Low-Temperature and Light Pretreatment Interactively Promote Rapid Flowering, Early Ripening, and Yield Accumulation of Winter Wheat
Abstract
1. Introduction
2. Results
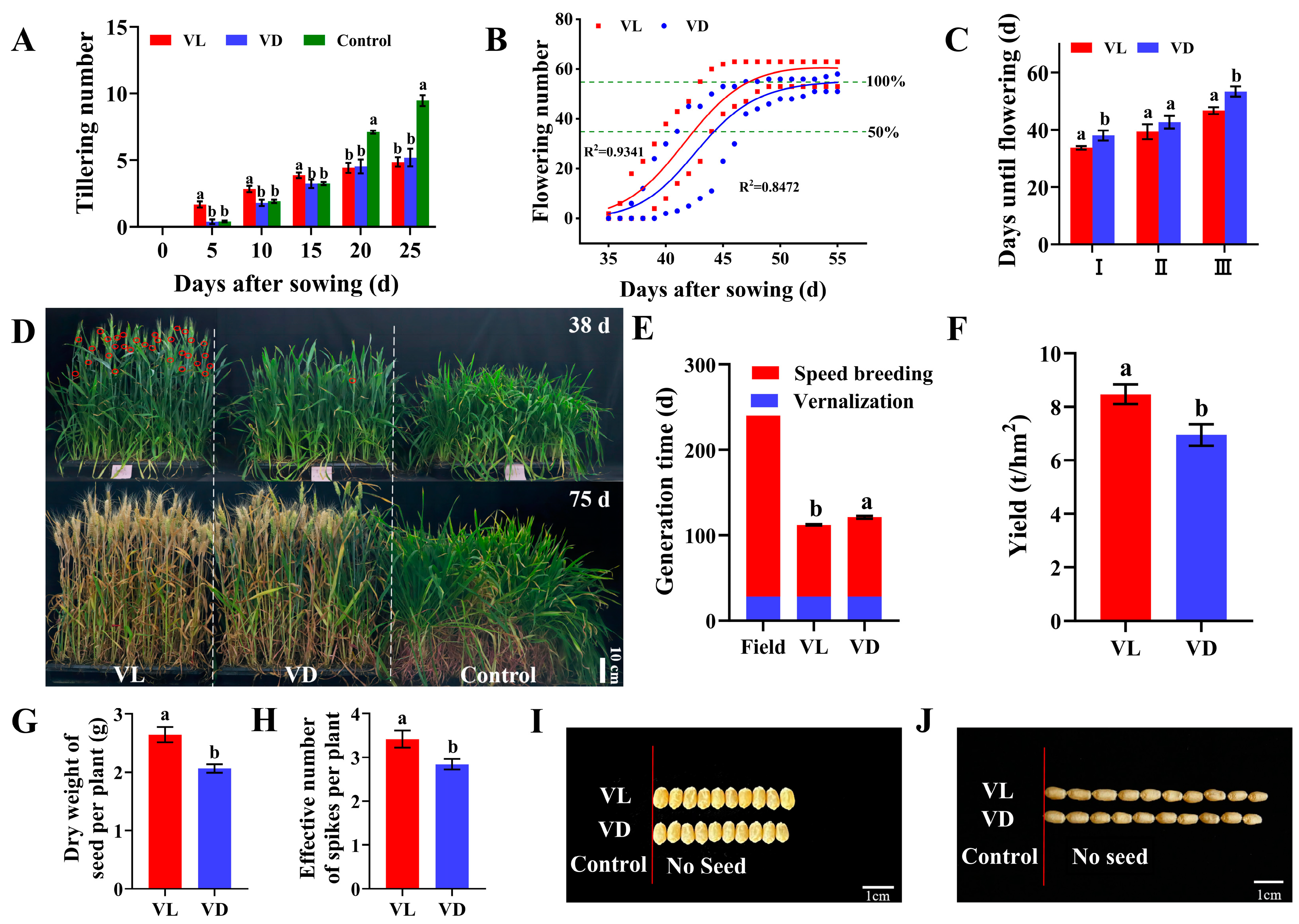
2.1. Combined Application of Low-Temperature and Light Pretreatment Accelerated the Growth and Yield of Winter Wheat
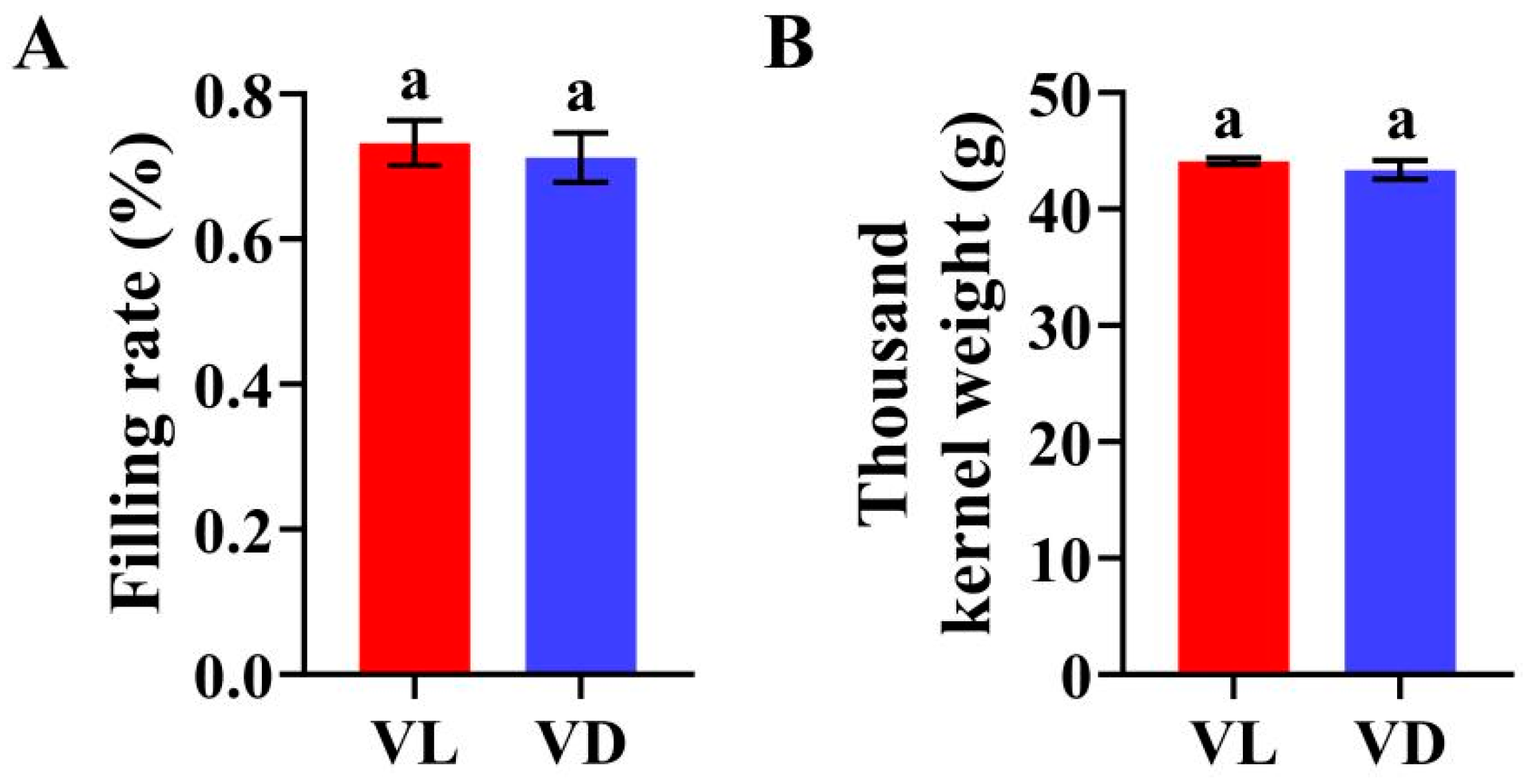
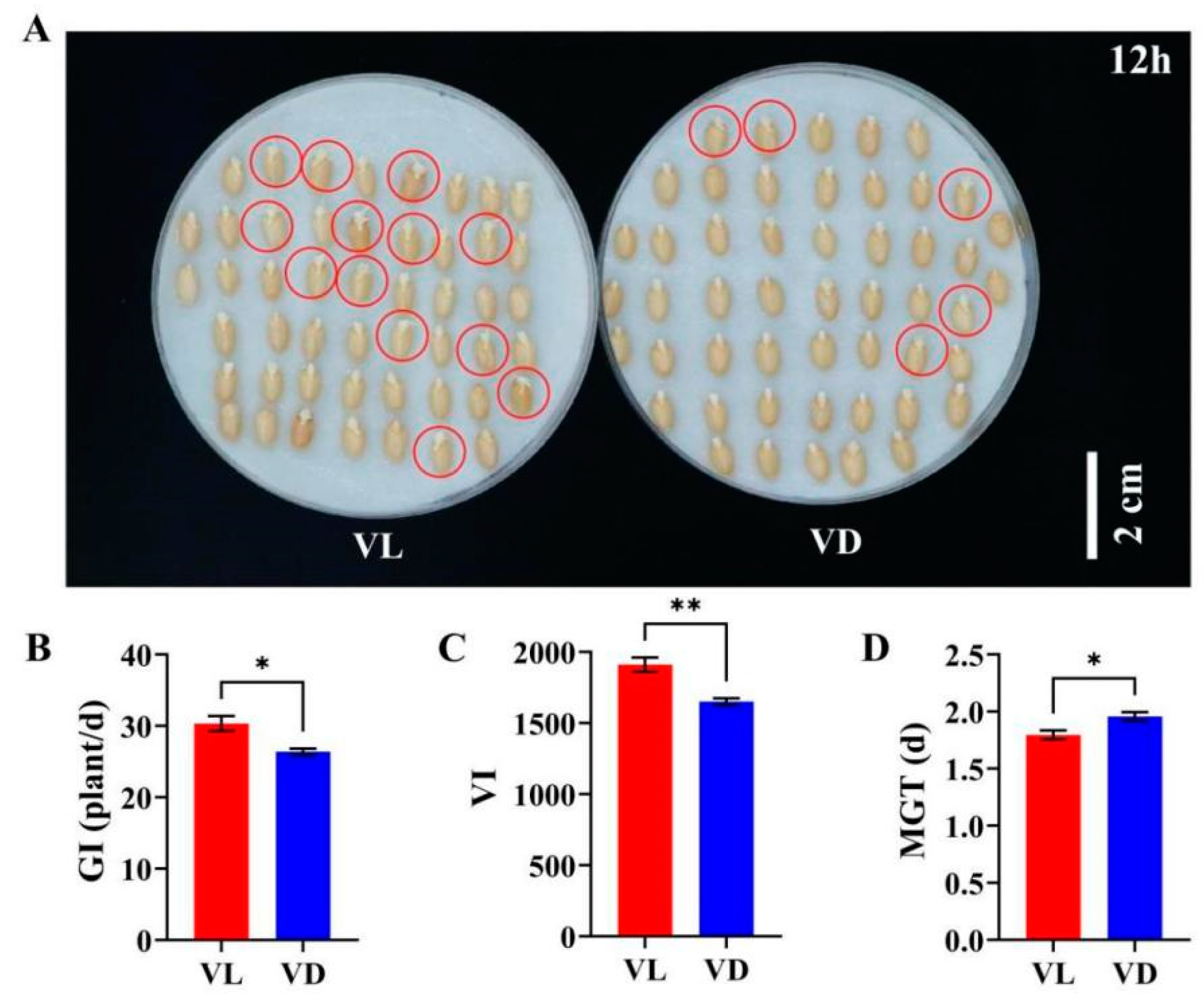
2.2. Combined Low-Temperature and Light Pretreatment Improved the Germination Capacity of Progeny Seeds of Speed Breeding
2.3. Gas Exchange Response to Combined Low-Temperature and Light Pretreatment
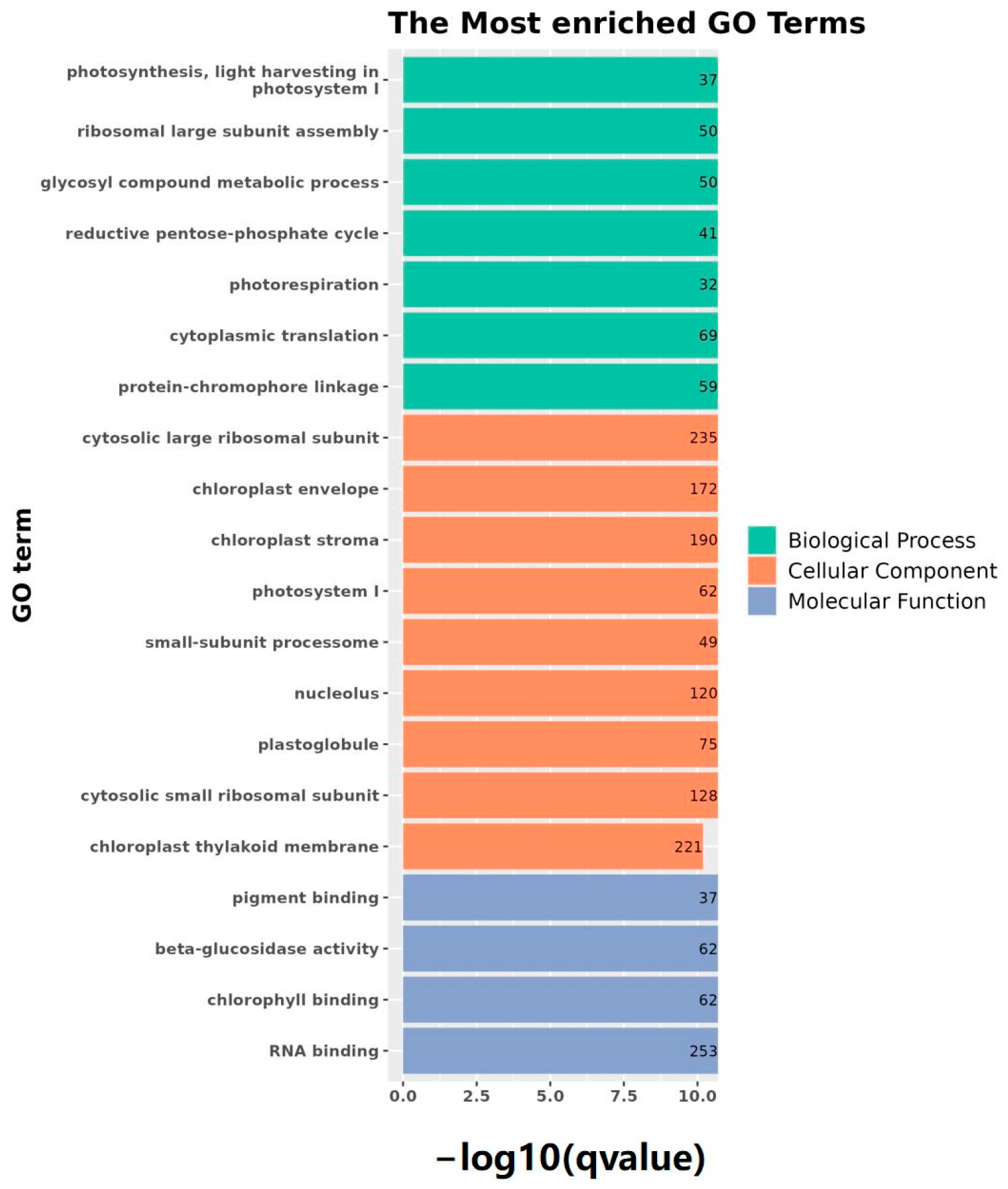
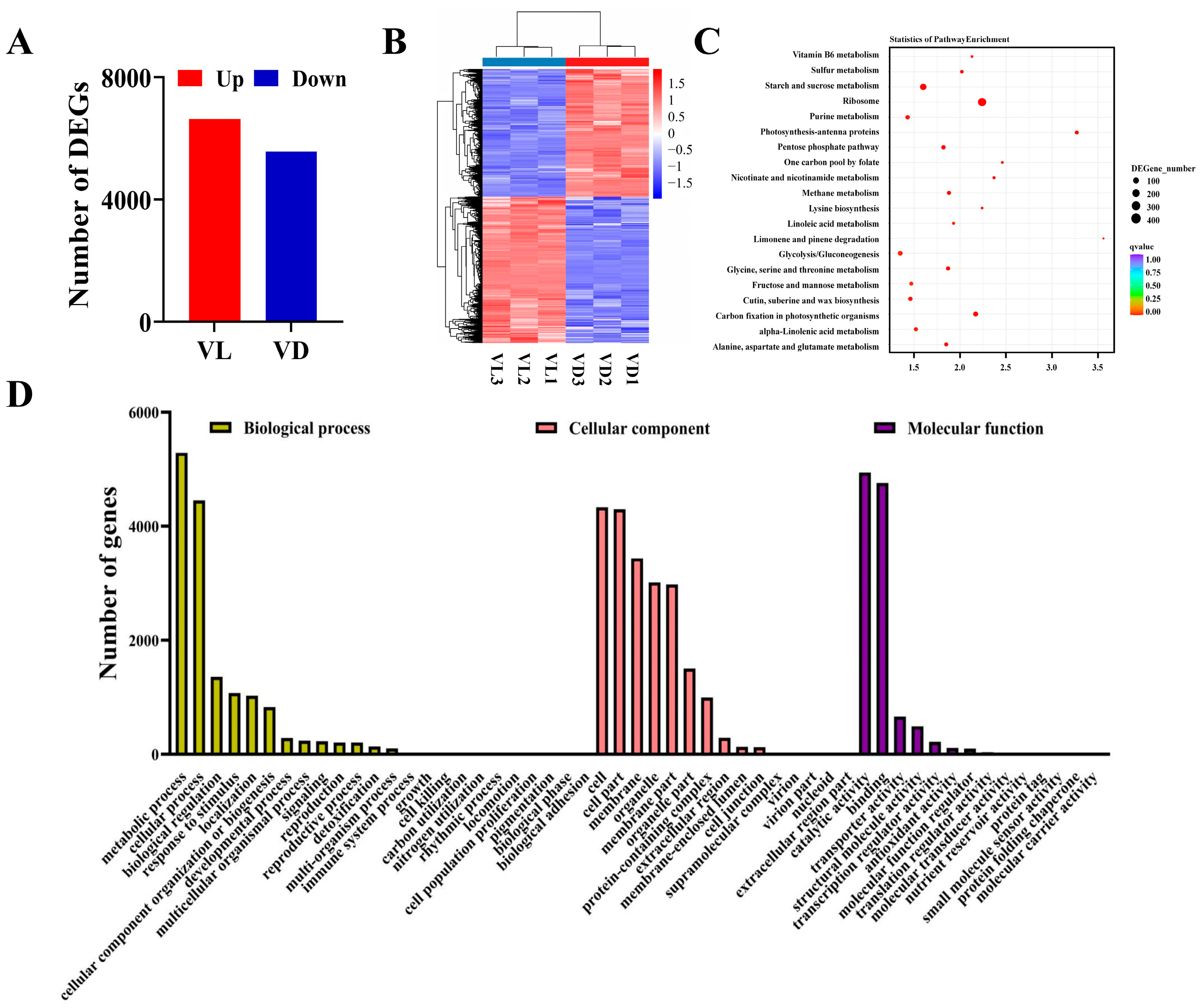
2.4. Identification and Analysis of Differently Expressed Genes
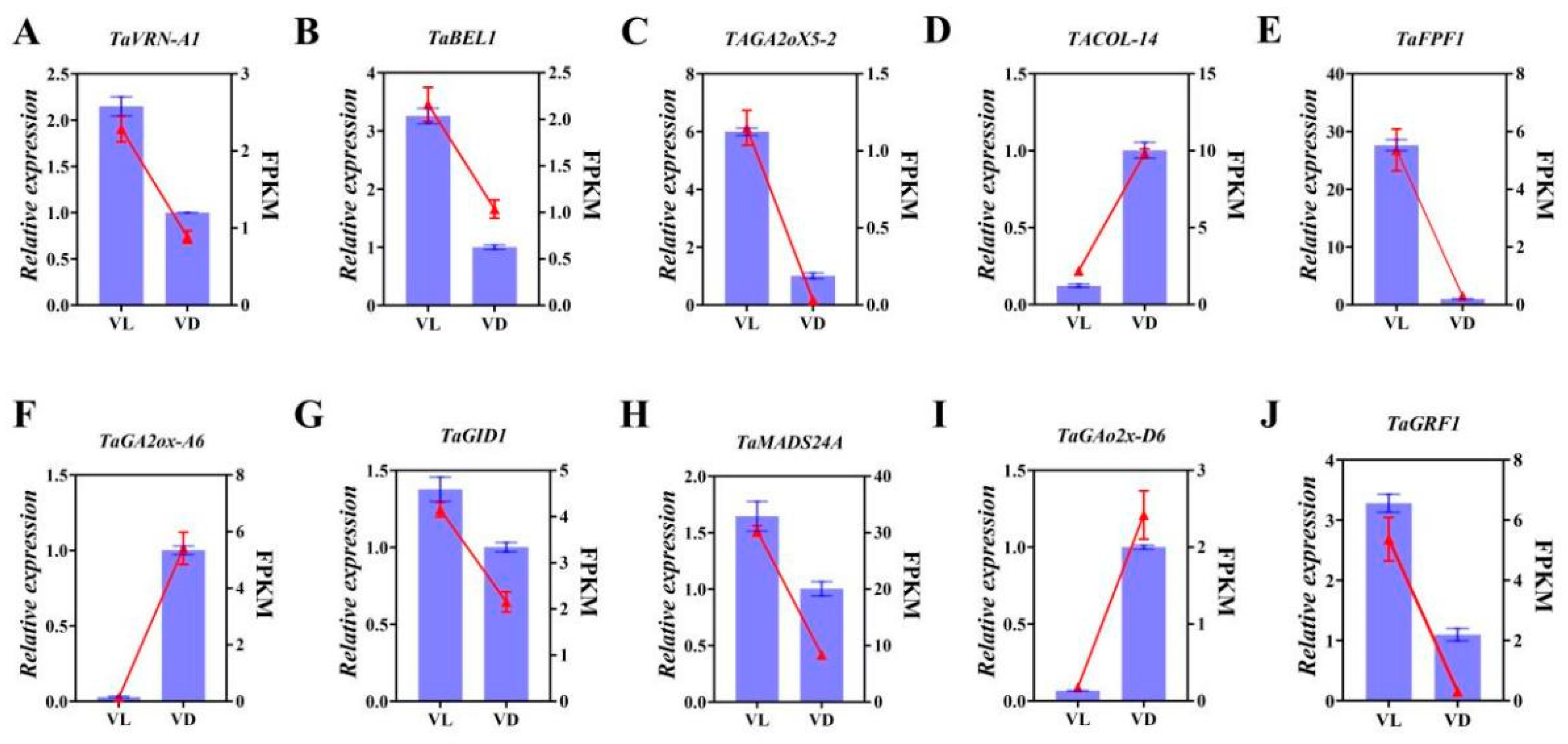
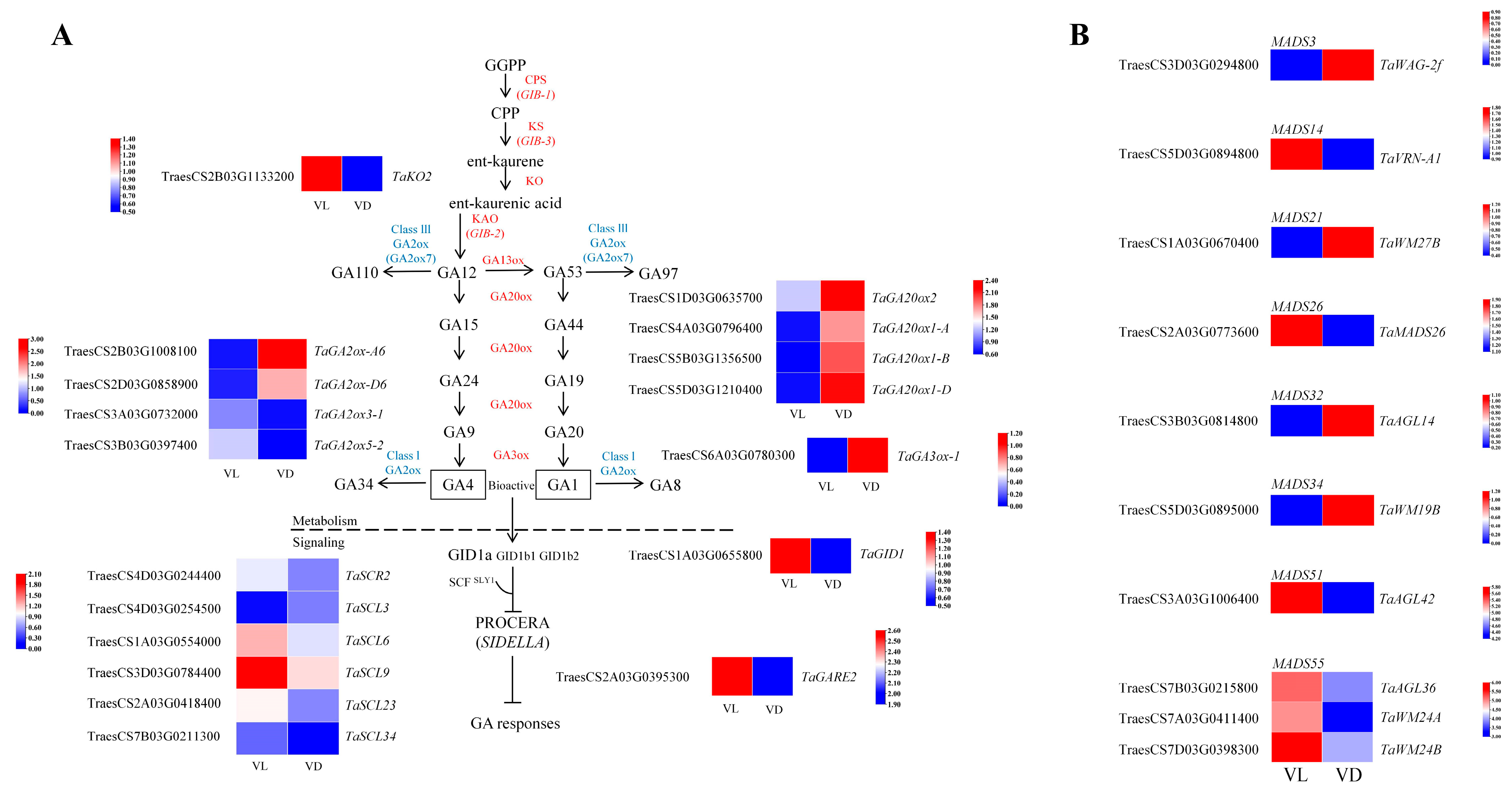
2.5. Expression Profiles of Flowering-Related Gene Response to Low Temperature and Different Light Conditions
2.6. Validation of Differently Expressed Genes by qPCR
3. Discussion
3.1. Low-Temperature and Light Exposure Speed up the Vernalization of Germinated Seeds and Promote Rapid Growth in Winter Wheat
3.2. Low-Temperature and Light Pretreatment Enhances the Vitality and Quality of Progeny Seeds in Speed Breeding
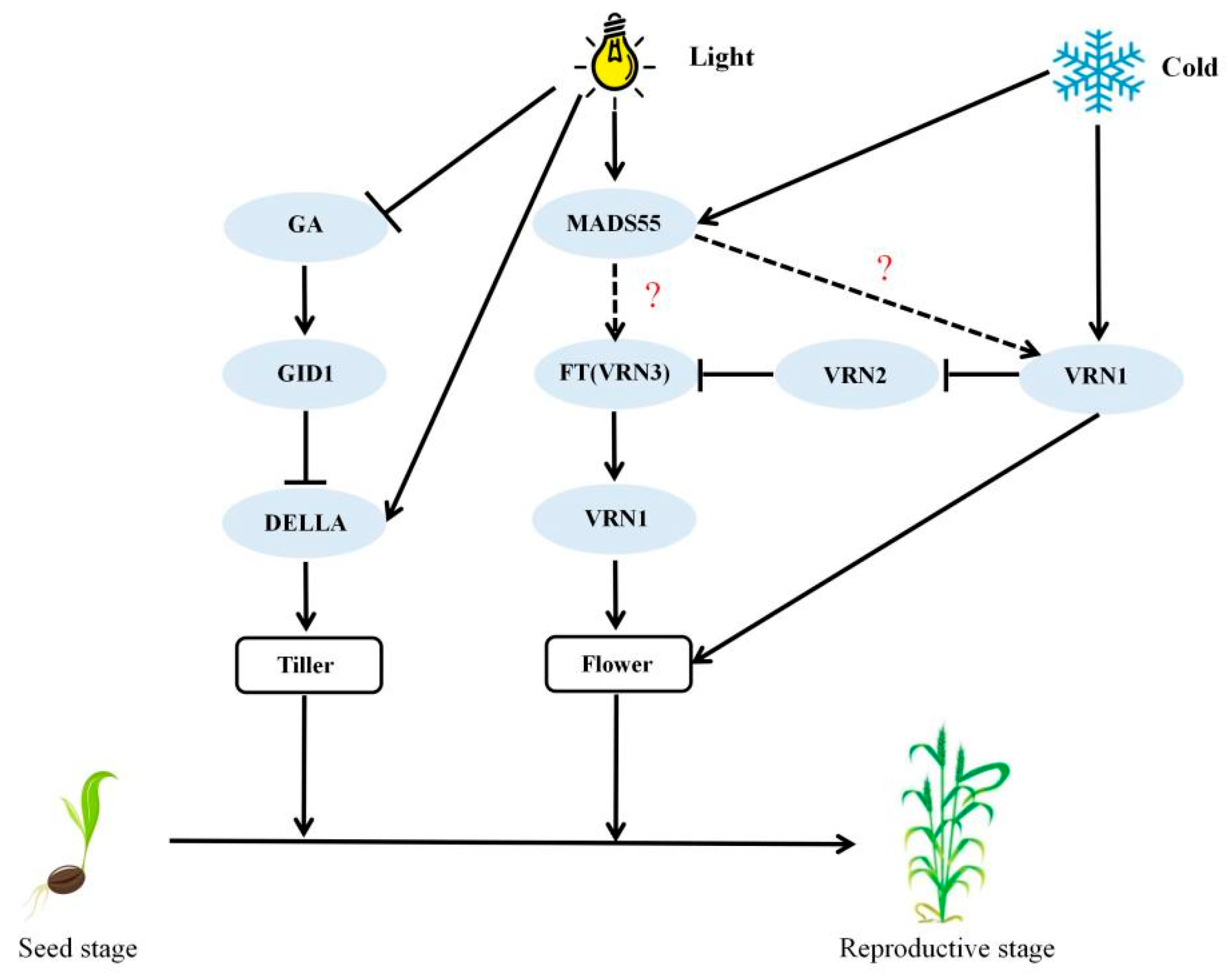
3.3. The Molecular Basis of Speed Vernalization Under Light and Low-Temperature Pretreatment
4. Materials and Methods
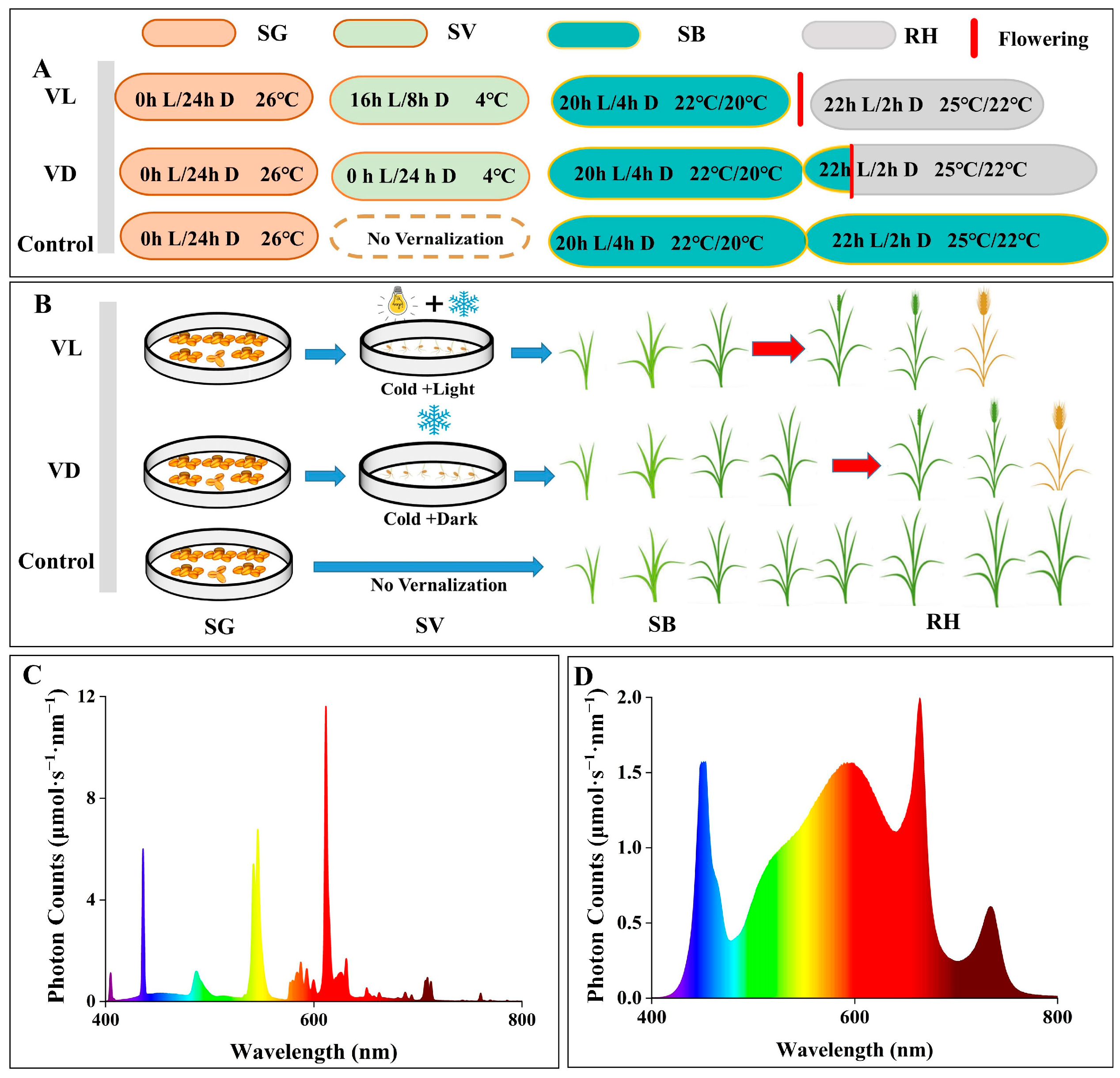
4.1. Experimental Materials and Growth Conditions
4.2. Morphological Indicators and Yield Determination
4.3. Gas Exchange Parameter Determination
4.4. Determination of Germination Capacity of Progeny Seeds
4.5. RNA Extraction and Transcriptome Sequencing
4.6. Transcriptomic Analysis
