Ectropis obliqua-Induced Secondary Metabolites Are Regulated by Methyl Jasmonate in a Threshold-Dependent Manner
Abstract
1. Introduction
2. Results
2.1. E. obliqua Infestation Alters the Metabolic Profile of Tea Plants
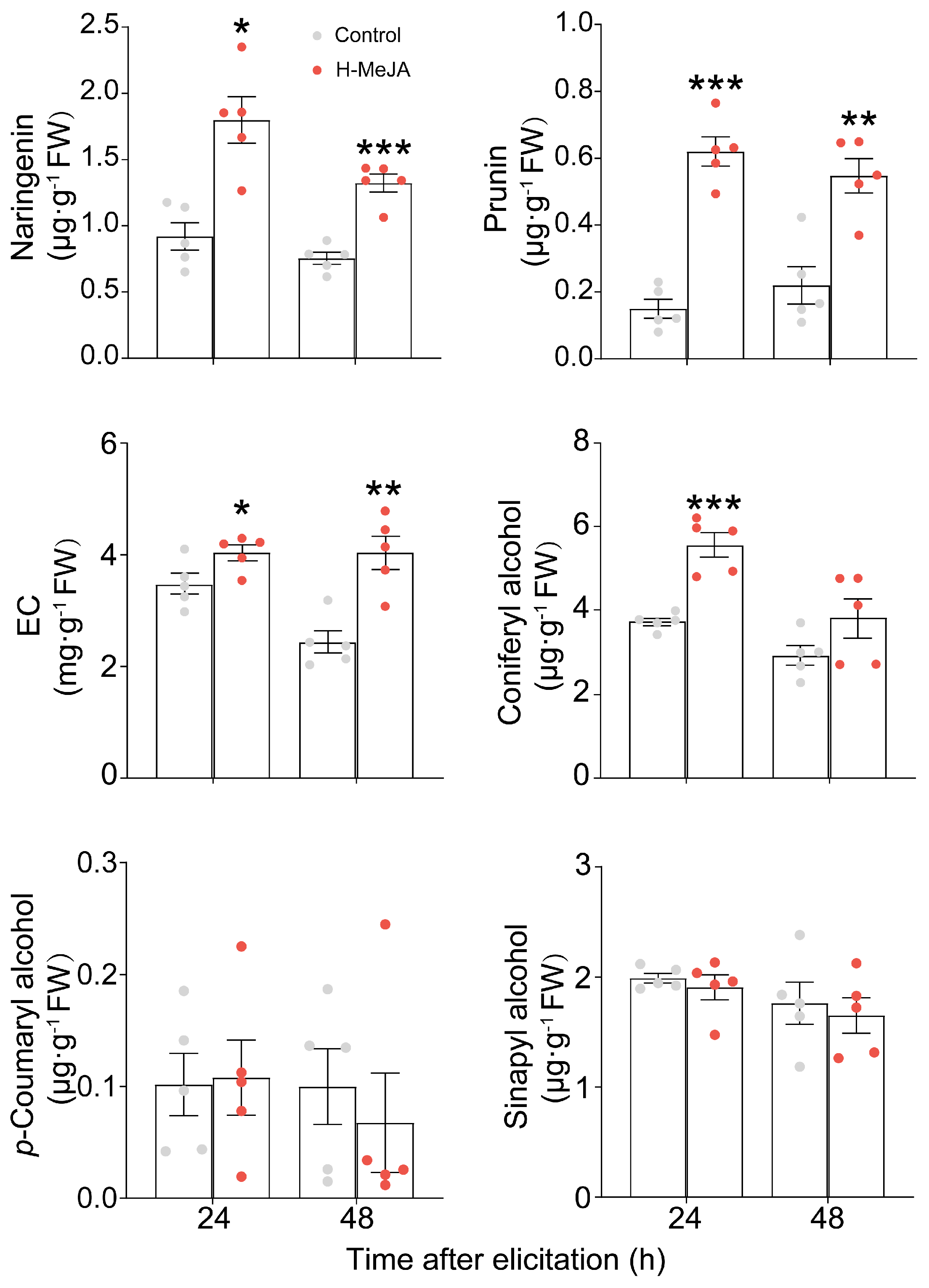
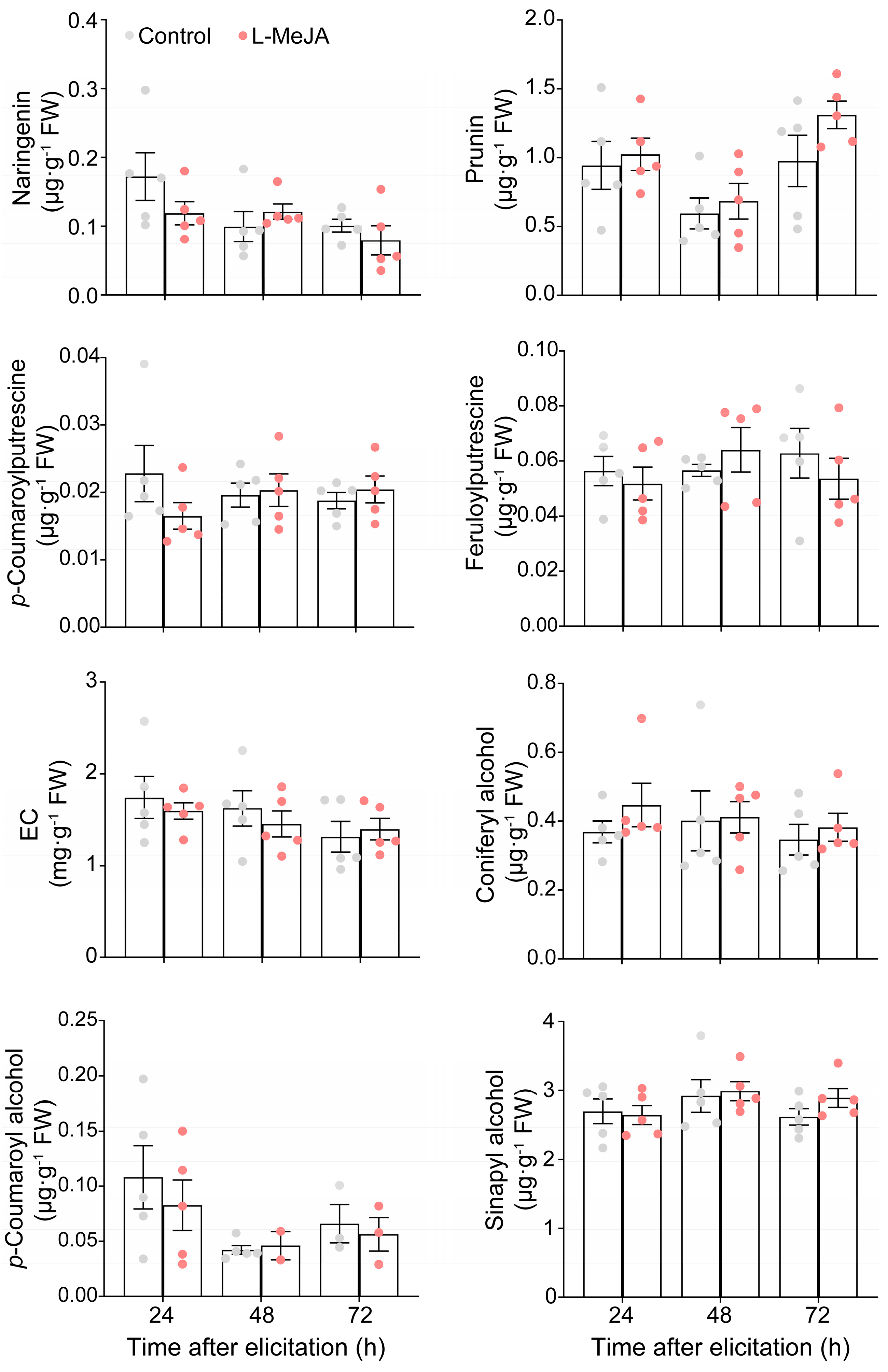
2.2. Effect of Exogenous Application of MeJA on E. obliqua-Induced Key Metabolites
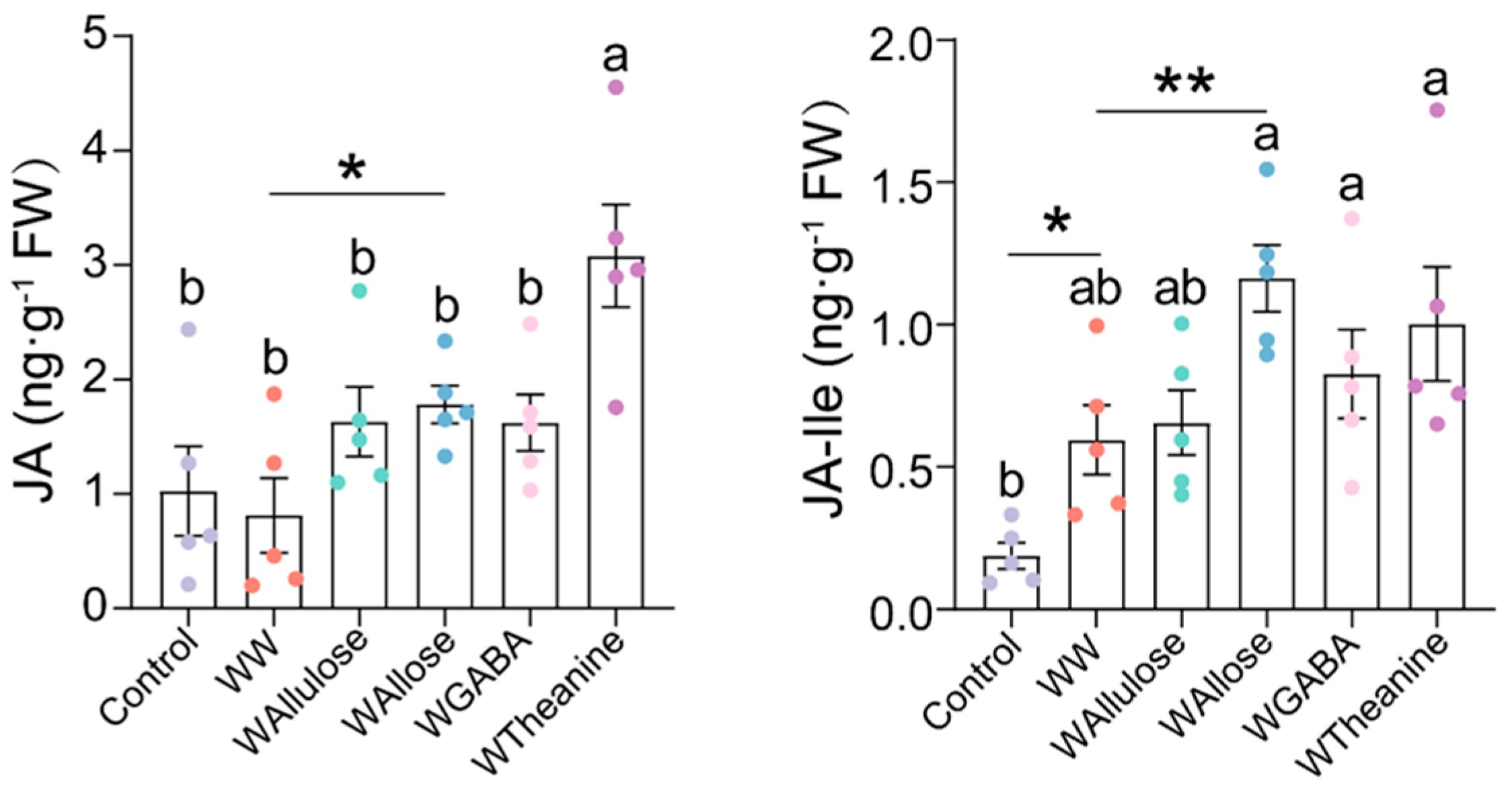
2.3. D-Allose and L-Theanine Induced Statistically Significant but Quantitatively Limited Increases in JA Levels
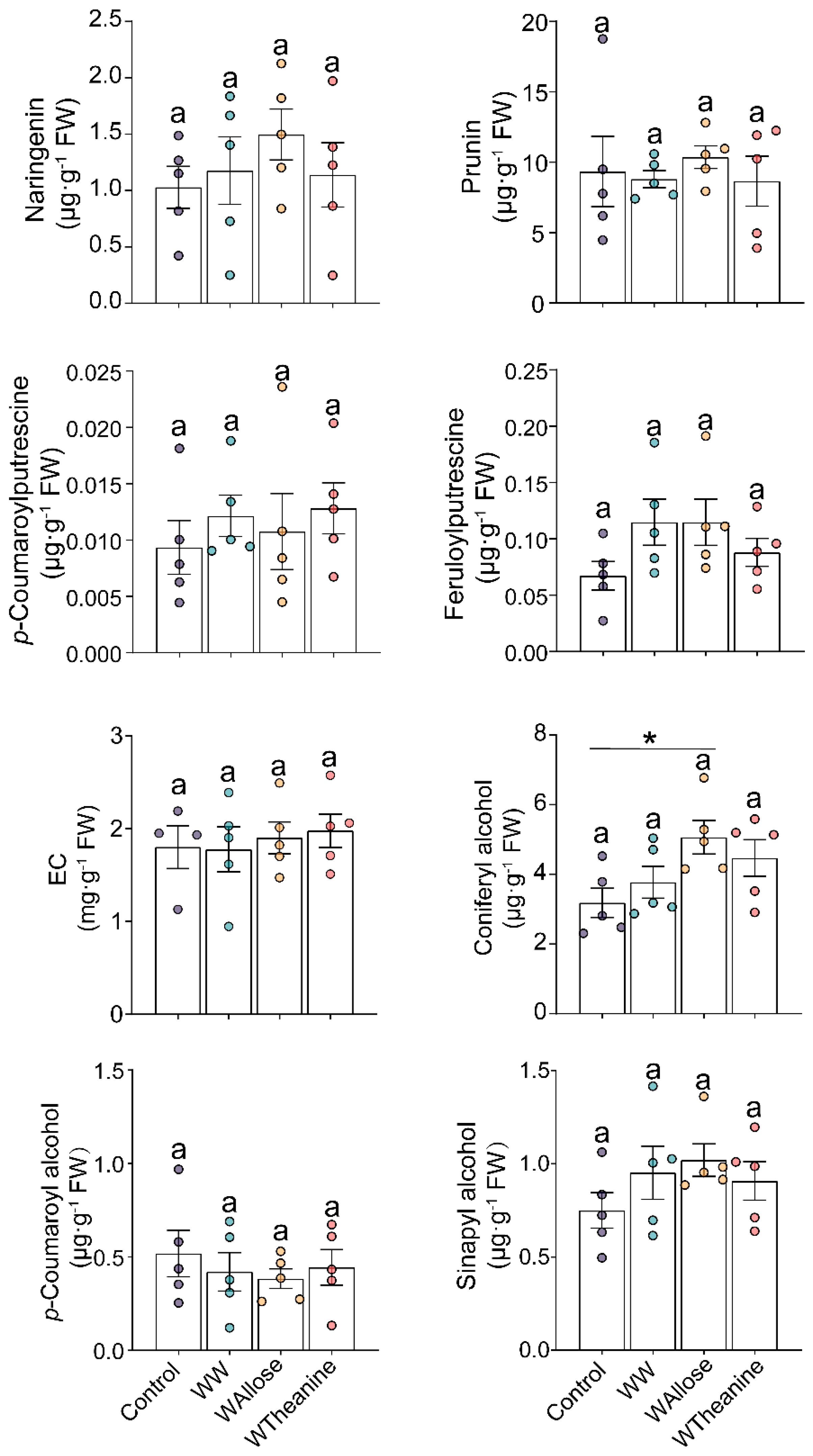
2.4. Neither D-Allose nor L-Theanine Altered the Target Metabolites
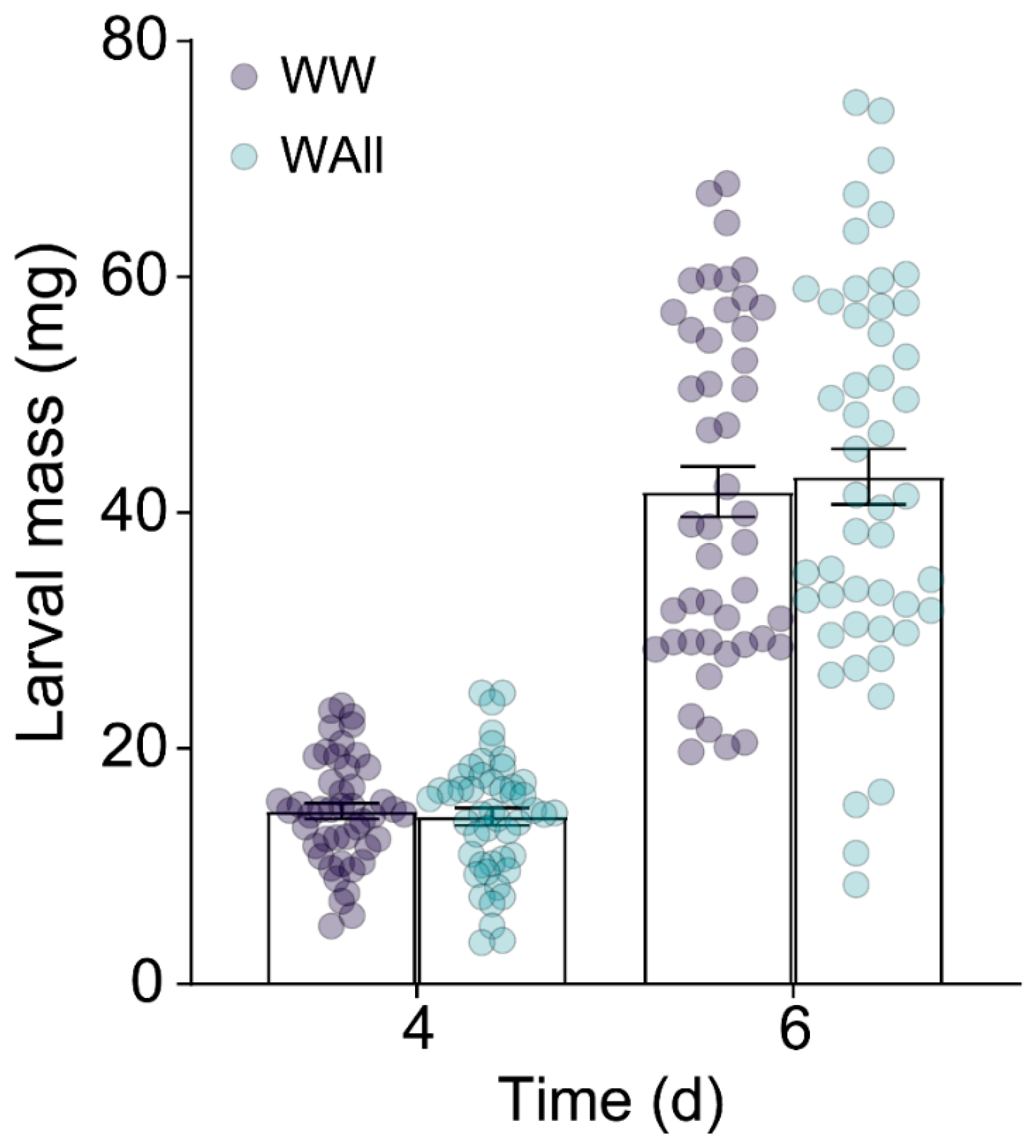
2.5. D-Allose Did Not Enhance Tea Pant Resistance to E. obliqua
3. Discussion
4. Materials and Methods
4.1. Plants and Herbivorous Insects
4.2. E. obliqua Infestation
4.3. MeJA Treatment
4.4. D-Allose, γ-Aminobutyric Acid, D-Allulose and L-Theanine Treatment
4.5. Flavonoid Extraction and Quantification
4.6. Phytohormone Quantification
4.7. Herbivore Bioassay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MeJA | Methyl jasmonate |
| JA | Jasmonate acid |
| EC | Epicatechin |
| GABA | γ-aminobutyric acid |
References
- Schuman, M.C.; Baldwin, I.T. The Layers of Plant Responses to Insect Herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, D.K.; Komal, J.; Samal, I.; Bhoi, T.K.; Kumar, P.V.D.; Mohapatra, S.; Athulya, R.; Majhi, P.K.; Mastinu, A. Plant Defense Responses to Insect Herbivores through Molecular Signaling, Secondary Metabolites, and Associated Epigenetic Regulation. Plant-Environ. Interact. 2025, 6, e70035. