Abstract
Bile acids and their corresponding intestinal epithelial receptors, the farnesoid X receptor (FXR), the G protein-coupled bile acid receptor (TGR5), play crucial roles in the physiological and pathological processes of intestinal epithelial cells. These acids and receptors are involved in the regulation of intestinal absorption, signal transduction, cellular proliferation and repair, cellular senescence, energy metabolism, and the modulation of gut microbiota. A comprehensive literature search was conducted using PubMed, employing keywords such as bile acid, bile acid receptor, FXR (nr1h4), TGR5 (gpbar1), intestinal epithelial cells, proliferation, differentiation, senescence, energy metabolism, gut microbiota, inflammatory bowel disease (IBD), colorectal cancer (CRC), and irritable bowel syndrome (IBS), with a focus on publications available in English. This review examines the diverse effects of bile acid signaling and bile receptor pathways on the proliferation, differentiation, senescence, and energy metabolism of intestinal epithelial cells. Additionally, it explores the interactions between bile acids, their receptors, and the microbiota, as well as the implications of these interactions for host health, particularly in relation to prevalent intestinal diseases. Finally, the review highlights the importance of developing highly specific ligands for FXR and TGR5 receptors in the context of metabolic and intestinal disorders.
1. Introduction
1.1. Physiological Functions of Bile Acids
Bile acids are steroid compounds synthesized through cholesterol metabolism and are integral to various physiological processes in humans [1]. Traditionally, the primary function attributed to bile acids has been their role in facilitating the digestion and absorption of lipids, due to their zwitterionic nature [2]. However, emerging research indicates that bile acids possess a broader and more intricate array of physiological functions [3,4,5,6,7]. Firstly, bile acids enhance the emulsification, digestion, and absorption of fat-soluble vitamins and lipids by forming micelles [8]. This mechanism is essential not only for nutrient absorption but also for the maintenance of cholesterol homeostasis [9]. Secondly, bile acids function as signaling molecules that regulate their own synthesis, transport, and metabolism through the activation of specific nuclear receptors, such as farnesoid X receptor (FXR), and membrane receptors, such as TGR5 [2,10]. Furthermore, bile acids are pivotal in the regulation of glucose and lipid metabolism. Research has demonstrated that bile acids influence liver glycogen synthesis, gluconeogenesis, and fatty acid oxidation via the FXR and TGR5 signaling pathways [11,12]. Bile acids were shown to stimulate enteroendocrine cells, leading to the secretion of intestinal hormones such as glucagon-like peptide-1 (GLP-1), thereby further influencing systemic metabolism [13]. Additionally, bile acids are crucial in maintaining intestinal barrier integrity and modulating intestinal immune responses [14]. They contribute to the preservation of intestinal epithelial integrity by modulating the expression and function of tight junction proteins [15]. Furthermore, bile acids are involved in regulating the composition and metabolic activities of the gut microbiota, thereby influencing host–microbe interactions [16,17]. Recent research elucidated the role of bile acids in regulating cellular processes such as proliferation, differentiation, and apoptosis [18]. These insights enhance our understanding of the physiological functions of bile acids as well as identify potential therapeutic targets for various diseases, including inflammatory bowel disease, metabolic syndrome, and certain cancers [11,14].
1.2. Importance of Intestinal Epithelial Cells
Enterocytes, as the principal constituents of the largest mucosal surface within the digestive tract, are crucial for sustaining intestinal functionality and overall systemic health [19]. These highly specialized cells establish a dynamic monolayer barrier that is essential for the selective absorption of nutrients also for defending against pathogenic invasions and preserving intestinal homeostasis [20]. The polarized structure and specialized membrane transporters of enterocytes facilitate the efficient uptake of nutrients [21]. These cells express a diverse array of specific transporters, including glucose transporters SGLT1 and GLUT2, amino acid transporters [22], and fatty acid transporters [23], thereby ensuring the effective absorption of carbohydrates, proteins, and lipids [24]. Furthermore, enterocytes are integral to the intestinal barrier, forming tight junctions, adherens junctions, and desmosome junctions to create a selectively permeable physical barrier [25]. Research has demonstrated that the expression and function of tight junction proteins, including claudins, occludin, and zonula occludens (ZO) proteins, are crucial for preserving the integrity of the intestinal barrier [26]. Furthermore, enterocytes contribute to innate immune defense by producing a range of antimicrobial peptides, such as defensins and cathelicidins, which establish a chemical barrier against pathogens [27]. Concurrently, these cells are capable of recognizing pathogen-associated molecular patterns (PAMPs) and initiating immune responses via pattern recognition receptors, including Toll-like receptors (TLRs) and NOD-like receptors (NLRs) [28,29].
In summary, intestinal epithelial cells are crucial for nutrient absorption, barrier defense, immune regulation, and the maintenance of microbial equilibrium through their diverse functions.
1.3. Bile Acids and Bile Acid Receptors
As the terminal products of cholesterol metabolism, bile acids are not only essential for lipid digestion and absorption but also serve as significant signaling molecules that regulate a variety of physiological processes. In humans, primary bile acids (such as cholic acid and chenodeoxycholic acid) are synthesized by the liver, whereas secondary bile acids (such as deoxycholic acid and lithocholic acid) are generated by the intestinal microbiota through the metabolism of primary bile acids. These bile acids exist either in a free state or conjugated with glycine or taurine, forming a complex bile acid pool within the body. The bile acid pool and its receptors constitute an intricate and sophisticated regulatory network, playing a pivotal role in maintaining homeostasis.
Bile acid receptors primarily encompass the farnesoid X receptor (FXR), the vitamin D receptor (VDR), the pregnane X receptor (PXR), constitutive androstane receptor (CAR), the G protein-coupled bile acid receptor (TGR5), sphingosine-1-phosphate receptor 2 (S1PR2), Mas-related G protein-coupled receptor X4 (MRGPRX4), etc. [30]. The expression patterns of these receptors differ across various intestinal segments and cell types, contributing to a complex signaling network. Bile acids and their receptors are integral in regulating intestinal absorption, signal transduction, cell proliferation and repair, cellular senescence, immune responses, energy metabolism, and the microbiota within intestinal epithelial cells. Among these, FXR and TGR5 are the predominant receptors expressed in intestinal epithelial cells. They are key regulatory factors in bile acid metabolism, innate immunity, energy metabolism, and inflammatory response. Therefore, this review will examine the diverse effects of bile acids and these two receptors on epithelial cells.
2. Effects of Bile Acids and Bile Acid Receptors on Intestinal Epithelial Cell (IEC) Proliferation
Bile acids and their receptors play a multifaceted and significant role in modulating the proliferation of intestinal epithelial cells. This regulatory effect is both concentration-dependent and receptor-specific, and it is crucial for maintaining the integrity and function of the intestinal epithelium [31]. Bile acids have the capacity to induce proliferation of intestinal epithelial cells while limiting apoptosis [18].
2.1. FXR-Mediated Proliferation Regulation
As the principal nuclear receptor for bile acids, FXR is integral to the regulation of IEC proliferation. The regulation of proliferation mediated by FXR encompasses a variety of molecular mechanisms and signaling pathways, with its role exhibiting complexity that is specific to cell type and contingent upon environmental factors.
FXR modulates the proliferation of intestinal epithelial cells through direct influence on the expression of genes associated with the cell cycle [32,33]. Specifically, FXR upregulates cell cycle inhibitors, notably increasing the expression of p21 (CDKN1A) [33,34], which subsequently inhibits epithelial cell proliferation. Empirical evidence indicated that FXR can directly bind to the FXR response element (FXRE) within the promoter region of the p21 gene, thereby enhancing its transcription [35]. Additionally, in certain cell types, FXR is capable of upregulating the expression of p16 (CDKN2A) [36]. The protein p16 functions by inhibiting CDK4/6 activity, thus preventing the transition of cells from the G1 phase to the S phase, effectively regulating cell proliferation [37]. Moreover, FXR activation triggers a pro-apoptotic program in both differentiated normal colon epithelium and transformed colonocytes [33].
FXR also exerts regulatory control over cell-cycle-promoting factors. It influences the expression of cyclin D1 [38,39], although its effects are contingent upon the cell type and environmental context. In certain scenarios, FXR activation may suppress cyclin D1 expression, thereby inhibiting the G1/S phase transition [39]. Furthermore, FXR impacts cell cycle progression by modulating the expression of other cell cycle proteins, such as cyclin E [40] (Figure 1). There is a complex interaction between FXR and the Wnt/β-catenin signaling pathway, which is crucial for the regulation of IEC proliferation [41]. FXR activation can inhibit the nuclear translocation of β-catenin, thereby weakening the activity of the Wnt signaling pathway [42]. This inhibitory effect is achieved by increasing the phosphorylation and degradation of β-catenin [43]. FXR can affect the expression of multiple Wnt target genes, such as c-Myc and cyclinD1, thereby regulating cell proliferation [44,45]. Studies have shown that FXR can directly interact with TCF4 (a key transcription factor in the Wnt signaling pathway) and affect its transcriptional activity [41,43,46,47] (Figure 1).
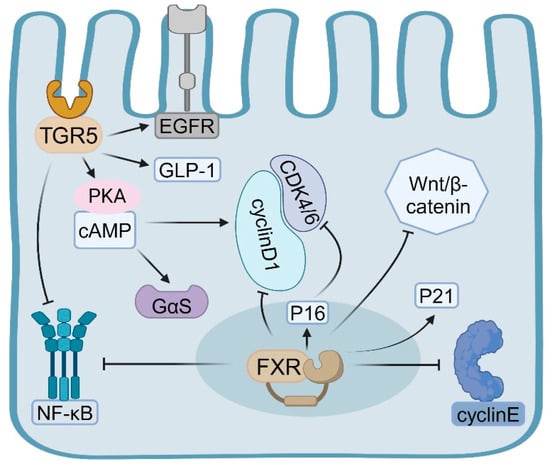
Figure 1.
The influence of bile acids and their associated intestinal epithelial receptors, FXR and TGR5, on the proliferation of intestinal epithelial cells.
The FXR plays a crucial role in modulating the self-renewal and differentiation of intestinal epithelial stem cells, thereby indirectly influencing the proliferation dynamics of the entire intestinal epithelium [48]. Research has demonstrated that FXR, inherent to intestinal macrophages, detects abnormal bile acids, resulting in the secretion of proinflammatory cytokines that subsequently promote the proliferation of intestinal stem cells. Mechanistically, the activation of FXR ameliorates intestinal inflammation and inhibits tumor growth associated with colitis by modulating the recruitment, polarization, and interaction of intestinal macrophages with Th17 cells. Conversely, the absence of FXR in bone marrow or intestinal macrophages exacerbates intestinal inflammation [49]. FXR influences the maintenance and function of stem cells by regulating the expression of stem cell markers such as Lgr5 and Olfm4 [50,51]. Additionally, FXR activation indirectly affects the maintenance of the stem cell niche by modulating the function of Paneth cells [18,52].
2.2. Role of TGR5 in the Cell Cycle
Activation of TGR5 has been shown to facilitate the activation of the EGFR signaling pathway, consequently stimulating cellular proliferation [53]. Research indicates that TGR5 signaling originates from plasma membrane rafts, which enhance EGFR interaction and transcriptional activation. In certain instances, TGR5 can advance cell cycle progression via the cAMP-PKA pathway [54]. Through this pathway, TGR5 modulates the expression of cell cycle proteins, such as Cyclin D1, promoting the transition of cells from the G1 phase to the S phase, thereby accelerating cellular proliferation [55]. Additionally, TGR5 activation can augment cellular resistance to oxidative stress and inflammatory damage, indirectly supporting cell proliferation. Bile acids have been observed to enhance IEC proliferation and mitigate mucosal damage by upregulating TGR5 expression [56,57].
In contrast to the nuclear receptor FXR, TGR5 primarily influences the cell cycle by activating intracellular second messenger systems and downstream signaling pathways. Specifically, TGR5-mediated activation of the cAMP-PKA signaling pathway predominantly stimulates adenylate cyclase through the Gαs protein, resulting in elevated intracellular cAMP levels [58]. Elevated levels of cyclic adenosine monophosphate (cAMP) activate protein kinase A (PKA), which subsequently phosphorylates various downstream target proteins, including those involved in cell cycle regulation [59,60]. PKA is capable of phosphorylating and activating the cAMP-response-element-binding protein (CREB), a crucial transcription factor that modulates the expression of numerous genes associated with the cell cycle [61] (Figure 1).
Activation of the TGR5 receptor facilitates the G1/S phase transition through multiple mechanisms, thereby promoting cellular proliferation [55]. Studies have shown that TGR5 activation upregulates the expression of cyclin D1, a pivotal regulator of the G1/S phase transition [55,62]. Furthermore, TGR5 activation results in the phosphorylation of the epidermal growth factor receptor (EGFR) transmembrane domain, leading to the activation of EGFR and its downstream signaling pathways [63]. Through EGFR transactivation, TGR5 can initiate the MAPK/ERK signaling cascade, which is integral to cell proliferation and survival [61] (Figure 1).
TGR5 indirectly affects cell cycle progression by affecting cellular energy metabolism. TGR5 activation can enhance mitochondrial function and ATP production, providing the necessary energy support for cell cycle progression [64]. Studies have shown that TGR5 reduces ROS generation by inhibiting the NF-κB pathway and activating Nrf2/HO-1 signaling, promoting the expression of antioxidant enzymes and thus protecting against bile duct ligation-induced cholestatic liver disease [65]. Therefore, TGR5 affects cell cycle progression and cell lifespan by regulating cellular antioxidant capacity.
TGR5 exerts an indirect influence on cell cycle progression through its modulation of cellular energy metabolism. Activation of TGR5 has been shown to enhance mitochondrial function and ATP production, thereby supplying the requisite energy for cell cycle advancement [64]. Research indicates that TGR5 mitigates reactive oxygen species (ROS) generation by inhibiting the NF-κB signaling pathway and activating the Nrf2/HO-1 pathway, which promotes the expression of antioxidant enzymes and offers protection against cholestatic liver disease induced by bile duct ligation [65]. Consequently, TGR5 plays a critical role in regulating cell cycle progression and cellular lifespan by modulating the antioxidant capacity of cells. The function of TGR5 varies across different types of intestinal epithelial cells. In intestinal endocrine cells, such as L cells, TGR5 activation primarily influences cellular function rather than proliferation. Notably, studies have demonstrated that ginsenoside compounds can modulate TGR5 activity in L cells, leading to increased expression of GLP-1 [66]. Additionally, bile acid signaling activates intestinal stem cells and promotes epithelial regeneration via TGR5 [18,67].
In addition to its direct influence on the cell cycle, TGR5 exerts an indirect impact on cell proliferation by modulating inflammation and cell survival. Activation of TGR5 can inhibit the NF-κB signaling pathway, thereby reducing the production of inflammatory mediators and fostering an environment conducive to cell proliferation [68]. In certain instances, TGR5 activation enhances cell survival by upregulating the expression of anti-apoptotic proteins, such as Bcl-2 [60,69]. Under specific pathological conditions, the function of TGR5 shifts, contributing to the onset and progression of disease. The role of TGR5 in inflammatory bowel disease is multifaceted; it predominantly exerts a protective effect [67,70], although it can also exacerbate inflammation in some cases [71]. The involvement of TGR5 in colon cancer remains a subject of debate [72]. Several studies have indicated that the activation of TGR5 may facilitate the progression of colon cancer. For instance, when TGR5 is activated by agonists such as INT-777, ursodeoxycholic acid (UDCA), and taurolithocholic acid (TLCA), varying effects are observed across different cancer cell types [73]. Furthermore, TGR5 activation can trigger several signaling pathways, including protein kinase B (AKT), nuclear factor κB (NF-κB), extracellular signal-regulated kinases (ERK1/2), signal transducer and activator of transcription 3 (STAT3), cyclic adenosine monophosphate (cAMP), and Ras homologous protein, all of which are intricately linked to tumorigenesis and cancer progression [72]. The effect of TGR5 activation is contingent upon the tumor stage and the surrounding microenvironment.
2.3. Phasic Regulatory Effect of Bile Acid Concentration on Cell Proliferation
The influence of bile acids on the proliferation of intestinal epithelial cells is evidently concentration-dependent, exhibiting a phasic regulatory effect wherein low concentrations stimulate proliferation, whereas high concentrations inhibit it [74,75]. This phasic effect underscores the complexity of bile acids as signaling molecules and highlights the body’s sophisticated regulatory mechanisms for maintaining intestinal homeostasis.
Within the physiological concentration range, bile acids facilitate the proliferation of intestinal epithelial cells through multiple mechanisms. At low concentrations, bile acids can subtly activate the epidermal growth factor receptor (EGFR) by either promoting ligand release or directly interacting with the receptor [76,77]. Specific bile acids, such as deoxycholic acid and chenodeoxycholic acid (CDCA), are capable of directly or indirectly activating the mitogen-activated protein kinase (MAPK) pathway, particularly the extracellular-signal-regulated kinases 1 and 2 (ERK1/2) [76,78]. Additionally, low concentrations of bile acids can elevate intracellular cyclic adenosine monophosphate (cAMP) levels by activating the TGR5 receptors [54,60]. Furthermore, low concentrations of bile acids induce mild oxidative stress. Research indicates that glycocholic acid (GCA) and glycoursodeoxycholic acid (GUDCA) protect retinal pigment epithelial (RPE) tight junctions from oxidative damage within the concentration range of 100–500 μM, whereas glycodeoxycholic acid (GDCA) offers protection in the range of 10–500 μM. This moderate oxidative stress activates the cell’s protective mechanisms, thereby promoting cell proliferation [79,80] (Figure 2, left). Moreover, in certain instances, moderate activation of the FXR indirectly stimulates cell proliferation by modulating the expression of specific genes, such as fibroblast growth factor 19 (FGF19).
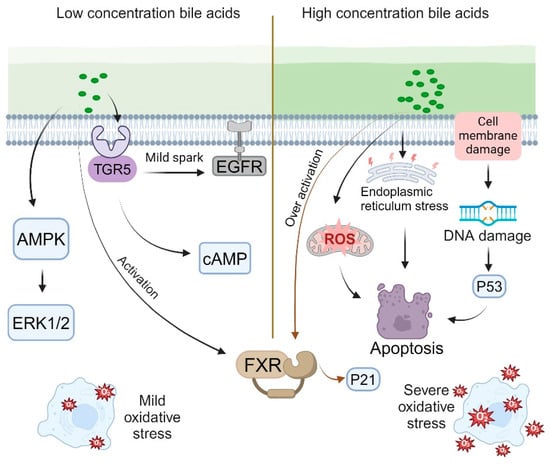
Figure 2.
The concentration of bile acids exerts phasic regulatory effects on cellular proliferation.
When bile acid concentrations surpass a specific threshold, their influence on cellular proliferation shifts to an inhibitory effect [81]. Elevated bile acid levels can directly inflict damage on cell membranes and DNA [82]. Such DNA damage may activate the p53 pathway, resulting in cell cycle arrest or apoptosis [83]. Additionally, high bile acid concentrations markedly increase the production of reactive oxygen species (ROS), culminating in severe oxidative stress [83,84]. This excessive oxidative stress can impair organelle function and trigger apoptotic pathways [83,85]. Furthermore, elevated bile acid levels induce endoplasmic reticulum (ER) stress and activate the unfolded protein response (UPR) [86,87,88]. Prolonged ER stress may also result in cell cycle arrest and apoptosis [89]. Mitochondrial dysfunction, another consequence of high bile acid concentrations, can lead to disruptions in energy metabolism and cell death [86,90]. Moreover, excessive bile acid concentrations excessively activate the FXR and upregulate the expression of cell cycle inhibitory factors such as p21 [35,91] (Figure 2, right).
The phasic regulatory effect of bile acids on cell proliferation is subject to a concentration threshold that varies according to cell type and the specific bile acid involved [92]. Various IEC types, including absorptive cells, goblet cells, and Paneth cells, exhibit differing sensitivities to bile acids [93,94,95]. Absorptive cells demonstrate heightened sensitivity to bile acids via specific transporters, such as OATP and ASBT [96,97]. Goblet cells play a crucial role in secreting mucus to establish a protective barrier within the intestine, and the presence of bile acids enhances mucus secretion, thereby safeguarding the intestinal epithelium from bile acid stimulation [98]. Research indicates that in rats subjected to a high-fat diet, there is an increase in intestinal bile acid secretion, which leads to the upregulation of the TGR5 in Paneth cells, consequently promoting the proliferation of intestinal epithelial cells [95]. Furthermore, intestinal epithelial stem cells exhibit particular sensitivity to fluctuations in bile acid concentrations [67]. Various bile acids, such as cholic acid, deoxycholic acid, and lithocholic acid, exhibit distinct concentration thresholds and exert differential effects [99,100,101] (Figure 2).
The phasic regulatory influence of bile acid concentrations on cellular proliferation holds significant physiological and pathological implications. This dual regulation is crucial for maintaining the normal renewal rate of intestinal epithelial cells, ensuring a balance between cell proliferation and shedding. Moreover, bile acids play a pivotal role in preserving intestinal health by modulating the proliferation of intestinal stem cells through the activation of the intestinal FXR [50]. Several research data have shown that fluctuations in bile acid concentrations are intricately linked to the proliferation and differentiation of intestinal stem cells, with elevated bile acid levels potentially increasing the risk of intestinal cancer [67]. Furthermore, bile acids influence the proliferation of intestinal epithelial cells by modulating the cell cycle and mitochondrial biogenesis [31].
Following intestinal injury, bile acids are released and facilitate the regeneration of the intestinal epithelium, primarily through the activation of the bile acid receptor TGR5 [56]. Concurrently, bile acids contribute to epithelial renewal by modulating intracellular energy metabolism [102]. Nevertheless, an excessive accumulation of bile acids can impede the proliferation of intestinal stem cells, thereby disrupting intestinal homeostasis [103]. Consequently, the dual regulatory role of bile acids within the intestine is crucial for sustaining the normal renewal rate of the intestinal epithelium. By maintaining optimal bile acid concentrations, a balance between cellular proliferation and homeostasis can be achieved, thereby enhancing intestinal health [31,103,104].
3. Role of Bile Acids and Bile Acid Receptors in Intestinal Epithelial Cell (IEC) Differentiation
3.1. Effect of FXR on Cell Fate Determination
As the primary bile acid nuclear receptor, FXR (nuclear receptor 1H4) is integral to the differentiation of intestinal epithelial cells [105]. FXR influences cell fate determination by modulating the expression of genes associated with differentiation, impacting the Notch signaling pathway, and regulating the differentiation of intestinal epithelial stem cells. Specifically, FXR can directly enhance the expression of several differentiation markers, including alkaline phosphatase (ALP) and sucrase-isomaltase (SI) [106,107]. Activation of FXR also leads to increased expression of CDX2, a key regulator of IEC differentiation [108]. Furthermore, research indicates that FXR modulates the Notch signaling pathway [109], which is crucial in determining the fate of absorptive and secretory cells [110]. The Notch signaling pathway is integral to the regulation of homeostasis and differentiation of intestinal stem cells. Notch1 and Notch2 receptors are expressed within the intestinal epithelium, with evidence indicating that Notch1 predominantly regulates the function and proliferation of these stem cells [111] (Figure 3). Disruption of Notch signaling results in a reduction of intestinal stem cells and impairs their regenerative capacity, underscoring its essential role in intestinal repair processes [112]. Furthermore, Notch signaling influences the function of intestinal epithelial stem cells by modulating the proliferation and differentiation of Lgr5+ precursor cells [50]. During the differentiation process of intestinal stem cells, the interplay between the FXR and Notch signaling pathways may influence cell fate decisions. Research suggests that activation of Notch signaling facilitates the differentiation of intestinal stem cells into absorptive cells, whereas FXR activation may modulate this process by regulating the expression of genes associated with differentiation [113]. Therefore, the coordinated interaction between the FXR and Notch signaling pathways plays a crucial role in determining the fate of intestinal stem cells.