4.7. Validation of RNA-Seq Data by qRT-PCR
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SV | Speed vernalization |
| SB | Speed breeding |
| VL | Vernalization with light |
| VD | Vernalization with dark |
| Control | No vernalization treatment |
| SG | Seed germinate |
| RH | Ripening and harvest |
Appendix A
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| VRN-A1 | GGCGCAGCAAGATCAAACTC | ATGTGGCTCACCATCCACG |
| BEL1 | GGCTATGGAAGCCCATGATA | GAAGCAGTCACCAGGGTTGT |
| GA2ox5-2 | GAGAACTAGCCAGCGACCAG | CGACAAAGAGGCCGAGATAG |
| COL14 | ACGACGACGAAGAGCAACTT | TTCAGCAGCTGCACCATATC |
| FPF1 | CAGCCAGCTAGAGAAGGGATG | CGATGTCGCGGTTCTCGTAG |
| GA2ox-A6 | AAGAGCGTGGAGCACAAAGT | GTGTGATTCATGCCGTCAAC |
| GID1 | CCACCATCGGCTTCTACCTGCTGTC | GGCGAGCTCATCCACGACGAGAC |
| MADS24A | AGCAGCCGGCTATTGATTTA | CCCTTGTGTTGGAGGTCACT |
| GA2ox-D6 | ACAGCGACTTCCTCACCATC | ACTTTGTGCTCCACGCTCTT |
| GRF1 | TACGCCATCGACCTGTCCAGC | ATGGAGATGGAGAGCTGCGTC |
| Actin | GTTGGTGATGAGGCCCAATC | GTGCTACACGGAGCTCATTG |
References
- Pardey, P.G.; Beddow, J.M.; Hurley, T.M.; Beatty, T.K.; Eidman, V.R. A Bounds Analysis of World Food Futures: Global Agriculture Through to 2050. Aust. J. Agric. Resour. Econ. 2014, 58, 571–589. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef]
- Paterson, G. Food Outlook: Biannual Report on Global Food Markets. Interaction 2024, 52. [Google Scholar] [CrossRef]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Hatta, M.A.M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Bugbee, B.; Koerner, G.; Albrechtsen, R.; Dewey, W.; Clawson, S. ‘USU-Apogee’ Wheat-Registration’. 1999. Available online: https://digitalcommons.usu.edu/cpl_dwarfcrops/11/ (accessed on 20 April 2025).