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from Chewing Insects—The Intersection of DAMPs, HAMPs, MAMPs and Effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular Interactions Between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in Jasmonate Signaling for Multistress Resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple Levels of Crosstalk in Hormone Networks Regulating Plant Defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Yang, D.-L.; Yang, Y.; He, Z. Roles of Plant Hormones and Their Interplay in Rice Immunity. Mol. Plant 2013, 6, 675–685. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Baldwin, I.T. Methyl Jasmonate-Elicited Herbivore Resistance: Does MeJA Function as a Signal without Being Hydrolyzed to JA? Planta 2008, 227, 1161–1168. [Google Scholar] [CrossRef]
- Jiang, D.; Yan, S. MeJA Is More Effective than JA in Inducing Defense Responses in Larix Olgensis. Arthropod-Plant Interact. 2018, 12, 49–56. [Google Scholar] [CrossRef]
- Jiao, L.; Bian, L.; Luo, Z.; Li, Z.; Xiu, C.; Fu, N.; Cai, X.; Chen, Z. Enhanced Volatile Emissions and Anti-Herbivore Functions Mediated by the Synergism between Jasmonic Acid and Salicylic Acid Pathways in Tea Plants. Hortic. Res. 2022, 9, uhac144. [Google Scholar] [CrossRef]
- Zhan, X.; Liu, Y.; Liang, X.; Wu, C.; Liu, X.; Shui, J.; Zhang, Y.; Wang, Y.; Chen, Q. Methyl Jasmonate-Treated Pepper (Capsicum annuum L.) Depresses Performance and Alters Activities of Protective, Detoxification and Digestive Enzymes of Green Peach Aphid [Myzus persicae (Sulzer) (Hemiptera: Aphididae)]. J. Insect Sci. 2022, 22, 11. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Han, W.-H.; Wang, J.-X.; Zhang, F.-B.; Ji, S.-X.; Zhong, Y.-W.; Liu, S.-S.; Wang, X.-W. Differential Induction of JA/SA Determines Plant Defense against Successive Leaf-Chewing and Phloem-Feeding Insects. J. Pest Sci. 2024, 1–16. [Google Scholar] [CrossRef]