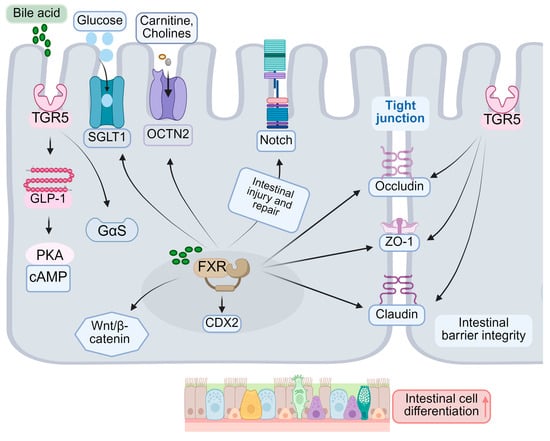
Figure 3.
The function of bile acids and bile acid receptors in the differentiation of intestinal epithelial cells.
FXR contributes to intestinal protection against harmful substances by enhancing the barrier function during the differentiation of intestinal epithelial cells. It regulates the expression of tight junction proteins, such as occludin and claudin [114], thereby strengthening the integrity of the intestinal epithelial barrier and reducing permeability. Although FXR is expressed at low levels in intestinal epithelial stem cells, its activation facilitates the differentiation of these stem cells into mature epithelial cells, partially through the modulation of the Wnt/β-catenin signaling pathway. In vivo, microbial-derived isoDCA enhances the immunostimulatory properties of dendritic cells by inhibiting FXR activity, which indirectly promotes the differentiation of colonic Tregs [18]. FXR is implicated in the maturation and differentiation of innate lymphoid cell (ILC) precursors [115] (Figure 3). Nonetheless, prior research has demonstrated that the nuclear receptor FXR plays a pivotal role in the functional maturation of hepatocyte-like cells (HLCs). Stem cell-derived hepatocytes exhibit a hybrid phenotype, possessing characteristics of both hepatocytes and intestinal cells. Investigations have revealed that FXR inhibits the intestinal traits of HLCs while promoting hepatic characteristics, thereby rendering stem-cell-derived cells more akin to primary hepatocytes [116]. Consequently, FXR facilitates the differentiation of intestinal epithelial cells into mature cells by modulating the expression of specific genes. Activation of FXR can enhance the differentiation of intestinal absorptive cells, such as intestinal epithelial cells, thereby augmenting their nutrient absorption capacity. This effect is typically associated with the upregulation of specific transporters, including the sodium-dependent glucose transporter SGLT1 [117] and the organic cation transporter OCTN2 [118].
3.2. Role of TGR5 in Cell Differentiation
While TGR5 is primarily recognized as a receptor involved in the regulation of metabolism and inflammation, recent research has demonstrated its role in the differentiation of intestinal epithelial cells [60]. Activation of TGR5 facilitates the differentiation of L cells and the secretion of GLP-1 [119,120]. In the intestinal environment, bile acids bind to TGR5, activating the Gαs signaling pathway and subsequently increasing intracellular cAMP levels [54,121] (Figure 3). This cascade further stimulates protein kinase A (PKA) and other downstream signaling pathways, thereby influencing cell differentiation. The activation of intestinal TGR5 enhances the expression of various differentiation markers, including intestinal-specific proteins such as MUC2, OCLN, and ZO-1, which are crucial for maintaining intestinal barrier function and promoting cell differentiation [122]. Moreover, studies indicate that TGR5 activation stimulates the proliferation and differentiation of intestinal epithelial cells as well as strengthens the integrity of the intestinal barrier by modulating the expression of tight junction proteins [123,124,125]. For instance, MUC2, the primary protein constituent of intestinal mucus, serves a protective role for the intestinal epithelium against pathogenic invasion, whereas OCLN and ZO-1 are critical components of tight junctions, essential for maintaining intercellular barrier integrity [126]. Furthermore, the activation of TGR5 is implicated in the remodeling of the intestinal microbiota, thereby supporting the preservation of intestinal health and immune function [61,127]. Under specific conditions, TGR5 influences the differentiation of enterochromaffin cells [128]. TGR5 contributes to the maintenance of barrier function in mature intestinal epithelial cells by enhancing the expression of tight junction proteins [129]. The involvement of TGR5 in intestinal processes presents a novel therapeutic target for a range of intestinal disorders. For example, research indicates that TGR5 activation may have therapeutic potential in the management of IBD, IBS, and other related conditions.
3.3. Effects of Bile Acids on Intestinal Epithelial Stem Cell Differentiation
Bile acids influence the functionality of Paneth cells via the activation of the FXR, which encompasses the secretion of antimicrobial peptides and the synthesis of growth factors [130,131]. The growth factors produced by Paneth cells are crucial for providing essential growth signals to intestinal stem cells, thereby facilitating their self-renewal and differentiation [132]. The normal functioning of Paneth cells, characterized by the adequate secretion of growth factors, is advantageous for the stability and maintenance of intestinal stem cell functions [133]. Furthermore, the antimicrobial activity of Paneth cells plays a pivotal role in regulating the intestinal microbiota and mitigating inflammatory responses induced by bacterial infections [134]. Inflammation adversely affects the functionality of intestinal stem cells, impeding their proliferation and differentiation [135,136]. Consequently, the integrity of Paneth cells is essential for sustaining the function of intestinal stem cells. Additionally, bile acids influence the expression and degradation of basement membrane proteins [137], thereby altering the physicochemical properties of the stem cell microenvironment [138,139,140]. Bile acids also indirectly modulate stem cell behavior by regulating the function of intestinal stromal cells, such as myofibroblasts [141,142].
Bile acids modulate β-catenin activity via the FXR, consequently influencing the Wnt signaling pathway, which is crucial for stem cell self-renewal and differentiation regulation [143]. Furthermore, bile acids impact the expression of Notch receptors or ligands, thereby modulating Notch signaling activity. The Wnt signaling pathway is integral to maintaining stem cell self-renewal and differentiation regulation. Research indicates that bile acids influence the Notch signaling pathway by altering the expression of Notch receptors or ligands [144]. The Notch signaling pathway is pivotal in various biological processes, including cell fate determination, tissue regeneration, and tumorigenesis [145,146]. It plays a significant role in liver development and regeneration, and the modulation of bile acids may affect intestinal stem cell function. Studies have demonstrated that the Notch signaling pathway is involved in liver development and regeneration by regulating the proliferation and differentiation of liver stem cells [144]. Furthermore, Notch signaling is essential for maintaining the integrity and functionality of intestinal epithelial cells, particularly in the context of intestinal inflammation and immune homeostasis [147]. Bile acids, as signaling molecules, can influence Notch signaling activity by modulating liver and intestinal metabolic processes, thereby impacting the fate of intestinal stem cells [148]. Alterations in the composition and concentration of bile acids are closely associated with changes in the intestinal microbiota, which may in turn affect the activation state of Notch signaling, influencing the self-renewal and differentiation of intestinal stem cells [149] (Figure 3). Notch signaling is pivotal in determining the fate of absorptive and secretory cells. Additionally, bile acids modulate the intensity of bone morphogenetic protein (BMP) signaling by affecting the expression of BMP ligands or receptors, which are critical for regulating stem cell differentiation and the formation of the crypt–villus axis.
4. Relationship Between Bile Acids, Bile Acid Receptors, and Intestinal Epithelial Cell (IEC) Aging
4.1. Bile-Acid-Induced Oxidative Stress and DNA Damage
Bile acids and their receptors play a multifaceted and significant role in the senescence of intestinal epithelial cells. This intricate relationship encompasses various dimensions, including oxidative stress, DNA damage, cell cycle regulation, and metabolic alterations. The aging process is frequently associated with inflammation, dysregulated bile acid (BAS) homeostasis, and intestinal dehydration [150]. Elevated concentrations of specific bile acids, such as deoxycholic acid, can enhance the production of reactive oxygen species (ROS) [151]. Persistent oxidative stress may expedite cellular aging and induce oxidative damage to proteins, lipids, and DNA [152]. Certain secondary bile acids have the potential to cause DNA damage directly or indirectly, including single-strand and double-strand breaks [153]. The accumulation of DNA damage is a hallmark of cellular aging and can activate aging-related pathways, such as p53 [154,155] (Figure 4).
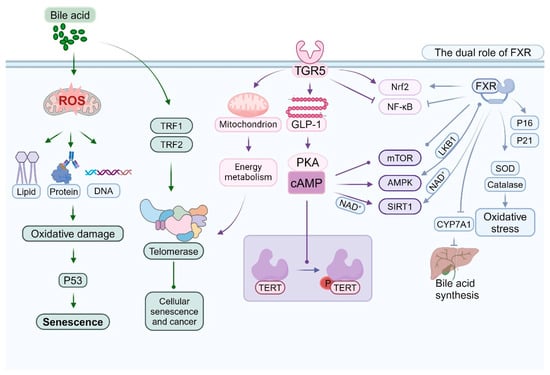
Figure 4.
The association between bile acids, bile acid receptors, and the senescence of intestinal epithelial cells.
As the principal nuclear receptor for bile acids, FXR exerts intricate regulatory effects during cellular aging. Dysfunction of the intestinal barrier is recognized as an evolutionarily conserved hallmark of aging [156]. In the context of anti-aging, activation of FXR can enhance the expression of antioxidant genes, including SOD and catalase, thereby mitigating oxidative stress [157,158]. FXR also attenuates the production of inflammatory mediators and alleviates aging-associated chronic inflammation by inhibiting the NF-κB signaling pathway [159]. Research indicates that FXR serves as a target for the prevention of diet- and aging-related metabolic disorders [160]. Our findings indicate that the down-regulation of FXR plays a pivotal role in the development of aging-induced fatty liver [161]. Additionally, FXR influences the cellular aging process by modulating the expression and activity of SIRT1 [162,163]. Regarding its potential role in promoting aging, under specific conditions, prolonged FXR activation induces cellular senescence by upregulating cell cycle inhibitors such as p16 and p21 [35,36,164]. Furthermore, excessive activation of FXR can disrupt cellular metabolism and indirectly expedite the aging process [165] (Figure 4).
TGR5 is critically involved in the regulation of cellular senescence [166]. Activation of TGR5 enhances cellular resistance to oxidative stress, partially through the activation of the Nrf2 signaling pathway [65,167,168]. Furthermore, TGR5 contributes to the maintenance of cellular energy homeostasis and delays the aging process by modulating mitochondrial function [166,169]. It also indirectly influences cellular aging by regulating GLP-1 secretion and energy metabolism. Certain bile acids modulate the mTOR signaling pathway via TGR5, thereby influencing cellular senescence and autophagy [170,171,172]. Recent studies have explored the interactions and potential mechanisms linking ileitis and metabolic-associated steatotic liver disease (MASLD). Changes in intestinal flora induced by MASLD result in elevated levels of secondary bile acids in the ileum. In the context of a compromised intestinal barrier, this leads to severe CD8+ T cell-mediated ileitis through the TGR5/mTOR/OXPHOS signaling pathway. Tissue damage induced by ileitis disrupts enterohepatic circulation, inhibits hepatic FXR activation, and exacerbates the MASLD phenotype [173]. This inflammatory milieu fosters enterocyte senescence by promoting oxidative stress and DNA damage [174,175]. Activation of TGR5 has been shown to influence the AMPK pathway, thereby modulating energy metabolism and cellular senescence [120,176] (Figure 4).
Activation of TGR5 has been shown to influence the AMPK pathway, thereby modulating energy metabolism and cellular senescence [120,176]. The intestinal microbiome plays a crucial role in bile acid metabolism and the aging of intestinal epithelial cells [16,177]. Specifically, the intestinal flora is capable of converting primary bile acids into secondary bile acids, which are more likely to promote cellular aging [150,178]. With advancing age, alterations in intestinal microbial diversity impact bile acid composition, subsequently influencing cellular aging processes [150,179].
Dysregulation of bile acid metabolism is linked to a range of aging-related gastrointestinal diseases [180]. Additionally, aging modifies bile acid metabolism and receptor functionality, thereby increasing the susceptibility to inflammatory bowel disease [15,150,172]. Prolonged exposure to elevated concentrations of certain bile acids has been associated with the development of colon cancer, a condition that correlates with cellular aging and the accumulation of DNA damage [181]. Consequently, anti-aging strategies that leverage bile acid signaling and elucidate the roles of bile acids and their receptors in cellular aging may provide a foundation for the development of novel anti-aging interventions.
4.2. Relationship Between Bile Acid and Telomerase Activity
Telomerase is a crucial enzyme responsible for maintaining telomere length, thereby playing a pivotal role in cellular aging and the regulation of lifespan. Recent research has demonstrated that bile acids and their receptors influence telomerase activity through various mechanisms, thus contributing to the regulation of cellular lifespan [182]. Specifically, certain bile acids have been shown to directly modulate telomerase activity by impacting the transcription of the telomerase reverse transcriptase (TERT) gene. Studies have shown that bile acids, at specific concentrations, can upregulate TERT expression, potentially enhancing telomerase activity. This observation is significantly linked to the role of telomerase in cellular proliferation and oncogenesis [183,184,185]. In certain cancer types, the reactivation of telomerase is considered a critical factor in tumor progression. For instance, mutations in the TERT promoter are commonly identified in urothelial carcinoma and are linked to increased telomerase activity and enhanced tumor invasiveness [183]. Furthermore, bile acids, such as ursodeoxycholic acid (UDCA), have been demonstrated to inhibit the proliferation of colorectal cancer cells by modulating the YAP signaling pathway, potentially through their impact on telomerase activity [184]. In hepatocellular carcinoma, alterations in bile acid concentrations are similarly associated with telomerase reactivation and increased tumor invasiveness [185].
The mechanism by which bile acids exert their effects may involve the regulation of TERT transcription, complementing other established regulatory mechanisms. Notably, TERT expression is influenced not only by transcription factors but also by epigenetic modifications. Recent research has indicated that the transcriptional activity of TERT is closely associated with the methylation status of its promoter region, particularly in cancer cells [186]. In thyroid cancer, TERT overexpression is linked to promoter mutations and epigenetic alterations, which collectively influence TERT transcriptional activity [187]. Furthermore, research has demonstrated that template activation factor I (TAF-I) plays a role in regulating TERT transcription by maintaining histone modifications and demethylated cytosine, both of which are associated with transcriptional activation [188]. These findings imply that bile acids may further facilitate cancer cell proliferation and survival by influencing the transcriptional regulatory network of TERT [189]. Additionally, studies have indicated that the atypical functions of TERT within cells may also contribute to its involvement in cancer, particularly through its roles in gene expression regulation and cell proliferation [190,191]. The identification of these non-canonical functions offers a more comprehensive perspective, enhancing our understanding of the multifaceted roles of TERT in cell biology.
The activation of the FXR indirectly modulates the expression of telomerase reverse transcriptase (TERT), primarily by influencing the activity of specific transcription factors. As a nuclear receptor, FXR is implicated in the regulation of various metabolic processes, including those related to bile acid, lipid, and glucose metabolism [192]. Scholars have conducted research indicating that FXR activation impacts hepatic and intestinal metabolism also plays a crucial role in cellular proliferation and survival [193]. Furthermore, FXR indirectly influences telomerase activity and assembly by modulating genes associated with the cell cycle and proliferation [194]. Recent research has highlighted the significance of the TGR5 in numerous physiological and pathological processes, such as metabolic diseases, inflammatory responses, and hepatic disorders [195]. Activation of TGR5 indirectly affects the phosphorylation and activity of TERT via the cAMP-PKA signaling pathway [68,196]. Additionally, TGR5 can modulate telomerase activity by altering the cellular energy metabolism state [195,197] (Figure 4).
Research has demonstrated that telomerase activity is modulated by various signaling pathways, with histone modification playing a pivotal role in this regulation [198]. Furthermore, while the chromatin structure of telomeres is traditionally classified as heterochromatin, recent investigations have revealed that telomeres in certain plant models may exhibit characteristics of true chromatin. This finding implies the existence of distinct regulatory mechanisms across different organisms [199]. High-resolution studies of telomerase structure enhance our comprehension of its interactions with substrates and pinpoint mutations that influence its activity [200]. Such structural insights are vital for the development of therapeutics aimed at effectively modulating telomerase activity, particularly in the context of diseases like cancer. Consequently, the impact of bile acids on the chromatin structure of the telomere region could further influence cell proliferation and tumor progression by modulating the accessibility and activity of telomerase.
Bile acids influence the expression and functionality of specific telomere-binding proteins, including TRF1 and TRF2. Alterations in these proteins subsequently impact the interaction between telomerase and telomeres. TRF1 and TRF2 are integral components of the telomere protection complex, known as shelterin, and are crucial for maintaining telomere length and safeguarding chromosome ends [201]. Research indicates that the binding affinity of TRF1 and TRF2 is modulated by nucleosome organization, which may further influence their functional roles at telomeres [202] (Figure 4). Moreover, TRF2 is significant in the differentiation and maintenance of neural progenitor cells, underscoring its multifaceted roles in cell fate determination [203]. Consequently, bile acids may indirectly modulate telomerase activity and telomere stability by regulating the expression and function of these telomere-binding proteins, thereby contributing to biological processes such as cellular aging and oncogenesis.
4.3. Interaction Between Bile Acid Receptors and Aging-Related Signaling Pathways (e.g., mTOR, AMPK, SIRT1)
Bile acid receptors, such as the FXR and the TGR5, engage in intricate interactions with several critical signaling pathways associated with aging. These interactions are pivotal in modulating cellular metabolism, stress responses, and lifespan regulation [7,204]. Activation of FXR influences the mechanistic target of rapamycin complex 1 (mTORC1) through various mechanisms [205,206,207], while TGR5 activation modulates mTOR activity via the cAMP-PKA signaling pathway [60,208]. This modulation is crucial for the regulation of autophagy and protein synthesis [209,210]. Alterations in mTOR activity subsequently impact the expression of genes involved in bile acid synthesis and transport [211], establishing a complex feedback loop between bile acid signaling and mTOR regulation [212] (Figure 4).
The activation of TGR5 indirectly stimulates AMPK by elevating intracellular cAMP levels [213,214]. The activation of AMPK constitutes a crucial mechanism through which TGR5 modulates energy metabolism [215]. FXR indirectly influences AMPK activation by modulating the expression or activity of LKB1 [216]. Subsequently, AMPK activation impacts the transcriptional activity of FXR [217]. AMPK further influences bile acid synthesis by regulating the expression of key enzymes, such as CYP7A1 [218]. This regulation establishes an additional feedback loop within the bile acid–AMPK signaling network [219] (Figure 4). FXR also indirectly modulates SIRT1 activity by affecting NAD+ metabolism [220]. SIRT1, in turn, regulates its transcriptional activity through the deacetylation of FXR [221]. TGR5 activation indirectly modulates SIRT1 activity by influencing mitochondrial function and NAD+ levels [222]. This interaction plays a significant role in the regulation of energy metabolism and anti-aging processes. Furthermore, SIRT1 affects the expression of genes associated with bile acid metabolism by modulating the activity of FXR and other transcription factors [223] (Figure 4).
Bile acid receptors are pivotal in cellular metabolic reprogramming through their interactions with mTOR, AMPK, and SIRT1 [224,225]. This integration influences cellular responses to nutritional and stress stimuli, thereby impacting the aging process [160]. FXR and TGR5 contribute to the regulation of autophagy by modulating the activities of mTOR and AMPK [173,226]. Precise regulation of autophagy is crucial for maintaining cellular homeostasis and mitigating the effects of aging [227]. Furthermore, the interaction of bile acid receptors with AMPK and SIRT1 plays a significant role in the regulation of mitochondrial biogenesis and function, which is essential for sustaining cellular energy balance and delaying the aging process. The interplay between bile acid receptors and key molecular targets, including mTOR, AMPK, and SIRT1, is integral to the pathogenesis of neurodegenerative disorders, particularly Alzheimer’s disease [228,229]. Furthermore, the interactions between bile acid receptors and aging-related signaling pathways significantly influence vascular function and cardiac metabolism, thereby impacting the aging process of the cardiovascular system [230,231]. Consequently, future therapeutic strategies are proposed to concurrently target bile acid receptors and critical aging pathways—such as employing mTOR inhibitors and AMPK activators—while integrating personalized treatment approaches and time-dependent interventions to achieve synergistic effects in combating aging.
5. Regulation of Energy Metabolism of Intestinal Epithelial Cells by Bile Acids and Bile Acid Receptors
5.1. Role of FXR in Lipid and Glucose Metabolism
The farnesoid X receptor (FXR), a nuclear receptor activated by bile acids, is integral not only to the regulation of bile acid metabolism but also to lipid and glucose metabolism [232]. FXR is pivotal in maintaining metabolic homeostasis through the direct regulation of gene expression and the indirect modulation of various metabolic pathways [9,233,234]. It exerts an inhibitory effect on the expression of key enzymes involved in fatty acid synthesis, such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) [235,236], thereby attenuating fatty acid synthesis. This inhibition is partially mediated by the downregulation of sterol regulatory element-binding protein-1c (SREBP-1c) [158,236,237] (Figure 5, left). Furthermore, research has demonstrated that the FXR-dependent reduction in polyunsaturated fatty acids is facilitated by decreased lipid absorption. Utilizing tissue-specific FXR knockout mice, researchers have shown that hepatic FXR regulates lipogenic genes, whereas intestinal FXR modulates lipid absorption, thereby delineating two distinct pathways through which FXR influences hepatic lipid regulation [238]. Activation of FXR has been shown to decrease triglyceride levels in both plasma and liver [239,240,241]. This phenomenon encompasses the inhibition of triglyceride synthesis and the enhancement of fatty acid oxidation. The FXR modulates the expression of apolipoproteins, including apoC [242], apoB [243], and apoAI [244] (Figure 5, left). Through this regulatory mechanism, FXR influences the composition and metabolism of lipoproteins. Furthermore, FXR indirectly impacts the conversion of cholesterol to bile acids by modulating the expression of CYP7A1 [245,246]. Additionally, FXR governs the enterohepatic circulation of cholesterol by affecting the expression of ABCG5/G8. The expression of FXR in adipose tissue plays a role in adipocyte differentiation and function [240,247,248,249,250], involving the regulation of lipogenesis-related genes within the peroxisome-proliferator-activated receptor (PPAR) family [251,252,253] (Figure 5, left).
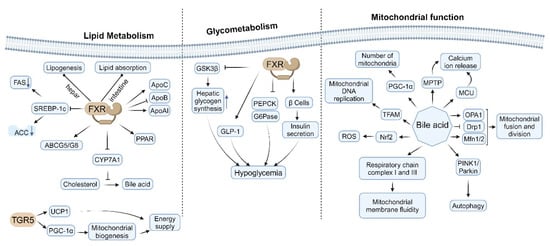
Figure 5.
The impact of bile acids and their corresponding receptors on glucose and lipid metabolism, as well as mitochondrial function.
The FXR plays a significant role in modulating hepatic glycogen synthesis through the regulation of glycogen synthase kinase 3β (GSK3β) expression [254,255]. Activation of FXR enhances hepatic glycogen storage and exerts inhibitory effects on hepatic lipogenesis and gluconeogenesis, thereby promoting lipid metabolism, glycogen synthesis, and insulin sensitivity, which collectively contribute to the reduction of blood glucose levels [256]. Notably, FXR suppresses the expression of critical gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) [236]. This suppression leads to a decrease in hepatic glucose output, thereby facilitating improved glycemic control [257] (Figure 5, middle). Additionally, FXR activation enhances insulin sensitivity through various mechanisms [258], such as reducing hepatic steatosis [259], enhancing lipid metabolism [248,260], and exerting anti-inflammatory effects [261]. Furthermore, FXR expression in pancreatic β cells influences insulin secretion and β cell viability [262] by modulating cellular metabolism and antioxidant defenses. FXR also indirectly impacts glucose metabolism by regulating the secretion of glucagon-like peptide-1 (GLP-1) [263], and it affects intestinal glucose absorption and metabolism [264] (Figure 5, middle).
The activation of FXR mitigates the progression of non-alcoholic fatty liver disease (NAFLD) by enhancing lipid metabolism and diminishing liver inflammation [265,266]. FXR agonists demonstrate potential in augmenting insulin sensitivity and regulating blood glucose levels. Consequently, FXR has emerged as a promising target for the treatment of metabolic syndrome, owing to its multifaceted roles in lipid and glucose metabolism. Notably, FXR and PPARα exhibit synergistic effects in the regulation of fatty acid oxidation and lipid metabolism [267]. In contrast, FXR and LXR exert antagonistic effects in the regulation of lipid and cholesterol metabolism [268,269]. Furthermore, FXR influences insulin signaling by modulating the expression or activity of insulin receptor substrates (IRS) [270].