- Levinskikh, M.; Sychev, V.; Derendyaeva, T.; Signalova, O.; Salisbury, F.; Campbell, W.; Bingham, G.; Bubenheim, D.; Jahns, G. Analysis of the Spaceflight Effects on Growth and Development of Super Dwarf Wheat Grown on the Space Station Mir. J. Plant Physiol. 2000, 156, 522–529. [Google Scholar] [CrossRef]
- Swami, P.; Deswal, K.; Rana, V.; Yadav, D.; Munjal, R. Speed breeding—A powerful tool to breed more crops in less time accelerating crop research. In Abiotic Stresses in Wheat; Elsevier: Amsterdam, The Netherlands, 2023; pp. 33–49. [Google Scholar] [CrossRef]
- Biesaga-Kościelniak, J.; Kościelniak, J.; Filek, M.; Marcińska, I.; Krekule, J.; Macháčková, I.; Kuboń, M. The effect of plant growth regulators and their interaction with electric current on winter wheat development. Acta Physiol. Plant. 2010, 32, 987–995. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, J.; Furbank, R.; Griffiths, S.; King, J.; Murchie, E.; Parry, M.; Slafer, G. Achieving yield gains in wheat. Plant Cell Environ. 2012, 35, 1799–1823. [Google Scholar] [CrossRef]
- Shewry, W. Darwin review. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Woods, D.P.; Li, K.; Li, C.; Dubcovsky, J. MiR172-APETALA2-like genes integrate vernalization and plant age to control flowering time in wheat. PLOS Genet. 2022, 18, e1010157. [Google Scholar] [CrossRef]
- Grogan, S.M.; Brown-Guedira, G.; Haley, S.D.; McMaster, G.S.; Reid, S.D.; Smith, J.; Byrne, P.F. Allelic Variation in Developmental Genes and Effects on Winter Wheat Heading Date in the U.S. Great Plains. PLOS ONE 2016, 11, e0152852. [Google Scholar] [CrossRef] [PubMed]