- Upadhyay, R.; Saini, R.; Shukla, P.K.; Tiwari, K.N. Role of Secondary Metabolites in Plant Defense Mechanisms: A Molecular and Biotechnological Insights. Phytochem. Rev. 2025, 24, 953–983. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Kalske, A.; Shiojiri, K.; Uesugi, A.; Sakata, Y.; Morrell, K.; Kessler, A. Insect Herbivory Selects for Volatile-Mediated Plant-Plant Communication. Curr. Biol. 2019, 29, 3128–3133.e3. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, R.; Sun, Y.; Zhang, M.; Li, S.; Xu, Y.; Song, J.; Li, J.; Qi, J.; Wang, L.; et al. ZmMYC2s Play Important Roles in Maize Responses to Simulated Herbivory and Jasmonate. J. Integr. Plant Biol. 2023, 65, 1041–1058. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Bredlau, J.; Kester, K.; Creighton, C.; Kaplan, I. Toxin or Medication? Immunotherapeutic Effects of Nicotine on a Specialist Caterpillar. Funct. Ecol. 2021, 35, 614–626. [Google Scholar] [CrossRef]
- Onkokesung, N.; Gaquerel, E.; Kotkar, H.; Kaur, H.; Baldwin, I.T.; Galis, I. MYB8 Controls Inducible Phenolamide Levels by Activating Three Novel Hydroxycinnamoyl-Coenzyme a: Polyamine Transferases in Nicotiana attenuata. Plant Physiol. 2012, 158, 389–407. [Google Scholar] [CrossRef]
- Schweizer, F.; Fernández-Calvo, P.; Zander, M.; Diez-Diaz, M.; Fonseca, S.; Glauser, G.; Lewsey, M.G.; Ecker, J.R.; Solano, R.; Reymond, P. Arabidopsis Basic Helix-Loop-Helix Transcription Factors MYC2, MYC3, and MYC4 Regulate Glucosinolate Biosynthesis, Insect Performance, and Feeding Behavior. Plant Cell 2013, 25, 3117–3132. [Google Scholar] [CrossRef]
