CD274 (PD-L1) Polymorphisms as Predictors of Efficacy in First-Line Platinum-Based Chemotherapy for Extensive-Stage Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics and Clinical Variants Analyses
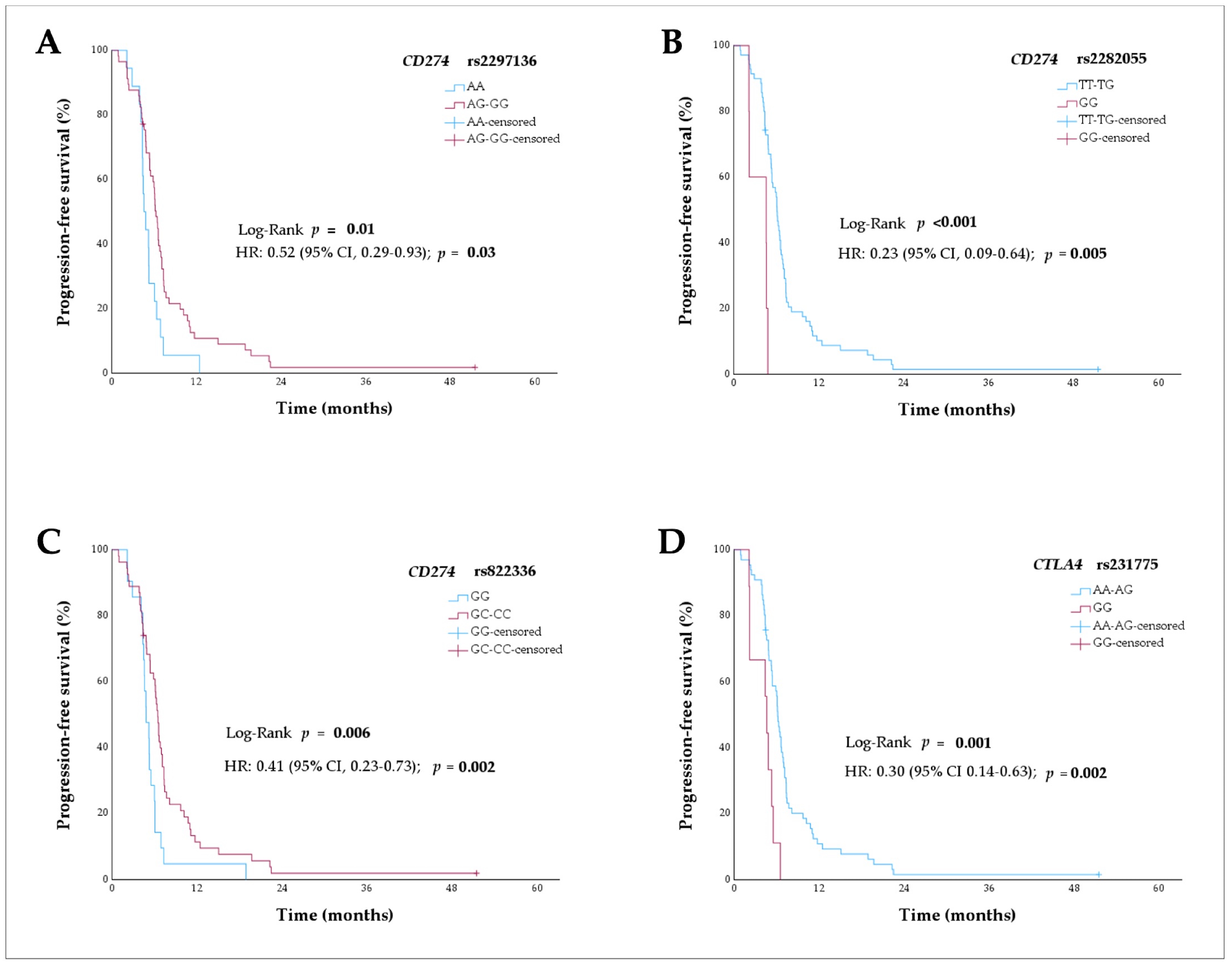
2.2. Polymorphisms of Immune Checkpoint Genes and Progression-Free Survival Analyses
2.3. Polymorphisms of Immune Checkpoint Genes and Overall Survival Analyses
2.4. Haplotype Analyses for CD274 Variants and Survival
2.5. Polymorphisms of Immune Checkpoint Genes and Platinum Sensitivity Analyses
2.6. Analyses of PD-L1 Protein Expression and CD274 Polymorphisms
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Definitions of Clinical Endpoints
4.3. Selection of Polymorphisms
4.4. Genotyping
4.5. Immunohistochemistry Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lung Cancer Statistics|How Common Is Lung Cancer? Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 12 February 2025).