- Palomino, C.; Cabrera, A. Evaluation of the Allelic Variations in Vernalisation (VRN1) and Photoperiod (PPD1) Genes and Genetic Diversity in a Spanish Spelt Wheat Collection. Int. J. Mol. Sci. 2023, 24, 16041. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, L.; Zhang, X.; Lv, G.; Pan, Y.; Chen, F. Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat. BMC Plant Biol. 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Gendall, A.R.; Levy, Y.Y.; Wilson, A.; Dean, C. The VERNALIZATION 2 Gene Mediates the Epigenetic Regulation of Vernalization in Arabidopsis. Cell 2001, 107, 525–535. [Google Scholar] [CrossRef]
- Griffith, M.; Ala, P.; Yang, D.S.C.; Hon, W.-C.; Moffatt, B.A. Antifreeze Protein Produced Endogenously in Winter Rye Leaves. Plant Physiol. 1992, 100, 593–596. [Google Scholar] [CrossRef]
- Agarwal, P.; Khurana, P. Overexpression of TaMADS from wheat promotes flowering by upregulating expression of floral promoters and provides protection against thermal stress. Plant Gene 2019, 17, 100168. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Tanaka, C.; Itoh, T.; Iwasaki, Y.; Mizuno, N.; Nasuda, S.; Murai, K. Direct interaction between VRN1 protein and the promoter region of the wheat FT gene. Genes Genet. Syst. 2018, 93, 25–29. [Google Scholar] [CrossRef]
- Woods, D.P.; McKeown, M.A.; Dong, Y.; Preston, J.C.; Amasino, R.M. Evolution of VRN2/Ghd7-Like Genes in Vernalization-Mediated Repression of Grass Flowering. Plant Physiol. 2016, 170, 2124–2135. [Google Scholar] [CrossRef]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2017, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-K.; O’connor, K.; Alahmad, S.; Lee, J.-H.; Dinglasan, E.; Park, H.; Lee, S.-M.; Hirsz, D.; Kwon, S.-W.; Kwon, Y.; et al. Speed vernalization to accelerate generation advance in winter cereal crops. Mol. Plant 2022, 15, 1300–1309. [Google Scholar] [CrossRef]