5.2. TGR5-Mediated Increase in Energy Expenditure
Recent research has elucidated that TGR5 plays a pivotal role in regulating energy expenditure, offering a novel perspective on the role of bile acids in metabolic regulation [271,272].
Upon activation, TGR5 stimulates adenylate cyclase via the Gαs protein, resulting in elevated intracellular cyclic adenosine monophosphate (cAMP) levels [58,273]. The increase in cAMP subsequently activates protein kinase A (PKA), which initiates a cascade of downstream effects [60,274]. Research indicates that TGR5 activation also modulates intracellular calcium ion concentrations, a process linked to the regulation of energy expenditure [275]. TGR5 ligands facilitate an increase in intracellular calcium concentrations by promoting calcium influx [276], thereby enhancing β-cell insulin secretion through the modulation of potassium and calcium currents, which affects the activation of acutely promoted stimulus–secretion coupling (SSC) [59].
Furthermore, TGR5 activation significantly upregulates the expression of uncoupling protein 1 (UCP1) in brown adipocytes [273,277]. UCP1 is essential for thermogenesis in brown adipose tissue, and its increased expression directly augments energy expenditure [278]. Additionally, TGR5 activation stimulates mitochondrial biogenesis via the activation of peroxisome-proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) [279]. The resultant increase in mitochondrial number and function enhances the thermogenic capacity of brown adipose tissue [169]. Moreover, TGR5 activation promotes fatty acid oxidation by influencing the expression or activity of key enzymes involved in this metabolic pathway [170,280] (Figure 5, left).
5.3. Effects of Bile Acids on Mitochondrial Function
Bile acids, as critical signaling molecules, play a significant role in lipid digestion and metabolic regulation, while also exerting diverse effects on mitochondrial function. These effects are dose-dependent and vary under physiological and pathological conditions. Certain bile acids can upregulate the expression of PGC-1α by activating TGR5 or FXR receptors [281], with PGC-1α serving as a key regulator of mitochondrial biogenesis, thereby promoting an increase in mitochondrial numbers. Additionally, bile acids influence the expression or activity of proteins involved in mitochondrial DNA replication [31], such as TFAM.
At low concentrations, bile acids enhance the activity of respiratory chain complexes, particularly complexes I and III [282]. Conversely, high concentrations inhibit respiratory chain function, leading to reduced ATP production [283]. Bile acids also modulate the efficiency of electron transfer by altering mitochondrial membrane fluidity [284] (Figure 5, right), with low concentrations proving beneficial and high concentrations detrimental. Physiological concentrations of bile acids play a crucial role in maintaining mitochondrial membrane potential and promoting energy metabolism. In contrast, elevated concentrations of bile acids result in the collapse of membrane potential, thereby inducing apoptosis [285,286] and inhibiting energy metabolism. Bile acids influence the function of ATP synthase (complex V), consequently affecting the proton gradient and membrane potential [287,288].
Furthermore, bile acids modulate calcium uptake by impacting the mitochondrial calcium uniporter (MCU). Certain bile acids influence the opening of the mitochondrial permeability transition pore (MPTP), thereby affecting calcium release [86,289,290] (Figure 5, right). At low concentrations, bile acids decrease reactive oxygen species (ROS) production by upregulating the expression of antioxidant enzymes [31]. Conversely, high concentrations of bile acids enhance ROS production, leading to oxidative damage [84]. Some bile acids can activate the Nrf2 pathway, thereby enhancing mitochondrial antioxidant defense [291,292]. Additionally, bile acids activate Nrf2 in intestinal cells, and the intestinal-cell-specific knockout of Nrf2 increases the susceptibility of fruit flies to bile-acid-induced toxicity [293]. Bile acids influence mitochondrial fusion and fission by modulating the expression or activity of proteins such as Mitofusin 1/2 (Mfn1/2) [294], dynamin-related protein 1 (Drp1) [295], and optic atrophy 1 (OPA1) [290]. Additionally, bile acids regulate the process of mitochondrial autophagy through their impact on the PINK1/Parkin pathway [295,296] (Figure 5, right).
5.4. Interaction Between Bile Acid Receptors and Metabolism-Related Hormones (Such as GLP-1)
The interactions between bile acid receptors and metabolism-related hormones are complex, as exemplified by glucagon-like peptide-1 (GLP-1). Bile acid receptors, particularly the FXR and the TGR5, engage in intricate interactions with various metabolism-related hormones. These interactions are crucial for maintaining overall metabolic homeostasis, with the interaction involving GLP-1 being especially significant.
The activation of TGR5 in intestinal L cells significantly enhances the secretion of GLP-1 [297,298], thereby facilitating improvements in glucose metabolism, lipid catabolism, and energy metabolism [299]. The activation of TGR5 leads to an increase in cAMP levels, activation of PKA, closure of potassium channels, cell depolarization, influx of calcium ions, and ultimately the secretion of GLP-1. Through the promotion of GLP-1 secretion, TGR5 indirectly enhances insulin sensitivity and glycemic control [300,301]. This mechanism constitutes a critical pathway through which bile acids enhance metabolic processes. FXR modulates the expression of GLP-1 receptors in intestinal tissues [263,302] and interacts with downstream PI3K/AKT via the FXR/GLP-1 axis. GLP-1 augments the TGR5-mediated increase in energy expenditure, and this synergistic interaction contributes to weight management and obesity prevention [195,299]. Bile acids and GLP-1 collaborate synergistically to enhance β-cell function and survival. Furthermore, FXR influences the expression of leptin receptors and modulates leptin sensitivity [303]. Bile acids indirectly regulate leptin secretion by impacting adipose tissue function [204,304]. Additionally, FXR affects the growth hormone axis by modulating the expression of IGF1 [305].
6. Interactions Between Bile Acids, Bile Acid Receptors, and the Gut Microbiome
Effects of Microorganisms on Bile Acid Metabolism
The intestinal microbiome is integral to bile acid metabolism, contributing also to the conversion of bile acids affecting their circulation, composition, and signal transduction. This interaction between microbes and bile acids significantly influences host metabolism and health [17,306]. Bacterial bile acid hydrolase (BSH) facilitates the deconjugation of bile acids [307] and is predominantly produced by Bifidobacterium, Lactobacillus, and Clostridium species [308]. The enzyme 7α-dehydroxylase is responsible for converting primary bile acids into secondary bile acids, such as the transformation of cholic acid (CA) into deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA) into lithocholic acid (LCA) [309]. Microorganisms are also capable of catalyzing the oxidation and reduction of bile acids through enzymes such as 3α-hydroxydehydrogenase and 7α-hydroxydehydrogenase [310]. Additionally, they can catalyze the isomerization of bile acids, exemplified by the formation of ursodeoxycholic acid (UDCA), with contributions from genera such as Bacteroides, Clostridium, Escherichia coli, Eubacterium, Peptostreptococcus, and Ruminococcus [311].
There exists a dynamic interaction between the microbiome and bile acids, wherein bile acids influence the composition of the intestinal microbiome, which in turn modulates the composition and size of the bile acid pool [312]. Microbial metabolism generates a diverse array of atypical bile acids, thereby enhancing the structural diversity of bile acids [6,9,313]. Intestinal microorganisms enzymatically convert bile acids synthesized in the liver into secondary bile acids, such as chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), and lithocholic acid (LCA), which can act as natural ligands for the FXR. Microbial activity influences the extent of FXR activation in both the intestine and liver [314]. Furthermore, products of microbial metabolism, including secondary bile acids such as LCA and DCA, serve as potent agonists of the TGR5, thereby impacting TGR5-mediated glucagon-like peptide-1 (GLP-1) secretion and energy metabolism [175,297,315]. Microbial-mediated conversion of bile acids plays a critical role in regulating glucose homeostasis, lipid metabolism, and energy balance through the activation of FXR and TGR5 signaling pathways [298,316]. Bile acids are not only vital for the digestion of fats but also function as signaling molecules that modulate various metabolic processes. Research indicates that the composition and functionality of the intestinal microbiota significantly influence bile acid metabolism, which subsequently affects the metabolic status of the host [317]. By activating FXR and TGR5 receptors in the liver and intestine, bile acids enhance the metabolism of glucose and lipids, thereby contributing to the regulation of systemic energy balance [264].
Under conditions of a high-fat diet, alterations in the composition and concentration of bile acids may contribute to metabolic disorders, including obesity and type 2 diabetes [318]. Modifications in the gut microbiota can influence insulin sensitivity and lipid metabolism by affecting the synthesis and conversion of bile acids, thereby impacting the activation states of the nuclear receptor FXR and the G-protein-coupled receptor TGR5 [319]. For instance, certain microorganisms possess the ability to modify bile acid structures through the action of bile salt hydrolase (BSH), enhancing their efficacy in activating FXR and TGR5, which in turn can improve glucose tolerance and lower blood lipid levels [302,320]. Furthermore, bile acid metabolites may exert regulatory effects on the host’s metabolic status by modulating the composition of the intestinal microbiota [18,321]. For instance, certain dietary components, such as polyphenols, have the potential to enhance metabolic health by altering the structure of the intestinal microbiota and facilitating the production of beneficial bile acids [322,323]. Consequently, microbial-mediated bile acid transformation influences the biological activity of bile acids also plays a significant role in host metabolism via the FXR and TGR5 signaling pathways. In conclusion, the conversion of bile acids mediated by the microbiota is pivotal in maintaining glucose homeostasis, lipid metabolism, and energy balance through the FXR and TGR5 signaling pathways, offering novel insights and targets for the treatment of metabolic diseases [324].
Microbial-mediated bile acid metabolism plays a crucial role in drug metabolism, encompassing drug activation and toxicity. Bile acids serve as essential components in lipid digestion also as signaling molecules that regulate diverse physiological processes. Research has demonstrated that intestinal microorganisms influence drug metabolic pathways by converting primary bile acids into secondary bile acids [325]. These secondary bile acids modulate the expression of drug-metabolizing enzymes through interactions with nuclear receptors, such as FXR and TGR5, thereby impacting the bioavailability and clearance of drugs [326,327]. Furthermore, bile acids metabolized by microorganisms can lead to drug activation or inactivation. For instance, certain drugs may be transformed by microorganisms into active forms within the intestine, thereby enhancing their efficacy [328]. Nevertheless, microbial metabolism has the potential to generate toxic metabolites, thereby exacerbating drug toxicity [329]. Consequently, it is imperative to comprehend the influence of microorganisms on bile acid metabolism and its subsequent effects on drug metabolism, as this knowledge is vital for advancing personalized medicine and drug design [330]. In the context of drug development, accounting for the effects of microorganisms on bile acid metabolism can enhance predictions of drug efficacy and safety. By incorporating metabolomics and microbiome methodologies, researchers can gain a more profound understanding of the role played by intestinal microorganisms in drug metabolism, thus offering novel insights for the development of new pharmaceutical agents [331].
7. Roles of Bile Acids and Bile Acid Receptors in Intestinal Diseases
7.1. Roles in Inflammatory Bowel Disease
In individuals with inflammatory bowel disease (IBD), disturbances in bile acid metabolism are characterized by impaired bile acid absorption [332,333], altered bile acid pool composition [334], and abnormal enterohepatic circulation [335].
In the context of IBD, the expression and functionality of the FXR are notably compromised. Some articles have reported that individuals with IBD exhibit diminished FXR activity, potentially linked to dysbiosis of the intestinal microbiota and irregularities in bile acid metabolism [336]. In murine models, the ablation of FXR has been associated with compromised intestinal barrier integrity and heightened intestinal permeability, which in turn exacerbates hepatic steatosis and the inflammatory response [337]. FXR is integral to the modulation of intestinal inflammation, serving a critical anti-inflammatory function. It mitigates intestinal inflammation by suppressing the expression of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) [338]. Furthermore, the activation of FXR has been shown to facilitate the proliferation of beneficial bacteria by modulating the composition of the intestinal microbiota, thereby augmenting the intestine’s anti-inflammatory capacity [339]. The FXR is integral to the maintenance of intestinal barrier function. Activation of FXR enhances the integrity of tight junctions in intestinal epithelial cells, thereby reducing intestinal permeability and preventing the translocation of harmful substances [340]. Empirical evidence suggested that FXR agonists bolster the integrity of these cells and mitigate damage associated with intestinal inflammation [341]. FXR is also involved in bile acid metabolism and hepatic metabolic regulation through its modulation of the intestinal hormone fibroblast growth factor 19 (FGF19). Following FXR activation, FGF19 is secreted as a principal intestinal hormone, which subsequently inhibits CYP7A1, a critical enzyme in hepatic bile acid synthesis, thus preserving bile acid homeostasis [342]. In the context of IBD, FGF19 expression is frequently downregulated, potentially leading to bile acid metabolism dysregulation and exacerbating intestinal inflammation [343]. FXR is pivotal in the pathophysiology of IBD, influencing inflammatory responses, maintaining intestinal barrier integrity, and modulating the FGF19 signaling pathway. Alterations in FXR functionality may therefore be a crucial factor in the disease process of IBD (Table 1).
The anti-inflammatory properties of TGR5 in the context of IBD encompass its roles in modulating intestinal motility, secretion, and immune regulation. Research indicates that TGR5 is integral not only to the regulation of intestinal motility and secretion but also to the modulation of immune responses, thereby influencing the anti-inflammatory outcomes in IBD [70]. Initially, TGR5 contributes to the progression of IBD by modulating intestinal motility. Proper intestinal motility is crucial for maintaining intestinal health, and TGR5 activation facilitates the contraction of intestinal smooth muscle, thereby enhancing intestinal peristalsis. This mechanism aids in the reduction of inflammatory substance accumulation within the intestine, consequently alleviating IBD symptoms [344]. Furthermore, TGR5 exerts anti-inflammatory effects through the promotion of intestinal secretion. Studies have demonstrated that TGR5 activation enhances the secretory function of intestinal epithelial cells, leading to increased release of anti-inflammatory mediators such as glucagon-like peptide-1 (GLP-1) and intestinal barrier proteins. These factors contribute to the repair of damaged intestinal barriers also to the inhibition of inflammatory responses, thereby reducing inflammation levels within the intestine [345]. Furthermore, the role of TGR5 in immune regulation is significant. TGR5 modulates immune cells in the intestine and promotes the generation of regulatory T cells (Treg), thereby enhancing intestinal immune tolerance. This immunomodulatory effect aids in preventing excessive immune responses and reducing the incidence of IBD [346]. Additionally, TGR5 inhibits the production of proinflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin (IL-6), further mitigating the inflammatory response in the intestine [347]. In summary, TGR5 demonstrates its anti-inflammatory potential in IBD by regulating intestinal motility, enhancing intestinal secretion, and modulating immune responses. These findings offer novel insights into IBD treatment, suggesting that future research could further explore the potential of TGR5 as a therapeutic target (Table 1).
In the context of IBD, the interplay between bile acids, microbial communities, and the host is of significant importance. Research has demonstrated that patients with IBD frequently exhibit dysbiosis, characterized by a reduction in secondary bile acids and alterations in short-chain fatty acids (SCFAs) [348]. Secondary bile acids, such as ursodeoxycholic acid (UDCA) and lithocholic acid (LCA), are recognized for their anti-inflammatory properties, and their synthesis is contingent upon the metabolic activities of intestinal microorganisms [349]. Alterations in the composition of the gut microbiota can influence the production of secondary bile acids, potentially exacerbating the pathological conditions associated with IBD [350]. Furthermore, microbial imbalance may result in diminished SCFA production, thereby compromising the integrity of the intestinal barrier and immune function [348].
Bile acids and their receptors exhibit significant potential in the therapeutic management of IBD. Firstly, the activation of the bile acid receptor FXR is regarded as a critical therapeutic approach. FXR is integral in the regulation of bile acid metabolism, lipid metabolism, and inflammatory responses [351]. Studies have shown that FXR agonists can ameliorate intestinal inflammation and enhance intestinal barrier function, thereby mitigating the symptoms associated with IBD [352]. Secondly, interventions targeting TGR5 (Takeda G protein-coupled receptor 5) also demonstrate considerable promise in IBD treatment. TGR5 is implicated in bile acid metabolism also in the regulation of energy balance and immune responses [353]. Activation of TGR5 can potentiate the intestinal anti-inflammatory response and improve the intestinal microenvironment, thereby offering novel therapeutic avenues for patients with IBD [349]. Furthermore, bile acid supplementation has been suggested as a potential therapeutic approach for IBD. Bile acids, functioning as signaling molecules, have the capacity to enhance intestinal health by modulating the gut microbiota and immune responses [18]. Scholars have conducted research indicating that appropriate bile acid supplementation can foster the proliferation of beneficial bacteria while suppressing pathogenic organisms, thereby ameliorating the intestinal microecological balance in patients with IBD [349,354]. Additionally, interventions targeting the microbiome represent a promising area of investigation. The gut microbiome plays a critical role in the pathogenesis of IBD and can influence its progression by modulating bile acid metabolism and signal transduction pathways [355]. Future research may focus on optimizing bile acid metabolism through microbiome interventions, thereby offering novel insights for IBD treatment [356,357]. In brief, bile acids and their receptors exhibit multiple mechanisms of action in the context of IBD treatment, and ongoing research is expected to further elucidate their potential, providing new directions for clinical applications.
7.2. Roles in Colon Cancer
The pro-oncogenic role of bile acids in colorectal cancer (CRC) development encompasses several critical mechanisms, including DNA damage and genomic instability, disruption of the balance between cell proliferation and apoptosis, modulation of the inflammatory microenvironment, and compromise of intestinal barrier integrity. Firstly, the accumulation of bile acids is intricately linked to DNA damage. Research indicates that specific bile acids, such as deoxycholic acid (DCA), can induce oxidative stress, resulting in DNA damage and mutations, thereby facilitating colorectal cancer development [358]. Furthermore, elevated concentrations of bile acids can lead to genomic instability within cells, a hallmark of cancer progression [359]. In colorectal cancer cells, persistent exposure to bile acids can result in aberrant cell cycle regulation, thus accelerating tumor progression [360]. Secondly, the role of bile acids is evident in the disruption of the balance between cell proliferation and apoptosis. A high-fat diet results in elevated bile acid levels, creating an environment that promotes the proliferation of intestinal epithelial cells while inhibiting apoptosis, thereby conferring a survival advantage to tumor cells [361]. This imbalance impairs the physiological functions of normal cells, establishing favorable conditions for the growth of cancer cells as well [362]. Additionally, the impact of the inflammatory microenvironment is a crucial aspect of the carcinogenic effects of bile acids. The accumulation of bile acids can activate the inflammatory response in the intestine, leading to a chronic inflammatory state, which is regarded as a significant driving factor in the development of colorectal cancer [363]. Chronic inflammation exacerbates DNA damage by releasing proinflammatory cytokines and reactive oxygen species (ROS), thereby promoting tumorigenesis [364].
In the context of colon cancer research, alterations in the expression of the FXR are closely associated with tumor suppression, anti-inflammatory effects, and metabolic regulation. As a nuclear receptor, FXR exerts multiple protective roles by modulating the metabolism of bile acids, glucose, and lipids, thereby inhibiting the development of hepatic and intestinal tumors [365]. In cases of colon cancer, FXR expression is typically suppressed, a condition that is strongly linked to tumor progression [108,137]. Empirical evidence indicated that FXR activation can impede the advancement of colon cancer by inhibiting the proliferation of intestinal cancer stem cells. For instance, certain bile acid components can antagonize FXR function, resulting in the proliferation and DNA damage of cancer stem cells, whereas selective FXR activation can mitigate this aberrant growth [50]. Furthermore, the FXR plays a significant role in modulating the growth and survival of tumor cells through the regulation of intracellular metabolic pathways. Notably, FXR influences autophagy by inhibiting the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway, thereby impacting the malignant progression of colon cancer cells [205]. The anti-inflammatory properties of FXR are equally crucial; FXR agonists mitigate inflammatory damage to hepatocytes by inducing the expression of suppressor of cytokine signaling 3 (SOCS3) [366]. In the context of colon cancer, FXR activation suppresses tumor cell proliferation, enhancing intestinal health by modulating the intestinal microbiota and inflammatory response as well [367]. For instance, FXR activation is linked to alterations in the composition of the intestinal microbiota, which may regulate the metabolic state of the intestine by influencing bile acid metabolism, thus inhibiting the development of colon cancer [367,368]. Furthermore, the significance of FXR in metabolic regulation is undeniable. FXR influences the intestinal metabolic environment by modulating lipid metabolism and bile acid synthesis, which plays a crucial role in the onset and progression of colon cancer [369]. For instance, the activation of FXR enhances the expression of antioxidant enzymes and mitigates oxidative damage, thereby suppressing intestinal inflammation and tumorigenesis [370]. Alterations in FXR expression in colon cancer are intricately linked to its tumor-suppressive, anti-inflammatory, and metabolic regulatory effects. Modulating FXR activity could offer novel therapeutic strategies for the treatment of colon cancer (Table 1).
In the context of colon cancer, bile acids exhibit a dual role in the carcinogenic process [371]. Alterations in the expression of TGR5 may significantly influence tumor cell proliferation and survival. Empirical evidence suggested that TGR5 is implicated not only in the regulation of cell proliferation but also in modulating the tumor microenvironment through its effects on immune responses and metabolic pathways. Specifically, the activation of TGR5 may facilitate the proliferation of colon cancer cells while inhibiting apoptosis, thereby enhancing tumor cell survival [72]. Consequently, TGR5 expression is upregulated in certain tumor tissues [372]. Furthermore, the immunomodulatory role of TGR5 in colon cancer warrants attention, as it may impact the immune evasion mechanisms of tumors by regulating immune cell infiltration and activity [373]. For instance, TGR5 expression is closely associated with the composition and functionality of immune cells within the tumor microenvironment, thereby influencing tumor progression and patient prognosis [371,374]. Regarding metabolic regulation, the activation of TGR5 could potentially enhance tumor cell proliferation by influencing energy metabolism and intracellular signaling pathways [375]. In conclusion, alterations in TGR5 expression in colon cancer may impact tumor cell proliferation, survival, immune regulation, and metabolism through various mechanisms, indicating its potential as a therapeutic target (Table 1).
Microbial dysbiosis can significantly influence bile acid metabolism, resulting in the enhanced production of carcinogenic secondary bile acids [17]. Research indicates that bile acids function as surfactants facilitating lipid digestion also play a crucial role in regulating the synthesis of short-chain fatty acids and modulating epigenetic mechanisms through interactions with host metabolic pathways, all of which may contribute to the progression of colorectal cancer [376,377]. Furthermore, alterations in bile acid profiles are intricately linked to the composition of the gut microbiota, with microbial metabolic activities capable of modifying the structure and function of bile acids, thereby impacting the host’s immune responses and metabolic state [378]. In the context of colorectal cancer, specific microbial communities may enhance the production of carcinogenic bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA), which have been implicated in the pathogenesis of this malignancy [379]. Concurrently, bile acids regulate the metabolism of short-chain fatty acids, which are vital for maintaining intestinal health and preventing tumorigenesis [16,380]. Understanding the interactions among bile acids, microbial communities, and the host is essential for developing innovative strategies to prevent and treat colorectal cancer (CRC) [381]. The use of FXR agonists is being explored for the chemoprevention of CRC [382]. Modulating bile acid metabolism could form a component of preventive strategies [383] and may exhibit synergistic effects when combined with conventional chemotherapeutic agents [384]. In contrast, TGR5 antagonists have the potential to inhibit TGR5-mediated tumor promotion [385]. It is important to consider the differential roles of TGR5 across various stages and subtypes of CRC [373,386]. Moreover, the targeted regulation of microorganisms involved in bile acid metabolism may influence CRC risk and progression, suggesting that probiotics or specific microbial metabolites could be employed in CRC prevention [387].