- Ou, S.H.I.; Ziogas, A.; Zell, J.A. Prognostic Factors for Survival in Extensive Stage Small Cell Lung Cancer (ED-SCLC): The Importance of Smoking History, Socioeconomic and Marital Statuses, and Ethnicity. J. Thorac. Oncol. 2009, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum-Etoposide versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients with Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Reck, M.; Peters, S.; Borghaei, H.; Herbst, R.; Siddiqui, M.; Cuchelkar, V.; Bhatt, K.; Chakrabarti, D.; Wang, L.; et al. IMporte: A Phase III Study of Lurbinectedin and Atezolizumab Versus Atezolizumab as Maintenance Therapy in ES-SCLC. In Proceedings of the Presented at World Conference on Lung Cancer (WCLC) 2022 (Abstract 2350), Vienna, Austria, 6–9 August 2022. [Google Scholar]
- Perol, M.; Ahn, M.-J.; Cheng, Y.; Clarke, J.; Dingemans, A.-M.; Gay, C.; Navarro, A.; Schuler, M.; Yoshida, T.; Martinez, P.; et al. Tarlatamab Plus Durvalumab as First-Line Maintenance in Extensive-Stage Small-Cell Lung Cancer: DeLLphi-305 Phase 3 Trial. In Proceedings of the Presented at World Conference on Lung Cancer (WCLC) 2024 (P1.13A.02), San Diego, CA, USA, 7–10 September 2024. [Google Scholar]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Dis. Prim. 2021, 7, 3. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular Subtypes of Small Cell Lung Cancer: A Synthesis of Human and Mouse Model Data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Keppens, C.; Dequeker, E.M.; Pauwels, P.; Ryska, A.; ‘t Hart, N.; von der Thüsen, J.H. PD-L1 Immunohistochemistry in Non-Small-Cell Lung Cancer: Unraveling Differences in Staining Concordance and Interpretation. Virchows Arch. 2021, 478, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA Repair by ERCC1 in Non-Small-Cell Lung Cancer and Cisplatin-Based Adjuvant Chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B. Pharmacogenetics of Platinum-Based Chemotherapy in Non-Small Cell Lung Cancer: Predictive Validity of Polymorphisms of ERCC1. Expert Opin. Drug Metab. Toxicol. 2018, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; López-Vilaró, L.; Ferre, M.; Majem, M.; Martinez-Recio, S.; Bell, O.; Arranz, M.J.; Salazar, J.; Sullivan, I. ERCC1 and ERCC2 Polymorphisms Predict the Efficacy and Toxicity of Platinum-Based Chemotherapy in Small Cell Lung Cancer. Pharmaceutics 2024, 16, 1121. [Google Scholar] [CrossRef]
- Todd, R.C.; Lippard, S.J. Inhibition of Transcription by Platinum Antitumor Compounds. Metallomics 2009, 1, 280–291. [Google Scholar] [CrossRef]
- De Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-Induced Antitumor Immunomodulation: A Review of Preclinical and Clinical Evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef] [PubMed]
- Nejad, E.B.; Van Der Sluis, T.C.; Van Duikeren, S.; Yagita, H.; Janssen, G.M.; Van Veelen, P.A.; Melief, C.J.M.; Van Der Burg, S.H.; Arens, R. Tumor Eradication by Cisplatin Is Sustained by CD80/86-Mediated Costimulation of CD8+ T Cells. Cancer Res. 2016, 76, 6017–6029. [Google Scholar] [CrossRef] [PubMed]
- Van Wigcheren, G.F.; De Haas, N.; Mulder, T.A.; Horrevorts, S.K.; Bloemendal, M.; Hins-Debree, S.; Mao, Y.; Kiessling, R.; van Herpen, C.M.L.; Flórez-Grau, G.; et al. Cisplatin Inhibits Frequency and Suppressive Activity of Monocytic Myeloid-Derived Suppressor Cells in Cancer Patients. Oncoimmunology 2021, 10, 1935557. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the Tumor Microenvironment in PD-L1/PD-1-Mediated Tumor Immune Escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Kula, A.; Dawidowicz, M.; Kiczmer, P.; Prawdzic Seńkowska, A.; Świętochowska, E. The Role of Genetic Polymorphism within PD-L1 Gene in Cancer. Review. Exp. Mol. Pathol. 2020, 116, 104494. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, D.K.; Choi, J.E.; Jin, C.C.; Hong, M.J.; Do, S.K.; Kang, H.G.; Lee, W.K.; Seok, Y.; Lee, E.B.; et al. PD-L1 Polymorphism Can Predict Clinical Outcomes of Non-Small Cell Lung Cancer Patients Treated with First-Line Paclitaxel-Cisplatin Chemotherapy. Sci. Rep. 2016, 6, 25952. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, D.K.; Choi, J.E.; Jin, C.C.; Hong, M.J.; Do, S.K.; Kang, H.G.; Lee, W.K.; Seok, Y.; Lee, E.B.; et al. Functional Polymorphisms in PD-L1 Gene Are Associated with the Prognosis of Patients with Early Stage Non-Small Cell Lung Cancer. Gene 2017, 599, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, J.; Chen, S.; Wang, Y.; Tian, Q. Influence of Programmed Death Ligand-1-Gene Polymorphism Rs822336 on the Prognosis and Safety of Postoperative Patients with NSCLC Who Received Platinum-Based Adjuvant Chemotherapy. Cancer Manag. Res. 2020, 12, 6755–6766. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoo, S.S.; Hong, M.J.; Choi, J.E.; Kim, S.; Kang, H.G.; Do, S.K.; Kim, J.H.; Baek, S.A.; Lee, W.K.; et al. Impact of Immune Checkpoint Gene CD155 Ala67Thr and CD226 Gly307Ser Polymorphisms on Small Cell Lung Cancer Clinical Outcome. Sci. Rep. 2021, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.S.; Rosenthal, A.; Nicholson, A.G.; Detterbeck, F.; Eberhardt, W.E.E.; Lievens, Y.; Lim, E.; Matilla, J.-M.; Yatabe, Y.; Filosso, P.L.; et al. The International Association for the Study of Lung Cancer Staging Project: The Database and Proposal for the Revision of the Staging of Pulmonary Neuroendocrine Carcinoma in the Forthcoming Ninth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2025. [Google Scholar] [CrossRef]
- Baize, N.; Monnet, I.; Greillier, L.; Geier, M.; Lena, H.; Janicot, H.; Vergnenegre, A.; Crequit, J.; Lamy, R.; Auliac, J.B.; et al. Carboplatin plus Etoposide versus Topotecan as Second-Line Treatment for Patients with Sensitive Relapsed Small-Cell Lung Cancer: An Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1224–1233. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Behera, M.; Chen, Z.; Bhimani, C.; Curran, W.J.; Khuri, F.R.; Ramalingam, S.S. A Systematic Analysis of Efficacy of Second-Line Chemotherapy in Sensitive and Refractory Small-Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 866–872. [Google Scholar] [CrossRef]
- Fabrizio, F.P.; Trombetta, D.; Rossi, A.; Sparaneo, A.; Castellana, S.; Muscarella, L.A. Gene Code CD274/PD-L1: From Molecular Basis toward Cancer Immunotherapy. Ther. Adv. Med. Oncol. 2018, 10, 1–18. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Rs2282055. 2025. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2282055 (accessed on 10 May 2024).
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of Functional Variation in Personal Genomes Using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Hausser, J.; Zavolan, M. Identification and Consequences of MiRNA-Target Interactions—Beyond Repression of Gene Expression. Nat. Rev. Genet. 2014, 15, 599–612. [Google Scholar] [CrossRef]
- Du, W.; Zhu, J.; Chen, Y.; Zeng, Y.; Shen, D.; Zhang, N.; Ning, W.; Liu, Z.; Huang, J.A. Variant SNPs at the MicroRNA Complementary Site in the B7-H1 3′-untranslated Region Increase the Risk of Non-small Cell Lung Cancer. Mol. Med. Rep. 2017, 16, 2682–2690. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Scheel, A.H.; Ozretić, L.; George, J.; Thomas, R.K.; Hagemann, T.; Zander, T.; Wolf, J.; Buettner, R. PD-L1 Expression in Small Cell Neuroendocrine Carcinomas. Eur. J. Cancer 2015, 51, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 Expression in the Tumor Microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Rs231775. 2025. Available online: https://www.ncbi.nlm.nih.gov/snp/rs231775 (accessed on 10 May 2024).