- Woldemariam, M.G.; Dinh, S.T.; Oh, Y.; Gaquerel, E.; Baldwin, I.T.; Galis, I. NaMYC2 Transcription Factor Regulates a Subset of Plant Defense Responses in Nicotiana attenuata. BMC Plant Biol. 2013, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hong, G.; Li, H.; Bing, X.; Chen, Y.; Jing, X.; Gershenzon, J.; Lou, Y.; Baldwin, I.T.; Li, R. Sakuranetin Protects Rice from Brown Planthopper Attack by Depleting Its Beneficial Endosymbionts. Proc. Natl. Acad. Sci. USA 2023, 120, e2305007120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Lin, S.; Xing, Y.; Zhang, X.; Ye, M.; Chang, Y.; Guo, H.; Sun, X. (+)-catechin, Epicatechin and Epigallocatechin Gallate Are Important Inducible Defensive Compounds Against Ectropis grisescens in Tea Plants. Plant Cell Environ. 2022, 45, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Dong, Y.; Li, X.; Xing, Y.; Liu, M.; Sun, X. JA-Ile-Macrolactone 5b Induces Tea Plant (Camellia sinensis) Resistance to Both Herbivore Ectropis Obliqua and Pathogen Colletotrichum camelliae. Int. J. Mol. Sci. 2020, 21, 1828. [Google Scholar] [CrossRef]
- Lin, S.; Ye, M.; Li, X.; Xing, Y.; Liu, M.; Zhang, J.; Sun, X. A Novel Inhibitor of the Jasmonic Acid Signaling Pathway Represses Herbivore Resistance in Tea Plants. Hortic. Res. 2022, 9, uhab038. [Google Scholar] [CrossRef]
- Xin, Z.; Zhang, Z.; Chen, Z.; Sun, X. Salicylhydroxamic Acid (SHAM) Negatively Mediates Tea Herbivore-Induced Direct and Indirect Defense Against the Tea Geometrid Ectropis obliqua. J. Plant Res. 2014, 127, 565–572. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Ye, M.; Li, X.-W.; Lin, S.-B.; Sun, X.-L. The Jasmonic Acid Pathway Positively Regulates the Polyphenol Oxidase-Based Defense Against Tea Geometrid Caterpillars in the Tea Plant (Camellia sinensis). J. Chem. Ecol. 2020, 46, 308–316. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Duan, X.-N.; Jin, S.; Li, X.-W.; Chen, Z.-M.; Ren, B.-Z.; Sun, X.-L. Regurgitant Derived from the Tea Geometrid Ectropis obliqua Suppresses Wound-Induced Polyphenol Oxidases Activity in Tea Plants. J. Chem. Ecol. 2013, 39, 744–751. [Google Scholar] [CrossRef]