- Gol, L.; Tomé, F.; von Korff, M. Floral transitions in wheat and barley: Interactions between photoperiod, abiotic stresses, and nutrient status. J. Exp. Bot. 2017, 68, 1399–1410. [Google Scholar] [CrossRef]
- Soleymani, A. Light response of sunflower and canola as affected by plant density, plant genotype and N fertilization. J. Photochem. Photobiol. B Biol. 2017, 173, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B.; Hemming, M.N.; Dennis, E.S.; Peacock, W.J. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 2007, 12, 352–357. [Google Scholar] [CrossRef]
- Shourbalal, S.K.S.; Soleymani, A.; Javanmard, H.R. Shortening vernalization in winter wheat (Triticum aestivum L.) using plant growth regulators and cold stratification. J. Clean. Prod. 2019, 219, 443–450. [Google Scholar] [CrossRef]
- Kabade, P.G.; Dixit, S.; Singh, U.M.; Alam, S.; Bhosale, S.; Kumar, S.; Singh, S.K.; Badri, J.; Varma, N.R.G.; Chetia, S.; et al. SpeedFlower: A comprehensive speed breeding protocol for indica and japonica rice. Plant Biotechnol. J. 2023, 22, 1051–1066. [Google Scholar] [CrossRef]
- Goggi, A.S.; Caragea, P.; Pollak, L.; McAndrews, G.; DeVries, M.; Montgomery, K. Seed Quality Assurance in Maize Breeding Programs: Tests to Explain Variations in Maize Inbreds and Populations. Agron. J. 2008, 100, 337–343. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef]
- González-Barrios, P.; Bhatta, M.; Halley, M.; Sandro, P.; Gutiérrez, L. Speed breeding and early panicle harvest accelerates oat (Avena sativa L.) breeding cycles. Crop. Sci. 2020, 61, 320–330. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef]
- Wang, B.; Smith, S.M.; Li, J. Genetic Regulation of Shoot Architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef]