7.3. Roles in Irritable Bowel Syndrome
In individuals with irritable bowel syndrome (IBS), approximately 25–30% of patients with diarrhea-predominant IBS (IBS-D) experience bile acid malabsorption [388], frequently accompanied by bile acid diarrhea (BAD), a chronic condition resulting from excessive bile acid influx into the colon [389,390]. Abnormal bile acid metabolism not only disrupts normal intestinal function but may also precipitate systemic inflammatory responses and metabolic disorders [9]. In IBS patients, there may be an upregulation of bile acid synthesis as a compensatory mechanism for bile acid deficiency due to malabsorption [391]. Furthermore, alterations in the composition of the bile acid pool can result in an imbalance between primary and secondary bile acids, potentially influencing the intestinal microbiota’s composition [392]. Research has demonstrated that bile acids play a role in regulating metabolism and immune responses through the activation of the FXR and TGR5 receptor in the intestine [9]. In patients with IBS, aberrant bile acid metabolism may lead to dysregulation of the FXR signaling pathway, consequently impacting bile acid synthesis and secretion in the liver [393]. Thus, interventions targeting bile acid metabolism, such as the use of bile acid binders or modulation of the intestinal microbiota, may offer novel therapeutic approaches for IBS [394].
Approximately 25% of patients with diarrhea-predominant IBS (IBS-D) exhibit bile acid diarrhea (BAD), which is associated with excessive bile acid excretion in the intestines [395]. Elevated bile acid levels can lead to increased intestinal fluid secretion, thereby inducing diarrhea [396]. Moreover, bile acid concentrations have been positively correlated with stool consistency and frequency, suggesting that abnormal bile acid metabolism may directly impact the bowel habits of IBS-D patients [397]. Additionally, bile acids are implicated in the pathogenesis of abdominal pain, as they can activate intestinal nerve receptors and affect sensory nerve function, thereby eliciting abdominal discomfort [398]. In patients with IBS, alterations in bile acid metabolism may contribute to heightened intestinal sensitivity, thereby exacerbating symptoms of abdominal pain [397]. Furthermore, dysregulated bile acid metabolism may influence intestinal permeability. Studies have shown that the intestinal barrier function in patients with diarrhea-predominant IBS (IBS-D) is compromised, resulting in increased intestinal permeability [399]. The integrity of the intestinal barrier is closely associated with bile acid alterations; excessive bile acids can inflict damage on intestinal epithelial cells, consequently enhancing intestinal permeability [400].
In the context of IBS, the FXR is integral to the regulation of intestinal barrier function, inflammation, and bile acid metabolism. Firstly, FXR is vital for the maintenance of intestinal barrier integrity. Empirical evidence suggested that the activation of FXR enhances the tight junctions of intestinal epithelial cells, thereby fortifying the intestinal barrier. This function is critical in preventing the translocation of harmful substances within the intestine, a process particularly relevant to IBS patients, where compromised barrier function may precipitate intestinal inflammation and associated discomfort [401]. Secondly, FXR is instrumental in modulating intestinal inflammation. It mitigates inflammatory responses by regulating the expression of genes associated with immune functions. In individuals with IBS, alterations in FXR expression may contribute to heightened inflammatory activity [400]. For instance, activation of the FXR can suppress the release of proinflammatory cytokines, thus mitigating intestinal inflammatory responses [402]. Moreover, FXR plays a crucial role in the regulation of bile acid metabolism. Bile acids are essential for lipid digestion also function as signaling molecules that influence the intestinal microbiota and various metabolic processes. In patients with IBS, bile acid metabolism may be disrupted, and FXR activation can enhance the synthesis and excretion of bile acids, thereby restoring metabolic balance [91,403]. Research has demonstrated that FXR activation can modulate the composition of the intestinal microbiota, consequently impacting bile acid metabolism and intestinal health [18,404] (Table 1).
TGR5 is purported to play a significant role in the pathophysiology of irritable bowel syndrome (IBS). IBS is a prevalent functional gastrointestinal disorder, characterized by symptoms such as abdominal pain, bloating, and altered bowel habits [405]. Research indicates that TGR5 is implicated in the regulation of intestinal motility also in visceral sensitivity and immune response [406]. The involvement of TGR5 in intestinal motility has been the subject of extensive investigation. Activation of TGR5 has been shown to facilitate the contraction of intestinal smooth muscle, thereby enhancing intestinal motility [407]. This mechanism may hold substantial clinical relevance for patients with IBS, particularly for those with constipation-predominant IBS (IBS-C), as these individuals frequently exhibit symptoms associated with intestinal hypomotility [408]. Secondly, TGR5 is intricately associated with visceral sensitivity, which denotes the capacity to perceive stimulation of visceral organs, such as the intestines. Patients with IBS frequently exhibit heightened visceral sensitivity [409]. Research indicates that the activation of TGR5 can influence visceral sensitivity by modulating neuronal excitability, thereby potentially mitigating abdominal pain and discomfort in IBS patients [410]. Furthermore, the significance of TGR5 in immune regulation warrants attention. The intestinal immune response is pivotal in the pathogenesis of IBS, and TGR5 activation can facilitate the release of anti-inflammatory cytokines, thus suppressing the intestinal inflammatory response [411]. This mechanism may hold substantial therapeutic promise for IBS patients, particularly those experiencing concurrent intestinal inflammation. To sum up, TGR5 is implicated in various aspects of IBS pathophysiology, including the regulation of intestinal motility, visceral sensitivity, and immune response. Future research may further elucidate the potential of TGR5 as a therapeutic target for IBS, offering novel strategies to enhance patient quality of life (Table 1).
The interplay between bile acids, microbial communities, and the host is crucial in the investigation of IBS. Dysbiosis is regarded as a pivotal factor in IBS, with alterations in the diversity and composition of the gut microbiota potentially influencing bile acid metabolism and function [9]. Bile acids are integral not only to lipid digestion but also act as signaling molecules that modulate host metabolic processes and immune responses [355]. Research has demonstrated that secondary bile acids are crucial in modulating the intestinal microbiota and host metabolism, particularly in sustaining intestinal health and preventing inflammation [412]. Furthermore, short-chain fatty acids (SCFAs), which are also byproducts of intestinal microbial metabolism, exert a considerable influence on the host’s metabolic and immune functions. SCFAs may contribute to the pathophysiology of IBS by enhancing intestinal barrier integrity and modulating immune responses [10,413]. Consequently, elucidating the interactions among bile acids, microbes, and the host, particularly in the context of IBS, could inform novel therapeutic strategies. Modulating intestinal microbiota and bile acid metabolism may ameliorate IBS symptoms and inspire innovative treatment approaches [348,414].

Table 1.
The Function of FXR and TGR5 in Traditional Intestinal Disorders. (The arrows: “↓” and “↑” represent the expression levels of bile acid receptor proteins, respectively).
Table 1.
The Function of FXR and TGR5 in Traditional Intestinal Disorders. (The arrows: “↓” and “↑” represent the expression levels of bile acid receptor proteins, respectively).
| Disease Type | Bile Acid Receptor | Receptor Function | Mechanism | References |
|---|---|---|---|---|
| IBD | FXR ↓ | FXR regulates inflammatory response, maintains intestinal barrier function, and affects FGF19 signaling pathway. | Dysbiosis of the gut microbiota and aberrant bile acid metabolism are interconnected phenomena. | [336,337,338,339,340,341,342,343] |
| TGR5 ↓ | TGR5 mitigates the accumulation of pro-inflammatory substances within the intestine. Activation of TGR5 can enhance the secretory functions of intestinal epithelial cells and promotes the release of anti-inflammatory mediators. | Diminished expression of TGR5 is associated with disruptions in intestinal motility, secretion, and immune regulation. | [70,344,345,346,347] | |
| CRC | FXR ↓ | Alterations in FXR expression are posited to be intricately associated with tumor suppressive effects, anti-inflammatory responses, and metabolic regulation. | A reduction in FXR expression levels diminishes its inhibitory impact on the proliferation of intestinal cancer stem cells, thereby facilitating the progression of colon cancer. | [50,108,137,366,367,368,369,370] |
| TGR5 ↑ | TGR5 is involved in the regulation of cell proliferation and may also influence the tumor microenvironment by modulating immune responses and metabolic pathways. | The activation of TGR5 has the potential to facilitate the proliferation of colon cancer cells while simultaneously inhibiting apoptosis, thereby augmenting the tumor’s survival capacity. | [371,372,373,374,375] | |
| IBS | FXR ↓ | Activation of the farnesoid X receptor (FXR) has been shown to strengthen the tight junctions of intestinal epithelial cells, consequently enhancing the integrity of the intestinal barrier. | In patients with IBS, the expression of FXR is altered, resulting in the exacerbation of inflammatory processes. | [18,91,401,402,403,404] |
| TGR5 ↓ | TGR5 is involved in the regulation of intestinal motility and also contributes to visceral sensitivity and immune response. | In patients with IBS, there is a downregulation of TGR5 expression levels, which is associated with diminished intestinal peristalsis, decreased neuronal excitability, altered visceral sensitivity, and a reduced release of anti-inflammatory factors. | [405,406,407,408,409,410,411] |
8. Conclusions
Over the past few decades, the field of bile acid receptor research has experienced significant advancements, transitioning from the initial identification of pivotal receptors such as FXR and TGR5 to a more comprehensive understanding of their intricate roles in metabolic regulation, inflammatory responses, and cellular signaling. These investigations underscore the significance of bile acids as signaling molecules as well as pave the way for novel therapeutic approaches for various metabolic, hepatobiliary, and inflammatory bowel diseases. Nevertheless, as research progresses, we encounter increasing challenges and opportunities. The development of effective and safe therapies based on bile acid signaling necessitates further basic and translational research to address several critical issues. Firstly, there is a need to bridge the gap between experimental data and clinical application, considering the substantial differences in bile acid composition and intestinal microbiota between humans and animal models. Secondly, it is essential to account for individual variations in intestinal microbiota and investigate how these microbial factors differentially impact disease states and responses to bile acid signaling therapies. Third, it is essential to consider the phasic effects of varying bile acid concentrations on the body. Given the reciprocal interaction between bile acids and microbial communities, it is imperative to elucidate how microbial populations regulate bile acid signaling and, conversely, how bile acid signaling influences gut microbial composition, health, and function. Understanding these dynamic interactions is crucial for designing effective therapeutic strategies.
The intricate cell-specific and spatiotemporal regulation of bile acid receptor function underscores the necessity for comprehensive research. To achieve this, it is vital to develop advanced research tools and methodologies, such as single-cell technologies, high-resolution imaging, and integrated multi-omics analyses, to thoroughly investigate the dynamic alterations in the bile acid signaling network across various physiological and pathological contexts. Grasping this complexity is fundamental for the development of more precise therapeutic interventions. The pivotal role of the gut microbiome in bile acid metabolism and signaling is widely acknowledged. Future research should aim to further elucidate the molecular mechanisms underlying microbial–bile acid–host interactions and investigate strategies to enhance health by modulating this axis. Such endeavors may pave the way for innovative microbiome-based interventions, serving as either supplements or alternatives to conventional pharmacological treatments. The design of highly specific ligands for receptors such as FXR and TGR5 remains a complex and challenging area of study. However, recent advancements in structural biology, computational chemistry, and artificial intelligence have introduced novel tools for ligand design. Future investigations should prioritize the development of new ligands characterized by tissue selectivity and regulatable activity, as well as the exploration of bifunctional or multifunctional ligands. These efforts are anticipated to yield more efficacious therapeutic agents with reduced side effects. Furthermore, as our comprehension of interindividual variations in bile acid signaling pathways deepens, the formulation of personalized treatment strategies becomes increasingly imperative. This encompasses the development of precise diagnostic and classification tools, the design of personalized dosing regimens, and the utilization of artificial intelligence and digital twin technology to forecast treatment outcomes. Attaining this objective necessitates interdisciplinary collaboration that synthesizes the strengths of fundamental research, clinical medicine, and data science.
Author Contributions
L.L. and D.L. conceptualized the framework of the article. X.L. wrote and edited the manuscript. L.X. and Y.Z. read the entire text and revised the sentence expression. J.X. and Q.T. provided expert opinion and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China, Project Number: 8247143079; the “Jie Bang Gua Shuai” Project of Hunan University of Chinese Medicine, Grant Number: Z2023JBGS05; the Key Research Foundation of Education Bureau of Hunan Province, Grant Number: 23A0293; and the National Natural Science Foundation of China, Grant Number: 82474115.
Institutional Review Board Statement
Written consent for participation was obtained. No ethics approval was required.
Informed Consent Statement
Consent for publication: Written consent for publication of images and necessary data was obtained, and all co-authors agree to submit the manuscript.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript. We thank all the peer reviewers for their opinions and suggestions. We express our gratitude to the Home for Researchers.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FXR | the farnesoid X receptor |
| TGR5 | G protein-coupled bile acid receptor |
| IBD | inflammatory bowel disease |
| CRC | colorectal cancer |
| IBS | irritable bowel syndrome |
| GLP-1 | glucagon-like peptide-1 |
| TLRs | Toll-like receptors |
| NLRs | NOD-like receptors |
| EGFR | epidermal growth factor receptor |
| ROS | reactive oxygen species |
| IEC | intestinal epithelial cell |
| MCU | mitochondrial calcium uniporter |
| BSH | bacterial bile acid hydrolase |
References
- Mohanty, I.; Allaband, C.; Mannochio-Russo, H.; El Abiead, Y.; Hagey, L.R.; Knight, R.; Dorrestein, P.C. The Changing Metabolic Landscape of Bile Acids—Keys to Metabolism and Immune Regulation. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 493–516. [Google Scholar] [CrossRef]
- Jones, H.; Alpini, G.; Francis, H. Bile Acid Signaling and Biliary Functions. Acta Pharm. Sin. B 2015, 5, 123–128. [Google Scholar] [CrossRef]
- VanHook, A.M. Bile Acids for Immune Tolerance. Sci. Signal 2021, 14, eabm3135. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Kheirbek, M.A. From Bile Acids to Melancholia. Neuron 2024, 112, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Gaskins, H.R. Another Renaissance for Bile Acid Gastrointestinal Microbiology. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Mannochio-Russo, H.; Schweer, J.V.; El Abiead, Y.; Bittremieux, W.; Xing, S.; Schmid, R.; Zuffa, S.; Vasquez, F.; Muti, V.B.; et al. The Underappreciated Diversity of Bile Acid Modifications. Cell 2024, 187, 1801–1818.e20. [Google Scholar] [CrossRef]
- Guan, B.; Tong, J.; Hao, H.; Yang, Z.; Chen, K.; Xu, H.; Wang, A. Bile Acid Coordinates Microbiota Homeostasis and Systemic Immunometabolism in Cardiometabolic Diseases. Acta Pharm. Sin. B 2022, 12, 2129–2149. [Google Scholar] [CrossRef]
- di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and Physical Chemistry of Bile Acids. Int. J. Mol. Sci. 2021, 22, 1780. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile Acid Metabolism and Signaling, the Microbiota, and Metabolic Disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of Bile Acids and Their Receptors in Gastrointestinal and Hepatic Pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Chen, B.; Bai, Y.; Tong, F.; Yan, J.; Zhang, R.; Zhong, Y.; Tan, H.; Ma, X. Glycoursodeoxycholic Acid Regulates Bile Acids Level and Alters Gut Microbiota and Glycolipid Metabolism to Attenuate Diabetes. Gut Microbes 2023, 15, 2192155. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Peng, Y.; Li, X. The Water Extract of Radix Scutellariae, Its Total Flavonoids and Baicalin Inhibited CYP7A1 Expression, Improved Bile Acid, and Glycolipid Metabolism in T2DM Mice. J. Ethnopharmacol. 2022, 293, 115238. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Albrechtsen, N.J.W.; Larsen, O.; Jepsen, S.L.; Balk-Moller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile Acids Are Important Direct and Indirect Regulators of the Secretion of Appetite- and Metabolism-Regulating Hormones from the Gut and Pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut Microbiota-Derived Bile Acids in Intestinal Immunity, Inflammation, and Tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jin, L.; Huang, W. Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases. Cells 2023, 12, 1888. [Google Scholar] [CrossRef]
- Fogelson, K.A.; Dorrestein, P.C.; Zarrinpar, A.; Knight, R. The Gut Microbial Bile Acid Modulation and Its Relevance to Digestive Health and Diseases. Gastroenterology 2023, 164, 1069–1085. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial Transformations of Human Bile Acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.B.; Masson, H.L.P.; Baumler, A.J. Bile Acids as Modulators of Gut Microbiota Composition and Function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of Intestinal Epithelial Cells in the Maintenance of Gut Homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Perrin, L.; Vignjevic, D.M. The Emerging Roles of the Cytoskeleton in Intestinal Epithelium Homeostasis. Semin. Cell Dev. Biol. 2023, 150–151, 23–27. [Google Scholar] [CrossRef]
- Cao, X.; Surma, M.A.; Simons, K. Polarized Sorting and Trafficking in Epithelial Cells. Cell Res. 2012, 22, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.T.; Homer, C.R.; Kemp, J.R.; Nickerson, K.P.; Deutschman, E.; Kim, Y.; West, G.; Sadler, T.; Stylianou, E.; Krokowski, D.; et al. Chromosome-Associated Protein D3 Promotes Bacterial Clearance in Human Intestinal Epithelial Cells by Repressing Expression of Amino Acid Transporters. Gastroenterology 2015, 148, 1405–1416. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Dray, C.; Sakar, Y.; Vinel, C.; Daviaud, D.; Masri, B.; Garrigues, L.; Wanecq, E.; Galvani, S.; Negre-Salvayre, A.; Barak, L.S.; et al. The Intestinal Glucose-Apelin Cycle Controls Carbohydrate Absorption in Mice. Gastroenterology 2013, 144, 771–780. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 21–22. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 Regulates Intestinal Epithelial Function by Affecting the Tight Junction Protein Claudin-1 via the ROCK Pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.F.; Peters, B.M.; La Torre, D.; Sidebottom, A.M.; Tao, Y.; Zhu, X.; Cham, C.M.; Wang, L.; Kambal, A.; Harris, K.G.; et al. Peptide YY: A Paneth Cell Antimicrobial Peptide That Maintains Candida Gut Commensalism. Science 2023, 381, 502–508. [Google Scholar] [CrossRef]
- Vlantis, K.; Polykratis, A.; Welz, P.-S.; van Loo, G.; Pasparakis, M.; Wullaert, A. TLR-Independent Anti-Inflammatory Function of Intestinal Epithelial TRAF6 Signalling Prevents DSS-Induced Colitis in Mice. Gut 2016, 65, 935–943. [Google Scholar] [CrossRef]
- Lei-Leston, A.C.; Murphy, A.G.; Maloy, K.J. Epithelial Cell Inflammasomes in Intestinal Immunity and Inflammation. Front. Immunol. 2017, 8, 1168. [Google Scholar] [CrossRef]
- Crosstalk Between Bile Acid-Activated Receptors and Microbiome in Entero-Hepatic Inflammation—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35074252/ (accessed on 22 April 2025).