- Anjos, S.; Nguyen, A.; Ounissi-Benkalha, H.; Tessier, M.C.; Polychronakos, C. A Common Autoimmunity Predisposing Signal Peptide Variant of the Cytotoxic T-Lymphocyte Antigen 4 Results in Inefficient Glycosylation of the Susceptibility Allele. J. Biol. Chem. 2002, 277, 46478–46486. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Wagner, M.; Jasek, M.; Karabon, L. Immune Checkpoint Molecules-Inherited Variations as Markers for Cancer Risk. Front. Immunol. 2021, 11, 606721. [Google Scholar] [CrossRef]
- Nomizo, T.; Ozasa, H.; Tsuji, T.; Funazo, T.; Yasuda, Y.; Yoshida, H.; Yagi, Y.; Sakamori, Y.; Nagai, H.; Hirai, T.; et al. Clinical Impact of Single Nucleotide Polymorphism in PD-L1 on Response to Nivolumab for Advanced Non-Small-Cell Lung Cancer Patients. Sci. Rep. 2017, 7, 45124. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, T.; Jia, Z.; Cao, D.; Cao, X.; Pan, Y.; Zhao, D.; Zhang, B.; Jiang, J. Polymorphism of the Programmed Death-Ligand 1 Gene Is Associated with Its Protein Expression and Prognosis in Gastric Cancer. J. Gastroenterol. Hepatol. 2019, 34, 1201–1207. [Google Scholar] [CrossRef]
- Funazo, T.Y.; Nomizo, T.; Ozasa, H.; Tsuji, T.; Yasuda, Y.; Yoshida, H.; Sakamori, Y.; Nagai, H.; Hirai, T.; Kim, Y.H. Clinical Impact of Low Serum Free T4 in Patients with Non-Small Cell Lung Cancer Treated with Nivolumab. Sci. Rep. 2019, 9, 17085. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Karami, S.; Sarabandi, S.; Moazeni-Roodi, A.; Małecki, A.; Ghavami, S.; Wiechec, E. Association between PD-1 and PD-L1 Polymorphisms and the Risk of Cancer: A Meta-Analysis of Case-Control Studies. Cancers 2019, 11, 1150. [Google Scholar] [CrossRef]
- de With, M.; Hurkmans, D.P.; De Hoop, E.O.; Lalouti, A.; Bins, S.; El Bouazzaoui, S.; van Brakel, M.; Debets, R.; Aerts, J.G.J.V.; van Schaik, R.H.N.; et al. Germline Variation in PDCD1 Is Associated with Overall Survival in Patients with Metastatic Melanoma Treated with Anti-PD-1 Monotherapy. Cancers 2021, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Sunakawa, Y.; Cao, S.; Volz, N.B.; Berger, M.D.; Yang, D.; Parekh, A.; Zhang, W.; Matsusaka, S.; Ning, Y.; Stremitzer, S.; et al. Genetic Variations in Immunomodulatory Pathways to Predict Survival in Patients with Locoregional Gastric Cancer. Pharmacogenom. J. 2017, 17, 528–534. [Google Scholar] [CrossRef]
- Wagner, M.; Tupikowski, K.; Jasek, M.; Tomkiewicz, A.; Witkowicz, A.; Ptaszkowski, K.; Karpinski, P.; Zdrojowy, R.; Halon, A.; Karabon, L. SNP-SNP Interaction in Genes Encoding PD-1/PD-L1 Axis as a Potential Risk Factor for Clear Cell Renal Cell Carcinoma. Cancers 2020, 12, 3521. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Musafer, A.; Paessler, S.; Witkowski, T.; Suen, C.S.N.L.W.; Tutuka, C.S.A.; Carlino, M.S.; Menzies, A.M.; Scolyer, R.A.; Cebon, J.; et al. PDCD1 Polymorphisms May Predict Response to Anti-PD-1 Blockade in Patients With Metastatic Melanoma. Front. Immunol. 2021, 12, 672521. [Google Scholar] [CrossRef] [PubMed]