- Zhang, X.; Ran, W.; Li, X.; Zhang, J.; Ye, M.; Lin, S.; Liu, M.; Sun, X. Exogenous Application of Gallic Acid Induces the Direct Defense of Tea Plant against Ectropis obliqua Caterpillars. Front. Plant Sci. 2022, 13, 833489. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, H.; Peng, Q.; Schreiner, M.; Baldermann, S.; Lin, Z. Integrated Proteomic and Metabolomic Analyses Reveal the Importance of Aroma Precursor Accumulation and Storage in Methyl Jasmonate-Primed Tea Leaves. Hortic. Res. 2021, 8, 95. [Google Scholar] [CrossRef]
- Jing, T.; Du, W.; Qian, X.; Wang, K.; Luo, L.; Zhang, X.; Deng, Y.; Li, B.; Gao, T.; Zhang, M.; et al. UGT89AC1-mediated Quercetin Glucosylation Is Induced upon Herbivore Damage and Enhances Camellia sinensis Resistance to Insect Feeding. Plant Cell Environ. 2024, 47, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fu, J.; Xu, Y.; Shen, Y.; Zhang, Y.; Ye, Z.; Tong, W.; Zeng, X.; Yang, J.; Tang, D.; et al. CsMYB1 Integrates the Regulation of Trichome Development and Catechins Biosynthesis in Tea Plant Domestication. New Phytol. 2022, 234, 902–917. [Google Scholar] [CrossRef]
- Liu, C.-F.; Yang, N.; Teng, R.-M.; Li, J.-W.; Chen, Y.; Hu, Z.-H.; Li, T.; Zhuang, J. Exogenous Methyl Jasmonate and Cytokinin Antagonistically Regulate Lignin Biosynthesis by Mediating CsHCT Expression in Camellia sinensis. Protoplasma 2023, 260, 869–884. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, J.; Lv, Z.; Shao, C.; Chen, Z.; Zhou, Y.; Shen, C. Tea-Derived Polyphenols Enhance Drought Resistance of Tea Plants (Camellia sinensis) by Alleviating Jasmonate–Isoleucine Pathway and Flavonoid Metabolism Flow. Int. J. Mol. Sci. 2024, 25, 3817. [Google Scholar] [CrossRef] [PubMed]