- Xu, L.; Li, Y.; Wei, Z.; Bai, R.; Gao, G.; Sun, W.; Jiang, X.; Wang, J.; Li, X.; Pi, Y. Chenodeoxycholic Acid (CDCA) Promoted Intestinal Epithelial Cell Proliferation by Regulating Cell Cycle Progression and Mitochondrial Biogenesis in IPEC-J2 Cells. Antioxidants 2022, 11, 2285. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, X.; Zhang, K.; Ma, J.; Li, M.; Jin, G.; Liu, X.; Wang, S.; Wang, B.; Wu, J.; et al. Microbial Metabolite Trimethylamine-N-Oxide Induces Intestinal Carcinogenesis through Inhibiting Farnesoid X Receptor Signaling. Cell. Oncol. 2024, 47, 1183–1199. [Google Scholar] [CrossRef]
- Modica, S.; Murzilli, S.; Salvatore, L.; Schmidt, D.R.; Moschetta, A. Nuclear Bile Acid Receptor FXR Protects against Intestinal Tumorigenesis. Cancer Res. 2008, 68, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Xu, Z.; Zhang, Y.; Jiang, P.; Huang, G.; Chen, S.; Lyu, X.; Zheng, P.; Zhao, X.; Zeng, Y.; et al. FXR Induces SOCS3 and Suppresses Hepatocellular Carcinoma. Oncotarget 2015, 6, 34606–34616. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Z.; Guo, J.; Zheng, J.; Sun, X.; Yu, J. Farnesoid X Receptor Activation Induces Antitumour Activity in Colorectal Cancer by Suppressing JAK2/STAT3 Signalling via Transactivation of SOCS3 Gene. J. Cell. Mol. Med. 2020, 24, 14549–14560. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Dong, R.; Huang, K.; Wang, X.; Wang, D.; Yue, N.; Wang, C.; Sun, P.; Gu, J.; Luo, H.; et al. Yangonin Modulates Lipid Homeostasis, Ameliorates Cholestasis and Cellular Senescence in Alcoholic Liver Disease via Activating Nuclear Receptor FXR. Phytomedicine 2021, 90, 153629. [Google Scholar] [CrossRef]
- Florencia Mansilla, S.; Belen De la Vega, M.; Luis Calzetta, N.; Omar Siri, S.; Gottifredi, V. CDK-Independent and PCNA-Dependent Functions of P21 in DNA Replication. Genes. 2020, 11, 593. [Google Scholar] [CrossRef]
- Liu, H.M.; Chang, Z.Y.; Yang, C.W.; Chang, H.H.; Lee, T.Y. Farnesoid X Receptor Agonist GW4064 Protects Lipopolysaccharide-Induced Intestinal Epithelial Barrier Function and Colorectal Tumorigenesis Signaling through the αKlotho/βKlotho/FGFs Pathways in Mice. Int. J. Mol. Sci. 2023, 24, 16932. [Google Scholar] [CrossRef]
- Maran, R.R.M.; Thomas, A.; Roth, M.; Sheng, Z.; Esterly, N.; Pinson, D.; Gao, X.; Zhang, Y.; Ganapathy, V.; Gonzalez, F.J.; et al. Farnesoid X Receptor Deficiency in Mice Leads to Increased Intestinal Epithelial Cell Proliferation and Tumor Development. J. Pharmacol. Exp. Ther. 2009, 328, 469–477. [Google Scholar] [CrossRef]
- Elpek, G.O. Molecular Pathways in Viral Hepatitis-Associated Liver Carcinogenesis: An Update. World J. Clin. Cases WJCC 2021, 9, 4890–4917. [Google Scholar] [CrossRef]
- Yu, J.; Li, S.; Guo, J.; Xu, Z.; Zheng, J.; Sun, X. Farnesoid X Receptor Antagonizes Wnt/β-Catenin Signaling in Colorectal Tumorigenesis. Cell Death Dis. 2020, 11, 640. [Google Scholar] [CrossRef]
- Dong, X.; Cai, C.; Fu, T. FXR Suppresses Colorectal Cancer by Inhibiting the Wnt/β-Catenin Pathway via Activation of TLE3. Genes. Diseases 2023, 10, 719–722. [Google Scholar] [CrossRef]
- Sun, D.-Q.; Yuan, F.; Fu, M.-Z.; Zhong, M.-Y.; Zhang, S.-L.; Lu, Y.; Targher, G.; Byrne, C.D.; Zheng, M.-H.; Yuan, W.-J. Farnesoid X Receptor Activation Protects against Renal Fibrosis via Modulation of β-Catenin Signaling. Mol. Metab. 2024, 79, 101841. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chen, J.; Drachenberg, C.B.; Raufman, J.-P.; Xie, G. Farnesoid X Receptor Represses Matrix Metalloproteinase 7 Expression, Revealing This Regulatory Axis as a Promising Therapeutic Target in Colon Cancer. J. Biol. Chem. 2019, 294, 8529–8542. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Su, G.; Ji, J.; Yang, X.; Miao, M.; Mo, P.; Li, M.; Xu, J.; Li, W.; Yu, C. Histone Demethylase JMJD1A Promotes Colorectal Cancer Growth and Metastasis by Enhancing Wnt/β-Catenin Signaling. J. Biol. Chem. 2018, 293, 10606–10619. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Esteghamat, F.; Chaube, B.K.; Gunawardhana, K.; Mani, M.; Thames, C.; Jain, D.; Ginsberg, H.N.; Fernandes-Hernando, C.; Mani, A. TCF7L2 Transcriptionally Regulates Fgf15 to Maintain Bile Acid and Lipid Homeostasis through Gut-Liver Crosstalk. FASEB J. 2022, 36, e22185. [Google Scholar] [CrossRef]
- Mao, J.; Chen, X.; Wang, C.; Li, W.; Li, J. Effects and Mechanism of the Bile Acid (Farnesoid X) Receptor on the Wnt/β-Catenin Signaling Pathway in Colon Cancer. Oncol. Lett. 2020, 20, 337–345. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Jiang, L.; Cai, W.; Yan, J. Impaired Intestinal FXR Signaling Is Involved in Aberrant Stem Cell Function Leading to Intestinal Failure-Associated Liver Disease in Pediatric Patients with Short Bowel Syndrome. FASEB J. 2024, 38, e23847. [Google Scholar] [CrossRef]
- Dong, X.; Qi, M.; Cai, C.; Zhu, Y.; Li, Y.; Coulter, S.; Sun, F.; Liddle, C.; Uboha, N.V.; Halberg, R.; et al. Farnesoid X Receptor Mediates Macrophage-Intrinsic Responses to Suppress Colitis-Induced Colon Cancer Progression. JCI Insight 2024, 9, e170428. [Google Scholar] [CrossRef]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.; Zhang, T.; Leblanc, M.; Liu, S.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 1098–1112.e18. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K.; et al. Hepatic Cytochrome P450 8B1 and Cholic Acid Potentiate Intestinal Epithelial Injury in Colitis by Suppressing Intestinal Stem Cell Renewal. Cell Stem Cell 2022, 29, 1366–1381.e9. [Google Scholar] [CrossRef]
- Hou, Q.; Dong, Y.; Yu, Q.; Wang, B.; Le, S.; Guo, Y.; Zhang, B. Regulation of the Paneth Cell Niche by Exogenous L-Arginine Couples the Intestinal Stem Cell Function. FASEB J. 2020, 34, 10299–10315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions. Front. Cell Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.D.; Godfrey, C.B.; Niklas, C.; Canals, M.; Kocan, M.; Poole, D.P.; Murphy, J.E.; Alemi, F.; Cottrell, G.S.; Korbmacher, C.; et al. The Bile Acid Receptor TGR5 Does Not Interact with β-Arrestins or Traffic to Endosomes but Transmits Sustained Signals from Plasma Membrane Rafts. J. Biol. Chem. 2013, 288, 22942–22960. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lei, H.; Yang, F.; Fan, X.; Dang, Q.; Li, Y. Activation of the Bile Acid Receptor GPBAR1 (TGR5) Ameliorates Interleukin-1β (IL-1β)- Induced Chondrocytes Senescence. Biomed. Pharmacother. 2018, 106, 1713–1719. [Google Scholar] [CrossRef]
- Ji, C.-G.; Xie, X.-L.; Yin, J.; Qi, W.; Chen, L.; Bai, Y.; Wang, N.; Zhao, D.-Q.; Jiang, X.-Y.; Jiang, H.-Q. Bile Acid Receptor TGR5 Overexpression Is Associated with Decreased Intestinal Mucosal Injury and Epithelial Cell Proliferation in Obstructive Jaundice. Transl. Res. 2017, 182, 88–102. [Google Scholar] [CrossRef]
- Sakanaka, T.; Inoue, T.; Yorifuji, N.; Iguchi, M.; Fujiwara, K.; Narabayashi, K.; Kakimoto, K.; Nouda, S.; Okada, T.; Kuramoto, T.; et al. The Effects of a TGR5 Agonist and a Dipeptidyl Peptidase IV Inhibitor on Dextran Sulfate Sodium-Induced Colitis in Mice. J. Gastroenterol. Hepatol. 2015, 30 (Suppl. S1), 60–65. [Google Scholar] [CrossRef]
- Reusswig, F.; Reich, M.; Wienands, L.; Herebian, D.; Keitel-Anselmino, V.; Elvers, M. The Bile Acid Receptor TGR5 Inhibits Platelet Activation and Thrombus Formation. Platelets 2024, 35, 2322733. [Google Scholar] [CrossRef]
- Maczewsky, J.; Kaiser, J.; Gresch, A.; Gerst, F.; Düfer, M.; Krippeit-Drews, P.; Drews, G. TGR5 Activation Promotes Stimulus-Secretion Coupling of Pancreatic β-Cells via a PKA-Dependent Pathway. Diabetes 2019, 68, 324–336. [Google Scholar] [CrossRef]
- Yang, W.; Han, F.; Gu, Y.; Qu, H.; Liu, J.; Shen, J.; Leng, Y. TGR5 Agonist Inhibits Intestinal Epithelial Cell Apoptosis via cAMP/PKA/c-FLIP/JNK Signaling Pathway and Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis. Acta Pharmacol. Sin. 2023, 44, 1649–1664. [Google Scholar] [CrossRef]
- Xue, C.; Jia, H.; Cao, R.; Cai, W.; Hong, W.; Tu, J.; Wang, S.; Jiang, Q.; Bi, C.; Shan, A.; et al. Oleanolic Acid Improved Intestinal Immune Function by Activating and Potentiating Bile Acids Receptor Signaling in E. Coli-Challenged Piglets. J. Animal Sci. Biotechnol. 2024, 15, 79. [Google Scholar] [CrossRef]
- Casaburi, I.; Avena, P.; Lanzino, M.; Sisci, D.; Giordano, F.; Maris, P.; Catalano, S.; Morelli, C.; Andò, S. Chenodeoxycholic Acid through a TGR5-Dependent CREB Signaling Activation Enhances Cyclin D1 Expression and Promotes Human Endometrial Cancer Cell Proliferation. Cell Cycle 2012, 11, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Nagathihalli, N.S.; Beesetty, Y.; Lee, W.; Washington, M.K.; Chen, X.; Lockhart, A.C.; Merchant, N.B. Novel Mechanistic Insights into Ectodomain Shedding of EGFR Ligands Amphiregulin and TGF-α: Impact on Gastrointestinal Cancers Driven by Secondary Bile Acids. Cancer Res. 2014, 74, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Keitel, V.; Häussinger, D. Perspective: TGR5 (Gpbar-1) in Liver Physiology and Disease. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 412–419. [Google Scholar] [CrossRef]
- Yang, H.; Luo, F.; Wei, Y.; Jiao, Y.; Qian, J.; Chen, S.; Gong, Y.; Tang, L. TGR5 Protects against Cholestatic Liver Disease via Suppressing the NF-ΚB Pathway and Activating the Nrf2/HO-1 Pathway. Ann. Transl. Med. 2021, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Xu, W.; Chen, L.; Chen, T.; Feng, X.; Chen, J.; Wei, D.; Huang, Q. Ginsenoside Compound K Increases Glucagon-like Peptide-1 Release and L-Cell Abundance in Db/Db Mice through TGR5/YAP Signaling. Int. Immunopharmacol. 2022, 113, 109405. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Sleiman, M.B.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, K.; Hao, H.; Zhao, Y.; Bao, L.; Qiu, M.; He, Y.; He, Z.; Zhang, N.; Hu, X.; et al. Gut Microbiota-Mediated Secondary Bile Acid Alleviates Staphylococcus Aureus-Induced Mastitis through the TGR5-cAMP-PKA-NF-κB/NLRP3 Pathways in Mice. NPJ Biofilms Microbiomes 2023, 9, 8. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, Y.; Niu, K.; Zeng, W.; Wang, R.; Guo, X.; Lin, C.; Hu, L. Oleanolic Acid Alleviate Intestinal Inflammation by Inhibiting Takeda G-Coupled Protein Receptor (TGR) 5 Mediated Cell Apoptosis. Food Funct. 2024, 15, 1963–1976. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin Improves DSS-Induced Colitis in Mice via Modulation of Fecal-Bacteria-Related Bile Acid Metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H. Probiotics Alleviate Inflammatory Bowel Disease in Mice by Regulating Intestinal Microorganisms-Bile Acid-NLRP3 Inflammasome Pathway. Acta Biochim. Pol. 2021, 68, 687–693. [Google Scholar] [CrossRef]
- Qi, Y.; Duan, G.; Wei, D.; Zhao, C.; Ma, Y. The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe? Molecules 2022, 27, 5292. [Google Scholar] [CrossRef]
- Sun, M.; Tan, Z.; Lin, K.; Li, X.; Zhu, J.; Zhan, L.; Zheng, H. Advanced Progression for the Heterogeneity and Homeostasis of Intestinal Stem Cells. Stem Cell Rev. Rep. 2023, 19, 2109–2119. [Google Scholar] [CrossRef]
- Meng, J.P.; Ceryak, S.; Aratsu, Z.; Jones, L.; Epstein, L.; Bouscarel, B. Biphasic Regulation by Bile Acids of Dermal Fibroblast Proliferation through Regulation of cAMP Production and COX-2 Expression Level. Am. J. Physiol. Cell Physiol. 2006, 291, C546–C554. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xiong, M.; Xu, X.; Wu, X.; Xu, J.; Cai, X.; Lu, L.; Zhou, H. Bile Acids Elevated by High-Fat Feeding Induce Endoplasmic Reticulum Stress in Intestinal Stem Cells and Contribute to Mucosal Barrier Damage. Biochem. Biophys. Res. Commun. 2020, 529, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Dossa, A.Y.; Escobar, O.; Golden, J.; Frey, M.R.; Ford, H.R.; Gayer, C.P. Bile Acids Regulate Intestinal Cell Proliferation by Modulating EGFR and FXR Signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G81–G92. [Google Scholar] [CrossRef]
- Golden, J.M.; Escobar, O.H.; Nguyen, M.V.L.; Mallicote, M.U.; Kavarian, P.; Frey, M.R.; Gayer, C.P. Ursodeoxycholic Acid Protects against Intestinal Barrier Breakdown by Promoting Enterocyte Migration via EGFR- and COX-2-Dependent Mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G259–G271. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.-P.; Studer, E.J.; Stravitz, R.T.; Gupta, S.; Qiao, L.; Dent, P.; Hylemon, P.B. Activation of the Raf-1/MEK/ERK Cascade by Bile Acids Occurs via the Epidermal Growth Factor Receptor in Primary Rat Hepatocytes. Hepatology 2002, 35, 307–314. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Boyer, J.L. The Role of Bile Acids in Cholestatic Liver Injury. Ann. Transl. Med. 2021, 9, atm-20-5110. [Google Scholar] [CrossRef]
- Warden, C.; Brantley, M.A. Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro. Biomolecules 2021, 11, 626. [Google Scholar] [CrossRef]
- Bartram, H.P.; Englert, S.; Scheppach, W.; Dusel, G.; Richter, F.; Richter, A.; Kasper, H. Antagonistic Effects of Deoxycholic Acid and Butyrate on Epithelial Cell Proliferation in the Proximal and Distal Human Colon. Z. Gastroenterol. 1994, 32, 389–392. [Google Scholar]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Moschetta, A. Bile Acids and Colon Cancer: Is FXR the Solution of the Conundrum? Mol. Aspects Med. 2017, 56, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ignacio Barrasa, J.; Olmo, N.; Pérez-Ramos, P.; Santiago-Gómez, A.; Lecona, E.; Turnay, J.; Antonia Lizarbe, M. Deoxycholic and Chenodeoxycholic Bile Acids Induce Apoptosis via Oxidative Stress in Human Colon Adenocarcinoma Cells. Apoptosis Int. J. Program. Cell Death 2011, 16, 1054–1067. [Google Scholar] [CrossRef]
- Ershad, M.; Shigenaga, M.K.; Bandy, B. Differential Protection by Anthocyanin-Rich Bilberry Extract and Resveratrol against Lipid Micelle-Induced Oxidative Stress and Monolayer Permeability in Caco-2 Intestinal Epithelial Cells. Food Funct. 2021, 12, 2950–2961. [Google Scholar] [CrossRef]
- Burban, A.; Sharanek, A.; Guguen-Guillouzo, C.; Guillouzo, A. Endoplasmic Reticulum Stress Precedes Oxidative Stress in Antibiotic-Induced Cholestasis and Cytotoxicity in Human Hepatocytes. Free Radic. Biol. Med. 2018, 115, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Pallagi-Kunstár, É.; Farkas, K.; Maléth, J.; Rakonczay, Z.; Nagy, F.; Molnár, T.; Szepes, Z.; Venglovecz, V.; Lonovics, J.; Rázga, Z.; et al. Bile Acids Inhibit Na+/H+ Exchanger and Cl−/HCO₃− Exchanger Activities via Cellular Energy Breakdown and Ca2+ Overload in Human Colonic Crypts. Pflugers Arch. 2015, 467, 1277–1290. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Zhao, J.; Song, G.; Weng, F.; Xu, X.; Li, F.; Jin, J.; Yan, D.; Huang, K.; et al. Glycyrrhetinic Acid Attenuates Endoplasmic Reticulum Stress-Induced Hepatocyte Apoptosis via CHOP/DR5/Caspase 8 Pathway in Cholestasis. Eur. J. Pharmacol. 2023, 961, 176193. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Haller, D. Structure-Function Analysis of the Tertiary Bile Acid TUDCA for the Resolution of Endoplasmic Reticulum Stress in Intestinal Epithelial Cells. Biochem. Biophys. Res. Commun. 2011, 409, 610–615. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Huang, Z.; Zhao, D.; Ganesh, B.S.; Lai, G.; Pandak, W.M.; Hylemon, P.B.; Bajaj, J.S.; Sanyal, A.J.; et al. C/EBP Homologous Protein-Induced Loss of Intestinal Epithelial Stemness Contributes to Bile Duct Ligation-Induced Cholestatic Liver Injury in Mice. Hepatology 2018, 67, 1441–1457. [Google Scholar] [CrossRef]
- Palmeira, C.M.; Rolo, A.P. Mitochondrially-Mediated Toxicity of Bile Acids. Toxicology 2004, 203, 1–15. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Bile Acid Receptors and Gastrointestinal Functions. Liver Res. 2019, 3, 31–39. [Google Scholar] [CrossRef]
- Kisthardt, S.C.; Thanissery, R.; Pike, C.M.; Foley, M.H.; Theriot, C.M. The Microbial-Derived Bile Acid Lithocholate and Its Epimers Inhibit Clostridioides Difficile Growth and Pathogenicity While Sparing Members of the Gut Microbiota. J. Bacteriol. 2023, 205, e00180-23. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Trammell, S.A.J.; Albrechtsen, N.J.W.; Schoonjans, K.; Albrechtsen, R.; Gillum, M.P.; Kuhre, R.E.; Holst, J.J. Bile Acids Drive Colonic Secretion of Glucagon-like-Peptide 1 and Peptide-YY in Rodents. Am. J. Physiol.-Gastroint. Liver Physiol. 2019, 316, G574–G584. [Google Scholar] [CrossRef]
- O’Leary, C.E.; Sbierski-Kind, J.; Kotas, M.E.; Wagner, J.C.; Liang, H.-E.; Schroeder, A.W.; de Tenorio, J.C.; von Moltke, J.; Ricardo-Gonzalez, R.R.; Eckalbar, W.L.; et al. Bile Acid-Sensitive Tuft Cells Regulate Biliary Neutrophil Influx. Sci. Immunol. 2022, 7, eabj1080. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, S.-Y.; Gillilland, M.; Li, J.-Y.; Lee, A.; Gao, J.; Zhang, G.; Xu, X.; Owyang, C. Bile Acid Toxicity in Paneth Cells Contributes to Gut Dysbiosis Induced by High-Fat Feeding. JCI Insight 2020, 5, e138881. [Google Scholar] [CrossRef]
- Oswald, S. Organic Anion Transporting Polypeptide (OATP) Transporter Expression, Localization and Function in the Human Intestine. Pharmacol. Ther. 2019, 195, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.H.; Leake, B.F.; Urquhart, B.L.; Gregor, J.C.; Dawson, P.A.; Kim, R.B. Functional Characterization of Genetic Variants in the Apical Sodium-Dependent Bile Acid Transporter (ASBT; SLC10A2). J. Gastroenterol. Hepatol. 2011, 26, 1740–1748. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, K.; Cai, X.; Wang, C.; Cao, Y.; Xiao, J. Rosmarinic Acid Restores Colonic Mucus Secretion in Colitis Mice by Regulating Gut Microbiota-Derived Metabolites and the Activation of Inflammasomes. J. Agric. Food Chem. 2023, 71, 4571–4585. [Google Scholar] [CrossRef]
- Song, P.; Rockwell, C.E.; Cui, J.Y.; Klaassen, C.D. Individual Bile Acids Have Differential Effects on Bile Acid Signaling in Mice. Toxicol. Appl. Pharmacol. 2015, 283, 57–64. [Google Scholar] [CrossRef]
- Májer, F.; Sharma, R.; Mullins, C.; Keogh, L.; Phipps, S.; Duggan, S.; Kelleher, D.; Keely, S.; Long, A.; Radics, G.; et al. New Highly Toxic Bile Acids Derived from Deoxycholic Acid, Chenodeoxycholic Acid and Lithocholic Acid. Bioorg Med. Chem. 2014, 22, 256–268. [Google Scholar] [CrossRef]
- Zheng, Y.; Yue, C.; Zhang, H.; Chen, H.; Liu, Y.; Li, J. Deoxycholic Acid and Lithocholic Acid Alleviate Liver Injury and Inflammation in Mice with Klebsiella Pneumoniae-Induced Liver Abscess and Bacteremia. J. Inflammation Res. 2021, 14, 777–789. [Google Scholar] [CrossRef]
- Zhou, W.; Ramachandran, D.; Mansouri, A.; Dailey, M.J. Glucose Stimulates Intestinal Epithelial Crypt Proliferation by Modulating Cellular Energy Metabolism. J. Cell. Physiol. 2018, 233, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Calibasi-Kocal, G.; Mashinchian, O.; Basbinar, Y.; Ellidokuz, E.; Cheng, C.-W.; Yilmaz, Ö.H. Nutritional Control of Intestinal Stem Cells in Homeostasis and Tumorigenesis. Trends Endocrinol. Metab. 2021, 32, 20–35. [Google Scholar] [CrossRef]
- Kim, K.-S.; Peck, B.C.; Hung, Y.-H.; Koch-Laskowski, K.; Wood, L.; Dedhia, P.H.; Spence, J.R.; Seeley, R.J.; Sethupathy, P.; Sandoval, D.A. Vertical Sleeve Gastrectomy Induces Enteroendocrine Cell Differentiation of Intestinal Stem Cells through Bile Acid Signaling. JCI Insight 2022, 7, e154302. [Google Scholar] [CrossRef]
- Id Boufker, H.; Lagneaux, L.; Fayyad-Kazan, H.; Badran, B.; Najar, M.; Wiedig, M.; Ghanem, G.; Laurent, G.; Body, J.-J.; Journé, F. Role of Farnesoid X Receptor (FXR) in the Process of Differentiation of Bone Marrow Stromal Cells into Osteoblasts. Bone 2011, 49, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.A.; Mennillo, E.; Yang, X.; van der Schoor, L.W.E.; Jonker, J.W.; Chen, S.; Tukey, R.H. Regulation of Intestinal UDP-Glucuronosyltransferase 1A1 by the Farnesoid X Receptor Agonist Obeticholic Acid Is Controlled by Constitutive Androstane Receptor through Intestinal Maturation. Drug Metab. Dispos. 2021, 49, 12–19. [Google Scholar] [CrossRef]
- Yu, J.; Yang, K.; Zheng, J.; Zhao, P.; Xia, J.; Sun, X.; Zhao, W. Activation of FXR and Inhibition of EZH2 Synergistically Inhibit Colorectal Cancer through Cooperatively Accelerating FXR Nuclear Location and Upregulating CDX2 Expression. Cell Death Dis. 2022, 13, 388. [Google Scholar] [CrossRef]
- Chen, M.; Lu, C.; Lu, H.; Zhang, J.; Qin, D.; Liu, S.; Li, X.; Zhang, L. Farnesoid X Receptor via Notch1 Directs Asymmetric Cell Division of Sox9+ Cells to Prevent the Development of Liver Cancer in a Mouse Model. Stem Cell Res. Ther. 2021, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, D.M.; Chen, B.; Iticovici, M.; Thaker, A.I.; Dai, N.; VanDussen, K.L.; Shaikh, N.; Lim, C.K.; Guillemin, G.J.; Tarr, P.I.; et al. Epithelial Indoleamine 2,3-Dioxygenase 1 Modulates Aryl Hydrocarbon Receptor and Notch Signaling to Increase Differentiation of Secretory Cells and Alter Mucus-Associated Microbiota. Gastroenterology 2019, 157, 1093–1108.e11. [Google Scholar] [CrossRef]