- Breunis, W.B.; Tarazona-Santos, E.; Chen, R.; Kiley, M.; Rosenberg, S.A.; Chanock, S.J. Influence of Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA4) Common Polymorphisms on Outcome in Treatment of Melanoma Patients with CTLA-4 Blockade. J. Immunother. 2008, 31, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Jiang, M.; Machado-Rugolo, J.; Yaegashi, L.B.; Prieto, T.; Farhat, C.; de Sá, V.K.; Nagai, M.A.; de Lima, V.C.C.; Takagaki, T.; et al. Variants in Epithelial-Mesenchymal Transition and Immune Checkpoint Genes Are Associated With Immune Cell Profiles and Predict Survival in Non-Small Cell Lung Cancer. Arch. Pathol. Lab. Med. 2020, 144, 1234–1244. [Google Scholar] [CrossRef]
- Houcken, J.; Degenhart, C.; Bender, K.; König, J.; Frommer, L.; Kahaly, G.J. PTPN22 and CTLA-4 Polymorphisms Are Associated With Polyglandular Autoimmunity. J. Clin. Endocrinol. Metab. 2018, 103, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Jiang, J.; Li, H.; Zhang, R. Role of CD226 Rs763361 Polymorphism in Susceptibility to Multiple Autoimmune Diseases. Immunol. Investig. 2020, 49, 926–942. [Google Scholar] [CrossRef]
- AL-Eitan, L.; Al Qudah, M.; Al Qawasmeh, M. Candidate Gene Association Analysis of Multiple Sclerosis in the Jordanian Arab Population: A Case-Control Study. Gene 2020, 758, 144959. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Leng, S.; Xu, Q.; Sheng, Z.; Zhang, Y.; Yu, J.; Feng, Q.; Hou, M.; Peng, J.; et al. Immune Checkpoint-Related Gene Polymorphisms Are Associated With Primary Immune Thrombocytopenia. Front. Immunol. 2021, 11, 615941. [Google Scholar] [CrossRef]
- Lee, K.M.; Baris, D.; Zhang, Y.; Hosgood, H.D.; Menashe, I.; Yeager, M.; Zahm, S.H.; Wang, S.S.; Purdue, M.P.; Chanock, S.; et al. Common Single Nucleotide Polymorphisms in Immunoregulatory Genes and Multiple Myeloma Risk among Women in Connecticut. Am. J. Hematol. 2010, 85, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Garassino, M.C.; Chen, Y.; Reinmuth, N.; Hotta, K.; Poltoratskiy, A.; Trukhin, D.; Hochmair, M.J.; Özgüroǧlu, M.; Ji, J.H.; et al. Durvalumab ± Tremelimumab + Platinum-Etoposide in Extensive-Stage Small Cell Lung Cancer (CASPIAN): Outcomes by PD-L1 Expression and Tissue Tumor Mutational Burden. Clin. Cancer Res. 2024, 30, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, E.; FAul, F.; Buchner, A.; Lang, A.G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
| Characteristic | n = 78 |
|---|---|
| Male (%) | 56 (71.8) |
| Age, median (range), years | 65 (38–86) |
| ECOG performance status (%) | |
| 0 | 5 (6.4) |
| 1 | 41 (56.2) |
| 2 | 22 (28.2) |
| 3–4 | 8 (10.3) |
| Unknown | 2 (2.6) |
| Smoking (%) | |
| Current | 52 (66.7) |
| Former | 25 (32.1) |
| Unknown | 1 (1.3) |
| Brain metastases (%) | 16 (21.3) |
| Liver metastases (%) | 35 (44.9) |
| Chemotherapy regimen selected (%) | |
| Cisplatin | 26 (33.3) |
| Carboplatin | 52 (66.7) |
| Median number of cycles (range) | 6 (2–6) |
| Consolidative thoracic radiation therapy (%) | 19 (24.4) |
| Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| SNP | n = 75 | mPFS (95% CI), Months | HR (95% CI) | pa Value | HR (95% CI) | pb Value |
| CD274 rs2297136 | ||||||
| AA | 18 | 4.6 (3.8–5.3) | Reference (1) | 0.007 | ||
| AG | 43 | 6.1 (5.2–6.9) | 0.58 (0.33–1.02) | |||
| GG | 14 | 6.8 (6.2–7.4) | 0.30 (0.14–0.66) | |||