- Lo, S.-F.; Yang, S.-Y.; Chen, K.-T.; Hsing, Y.-I.; Zeevaart, J.A.; Chen, L.-J.; Yu, S.-M. A Novel Class of Gibberellin 2-Oxidases Control Semidwarfism, Tillering, and Root Development in Rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef]
- Assuero, S.; Lorenzo, M.; Ramírez, N.P.; Velázquez, L.; Tognetti, J. Tillering promotion by paclobutrazol in wheat and its relationship with plant carbohydrate status. N. Z. J. Agric. Res. 2012, 55, 347–358. [Google Scholar] [CrossRef]
- Luo, X.; Liu, B.; Xie, L.; Wang, K.; Xu, D.; Tian, X.; Xie, L.; Li, L.; Ye, X.; He, Z.; et al. The TaSOC1-TaVRN1 module integrates photoperiod and vernalization signals to regulate wheat flowering. Plant Biotechnol. J. 2023, 22, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Nagarajan, R.; Liu, S.; Luzuriaga, J.C.; Zhai, W.; Cao, S.; Jia, H.; Carver, B.F.; Yan, L. The E3 ligase TaE3V-B1 ubiquitinates proteins encoded by the vernalization gene TaVRN1 and regulates developmental processes in wheat. Plant Physiol. 2024, 197. [Google Scholar] [CrossRef] [PubMed]
- von Zitzewitz, J.; Szűcs, P.; Dubcovsky, J.; Yan, L.; Francia, E.; Pecchioni, N.; Casas, A.; Chen, T.H.; Hayes, P.M.; Skinner, J.S. Molecular and Structural Characterization of Barley Vernalization Genes. Plant Mol. Biol. 2005, 59, 449–467. [Google Scholar] [CrossRef]
- de Folter, S.; Immink, R.G.; Kieffer, M.; Pařenicová, L.; Henz, S.R.; Weigel, D.; Busscher, M.; Kooiker, M.; Colombo, L.; Kater, M.M.; et al. Comprehensive Interaction Map of the Arabidopsis MADS Box Transcription Factors. Plant Cell 2005, 17, 1424–1433. [Google Scholar] [CrossRef]