- Carulli, A.J.; Keeley, T.M.; Demitrack, E.S.; Chung, J.; Maillard, I.; Samuelson, L.C. Notch Receptor Regulation of Intestinal Stem Cell Homeostasis and Crypt Regeneration. Dev. Biol. 2015, 402, 98–108. [Google Scholar] [CrossRef]
- Liu, J.; Liu, K.; Wang, Y.; Shi, Z.; Xu, R.; Zhang, Y.; Li, J.; Liu, C.; Xue, B. Death Receptor 5 Is Required for Intestinal Stem Cell Activity during Intestinal Epithelial Renewal at Homoeostasis. Cell Death Dis. 2024, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Verzi, M.P.; Shivdasani, R.A. Epigenetic Regulation of Intestinal Stem Cell Differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G189–G196. [Google Scholar] [CrossRef]
- Pope, J.L.; Bhat, A.A.; Sharma, A.; Ahmad, R.; Krishnan, M.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-1 Regulates Intestinal Epithelial Homeostasis through the Modulation of Notch-Signalling. Gut 2014, 63, 622–634. [Google Scholar] [CrossRef]
- Fu, T.; Li, Y.; Oh, T.G.; Cayabyab, F.; He, N.; Tang, Q.; Coulter, S.; Truitt, M.; Medina, P.; He, M.; et al. FXR Mediates ILC-Intrinsic Responses to Intestinal Inflammation. Proc. Natl. Acad. Sci. USA 2022, 119, e2213041119. [Google Scholar] [CrossRef] [PubMed]
- Nell, P.; Kattler, K.; Feuerborn, D.; Hellwig, B.; Rieck, A.; Salhab, A.; Lepikhov, K.; Gasparoni, G.; Thomitzek, A.; Belgasmi, K.; et al. Identification of an FXR-Modulated Liver-Intestine Hybrid State in iPSC-Derived Hepatocyte-like Cells. J. Hepatol. 2022, 77, 1386–1398. [Google Scholar] [CrossRef]
- Zhao, L.; Xuan, Z.; Song, W.; Zhang, S.; Li, Z.; Song, G.; Zhu, X.; Xie, H.; Zheng, S.; Song, P. A Novel Role for Farnesoid X Receptor in the Bile Acid-mediated Intestinal Glucose Homeostasis. J. Cell. Mol. Med. 2020, 24, 12848–12861. [Google Scholar] [CrossRef]
- Maeda, T.; Miyata, M.; Yotsumoto, T.; Kobayashi, D.; Nozawa, T.; Toyama, K.; Gonzalez, F.J.; Yamazoe, Y.; Tamai, I. Regulation of Drug Transporters by the Farnesoid X Receptor in Mice. Mol. Pharm. 2004, 1, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Huang, S.; Xu, W.; Chen, L.; Su, J.; Ni, H.; Feng, X.; Chen, J.; Wang, X.; Huang, Q. Compound K Attenuates Hyperglycemia by Enhancing Glucagon-like Peptide-1 Secretion through Activating TGR5 via the Remodeling of Gut Microbiota and Bile Acid Metabolism. J. Ginseng Res. 2022, 46, 780–789. [Google Scholar] [CrossRef]
- Guo, Q.; Hou, X.; Cui, Q.; Li, S.; Shen, G.; Luo, Q.; Wu, H.; Chen, H.; Liu, Y.; Chen, A.; et al. Pectin Mediates the Mechanism of Host Blood Glucose Regulation through Intestinal Flora. Crit. Rev. Food Sci. Nutr. 2024, 64, 6714–6736. [Google Scholar] [CrossRef]
- Bunnett, N.W. Neuro-Humoral Signalling by Bile Acids and the TGR5 Receptor in the Gastrointestinal Tract. J. Physiol. 2014, 592, 2943–2950. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. AMPK Improves Gut Epithelial Differentiation and Barrier Function via Regulating Cdx2 Expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Chang, Z.-Y.; Liu, H.-M.; Leu, Y.-L.; Hsu, C.-H.; Lee, T.-Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, F.; Fu, Y.; Yi, X.; Feng, S.; Liu, Z.; Deng, D.; Yang, Q.; Yu, M.; Zhu, C.; et al. Tauroursodeoxycholic Acid (TUDCA) Improves Intestinal Barrier Function Associated with TGR5-MLCK Pathway and the Alteration of Serum Metabolites and Gut Bacteria in Weaned Piglets. J. Anim. Sci. Biotechnol. 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lv, X.; Liu, G.; Li, S.; Fan, J.; Chen, L.; Huang, Z.; Lin, G.; Xu, X.; Huang, Z.; et al. Gut Microbiota-Related Bile Acid Metabolism-FXR/TGR5 Axis Impacts the Response to Anti-A4β7-Integrin Therapy in Humanized Mice with Colitis. Gut Microbes 2023, 15, 2232143. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shang, S.; Luan, W.; Ma, J.; Yang, H.; Qian, Q.; Wu, Z.; Li, X. Apple Polyphenol Extracts Attenuated Depressive-Like Behaviors of High-Sucrose Diet Feeding Mice by Farnesoid X Receptor-Mediated Modulation of Bile Acid Circulation within the Liver-Gut-Brain Axis. J. Agric. Food Chem. 2024, 72, 25118–25134. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile Acid-Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT Cells—A Major Target for GLP-1 and Gut Microbial Metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Merlen, G.; Kahale, N.; Ursic-Bedoya, J.; Bidault-Jourdainne, V.; Simerabet, H.; Doignon, I.; Tanfin, Z.; Garcin, I.; Péan, N.; Gautherot, J.; et al. TGR5-Dependent Hepatoprotection through the Regulation of Biliary Epithelium Barrier Function. Gut 2020, 69, 146–157. [Google Scholar] [CrossRef]
- Liu, T.-C.; Kern, J.T.; Jain, U.; Sonnek, N.M.; Xiong, S.; Simpson, K.F.; VanDussen, K.L.; Winkler, E.S.; Haritunians, T.; Malique, A.; et al. Western Diet Induces Paneth Cell Defects through Microbiome Alterations and Farnesoid X Receptor and Type I Interferon Activation. Cell Host Microbe 2021, 29, 988–1001.e6. [Google Scholar] [CrossRef]
- Hung, C.-T.; Ma, C.; Panda, S.K.; Trsan, T.; Hodel, M.; Frein, J.; Foster, A.; Sun, S.; Wu, H.-T.; Kern, J.; et al. Western Diet Reduces Small Intestinal Intraepithelial Lymphocytes via FXR-Interferon Pathway. Mucosal Immunol. 2024, 17, 1019–1028. [Google Scholar] [CrossRef]
- Wei, W.; Pan, S.; Ma, Y.; Xiao, Y.; Yang, Y.; He, S.; Bravo, A.; Soberón, M.; Liu, K. GATAe Transcription Factor Is Involved in Bacillus Thuringiensis Cry1Ac Toxin Receptor Gene Expression Inducing Toxin Susceptibility. Insect Biochem. Mol. Biol. 2020, 118, 103306. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Paerregaard, S.I.; Brandum, E.P.; Jorgensen, A.S.; Hjorto, G.M.; Jensen, B.A.H. Rewiring Host-Microbe Interactions and Barrier Function during Gastrointestinal Inflammation. Gastroenterol. Rep. 2022, 10, goac008. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.; Gulati, A.S. Implications of Paneth Cell Dysfunction on Gastrointestinal Health and Disease. Curr. Opin. Gastroenterol. 2022, 38, 535–540. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Zhou, Y.; Li, X.; Xu, S.; Jin, Q.; Li, W. Bacillus Amyloliquefaciens SC06 Relieving Intestinal Inflammation by Modulating Intestinal Stem Cells Proliferation and Differentiation via AhR/STAT3 Pathway in LPS-Challenged Piglets. J. Agric. Food Chem. 2024, 72, 6096–6109. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Tang, L.; Wang, Y.; Zhou, Y.; Li, X.; Jin, M.; Fu, A.; Li, W. Bacillus Amyloliquefaciens SC06 Alleviated Intestinal Damage Induced by Inflammatory via Modulating Intestinal Microbiota and Intestinal Stem Cell Proliferation and Differentiation. Int. Immunopharmacol. 2024, 130, 111675. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xi, Y.; Kim, J.-W.; Zhu, J.; Zhang, M.; Xu, M.; Ren, S.; Yang, D.; Ma, X.; Xie, W. Sulfation of Chondroitin and Bile Acids Converges to Antagonize Wnt/β-Catenin Signaling and Inhibit APC Deficiency-Induced Gut Tumorigenesis. Acta Pharm. Sin. B 2024, 14, 1241–1256. [Google Scholar] [CrossRef]
- Pineiro-Llanes, J.; da Silva, L.; Huang, J.; Cristofoletti, R. Comparative Study of Basement-Membrane Matrices for Human Stem Cell Maintenance and Intestinal Organoid Generation. J. Vis. Exp. JoVE 2024, 205, e66277. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Falcone, F.H.; Stolnik, S.; Garnett, M. Basement Membrane Influences Intestinal Epithelial Cell Growth and Presents a Barrier to the Movement of Macromolecules. Exp. Cell Res. 2014, 323, 218–231. [Google Scholar] [CrossRef]
- Hahn, U.; Stallmach, A.; Hahn, E.G.; Riecken, E.O. Basement Membrane Components Are Potent Promoters of Rat Intestinal Epithelial Cell Differentiation in Vitro. Gastroenterology 1990, 98, 322–335. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Kim, S.; Kim, Y.; Lee, Y.-S.; Lee, S.; Lee, S.-H.; Kweon, M.-N. A High-Fat Diet Activates the BAs-FXR Axis and Triggers Cancer-Associated Fibroblast Properties in the Colon. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1141–1159. [Google Scholar] [CrossRef]
- Wu, N.; Sun, H.; Zhao, X.; Zhang, Y.; Tan, J.; Qi, Y.; Wang, Q.; Ng, M.; Liu, Z.; He, L.; et al. MAP3K2-Regulated Intestinal Stromal Cells Define a Distinct Stem Cell Niche. Nature 2021, 592, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, M.; Caron, S.; Duhem, C.; Prawitt, J.; Dumont, J.; Lucas, A.; Bouchaert, E.; Briand, O.; Brozek, J.; Kuipers, F.; et al. The Farnesoid X Receptor Regulates Adipocyte Differentiation and Function by Promoting Peroxisome Proliferator-Activated Receptor-Gamma and Interfering with the Wnt/Beta-Catenin Pathways. J. Biol. Chem. 2010, 285, 36759–36767. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.L.; Elghajiji, A.; Grimaldos Rodriguez, C.; Weston, S.D.; Burke, Z.D.; Tosh, D. The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal. Genes 2020, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Previs, R.A.; Coleman, R.L.; Harris, A.L.; Sood, A.K. Molecular Pathways: Translational and Therapeutic Implications of the Notch Signaling Pathway in Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 955–961. [Google Scholar] [CrossRef]
- Pajcini, K.V.; Speck, N.A.; Pear, W.S. Notch Signaling in Mammalian Hematopoietic Stem Cells. Leukemia 2011, 25, 1525–1532. [Google Scholar] [CrossRef]
- Obata, Y.; Takahashi, D.; Ebisawa, M.; Kakiguchi, K.; Yonemura, S.; Jinnohara, T.; Kanaya, T.; Fujimura, Y.; Ohmae, M.; Hase, K.; et al. Epithelial Cell-Intrinsic Notch Signaling Plays an Essential Role in the Maintenance of Gut Immune Homeostasis. J. Immunol. 2012, 188, 2427–2436. [Google Scholar] [CrossRef]
- Dawson, P.A. Roles of Ileal ASBT and OSTα-OSTβ in Regulating Bile Acid Signaling. Dig. Dis. 2017, 35, 261–266. [Google Scholar] [CrossRef]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic Profiling and Populational Patterns of Bacterial Bile Salt Hydrolase (BSH) Genes Based on Worldwide Human Gut Microbiome. Microbiome 2019, 7, 9. [Google Scholar] [CrossRef]
- Ma, J.; Hong, Y.; Zheng, N.; Xie, G.; Lyu, Y.; Gu, Y.; Xi, C.; Chen, L.; Wu, G.; Li, Y.; et al. Gut Microbiota Remodeling Reverses Aging-Associated Inflammation and Dysregulation of Systemic Bile Acid Homeostasis in Mice Sex-Specifically. Gut Microbes 2020, 11, 1450–1474. [Google Scholar] [CrossRef]
- Su, J.; He, Z.; Yu, Y.; Lu, M.; Wu, Z.; Zhang, D. Gualou Xiebai Decoction Ameliorates Increased Caco-2 Monolayer Permeability Induced by Bile Acids via Tight Junction Regulation, Oxidative Stress Suppression and Apoptosis Reduction. J. Bioenerg. Biomembr. 2022, 54, 45–57. [Google Scholar] [CrossRef]
- Liu, Z.; Han, K.; Huo, X.; Yan, B.; Gao, M.; Lv, X.; Yu, P.; Gao, G.; Chang, Y.-Z. Nrf2 Knockout Dysregulates Iron Metabolism and Increases the Hemolysis through ROS in Aging Mice. Life Sci. 2020, 255, 117838. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Hara, E. Gut-Liver Axis-Mediated Mechanism of Liver Cancer: A Special Focus on the Role of Gut Microbiota. Cancer Sci. 2021, 112, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Vaddavalli, P.L.; Schumacher, B. The P53 Network: Cellular and Systemic DNA Damage Responses in Cancer and Aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Chesnokova, V.; Zonis, S.; Apostolou, A.; Estrada, H.Q.; Knott, S.; Wawrowsky, K.; Michelsen, K.; Ben-Shlomo, A.; Barrett, R.; Gorbunova, V.; et al. Local Non-Pituitary Growth Hormone Is Induced with Aging and Facilitates Epithelial Damage. Cell Rep. 2021, 37, 110068. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shi, L.; Huang, W. The Role of Bile Acids in Human Aging. Med. Rev. 2024, 4, 154–157. [Google Scholar] [CrossRef]
- Xue, Y.; Wei, Y.; Cao, L.; Shi, M.; Sheng, J.; Xiao, Q.; Cheng, Z.; Luo, T.; Jiao, Q.; Wu, A.; et al. Protective Effects of Scutellaria-Coptis Herb Couple against Non-Alcoholic Steatohepatitis via Activating NRF2 and FXR Pathways in Vivo and in Vitro. J. Ethnopharmacol. 2024, 318, 116933. [Google Scholar] [CrossRef]
- Duan, Y.; Guo, F.; Li, C.; Xiang, D.; Gong, M.; Yi, H.; Chen, L.; Yan, L.; Zhang, D.; Dai, L.; et al. Aqueous Extract of Fermented Eucommia Ulmoides Leaves Alleviates Hyperlipidemia by Maintaining Gut Homeostasis and Modulating Metabolism in High-Fat Diet Fed Rats. Phytomedicine 2024, 128, 155291. [Google Scholar] [CrossRef]
- Ha, S.; Yang, Y.; Won Kim, J.; Son, M.; Kim, D.; Kim, M.-J.; Im, D.-S.; Young Chung, H.; Chung, K.W. Diminished Tubule Epithelial Farnesoid X Receptor Expression Exacerbates Inflammation and Fibrosis Response in Aged Rat Kidney. J. Gerontol. A 2023, 78, 60–68. [Google Scholar] [CrossRef]
- Yang, G.; Jena, P.K.; Hu, Y.; Sheng, L.; Chen, S.-Y.; Slupsky, C.M.; Davis, R.; Tepper, C.G.; Wan, Y.-J.Y. The Essential Roles of FXR in Diet and Age Influenced Metabolic Changes and Liver Disease Development: A Multi-Omics Study. Biomark. Res. 2023, 11, 20. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, X.; Lu, Y.; Wang, E.; Zhang, Z.; Yang, J.; Zhang, H.; Li, X. Hepatic Steatosis Exacerbated by Endoplasmic Reticulum Stress-Mediated Downregulation of FXR in Aging Mice. J. Hepatol. 2014, 60, 847–854. [Google Scholar] [CrossRef]
- Cui, S.; Hu, H.; Chen, A.; Cui, M.; Pan, X.; Zhang, P.; Wang, G.; Wang, H.; Hao, H. SIRT1 Activation Synergizes with FXR Agonism in Hepatoprotection via Governing Nucleocytoplasmic Shuttling and Degradation of FXR. Acta Pharm. Sin. B 2023, 13, 559–576. [Google Scholar] [CrossRef]
- Rogina, B.; Tissenbaum, H.A. SIRT1, Resveratrol and Aging. Front. Genet. 2024, 15, 1393181. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, X.; Wang, L.; Wang, C.; Huang, K.; Fu, T.; Liu, K.; Wu, J.; Sun, H.; Meng, Q. Yangonin Inhibits Ethanol-Induced Hepatocyte Senescence via miR-194/FXR Axis. Eur. J. Pharmacol. 2021, 890, 173653. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, Y.; Che, M.; Li, C.; Wu, Q.; Sun, C. Melatonin Relieves Hepatic Lipid Dysmetabolism Caused by Aging via Modifying the Secondary Bile Acid Pattern of Gut Microbes. Cell Mol. Life Sci. 2022, 79, 527. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Luo, Y.; Wang, D.; Adorini, L.; Pruzanski, M.; Dobrinskikh, E.; Levi, M. A Dual Agonist of Farnesoid X Receptor (FXR) and the G Protein-Coupled Receptor TGR5, INT-767, Reverses Age-Related Kidney Disease in Mice. J. Biol. Chem. 2017, 292, 12018–12024. [Google Scholar] [CrossRef]
- Zhuang, L.; Ding, W.; Zhang, Q.; Ding, W.; Xu, X.; Yu, X.; Xi, D. TGR5 Attenuated Liver Ischemia-Reperfusion Injury by Activating the Keap1-Nrf2 Signaling Pathway in Mice. Inflammation 2021, 44, 859–872. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the Liver-Gut System: Pharmacological Bases Explaining Its Therapeutic Effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal Hypoxia-Inducible Factor 2α Regulates Lactate Levels to Shape the Gut Microbiome and Alter Thermogenesis. Cell Metab. 2021, 33, 1988–2003.e7. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Li, J.; Chavan, H.; Matye, D.; Ni, H.-M.; Chiang, J.Y.L.; Krishnamurthy, P.; Ding, W.-X.; Li, T. Targeting the Enterohepatic Bile Acid Signaling Induces Hepatic Autophagy via a CYP7A1-AKT-mTOR Axis in Mice. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 245–260. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, M.; Hampton, K.; Ruiz, P.; Beasy, G.; Nagies, F.S.; Parker, A.; Lazenby, J.; Bone, C.; Alava-Arteaga, A.; Patel, M.; et al. Regulation of Intestinal Senescence during Cholestatic Liver Disease Modulates Barrier Function and Liver Disease Progression. JHEP Rep. 2024, 6, 101159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, L.; Zou, T.; Lian, S.; Luo, J.; Lu, Y.; Hao, H.; Xu, Y.; Xiang, Y.; Zhang, X.; et al. Ileitis Promotes MASLD Progression via Bile Acid Modulation and Enhanced TGR5 Signaling in Ileal CD8+ T Cells. J. Hepatol. 2024, 80, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ruan, Z.; Lin, X.; Wang, H.; Xin, Y.; Tang, H.; Hu, Z.; Zhou, Y.; Wu, Y.; Wang, J.; et al. NAD+ Dependent UPRmt Activation Underlies Intestinal Aging Caused by Mitochondrial DNA Mutations. Nat. Commun. 2024, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Zhao, F.; Guo, Y.; Zhang, Y.; Chen, Y.; He, L.; Li, L. Lactobacillus Reuteri JCM 1112 Ameliorates Chronic Acrylamide-Induced Glucose Metabolism Disorder via the Bile Acid-TGR5-GLP-1 Axis and Modulates Intestinal Oxidative Stress in Mice. Food Funct. 2024, 15, 6450–6458. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Feng, X.; Lin, Q.; Deng, J.; Yuan, Y.; Li, M.; Zhai, B.; Chen, J. Folic Acid Supplementation Prevents High Body Fat-Induced Bone Loss through TGR5 Signaling Pathways. Food Funct. 2024, 15, 4193–4206. [Google Scholar] [CrossRef]
- Chu, C.; Li, T.; Yu, L.; Li, Y.; Li, M.; Guo, M.; Zhao, J.; Zhai, Q.; Tian, F.; Chen, W. A Low-Protein, High-Carbohydrate Diet Exerts a Neuroprotective Effect on Mice with 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease by Regulating the Microbiota-Metabolite-Brain Axis and Fibroblast Growth Factor 21. J. Agric. Food Chem. 2023, 71, 8877–8893. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, Z.; Liu, X.; Jin, G.; Wang, Y.; He, L.; Li, M.; Pang, X.; Yan, B.; Jia, Z.; et al. Dietary Emulsifier Polysorbate 80 Exposure Accelerates Age-Related Cognitive Decline. Brain. Behav. Immun. 2024, 119, 171–187. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.; Bao, Y.; Huang, W.; He, X.; Hong, Y.; Wei, W.; Liu, Z.; Gao, X.; Yang, Y.; et al. Gut Microbiota-Brain Bile Acid Axis Orchestrates Aging-Related Neuroinflammation and Behavior Impairment in Mice. Pharmacol. Res. 2024, 208, 107361. [Google Scholar] [CrossRef]
- Hu, X.; Jiao, F.; Deng, J.; Zhou, Z.; Chen, S.; Liu, C.; Liu, Z.; Guo, F. Intestinal Epithelial Cell-Specific Deletion of Cytokine-Inducible SH2-Containing Protein Alleviates Experimental Colitis in Ageing Mice. J. Crohn’s Colitis 2023, 17, 1278–1290. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Durand, S.; Daillère, R.; Iribarren, K.; Lemaitre, F.; Derosa, L.; Aprahamian, F.; Bossut, N.; Nirmalathasan, N.; Madeo, F.; et al. Oral Administration of Akkermansia Muciniphila Elevates Systemic Antiaging and Anticancer Metabolites. Aging 2021, 13, 6375–6405. [Google Scholar] [CrossRef]
- Sonoda, S.; Yamaza, T. A New Target of Dental Pulp-Derived Stem Cell-Based Therapy on Recipient Bone Marrow Niche in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2022, 23, 3479. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. Cancer. TERT Promoter Mutations and Telomerase Reactivation in Urothelial Cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Zhang, C.; Tang, Q.; Bi, F. Ursodeoxycholic Acid Suppresses the Malignant Progression of Colorectal Cancer through TGR5-YAP Axis. Cell Death Discov. 2021, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Le Grazie, M.; Roselli, J.; Polvani, S.; Galli, A.; Tovoli, F.; Tarocchi, M. Telomerase Reactivation Is Associated with Hepatobiliary and Pancreatic Cancers. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 420–428. [Google Scholar] [CrossRef]
- McKelvey, B.A.; Umbricht, C.B.; Zeiger, M.A. Telomerase Reverse Transcriptase (TERT) Regulation in Thyroid Cancer: A Review. Front. Endocrinol. 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, J.; Jiang, K.; Chen, Y.; Zhu, L.; Liu, R. TERT Promoter Methylation Is Associated with High Expression of TERT and Poor Prognosis in Papillary Thyroid Cancer. Front. Oncol. 2024, 14, 1325345. [Google Scholar] [CrossRef]
- Kato, K.; Kawaguchi, A.; Nagata, K. Template Activating Factor-I Epigenetically Regulates the TERT Transcription in Human Cancer Cells. Sci. Rep. 2021, 11, 17726. [Google Scholar] [CrossRef]
- Saretzki, G. Extra-Telomeric Functions of Human Telomerase: Cancer, Mitochondria and Oxidative Stress. Curr. Pharm. Des. 2014, 20, 6386–6403. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, P.; Sun, X.; Zheng, J.; Wei, G.; Zhang, L.; Wang, H.; Yao, J.; Lu, S.; Jia, P. Acidified Bile Acids Increase hTERT Expression via C-Myc Activation in Human Gastric Cancer Cells. Oncol. Rep. 2015, 33, 3038–3044. [Google Scholar] [CrossRef]
- Li, Y.; Tergaonkar, V. Noncanonical Functions of Telomerase: Implications in Telomerase-Targeted Cancer Therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef]
- Han, C.Y.; Kim, T.H.; Koo, J.H.; Kim, S.G. Farnesoid X Receptor as a Regulator of Fuel Consumption and Mitochondrial Function. Arch. Pharm. Res. 2016, 39, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Cariello, M.; Morgano, A.; Gross, I.; Vegliante, M.C.; Murzilli, S.; Salvatore, L.; Freund, J.-N.; Sabbà, C.; Moschetta, A. Transcriptional Regulation of the Intestinal Nuclear Bile Acid Farnesoid X Receptor (FXR) by the Caudal-Related Homeobox 2 (CDX2). J. Biol. Chem. 2014, 289, 28421–28432. [Google Scholar] [CrossRef]
- Zhao, K.; He, J.; Zhang, Y.; Xu, Z.; Xiong, H.; Gong, R.; Li, S.; Chen, S.; He, F. Activation of FXR Protects against Renal Fibrosis via Suppressing Smad3 Expression. Sci. Rep. 2016, 6, 37234. [Google Scholar] [CrossRef]
- Jin, W.; Zheng, M.; Chen, Y.; Xiong, H. Update on the Development of TGR5 Agonists for Human Diseases. Eur. J. Med. Chem. 2024, 271, 116462. [Google Scholar] [CrossRef]