| AA | 18 | 4.6 (3.8–5.3) | Reference (1) | |||
| AG-GG | 57 | 6.3 (5.7–6.8) | 0.50 (0.29–0.87) | 0.01 * | 0.52 (0.29–0.93) | 0.03 |
| CD274 rs2282055 | ||||||
| TT | 42 | 6.1 (5.1–7.0) | Reference (1) | 0.001 | ||
| TG | 28 | 6.1 (4.4–7.8) | 0.79 (0.48–1.28) | |||
| GG | 5 | 4.6 (0.0–9.7) | 4.48 (1.64–12.23) | |||
| GG | 5 | 4.6 (0.0–9.7) | Reference (1) | |||
| TT-TG | 70 | 6.1 (5.6–6.6) | 0.20 (0.08–0.55) | <0.001 ** | 0.23 (0.09–0.64) | 0.005 |
| CD274 rs822336 | ||||||
| GG | 21 | 4.8 (3.9–5.8) | Reference (1) | 0.02 | ||
| GC | 35 | 6.5 (5.9–7.0) | 0.48 (0.27–0.84) | |||
| CC | 19 | 6.6 (5.9–7.3) | 0.50 (0.26–0.96) | |||
| GG | 21 | 4.8 (3.9–5.8) | Reference (1) | |||
| GC-CC | 54 | 6.5 (6.0–7.0) | 0.48 (0.29–0.82) | 0.006 * | 0.41 (0.23–0.73) | 0.002 |
| CTLA4 rs231775 | ||||||
| AA | 34 | 6.1 (5.9–6.3) | Reference (1) | 0.005 | ||
| AG | 32 | 6.3 (4.6–7.9) | 0.94 (0.58–1.55) | |||
| GG | 9 | 4.6 (3.9–5.3) | 3.10 (1.43–6.72) | |||
| GG | 9 | 4.6 (3.9–5.3) | Reference (1) | |||
| AA-AG | 66 | 6.1 (5.7–6.5) | 0.31 (0.15–0.07) | 0.001 ** | 0.30 (0.14–0.63) | 0.002 |
| Gene Symbol | Reference SNP | Variant Description | References for Rationale |
|---|---|---|---|
| CD274 (PD-L1) | rs4143815 | 3′-UTR | [23,24,34,42] |
| rs2297136 | 3′-UTR | [23,34,43] | |
| rs2282055 | Intron | [42,44] | |
| rs822336 | 2 KB Upstream | [24,25,43] | |
| PDCD1 | rs2227981 | Synonymous | [45,46] |
| rs10204525 | 3′-UTR | [47,48] | |
| rs11568821 | Intron | [45,49] | |
| rs7421861 | Intron | [45,48] | |
| CTLA4 | rs4553808 | 2 KB Upstream | [50] |
| rs231775 | Missense | [50,51] | |
| rs3087243 | 500B Downstream | [52] | |
| CD226 | rs763361 | Missense | [26,53] |
| LAG3 | rs2365095 | Intron | [54] |
| rs870849 | Missense | [55] | |
| rs3782735 | Intron | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba, A.; López-Vilaró, L.; Ferre, M.; Martinez-Recio, S.; Majem, M.; Sullivan, I.; Salazar, J. CD274 (PD-L1) Polymorphisms as Predictors of Efficacy in First-Line Platinum-Based Chemotherapy for Extensive-Stage Small Cell Lung Cancer. Int. J. Mol. Sci. 2025, 26, 4245. https://doi.org/10.3390/ijms26094245
Barba A, López-Vilaró L, Ferre M, Martinez-Recio S, Majem M, Sullivan I, Salazar J. CD274 (PD-L1) Polymorphisms as Predictors of Efficacy in First-Line Platinum-Based Chemotherapy for Extensive-Stage Small Cell Lung Cancer. International Journal of Molecular Sciences. 2025; 26(9):4245. https://doi.org/10.3390/ijms26094245
Chicago/Turabian StyleBarba, Andrés, Laura López-Vilaró, Malena Ferre, Sergio Martinez-Recio, Margarita Majem, Ivana Sullivan, and Juliana Salazar. 2025. "CD274 (PD-L1) Polymorphisms as Predictors of Efficacy in First-Line Platinum-Based Chemotherapy for Extensive-Stage Small Cell Lung Cancer" International Journal of Molecular Sciences 26, no. 9: 4245. https://doi.org/10.3390/ijms26094245
APA StyleBarba, A., López-Vilaró, L., Ferre, M., Martinez-Recio, S., Majem, M., Sullivan, I., & Salazar, J. (2025). CD274 (PD-L1) Polymorphisms as Predictors of Efficacy in First-Line Platinum-Based Chemotherapy for Extensive-Stage Small Cell Lung Cancer. International Journal of Molecular Sciences, 26(9), 4245. https://doi.org/10.3390/ijms26094245