- Chai, S.; Yao, Q.; Liu, R.; Xiang, W.; Xiao, X.; Fan, X.; Zeng, J.; Sha, L.; Kang, H.; Zhang, H.; et al. Identification and validation of a major gene for kernel length at the P1 locus in Triticum polonicum. Crop. J. 2021, 10, 387–396. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Y.; Wang, K.; Luo, X.; Xu, D.; Tian, X.; Li, L.; Ye, X.; Xia, X.; Li, W.; et al. TaVrt2, an SVP-like gene, cooperates with TaVrn1 to regulate vernalization-induced flowering in wheat. New Phytol. 2019, 231, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Tsoularis, A.; Wallace, J. Analysis of logistic growth models. Math. Biosci. 2002, 179, 21–55. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Wang, Y.; Zhang, X.; Grundy, S.; Hardy, K.; Yang, Q.; Lu, C. A Transcriptome Analysis Revealing the New Insight of Green Light on Tomato Plant Growth and Drought Stress Tolerance. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Thornley, J.H. Mathematical Models in Plant Physiology; Academic Press (Inc.): London, UK, 1976. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Lian, Y.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manag. 2022, 317, 115441. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Xu, S.; Ji, X.; Han, H.; Zhang, J.; Zhou, S.; Guo, B.; Yang, X.; Li, X.; Guo, X.; Liu, T.; et al. AcRLK2P-1, an LRR receptor protein kinase gene from Agropyron cristatum, confers leaf rust resistance in wheat. Plant Commun. 2024, 5, 101132. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
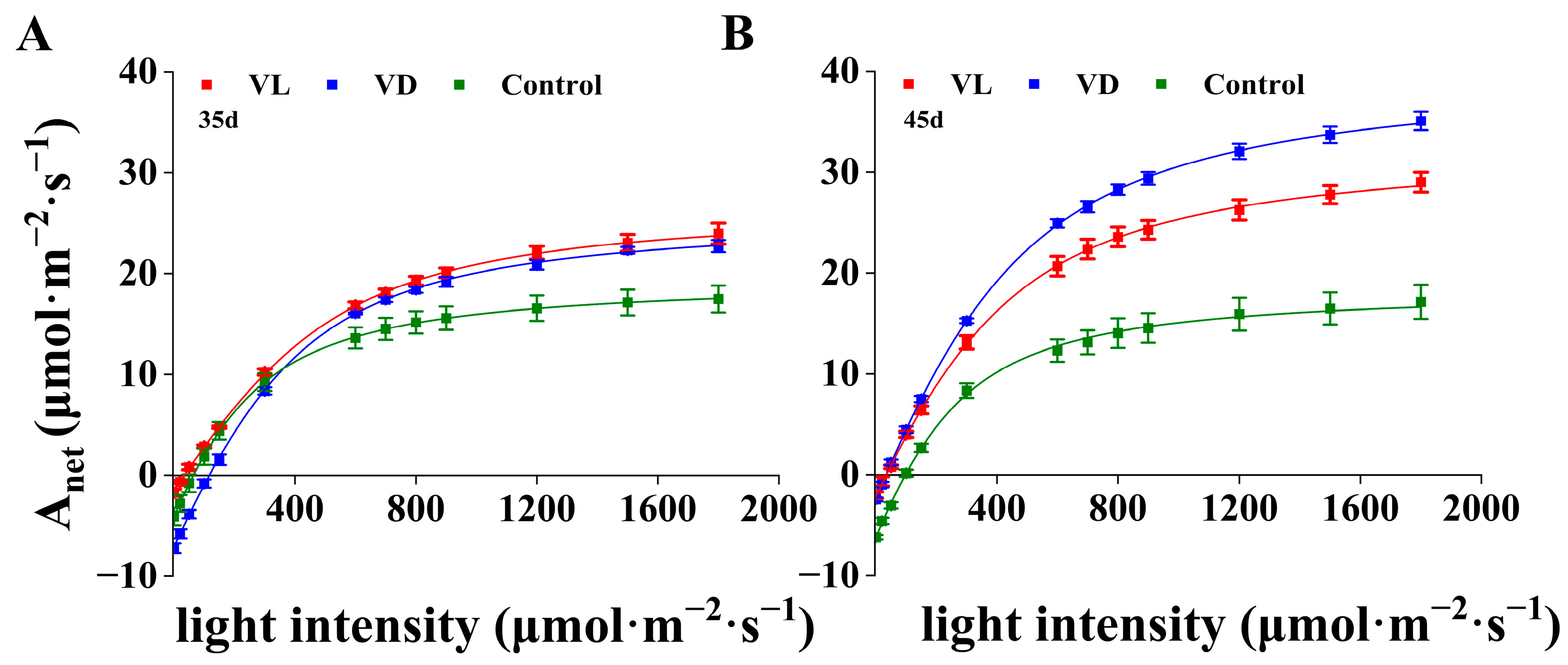
| Days After Sowing | Treatment | Measured | Fitting | |||
|---|---|---|---|---|---|---|
| Anet370 (umol m−2·s−1) | Fv/Fm | Amax (umol m−2·s−1) | AQE | Rdark (umol·m−2·s−1) | ||
| 35 d | Control | 9.40 ± 0.38 b | 0.818 ± 0.02 a | 23.60 ± 0.16 c | 0.07± 0.01 a | 4.11 ±0.05 b |
| VL | 10.22 ± 0.62 a | 0.835 ± 0.01 a | 32.92 ± 0.35 a | 0.05 ± 0.01 a | 1.58 ± 0.06 c | |