- Sung, J.Y.; Kim, S.G.; Cho, D.H.; Kim, J.-R.; Choi, H.C. SRT1720-Induced Activation of SIRT1 Alleviates Vascular Smooth Muscle Cell Senescence through PKA-Dependent Phosphorylation of AMPKα at Ser485. FEBS Open Bio 2020, 10, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the Hallmarks of Cancer: Galectins as Multifunctional Mediators of Tumor Progression. J. Exp. Med. 2020, 217, e20182041. [Google Scholar] [CrossRef] [PubMed]
- Vaquero-Sedas, M.I.; Vega-Palas, M.Á. Targeting Cancer through the Epigenetic Features of Telomeric Regions. Trends Cell Biol. 2019, 29, 281–290. [Google Scholar] [CrossRef]
- Vaquero-Sedas, M.I.; Vega-Palas, M.A. On the Chromatin Structure of Eukaryotic Telomeres. Epigenetics 2011, 6, 1055–1058. [Google Scholar] [CrossRef]
- Ghanim, G.E.; Fountain, A.J.; van Roon, A.-M.M.; Rangan, R.; Das, R.; Collins, K.; Nguyen, T.H.D. Structure of Human Telomerase Holoenzyme with Bound Telomeric DNA. Nature 2021, 593, 449–453. [Google Scholar] [CrossRef]
- Soohoo, C.Y.; Shi, R.; Lee, T.H.; Huang, P.; Lu, K.P.; Zhou, X.Z. Telomerase Inhibitor PinX1 Provides a Link between TRF1 and Telomerase to Prevent Telomere Elongation. J. Biol. Chem. 2011, 286, 3894–3906. [Google Scholar] [CrossRef]
- Galati, A.; Micheli, E.; Alicata, C.; Ingegnere, T.; Cicconi, A.; Pusch, M.C.; Giraud-Panis, M.-J.; Gilson, E.; Cacchione, S. TRF1 and TRF2 Binding to Telomeres Is Modulated by Nucleosomal Organization. Nucleic Acids Res. 2015, 43, 5824–5837. [Google Scholar] [CrossRef] [PubMed]
- Ovando-Roche, P.; Yu, J.S.L.; Testori, S.; Ho, C.; Cui, W. TRF2-Mediated Stabilization of hREST4 Is Critical for the Differentiation and Maintenance of Neural Progenitors. Stem Cells 2014, 32, 2111–2122. [Google Scholar] [CrossRef]
- Ding, L.; Yang, Q.; Zhang, E.; Wang, Y.; Sun, S.; Yang, Y.; Tian, T.; Ju, Z.; Jiang, L.; Wang, X.; et al. Notoginsenoside Ft1 Acts as a TGR5 Agonist but FXR Antagonist to Alleviate High Fat Diet-Induced Obesity and Insulin Resistance in Mice. Acta Pharm. Sin. B 2021, 11, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, L.; Qin, S.; Geng, H.; Xia, C.; Zheng, Y.; Lei, X.; Zhang, J.; Wu, S.; Yao, J.; et al. Farnesoid X Receptor (FXR) Regulates mTORC1 Signaling and Autophagy by Inhibiting SESN2 Expression. Mol. Nutr. Food Res. 2023, 67, e2200517. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, M.; So, J.; Lee, S.-H.; Ko, S.; Shin, D. Farnesoid X Receptor Activation Impairs Liver Progenitor Cell-Mediated Liver Regeneration via the PTEN-PI3K-AKT-mTOR Axis in Zebrafish. Hepatology 2021, 74, 397–410. [Google Scholar] [CrossRef]
- Roglans, N.; Fauste, E.; Bentanachs, R.; Velázquez, A.M.; Pérez-Armas, M.; Donis, C.; Panadero, M.I.; Alegret, M.; Otero, P.; Bocos, C.; et al. Bempedoic Acid Restores Liver H2S Production in a Female Sprague-Dawley Rat Dietary Model of Non-Alcoholic Fatty Liver. Int. J. Mol. Sci. 2022, 24, 473. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, Q.; Yang, H.; Ren, A.; He, Z.; Tan, Z. Maternal Intake Restriction Programs the Energy Metabolism, Clock Circadian Regulator and mTOR Signals in the Skeletal Muscles of Goat Offspring Probably via the Protein Kinase A-cAMP-Responsive Element-Binding Proteins Pathway. Anim. Nutr. 2021, 7, 1303–1314. [Google Scholar] [CrossRef]
- Shang, J.-N.; Yu, C.-G.; Li, R.; Xi, Y.; Jian, Y.J.; Xu, N.; Chen, S. The Nonautophagic Functions of Autophagy-Related Proteins. Autophagy 2024, 20, 720–734. [Google Scholar] [CrossRef]
- Wu, N.; Zheng, W.; Zhou, Y.; Tian, Y.; Tang, M.; Feng, X.; Ashrafizadeh, M.; Wang, Y.; Niu, X.; Tambuwala, M.; et al. Autophagy in Aging-Related Diseases and Cancer: Principles, Regulatory Mechanisms and Therapeutic Potential. Ageing Res. Rev. 2024, 100, 102428. [Google Scholar] [CrossRef]
- García-Rodríguez, J.L.; Barbier-Torres, L.; Fernández-Álvarez, S.; Gutiérrez-de Juan, V.; Monte, M.J.; Halilbasic, E.; Herranz, D.; Álvarez, L.; Aspichueta, P.; Marín, J.J.G.; et al. SIRT1 Controls Liver Regeneration by Regulating Bile Acid Metabolism through Farnesoid X Receptor and Mammalian Target of Rapamycin Signaling. Hepatology 2014, 59, 1972–1983. [Google Scholar] [CrossRef]
- Wang, Y.; Gunewardena, S.; Li, F.; Matye, D.J.; Chen, C.; Chao, X.; Jung, T.; Zhang, Y.; Czerwiński, M.; Ni, H.-M.; et al. An FGF15/19-TFEB Regulatory Loop Controls Hepatic Cholesterol and Bile Acid Homeostasis. Nat. Commun. 2020, 11, 3612. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Gao, R.; Zhao, F.; Wang, J.; Zhu, W. Galacto-Oligosaccharides Alleviate LPS-Induced Immune Imbalance in Small Intestine through Regulating Gut Microbe Composition and Bile Acid Pool. J. Agric. Food Chem. 2023, 71, 17615–17626. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; Wen, P.; Chen, Y.; Cao, J.; Sun, X.; Dong, Y. Chronic Stress-Induced Serotonin Impairs Intestinal Epithelial Cell Mitochondrial Biogenesis via the AMPK-PGC-1α Axis. Int. J. Biol. Sci. 2024, 20, 4476–4495. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sheng, F.; Zou, L.; Xiao, J.; Li, P. Hyperoside Attenuates Non-Alcoholic Fatty Liver Disease in Rats via Cholesterol Metabolism and Bile Acid Metabolism. J. Adv. Res. 2021, 34, 109–122. [Google Scholar] [CrossRef]
- Huang, J.; Liao, S.; Fu, X.; Wang, Y.; Zhou, S.; Lu, Y. AMP-Activated Protein Kinase-Farnesoid X Receptor Pathway Contributes to Oleanolic Acid-Induced Liver Injury. J. Appl. Toxicol. 2023, 43, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Yu, L.; Yuan, Z.; Sun, R.; Yang, H.; Zhang, L.; Jiang, Z. Alpha-Naphthylisothiocyanate Impairs Bile Acid Homeostasis through AMPK-FXR Pathways in Rat Primary Hepatocytes. Toxicology 2016, 370, 106–115. [Google Scholar] [CrossRef]
- Lee, E.; Lee, M.-S.; Chang, E.; Kim, C.-T.; Choi, A.-J.; Kim, I.-H.; Kim, Y. High Hydrostatic Pressure Extract of Mulberry Leaves Ameliorates Hypercholesterolemia via Modulating Hepatic microRNA-33 Expression and AMPK Activity in High Cholesterol Diet Fed Rats. Food Nutr. Res. 2021, 65, 10-29219. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple Polyphenol Extract Improves High-Fat Diet-Induced Hepatic Steatosis by Regulating Bile Acid Synthesis and Gut Microbiota in C57BL/6 Male Mice. J. Agric. Food Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef]
- Rodríguez-Agudo, R.; González-Recio, I.; Serrano-Maciá, M.; Bravo, M.; Petrov, P.; Blaya, D.; Herranz, J.M.; Mercado-Gómez, M.; Rejano-Gordillo, C.M.; Lachiondo-Ortega, S.; et al. Anti-miR-873-5p Improves Alcohol-Related Liver Disease by Enhancing Hepatic Deacetylation via SIRT1. JHEP Rep. 2024, 6, 100918. [Google Scholar] [CrossRef]
- Qin, T.; Hasnat, M.; Wang, Z.; Hassan, H.M.; Zhou, Y.; Yuan, Z.; Zhang, W. Geniposide Alleviated Bile Acid-Associated NLRP3 Inflammasome Activation by Regulating SIRT1/FXR Signaling in Bile Duct Ligation-Induced Liver Fibrosis. Phytomedicine 2023, 118, 154971. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L. Circadian Rhythms in Liver Metabolism and Disease. Acta Pharm. Sin. B 2015, 5, 113–122. [Google Scholar] [CrossRef]
- Lai, J.; Li, F.; Li, H.; Huang, R.; Ma, F.; Gu, X.; Cai, Y.; Huang, D.; Li, S.; Xiao, S.; et al. Melatonin Alleviates Necrotizing Enterocolitis by Reducing Bile Acid Levels through the SIRT1/FXR Signalling Axis. Int. Immunopharmacol. 2024, 128, 111360. [Google Scholar] [CrossRef]
- Liu, C.-X.; Gao, Y.; Xu, X.-F.; Jin, X.; Zhang, Y.; Xu, Q.; Ding, H.-X.; Li, B.-J.; Du, F.-K.; Li, L.-C.; et al. Bile Acids Inhibit Ferroptosis Sensitivity through Activating Farnesoid X Receptor in Gastric Cancer Cells. World J. Gastroenterol. 2024, 30, 485–498. [Google Scholar] [CrossRef]
- Adam, A.A.A.; Jongejan, A.; Moerland, P.D.; van der Mark, V.A.; Oude Elferink, R.P.; Chamuleau, R.A.F.M.; Hoekstra, R. Genome-Wide Expression Profiling Reveals Increased Stability and Mitochondrial Energy Metabolism of the Human Liver Cell Line HepaRG-CAR. Cytotechnology 2020, 72, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Shi, J.; Wang, L.; Wang, D.; Wang, Y.; Song, J.; Xin, L.; Jiang, F. Maternal PFOS Exposure in Mice Induces Hepatic Lipid Accumulation and Inflammation in Adult Female Offspring: Involvement of Microbiome-Gut-Liver Axis and Autophagy. J. Hazard. Mater. 2024, 470, 134177. [Google Scholar] [CrossRef]
- Luo, T.; Zhao, L.; Feng, C.; Yan, J.; Yuan, Y.; Chen, H. Asparagine Prevents Intestinal Stem Cell Aging via the Autophagy-Lysosomal Pathway. Aging Cell 2024, 24, e14423. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, L.; Zhang, Y.; Chen, T.; Shi, H.; Sun, H.; Ding, S.; Chen, S.; Cao, H.; Zhang, G.; et al. BIN1 Deficiency Enhances ULK3-Dependent Autophagic Flux and Reduces Dendritic Size in Mouse Hippocampal Neurons. Autophagy 2024, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Alcázar, A.; Portillo-Carrasquer, M.; Delaspre, F.; Pazos-Gil, M.; Tamarit, J.; Ros, J.; Cabiscol, E. Deciphering the Ferroptosis Pathways in Dorsal Root Ganglia of Friedreich Ataxia Models. The Role of LKB1/AMPK, KEAP1, and GSK3β in the Impairment of the NRF2 Response. Redox Biol. 2024, 76, 103339. [Google Scholar] [CrossRef]
- Velagapudi, S.; Karsai, G.; Karsai, M.; Mohammed, S.A.; Montecucco, F.; Liberale, L.; Lee, H.; Carbone, F.; Adami, G.F.; Yang, K.; et al. Inhibition of de Novo Ceramide Synthesis by Sirtuin-1 Improves Beta-Cell Function and Glucose Metabolism in Type 2 Diabetes. Cardiovasc. Res. 2024, 120, 1265–1278. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, L.; Guo, X.; Tao, H.; Liu, Y.; Liu, X.; Zhang, Y.; Meng, X. The Potential of Herbal Drugs to Treat Heart Failure: The Roles of Sirt1/AMPK. J. Pharm. Anal. 2024, 14, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Liu, H.; Wang, P.; Li, L.; Bionaz, M.; Lin, P.; Yao, J. Altered Bile Acid and Correlations with Gut Microbiome in Transition Dairy Cows with Different Glucose and Lipid Metabolism Status. J. Dairy. Sci. 2024, 107, 9915–9933. [Google Scholar] [CrossRef]
- Song, Z.; Chen, J.; Ji, Y.; Yang, Q.; Chen, Y.; Wang, F.; Wu, Z. Amuc Attenuates High-Fat Diet-Induced Metabolic Disorders Linked to the Regulation of Fatty Acid Metabolism, Bile Acid Metabolism, and the Gut Microbiota in Mice. Int. J. Biol. Macromol. 2023, 242, 124650. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jiang, C.; Shi, J.; Gao, X.; Sun, D.; Sun, L.; Wang, T.; Takahashi, S.; Anitha, M.; Krausz, K.W.; et al. An Intestinal Farnesoid X Receptor-Ceramide Signaling Axis Modulates Hepatic Gluconeogenesis in Mice. Diabetes 2017, 66, 613–626. [Google Scholar] [CrossRef]
- Mencarelli, A.; Renga, B.; D’Amore, C.; Santorelli, C.; Graziosi, L.; Bruno, A.; Monti, M.C.; Distrutti, E.; Cipriani, S.; Donini, A.; et al. Dissociation of Intestinal and Hepatic Activities of FXR and LXRα Supports Metabolic Effects of Terminal Ileum Interposition in Rodents. Diabetes 2013, 62, 3384–3393. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhou, W.; Yan, X.; Qiao, Y.; Guan, L.; Zhang, Z.; Liu, H.; Jiang, J.; Liu, J.; Peng, L. Astragaloside IV Ameliorates Diet-Induced Hepatic Steatosis in Obese Mice by Inhibiting Intestinal FXR via Intestinal Flora Remodeling. Phytomedicine 2022, 107, 154444. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR Activation Protects against NAFLD via Bile-Acid-Dependent Reductions in Lipid Absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Dou, X.; Huo, T.; Liu, Y.; Pang, Z.; Su, L.; Zhao, X.; Peng, X.; Liu, Z.; Zhang, L.; Jiao, N. Discovery of Novel and Selective Farnesoid X Receptor Antagonists through Structure-Based Virtual Screening, Preliminary Structure-Activity Relationship Study, and Biological Evaluation. Eur. J. Med. Chem. 2024, 269, 116323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, R.; Lu, X.; Zhang, Y.; Yang, M.; Su, Y.; Jiang, Y.; Man, C. Lactobacillus Reuteri J1 Prevents Obesity by Altering the Gut Microbiota and Regulating Bile Acid Metabolism in Obese Mice. Food Funct. 2022, 13, 6688–6701. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Chen, W.-D.; Xu, Y.; Yin, L.; Ge, X.; Jadhav, K.; Adorini, L.; Zhang, Y. Hepatic Carboxylesterase 1 Is Essential for Both Normal and Farnesoid X Receptor-Controlled Lipid Homeostasis. Hepatology 2014, 59, 1761–1771. [Google Scholar] [CrossRef]
- Ghosh Laskar, M.; Eriksson, M.; Rudling, M.; Angelin, B. Treatment with the Natural FXR Agonist Chenodeoxycholic Acid Reduces Clearance of Plasma LDL Whilst Decreasing Circulating PCSK9, Lipoprotein(a) and Apolipoprotein C-III. J. Intern. Med. 2017, 281, 575–585. [Google Scholar] [CrossRef]
- Handelman, S.K.; Puentes, Y.M.; Kuppa, A.; Chen, Y.; Du, X.; Feitosa, M.F.; Palmer, N.D.; Speliotes, E.K. Population-Based Meta-Analysis and Gene-Set Enrichment Identifies FXR/RXR Pathway as Common to Fatty Liver Disease and Serum Lipids. Hepatol. Commun. 2022, 6, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, M.-S.; Kang, S.-A.; Kim, C.-T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14, 3330. [Google Scholar] [CrossRef]
- Modica, S.; Petruzzelli, M.; Bellafante, E.; Murzilli, S.; Salvatore, L.; Celli, N.; Di Tullio, G.; Palasciano, G.; Moustafa, T.; Halilbasic, E.; et al. Selective Activation of Nuclear Bile Acid Receptor FXR in the Intestine Protects Mice against Cholestasis. Gastroenterology 2012, 142, 355–365.e4. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, M.; Zhang, L.; Wei, R.; Meng, K.; Bao, Y.; Zhang, L.; Zheng, J. Four Citrus Flavanones Exert Atherosclerosis Alleviation Effects in ApoE-/- Mice via Different Metabolic and Signaling Pathways. J. Agric. Food Chem. 2021, 69, 5226–5237. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Karrasch, T.; Schäffler, A. The Emerging Role of Bile Acids in White Adipose Tissue. Trends Endocrinol. Metab. 2023, 34, 718–734. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine Farnesoid X Receptor Agonist and the Gut Microbiota Activate G-Protein Bile Acid Receptor-1 Signaling to Improve Metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR Agonism Promotes Adipose Tissue Browning and Reduces Obesity and Insulin Resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-Induced Obesity Requires Farnesoid X Receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef]
- Dean, A.E.; Reichardt, F.; Anakk, S. Sex Differences Feed into Nuclear Receptor Signaling along the Digestive Tract. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166211. [Google Scholar] [CrossRef]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An Integrated View of Anti-Inflammatory and Antifibrotic Targets for the Treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Trauner, M. Current Treatment of Non-Alcoholic Fatty Liver Disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.; He, A.; Wang, S.; Chen, Y.; Lu, J.; Lv, J.; Li, S.; Wang, J.; Qian, M.; et al. Mebhydrolin Ameliorates Glucose Homeostasis in Type 2 Diabetic Mice by Functioning as a Selective FXR Antagonist. Metabolism 2021, 119, 154771. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, W.; Dong, W.; Chen, G.; Sun, Y.; Zeng, X. Anti-Diabetic Effect of Dicaffeoylquinic Acids Is Associated with the Modulation of Gut Microbiota and Bile Acid Metabolism. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Dong, R.; Yang, X.; Wang, C.; Liu, K.; Liu, Z.; Ma, X.; Sun, H.; Huo, X.; Fu, T.; Meng, Q. Yangonin Protects against Non-Alcoholic Fatty Liver Disease through Farnesoid X Receptor. Phytomedicine 2019, 53, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, X.; Zhou, L.; Zhou, S.; Zheng, Y.; Yu, J.; Fan, W.; Zhu, Y.; Han, X. Type 2 Diabetes Mitigation in the Diabetic Goto-Kakizaki Rat by Elevated Bile Acids Following a Common-Bile-Duct Surgery. Metabolism 2016, 65, 78–88. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Gonzalez, F.J. The Role of Farnesoid X Receptor in Metabolic Diseases, and Gastrointestinal and Liver Cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Yang, X.; Shen, C.; Huang, J.; Zhang, D.; Liu, N.; Liu, C.; Zhong, Y.; Chen, Y.; et al. Hepatic Zbtb18 (Zinc Finger and BTB Domain Containing 18) Alleviates Hepatic Steatohepatitis via FXR (Farnesoid X Receptor). Signal Transduction Targeted Ther. 2024, 9, 20. [Google Scholar] [CrossRef]
- González-Regueiro, J.A.; Moreno-Castañeda, L.; Uribe, M.; Chávez-Tapia, N.C. The Role of Bile Acids in Glucose Metabolism and Their Relation with Diabetes. Ann. Hepatol. 2017, 16, 16–21. [Google Scholar] [CrossRef]
- Vitulo, M.; Gnodi, E.; Rosini, G.; Meneveri, R.; Giovannoni, R.; Barisani, D. Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD. Int. J. Mol. Sci. 2023, 24, 12748. [Google Scholar] [CrossRef]
- Kong, X.; Yang, C.; Li, B.; Yan, D.; Yang, Y.; Cao, C.; Xing, B.; Ma, X. FXR/Menin-Mediated Epigenetic Regulation of E2F3 Expression Controls β-Cell Proliferation and Is Increased in Islets from Diabetic GK Rats after RYGB. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167136. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Chen, Y.; Dong, Q.; Li, D.; Guo, M.; Zhang, L.; Shi, Y.; Wu, H.; Li, L.; Sun, Z. Acrylamide Induced Glucose Metabolism Disorder in Rats Involves Gut Microbiota Dysbiosis and Changed Bile Acids Metabolism. Food Res. Int. 2022, 157, 111405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Xia, J.; Dong, C.; Luo, X. Human Microbiome and Its Medical Applications. Front. Mol. Biosci. 2021, 8, 703585. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Gao, X.; Lei, L.; Feng, J.; Zhang, W.; Hu, Y.; Shen, Z.; Liu, Z.; Huan, Y.; Wu, S.; et al. Machine Learning- and Structure-Based Discovery of a Novel Chemotype as FXR Agonists for Potential Treatment of Nonalcoholic Fatty Liver Disease. Eur. J. Med. Chem. 2023, 252, 115307. [Google Scholar] [CrossRef]
- Sun, J.; Fan, J.; Li, T.; Yan, X.; Jiang, Y. Nuciferine Protects Against High-Fat Diet-Induced Hepatic Steatosis via Modulation of Gut Microbiota and Bile Acid Metabolism in Rats. J. Agric. Food Chem. 2022, 70, 12014–12028. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Cai, W.; Wang, Q.; Chang, Y.; Sun, Y.; Dong, P.; Wang, J. Novel ι-Carrageenan Tetrasaccharide Alleviates Liver Lipid Accumulation via the Bile Acid-FXR-SHP/PXR Pathway to Regulate Cholesterol Conversion and Fatty Acid Metabolism in Insulin-Resistant Mice. J. Agric. Food Chem. 2021, 69, 9813–9821. [Google Scholar] [CrossRef]
- Fan, L.; Lai, R.; Ma, N.; Dong, Y.; Li, Y.; Wu, Q.; Qiao, J.; Lu, H.; Gong, L.; Tao, Z.; et al. miR-552-3p Modulates Transcriptional Activities of FXR and LXR to Ameliorate Hepatic Glycolipid Metabolism Disorder. J. Hepatol. 2021, 74, 8–19. [Google Scholar] [CrossRef]
- Gaillard, D.; Masson, D.; Garo, E.; Souidi, M.; Pais de Barros, J.-P.; Schoonjans, K.; Grober, J.; Besnard, P.; Thomas, C. Muricholic Acids Promote Resistance to Hypercholesterolemia in Cholesterol-Fed Mice. Int. J. Mol. Sci. 2021, 22, 7163. [Google Scholar] [CrossRef]
- Ruan, G.; Wu, F.; Shi, D.; Sun, H.; Wang, F.; Xu, C. Metformin: Update on Mechanisms of Action on Liver Diseases. Front. Nutr. 2023, 10, 1327814. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with Camu Camu (Myrciaria Dubia) Prevents Obesity by Altering the Gut Microbiota and Increasing Energy Expenditure in Diet-Induced Obese Mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Broeders, E.P.M.; Nascimento, E.B.M.; Havekes, B.; Brans, B.; Roumans, K.H.M.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yao, L.; Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Fan, S.; Zhang, Z.; Gong, S.; Chang, S.; et al. Gut Microbiota Mediates the Effects of Curcumin on Enhancing Ucp1-Dependent Thermogenesis and Improving High-Fat Diet-Induced Obesity. Food Funct. 2021, 12, 6558–6575. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, C.; Huang, X.; Yi, S.; Pan, S.; Zhang, Y.; Yuan, G.; Cao, Q.; Ye, X.; Li, H. Gut Microbiota-Mediated Secondary Bile Acids Regulate Dendritic Cells to Attenuate Autoimmune Uveitis through TGR5 Signaling. Cell Rep. 2021, 36, 109726. [Google Scholar] [CrossRef] [PubMed]
- Halkias, C.; Darby, W.G.; Feltis, B.N.; McIntyre, P.; Macrides, T.A.; Wright, P.F.A. Marine Bile Natural Products as Agonists of the TGR5 Receptor. J. Nat. Prod. 2021, 84, 1507–1514. [Google Scholar] [CrossRef]
- Lo, S.-H.; Cheng, K.-C.; Li, Y.-X.; Chang, C.-H.; Cheng, J.-T.; Lee, K.-S. Development of Betulinic Acid as an Agonist of TGR5 Receptor Using a New in Vitro Assay. Drug Des. Devel Ther. 2016, 10, 2669–2676. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, H.; Wu, J.; Zhou, Y.; Tang, F.; Liu, J.; Feng, W.; Peng, C. The Energy Metabolism-Promoting Effect of Aconite Is Associated with Gut Microbiota and Bile Acid Receptor TGR5-UCP1 Signaling. Front. Pharmacol. 2024, 15, 1392385. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.-Y.; Wei, Y.-L.; Hao, J.-Y.; Lei, Y.-Q.; Zhao, W.-B.; Xiao, Y.-H.; Sun, A.-D. The Polyphenol-Rich Extract from Chokeberry (Aronia Melanocarpa L.) Modulates Gut Microbiota and Improves Lipid Metabolism in Diet-Induced Obese Rats. Nutr. Metab. 2020, 17, 54. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, D.; Luo, Y.; Myakala, K.; Dobrinskikh, E.; Rosenberg, A.Z.; Levi, J.; Kopp, J.B.; Field, A.; Hill, A.; et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 2018, 29, 118–137. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 Signalling Promotes Mitochondrial Fission and Beige Remodelling of White Adipose Tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef]