- Kano, A.; Gomi, K.; Yamasaki-Kokudo, Y.; Satoh, M.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; Ishida, Y.; et al. A Rare Sugar, d -Allose, Confers Resistance to Rice Bacterial Blight with Upregulation of Defense-Related Genes in Oryza sativa. Phytopathology 2010, 100, 85–90. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Song, F. D-Allose Is a Critical Regulator of Inducible Plant Immunity in Tomato. Physiol. Mol. Plant Pathol. 2020, 111, 101507. [Google Scholar] [CrossRef]
- Mochizuki, S.; Fukumoto, T.; Ohara, T.; Ohtani, K.; Yoshihara, A.; Shigematsu, Y.; Tanaka, K.; Ebihara, K.; Tajima, S.; Gomi, K.; et al. The Rare Sugar D-Tagatose Protects Plants from Downy Mildews and Is a Safe Fungicidal Agrochemical. Commun. Biol. 2020, 3, 423. [Google Scholar] [CrossRef]
- Kano, A.; Hosotani, K.; Gomi, K.; Yamasaki-Kokudo, Y.; Shirakawa, C.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; et al. D-Psicose Induces Upregulation of Defense-Related Genes and Resistance in Rice Against Bacterial Blight. J. Plant Physiol. 2011, 168, 1852–1857. [Google Scholar] [CrossRef]
- Kano, A.; Fukumoto, T.; Ohtani, K.; Yoshihara, A.; Ohara, T.; Tajima, S.; Izumori, K.; Tanaka, K.; Ohkouchi, T.; Ishida, Y.; et al. The Rare Sugar D-Allose Acts as a Triggering Molecule of Rice Defence via ROS Generation. J. Exp. Bot. 2013, 64, 4939–4951. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, X.; Guo, R.; Xia, X.; Liu, L.; An, Y.; Yan, X.; Wang, S.; Guo, L.; Wei, C. The Biosynthesis of Main Taste Compounds Is Coordinately Regulated by miRNAs and Phytohormones in Tea Plant (Camellia sinensis). J. Agric. Food Chem. 2020, 68, 6221–6236. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of Gamma-Aminobutyric Acid Increased the Level of Phytohormones in Citrus Sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Li, J.; Ali, M.; Wang, Y.; Liu, X.; Li, F.; Li, X. GABA Primes Defense Responses Against Botrytis cinerea in Tomato Fruit by Modulating Ethylene and JA Signaling Pathways. Postharvest Biol. Technol. 2024, 208, 112665. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Y.; Ma, L.; Cao, D.; Jin, X. Physiological and Metabolomic Analyses Reveal the Resistance Response Mechanism to Tea Aphid Infestation in New Shoots of Tea Plants (Camellia sinensis). Plant Stress 2024, 13, 100545. [Google Scholar] [CrossRef]
- Liao, Y.; Yu, Z.; Liu, X.; Zeng, L.; Cheng, S.; Li, J.; Tang, J.; Yang, Z. Effect of Major Tea Insect Attack on Formation of Quality-Related Nonvolatile Specialized Metabolites in Tea (Camellia sinensis) Leaves. J. Agric. Food Chem. 2019, 67, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, Y.; Yan, X.; Liu, L.; Guo, R.; Xia, X.; Cheng, D.; Mi, X.; Samarina, L.; Liu, S.; et al. Duplication and Transcriptional Divergence of Three Kunitz Protease Inhibitor Genes That Modulate Insect and Pathogen Defenses in Tea Plant (Camellia sinensis). Hortic. Res. 2019, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Lin, H. Contribution of Phenylpropanoid Metabolism to Plant Development and Plant–Environment Interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, M.; Fan, J.; Lu, C.; Dong, W.; Chen, W.; Chen, Z.; Dai, X.; He, Y.; Niu, S. Integrated Metabolomic and Transcriptomic Profiling Reveals the Defense Response of Tea Plants (Camellia sinensis) to Toxoptera aurantii. J. Agric. Food Chem. 2024, 72, 27125–27138. [Google Scholar] [CrossRef]
