.; Vandijck, M.; Le Guen, Y.; Kasireddy, N.M.; Shahid, M.; Corso, N.K.; et al. Performance of a fully-automated Lumipulse plasma phospho-tau181 assay for Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 172. [Google Scholar] [CrossRef]
- Martínez-Dubarbie, F.; Guerra-Ruiz, A.; López-García, S.; Irure-Ventura, J.; Lage, C.; Fernández-Matarrubia, M.; Pozueta-Cantudo, A.; García-Martínez, M.; Corrales-Pardo, A.; Bravo, M.; et al. Influence of Physiological Variables and Comorbidities on Plasma Aβ40, Aβ42, and p-tau181 Levels in Cognitively Unimpaired Individuals. Int. J. Mol. Sci. 2024, 25, 1481. [Google Scholar] [CrossRef]
- Verde, F.; Milone, I.; Dubini, A.; Colombrita, C.; Perego, A.; Solca, F.; Maranzano, A.; Ciusani, E.; Poletti, B.; Ratti, A.; et al. Influence of kidney function and CSF/serum albumin ratio on plasma Aβ42 and Aβ40 levels measured on a fully automated platform in patients with Alzheimer’s disease. Neurol. Sci. 2023, 44, 3287–3290. [Google Scholar] [CrossRef]
- Binette, A.P.; Janelidze, S.; Cullen, N.; Dage, J.L.; Bateman, R.J.; Zetterberg, H.; Blennow, K.; Stomrud, E.; Mattsson-Carlgren, N.; Hansson, O. Confounding factors of Alzheimer’s disease plasma biomarkers and their impact on clinical performance. Alzheimer’s Dement. 2022, 19, 1403–1414. [Google Scholar] [CrossRef]
- Fabrizi, E.; Ancidoni, A.; Locuratolo, N.; Piscopo, P.; Della Gatta, F.; Salemme, S.; Pani, S.M.; Marconi, D.; Vignatelli, L.; Sagliocca, L.; et al. The Italian guideline on diagnosis and treatment of dementia and mild cognitive impairment. Age and Ageing 2024, 53, afae250. [Google Scholar] [CrossRef]
- Catania, M.; Battipaglia, C.; Perego, A.; Salvi, E.; Maderna, E.; Cazzaniga, F.A.; Rossini, P.M.; Marra, C.; Vanacore, N.; Redolfi, A.; et al. Exploring the ability of plasma pTau217, pTau181 and beta-amyloid in mirroring cerebrospinal fluid biomarker profile of Mild Cognitive Impairment by the fully automated Lumipulse((R)) platform. Fluids Barriers CNS 2025, 22, 9. [Google Scholar] [CrossRef]
| n = 51 | |
|---|---|
| Characteristics | |
| Males, n [%] | 28 [55%] |
| Age, mean ± SD | 70 ± 8 |
| Diagnosis | |
| AD | 28 [55%] |
| FTD | 9 [17%] |
| MCI | 4 [8%] |
| VAD | 3 [6%] |
| PSP | 2 [4%] |
| Parkinsonism | 5 [10%] |
| CSF biomarkers | |
| Aβ42, mean ± SD, pg/mL | 831 ± 427 |
| Aβ40, mean ± SD, pg/mL | 12,301 ± 3867 |
| Aβ42/Aβ40, mean ± SD | 0.069 ± 0.028 |
| Total tau, mean ± SD, pg/mL | 469 ± 319 |
| pTau 181, mean ± SD, pg/mL | 71.7 ± 62.8 |
| Plasma biomarkers | |
| Aβ42, mean ± SD, pg/mL | 26.77 ± 6.16 |
| Aβ40, mean ± SD, pg/mL | 331.07 ± 61.25 |
| Aβ42/Aβ40, mean ± SD | 0.081 ± 0.015 |
| pTau 181, mean ± SD, pg/mL | 1.89 ± 1.66 |
| pTau 181/Aβ42, mean ± SD | 0.077 ± 0.070 |
| A classification, n [%] | |
| A+ | 28 [55%] |
| A− | 23 [45%] |
| AT classification, n [%] | |
| A+T− | 5 [10%] |
| A+T+ | 23 [45%] |
| A−T− | 21 [41%] |
| A-T+ | 2 [4%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rino, F.; Rispoli, F.; Zuffi, M.; Matteucci, E.; Gavazzi, A.; Salvatici, M.; Sansico, D.F.; Pollaroli, G.; Drago, L. Assessment of Plasma and Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease and Other Dementias: A Center-Based Study. Int. J. Mol. Sci. 2025, 26, 4308. https://doi.org/10.3390/ijms26094308
De Rino F, Rispoli F, Zuffi M, Matteucci E, Gavazzi A, Salvatici M, Sansico DF, Pollaroli G, Drago L. Assessment of Plasma and Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease and Other Dementias: A Center-Based Study. International Journal of Molecular Sciences. 2025; 26(9):4308. https://doi.org/10.3390/ijms26094308
Chicago/Turabian StyleDe Rino, Francesca, Francesca Rispoli, Marta Zuffi, Eleonora Matteucci, Armando Gavazzi, Michela Salvatici, Delia Francesca Sansico, Giulia Pollaroli, and Lorenzo Drago. 2025. "Assessment of Plasma and Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease and Other Dementias: A Center-Based Study" International Journal of Molecular Sciences 26, no. 9: 4308. https://doi.org/10.3390/ijms26094308
APA StyleDe Rino, F., Rispoli, F., Zuffi, M., Matteucci, E., Gavazzi, A., Salvatici, M., Sansico, D. F., Pollaroli, G., & Drago, L. (2025). Assessment of Plasma and Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease and Other Dementias: A Center-Based Study. International Journal of Molecular Sciences, 26(9), 4308. https://doi.org/10.3390/ijms26094308