| VD | 8.36 ± 0.70 c | 0.826 ± 0.01 a | 28.44 ± 0.56 b | 0.07 ± 0.01 a | 7.13 ± 0.13 a | |
| 45 d | Control | 8.89 ± 1.57 c | 0.834 ± 0.02 a | 24.69 ± 0.80 c | 0.07 ± 0.01 a | 6.18 ±0.09 a |
| VL | 13.13 ± 1.61 b | 0.829 ± 0.02 a | 34.77 ± 0.91 b | 0.06 ± 0.01 a | 2.18 ± 0.12 b | |
| VD | 15.26 ± 0.58 a | 0.839 ± 0.01 a | 42.32 ± 0.35 a | 0.07 ± 0.01 a | 2.43 ± 0.04 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Shuai, R.; Zhan, X.; Wang, Q.; Tang, S.; Gao, T.; Zhao, Y.; Yang, Q.; Bian, Z. Low-Temperature and Light Pretreatment Interactively Promote Rapid Flowering, Early Ripening, and Yield Accumulation of Winter Wheat. Int. J. Mol. Sci. 2025, 26, 4280. https://doi.org/10.3390/ijms26094280
Wu Y, Shuai R, Zhan X, Wang Q, Tang S, Gao T, Zhao Y, Yang Q, Bian Z. Low-Temperature and Light Pretreatment Interactively Promote Rapid Flowering, Early Ripening, and Yield Accumulation of Winter Wheat. International Journal of Molecular Sciences. 2025; 26(9):4280. https://doi.org/10.3390/ijms26094280
Chicago/Turabian StyleWu, Yuanlong, Runnan Shuai, Xiaoxu Zhan, Qiangui Wang, Si Tang, Tingting Gao, Yanyan Zhao, Qichang Yang, and Zhonghua Bian. 2025. "Low-Temperature and Light Pretreatment Interactively Promote Rapid Flowering, Early Ripening, and Yield Accumulation of Winter Wheat" International Journal of Molecular Sciences 26, no. 9: 4280. https://doi.org/10.3390/ijms26094280
APA StyleWu, Y., Shuai, R., Zhan, X., Wang, Q., Tang, S., Gao, T., Zhao, Y., Yang, Q., & Bian, Z. (2025). Low-Temperature and Light Pretreatment Interactively Promote Rapid Flowering, Early Ripening, and Yield Accumulation of Winter Wheat. International Journal of Molecular Sciences, 26(9), 4280. https://doi.org/10.3390/ijms26094280