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Inzé, D.; Goossens, A. Mapping Methyl Jasmonate-Mediated Transcriptional Reprogramming of Metabolism and Cell Cycle Progression in Cultured Arabidopsis Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef]
- Hyde, L.S.; Pellny, T.K.; Freeman, J.; Michaelson, L.V.; Simister, R.; McQueen-Mason, S.J.; Mitchell, R.A.C. Response of Cell-Wall Composition and RNA-Seq Transcriptome to Methyl-Jasmonate in Brachypodium distachyon Callus. Planta 2018, 248, 1213–1229. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Qian, X.; Li, X.; Chai, Z.; Ni, D.; Sun, X. Ectropis obliqua-Induced Secondary Metabolites Are Regulated by Methyl Jasmonate in a Threshold-Dependent Manner. Int. J. Mol. Sci. 2025, 26, 4248. https://doi.org/10.3390/ijms26094248
Yu Y, Qian X, Li X, Chai Z, Ni D, Sun X. Ectropis obliqua-Induced Secondary Metabolites Are Regulated by Methyl Jasmonate in a Threshold-Dependent Manner. International Journal of Molecular Sciences. 2025; 26(9):4248. https://doi.org/10.3390/ijms26094248
Chicago/Turabian StyleYu, Yongchen, Xiaona Qian, Xiwang Li, Zhichao Chai, Dejiang Ni, and Xiaoling Sun. 2025. "Ectropis obliqua-Induced Secondary Metabolites Are Regulated by Methyl Jasmonate in a Threshold-Dependent Manner" International Journal of Molecular Sciences 26, no. 9: 4248. https://doi.org/10.3390/ijms26094248
APA StyleYu, Y., Qian, X., Li, X., Chai, Z., Ni, D., & Sun, X. (2025). Ectropis obliqua-Induced Secondary Metabolites Are Regulated by Methyl Jasmonate in a Threshold-Dependent Manner. International Journal of Molecular Sciences, 26(9), 4248. https://doi.org/10.3390/ijms26094248