- Wang, X.X.; Xie, C.; Libby, A.E.; Ranjit, S.; Levi, J.; Myakala, K.; Bhasin, K.; Jones, B.A.; Orlicky, D.J.; Takahashi, S.; et al. The Role of FXR and TGR5 in Reversing and Preventing Progression of Western Diet-Induced Hepatic Steatosis, Inflammation, and Fibrosis in Mice. J. Biol. Chem. 2022, 298, 102530. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, J.; Wang, J.; Ma, Y.; Zhang, Y.; Zhang, Q.; Zhang, Z.; Yin, H. The Interaction between Intracellular Energy Metabolism and Signaling Pathways during Osteogenesis. Front. Mol. Biosci. 2021, 8, 807487. [Google Scholar] [CrossRef] [PubMed]
- Sarker, R.; Lin, R.; Singh, V.; Donowitz, M.; Tse, C.-M. SLC26A3 (DRA) Is Stimulated in a Synergistic, Intracellular Ca2+-Dependent Manner by cAMP and ATP in Intestinal Epithelial Cells. Am. J. Physiol. Cell Physiol. 2023, 324, C1263–C1273. [Google Scholar] [CrossRef]
- Oleynikov, I.P.; Sudakov, R.V.; Azarkina, N.V.; Vygodina, T.V. Direct Interaction of Mitochondrial Cytochrome c Oxidase with Thyroid Hormones: Evidence for Two Binding Sites. Cells 2022, 11, 908. [Google Scholar] [CrossRef]
- Wang, Z.; Qiang, X.; Peng, Y.; Wang, Y.; Zhao, Q.; He, D. Design and Synthesis of Bile Acid Derivatives and Their Activity against Colon Cancer. RSC Med. Chem. 2022, 13, 1391–1409. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, J.; Zhang, J.; Li, A.; Wang, G. Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2. Toxins 2024, 16, 381. [Google Scholar] [CrossRef]
- Xu, Y.; Feingold, P.L.; Surman, D.R.; Brown, K.; Xi, S.; Davis, J.L.; Hernandez, J.; Schrump, D.S.; Ripley, R.T. Bile Acid and Cigarette Smoke Enhance the Aggressive Phenotype of Esophageal Adenocarcinoma Cells by Downregulation of the Mitochondrial Uncoupling Protein-2. Oncotarget 2017, 8, 101057–101071. [Google Scholar] [CrossRef] [PubMed]
- Takanaga, H.; Maeda, H.; Yabuuchi, H.; Tamai, I.; Higashida, H.; Tsuji, A. Nicotinic Acid Transport Mediated by pH-Dependent Anion Antiporter and Proton Cotransporter in Rabbit Intestinal Brush-Border Membrane. J. Pharm. Pharmacol. 1996, 48, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Y.; Jia, W.; Can, C.; Wang, R.; Yang, X.; Gu, C.; Liu, F.; Ji, C.; Ma, D. Chenodeoxycholic Acid Suppresses AML Progression through Promoting Lipid Peroxidation via ROS/P38 MAPK/DGAT1 Pathway and Inhibiting M2 Macrophage Polarization. Redox Biol. 2022, 56, 102452. [Google Scholar] [CrossRef]
- Da Dalt, L.; Moregola, A.; Svecla, M.; Pedretti, S.; Fantini, F.; Ronzio, M.; Uboldi, P.; Dolfini, D.; Donetti, E.; Baragetti, A.; et al. The Inhibition of Inner Mitochondrial Fusion in Hepatocytes Reduces Non-Alcoholic Fatty Liver and Improves Metabolic Profile during Obesity by Modulating Bile Acid Conjugation. Cardiovasc. Res. 2024, 119, 2917–2929. [Google Scholar] [CrossRef]
- Yao, B.; He, J.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The Protective Effect of Lithocholic Acid on the Intestinal Epithelial Barrier Is Mediated by the Vitamin D Receptor via a SIRT1/Nrf2 and NF-κB Dependent Mechanism in Caco-2 Cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Liu, J.; Zuo, J.; Yan, L.; Thring, R.W.; Ba, X.; Qi, D.; Wu, M.; Gao, Y.; et al. Tauroursodeoxycholic Acid Functions as a Critical Effector Mediating Insulin Sensitization of Metformin in Obese Mice. Redox Biol. 2022, 57, 102481. [Google Scholar] [CrossRef]
- Ferrebee, C.B.; Li, J.; Haywood, J.; Pachura, K.; Robinson, B.S.; Hinrichs, B.H.; Jones, R.M.; Rao, A.; Dawson, P.A. Organic Solute Transporter α-β Protects Ileal Enterocytes from Bile Acid-Induced Injury. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Xu, W.; Ding, C.; He, T.; Xu, X.; Shuai, Y.; Huang, H.; Wu, J.; Wang, Y.; Wang, C.; et al. Bile Acids Target Mitofusin 2 to Differentially Regulate Innate Immunity in Physiological versus Cholestatic Conditions. Cell Rep. 2023, 42, 112011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Zhu, L.; Zheng, X.; Xie, T.-H.; Wang, W.; Zou, J.; Li, Y.; Li, H.-Y.; Cai, J.; Gu, S.; et al. TGR5 Activation Ameliorates Mitochondrial Homeostasis via Regulating the PKCδ/Drp1-HK2 Signaling in Diabetic Retinopathy. Front. Cell Dev. Biol. 2021, 9, 759421. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shen, D.; Jiang, C.; Wang, H.; Chang, M. Ursodeoxycholic Acid Protects Dopaminergic Neurons from Oxidative Stress via Regulating Mitochondrial Function, Autophagy, and Apoptosis in MPTP/MPP+-Induced Parkinson’s Disease. Neurosci. Lett. 2021, 741, 135493. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, H.; Shen, C.; Zhang, M.; Wang, X.; Yuan, M.; Yuan, M.; Jia, S.; Cao, Z.; Wu, C.; et al. Gut Microbiota Regulates Postprandial GLP-1 Response via Ileal Bile Acid-TGR5 Signaling. Gut Microbes 2023, 15, 2274124. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Jiang, R.; Zhao, A.; Wu, Q.; Kuang, J.; Sun, D.; Ren, Z.; Li, M.; Zhao, M.; et al. Hyocholic Acid Species Improve Glucose Homeostasis through a Distinct TGR5 and FXR Signaling Mechanism. Cell Metab. 2021, 33, 791–803.e7. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, T.; Wang, Z.; Wang, X.; Liu, L.; Luo, Y.; Wang, R.; Li, L.; Huang, W.; Wang, Z.; et al. Red Ginseng Extracts Ameliorate High-Fat Diet-Induced Obesity and Insulin Resistance by Activating the Intestinal TGR5-Mediated Bile Acids Signaling Pathway. Phytomedicine 2023, 119, 154982. [Google Scholar] [CrossRef]
- Huang, S.; Ma, S.; Ning, M.; Yang, W.; Ye, Y.; Zhang, L.; Shen, J.; Leng, Y. TGR5 Agonist Ameliorates Insulin Resistance in the Skeletal Muscles and Improves Glucose Homeostasis in Diabetic Mice. Metabolism 2019, 99, 45–56. [Google Scholar] [CrossRef]
- Hou, Y.; Zhai, X.; Wang, X.; Wu, Y.; Wang, H.; Qin, Y.; Han, J.; Meng, Y. Research Progress on the Relationship between Bile Acid Metabolism and Type 2 Diabetes Mellitus. Diabetol. Metab. Syndr. 2023, 15, 235. [Google Scholar] [CrossRef]
- Tawulie, D.; Jin, L.; Shang, X.; Li, Y.; Sun, L.; Xie, H.; Zhao, J.; Liao, J.; Zhu, Z.; Cui, H.; et al. Jiang-Tang-San-Huang Pill Alleviates Type 2 Diabetes Mellitus through Modulating the Gut Microbiota and Bile Acids Metabolism. Phytomedicine 2023, 113, 154733. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Feng, Y.; Chen, Y.; Fan, S.; Huang, C. Deoxyschizandrin Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease: Involvement of Dual Farnesyl X Receptor/G Protein-Coupled Bile Acid Receptor 1 Activation and Leptin Sensitization. Phytother. Res. 2023, 37, 2771–2786. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut Microbiota Turicibacter Strains Differentially Modify Bile Acids and Host Lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Tamai, Y.; Eguchi, A.; Shigefuku, R.; Kitamura, H.; Tempaku, M.; Sugimoto, R.; Kobayashi, Y.; Iwasa, M.; Takei, Y.; Nakagawa, H. Association of Lithocholic Acid with Skeletal Muscle Hypertrophy through TGR5-IGF-1 and Skeletal Muscle Mass in Cultured Mouse Myotubes, Chronic Liver Disease Rats and Humans. eLife 2022, 11, e80638. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile Acids and the Gut Microbiota: Metabolic Interactions and Impacts on Disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Jia, B.; Park, D.; Chun, B.H.; Hahn, Y.; Jeon, C.O. Diet-Related Alterations of Gut Bile Salt Hydrolases Determined Using a Metagenomic Analysis of the Human Microbiome. Int. J. Mol. Sci. 2021, 22, 3652. [Google Scholar] [CrossRef]
- Su, M.; Hu, R.; Tang, T.; Tang, W.; Huang, C. Review of the Correlation between Chinese Medicine and Intestinal Microbiota on the Efficacy of Diabetes Mellitus. Front. Endocrinol. 2022, 13, 1085092. [Google Scholar] [CrossRef]
- Li, J.; Dawson, P.A. Animal Models to Study Bile Acid Metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef]
- Doden, H.L.; Ridlon, J.M. Microbial Hydroxysteroid Dehydrogenases: From Alpha to Omega. Microorganisms 2021, 9, 469. [Google Scholar] [CrossRef]
- Paik, D.; Yao, L.; Zhang, Y.; Bae, S.; D’Agostino, G.D.; Zhang, M.; Kim, E.; Franzosa, E.A.; Avila-Pacheco, J.; Bisanz, J.E.; et al. Human Gut Bacteria Produce ΤH17-Modulating Bile Acid Metabolites. Nature 2022, 603, 907–912. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic Gut Microbial Modulation of Bile Acid Metabolism in Host Tissue Compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4523–4530. [Google Scholar] [CrossRef]
- Taoka, H.; Yokoyama, Y.; Morimoto, K.; Kitamura, N.; Tanigaki, T.; Takashina, Y.; Tsubota, K.; Watanabe, M. Role of Bile Acids in the Regulation of the Metabolic Pathways. World J. Diabetes 2016, 7, 260–270. [Google Scholar] [CrossRef]
- He, Q.; Liu, L.; Wei, J.; Jiang, J.; Rong, Z.; Chen, X.; Zhao, J.; Jiang, K. Roles and Action Mechanisms of Bile Acid-Induced Gastric Intestinal Metaplasia: A Review. Cell Death Discov. 2022, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Münzker, J.; Haase, N.; Till, A.; Sucher, R.; Haange, S.-B.; Nemetschke, L.; Gnad, T.; Jäger, E.; Chen, J.; Riede, S.J.; et al. Functional Changes of the Gastric Bypass Microbiota Reactivate Thermogenic Adipose Tissue and Systemic Glucose Control via Intestinal FXR-TGR5 Crosstalk in Diet-Induced Obesity. Microbiome 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Jeong, J.-J.; Kim, D.J.; Suk, K.T. Recent Trends of Microbiota-Based Microbial Metabolites Metabolism in Liver Disease. Front. Med. 2022, 9, 841281. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Q.; Hu, R.; Yang, X.; Fang, C.; Yao, L.; Lv, J.; Wang, L.; Shi, M.; Zhang, W.; et al. Effects of Prevotella Copri on Insulin, Gut Microbiota and Bile Acids. Gut Microbes 2024, 16, 2340487. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Abdullah, N.; Hafeez, M.A.; Guan, R.; Feng, F. Lactobacillus Plantarum ZJUFB2 Prevents High Fat Diet-Induced Insulin Resistance in Association with Modulation of the Gut Microbiota. Front. Nutr. 2021, 8, 754222. [Google Scholar] [CrossRef]
- Xu, J.; Xie, S.; Chi, S.; Zhang, S.; Cao, J.; Tan, B. Protective Effects of Taurocholic Acid on Excessive Hepatic Lipid Accumulation via Regulation of Bile Acid Metabolism in Grouper. Food Funct. 2022, 13, 3050–3062. [Google Scholar] [CrossRef]
- Roager, H.M.; Stanton, C.; Hall, L.J. Microbial Metabolites as Modulators of the Infant Gut Microbiome and Host-Microbial Interactions in Early Life. Gut Microbes 2023, 15, 2192151. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Vos, J.; Blom, B.; Hazenberg, M.D. Allogeneic Hematopoietic Cell Transplantation, the Microbiome, and Graft-versus-Host Disease. Gut Microbes 2023, 15, 2178805. [Google Scholar] [CrossRef]
- Gao, J.; Sadiq, F.A.; Zheng, Y.; Zhao, J.; He, G.; Sang, Y. Biofilm-Based Delivery Approaches and Specific Enrichment Strategies of Probiotics in the Human Gut. Gut Microbes 2022, 14, 2126274. [Google Scholar] [CrossRef]
- Fleishman, J.S.; Kumar, S. Bile Acid Metabolism and Signaling in Health and Disease: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Targeted Ther. 2024, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y.L. Bile Acid Signaling in Metabolic Disease and Drug Therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile Acids in Glucose Metabolism in Health and Disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Halilbasic, E.; Claudel, T.; Trauner, M. Bile Acid Transporters and Regulatory Nuclear Receptors in the Liver and Beyond. J. Hepatol. 2013, 58, 155–168. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Jia, W. The Influence of Gut Microbiota on Drug Metabolism and Toxicity. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef]
- Li, H.; Jia, W. Cometabolism of Microbes and Host: Implications for Drug Metabolism and Drug-Induced Toxicity. Clin. Pharmacol. Ther. 2013, 94, 574–581. [Google Scholar] [CrossRef]
- Tan, J.; Fu, B.; Zhao, X.; Ye, L. Novel Techniques and Models for Studying the Role of the Gut Microbiota in Drug Metabolism. Eur. J. Drug Metab. Pharmacokinet. 2024, 49, 131–147. [Google Scholar] [CrossRef]
- Ervin, S.M.; Simpson, J.B.; Gibbs, M.E.; Creekmore, B.C.; Lim, L.; Walton, W.G.; Gharaibeh, R.Z.; Redinbo, M.R. Structural Insights into Endobiotic Reactivation by Human Gut Microbiome-Encoded Sulfatases. Biochemistry 2020, 59, 3939–3950. [Google Scholar] [CrossRef]
- Mena Bares, L.M.; Carmona Asenjo, E.; García Sánchez, M.V.; Moreno Ortega, E.; Maza Muret, F.R.; Guiote Moreno, M.V.; Santos Bueno, A.M.; Iglesias Flores, E.; Benítez Cantero, J.M.; Vallejo Casas, J.A. 75SeHCAT Scan in Bile Acid Malabsorption in Chronic Diarrhoea. Rev. Esp. Med. Nucl. Imagen Mol. 2017, 36, 37–47. [Google Scholar] [CrossRef]
- Li, N.; Zhan, S.; Tian, Z.; Liu, C.; Xie, Z.; Zhang, S.; Chen, M.; Zeng, Z.; Zhuang, X. Alterations in Bile Acid Metabolism Associated With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1525–1540. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Gonzalez-Freire, M.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Khadeer, M.; Ordiz, M.I.; Ferrucci, L.; Manary, M.J. Environmental Enteric Dysfunction Is Associated With Altered Bile Acid Metabolism. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Liu, Y.; Wang, Y.; Liu, Y.; Chai, L.; Wang, H. Impairment of Bile Acid Metabolism and Altered Composition by Lead and Copper in Bufo Gargarizans Tadpoles. Sci. Total Environ. 2023, 900, 165901. [Google Scholar] [CrossRef]
- Wilson, A.; Almousa, A.; Teft, W.A.; Kim, R.B. Attenuation of Bile Acid-Mediated FXR and PXR Activation in Patients with Crohn’s Disease. Sci. Rep. 2020, 10, 1866. [Google Scholar] [CrossRef]
- Huang, M.; Kong, B.; Zhang, M.; Rizzolo, D.; Armstrong, L.E.; Schumacher, J.D.; Chow, M.D.; Lee, Y.-H.; Joseph, L.B.; Stofan, M.; et al. Enhanced Alcoholic Liver Disease in Mice with Intestine-Specific Farnesoid X Receptor Deficiency. Lab. Investig. 2020, 100, 1158–1168. [Google Scholar] [CrossRef]
- Liu, H.-M.; Liao, J.-F.; Lee, T.-Y. Farnesoid X Receptor Agonist GW4064 Ameliorates Lipopolysaccharide-Induced Ileocolitis through TLR4/MyD88 Pathway Related Mitochondrial Dysfunction in Mice. Biochem. Biophys. Res. Commun. 2017, 490, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Cariello, M.; Scialpi, N.; Peres, C.; Vetrano, S.; Fiorino, G.; Danese, S.; Ko, B.; Luo, J.; et al. Fibroblast Growth Factor 19 Modulates Intestinal Microbiota and Inflammation in Presence of Farnesoid X Receptor. EBioMedicine 2020, 54, 102719. [Google Scholar] [CrossRef]
- Yan, M.; Hou, L.; Cai, Y.; Wang, H.; Ma, Y.; Geng, Q.; Jiang, W.; Tang, W. Effects of Intestinal FXR-Related Molecules on Intestinal Mucosal Barriers in Biliary Tract Obstruction. Front. Pharmacol. 2022, 13, 906452. [Google Scholar] [CrossRef]
- Li, Z.; Dong, H.; Bian, S.; Wu, H.; Song, W.; Jia, X.; Chen, J.; Zhu, X.; Zhao, L.; Xuan, Z.; et al. FXR Maintains the Intestinal Barrier and Stemness by Regulating CYP11A1-Mediated Corticosterone Synthesis in Biliary Obstruction Diseases. Int. J. Mol. Sci. 2023, 24, 13494. [Google Scholar] [CrossRef]
- Yu Cai Lim, M.; Kiat Ho, H. Pharmacological Modulation of Cholesterol 7α-Hydroxylase (CYP7A1) as a Therapeutic Strategy for Hypercholesterolemia. Biochem. Pharmacol. 2024, 220, 115985. [Google Scholar] [CrossRef] [PubMed]
- Meadows, V.; Kennedy, L.; Ekser, B.; Kyritsi, K.; Kundu, D.; Zhou, T.; Chen, L.; Pham, L.; Wu, N.; Demieville, J.; et al. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis Through Farnesoid X Receptor Signaling. Hepatology 2021, 74, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-H.; Yuece, B.; Li, Y.-Y.; Feng, Y.-J.; Feng, J.-Y.; Yu, L.-Y.; Li, K.; Li, Y.-N.; Storr, M. A Novel CB Receptor GPR55 and Its Ligands Are Involved in Regulation of Gut Movement in Rodents. Neurogastroenterol. Motil. 2011, 23, 862-e342. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-B.; Gong, W.; Hou, Y.-Y.; He, W.-Y.; Wang, R.; Wang, X.-C.; Hu, J.-N. Mucoadhesive Hydrogel with Anti-Gastric Acid and Sustained-Release Functions for Amelioration of DSS-Induced Ulcerative Colitis. J. Agric. Food Chem. 2023, 71, 4016–4028. [Google Scholar] [CrossRef]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory Immune Cells in Regulation of Intestinal Inflammatory Response to Microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, W.; Zhou, G.; Lin, J.; Chu, F.; Wu, H.; Liu, Z. Anti-TNF-α Therapy Suppresses Proinflammatory Activities of Mucosal Neutrophils in Inflammatory Bowel Disease. Mediators Inflamm. 2018, 2018, 3021863. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Li, M.; He, T.; Guo, S.; Zhu, L.; Tan, J.; Wang, B. Novel Approaches in IBD Therapy: Targeting the Gut Microbiota-Bile Acid Axis. Gut Microbes 2024, 16, 2356284. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Li, C.; Xie, Z.; Dai, L. Amelioration of Colitis by a Gut Bacterial Consortium Producing Anti-Inflammatory Secondary Bile Acids. Microbiol. Spectr. 2023, 11, e0333022. [Google Scholar] [CrossRef]
- Boesjes, M.; Brufau, G. Metabolic Effects of Bile Acids in the Gut in Health and Disease. Curr. Med. Chem. 2014, 21, 2822–2829. [Google Scholar] [CrossRef]
- Sonne, D.P. MECHANISMS IN ENDOCRINOLOGY: FXR Signalling: A Novel Target in Metabolic Diseases. Eur. J. Endocrinol. 2021, 184, R193–R205. [Google Scholar] [CrossRef]
- Corpechot, C. Primary Biliary Cirrhosis and Bile Acids. Clin. Res. Hepatol. Gastroenterol. 2012, 36 (Suppl. S1), S13–S20. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Yao, Z.; Gao, Z.; Jiang, Y.; Chen, X.; Peng, L. Insulin Alleviates Murine Colitis through Microbiome Alterations and Bile Acid Metabolism. J. Transl. Med. 2023, 21, 498. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile Acid-Gut Microbiota Axis in Inflammatory Bowel Disease: From Bench to Bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y.L. Bile Acid Signaling in Metabolic and Inflammatory Diseases and Drug Development. Pharmacol. Rev. 2024, 76, 1221–1253. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Whelan, K.; Allegretti, J.R.; Sokol, H. Diet and Microbiome-Directed Therapy 2.0 for IBD. Clin. Gastroenterol. Hepatol. 2025, 23, 406–418. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Q.; Zhang, M.; Liu, C.; Su, Q.; Zhang, L.; Xu, Z.; Lu, W.; Ching, J.; Tang, W.; et al. Noninvasive, Microbiome-Based Diagnosis of Inflammatory Bowel Disease. Nat. Med. 2024, 30, 3555–3567. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary Bile Acids: An Underrecognized Cause of Colon Cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Payne, C.M.; Bernstein, C.; Dvorak, K.; Bernstein, H. Hydrophobic Bile Acids, Genomic Instability, Darwinian Selection, and Colon Carcinogenesis. Clin. Exp. Gastroenterol. 2008, 1, 19–47. [Google Scholar] [CrossRef]
- Li, G.; Zhu, J.; Zhai, L. Exploring Molecular Markers and Drug Candidates for Colorectal Cancer through Comprehensive Bioinformatics Analysis. Aging 2023, 15, 7038–7055. [Google Scholar] [CrossRef]
- Ye, C.; Wu, C.; Li, Y.; Chen, C.; Li, X.; Zhang, J.; Xu, Z.; Chen, H.; Guo, Y. Traditional Medicine Xianglian Pill Suppresses High-Fat Diet-Related Colorectal Cancer via Inactivating TLR4/MyD88 by Remodeling Gut Microbiota Composition and Bile Acid Metabolism. J. Ethnopharmacol. 2024, 333, 118411. [Google Scholar] [CrossRef]
- Barrasa, J.I.; Olmo, N.; Lizarbe, M.A.; Turnay, J. Bile Acids in the Colon, from Healthy to Cytotoxic Molecules. Toxicol. Vitro 2013, 27, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; von Ehrlich-Treuenstätt, V.; Schardey, J.; Wirth, U.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Kühn, F. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J. Gastrointest. Surg. 2023, 27, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-B.; An, S.-Y.; Pyo, S.-S.; Kim, J.; Kim, S.-W.; Yoon, D.-W. Sleep Fragmentation Accelerates Carcinogenesis in a Chemical-Induced Colon Cancer Model. Int. J. Mol. Sci. 2023, 24, 4547. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, W.; Huang, W. FXR and Liver Carcinogenesis. Acta Pharmacol. Sin. 2015, 36, 37–43. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, G.; Gong, W.; Zhou, P.; Zhao, Y.; Zhang, Y.; Zeng, Y.; Gao, M.; Pan, Z.; He, F. FXR Ligands Protect against Hepatocellular Inflammation via SOCS3 Induction. Cell Signal 2012, 24, 1658–1664. [Google Scholar] [CrossRef]
- Zou, B.; Yang, W.; Tang, Y.; Hou, Y.; Tang, T.; Qu, S. Intestinal Microbiota-Farnesoid X Receptor Axis in Metabolic Diseases. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 509, 167–171. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef]
- Ocvirk, S.; O’Keefe, S.J.D. Dietary Fat, Bile Acid Metabolism and Colorectal Cancer. Semin. Cancer Biol. 2021, 73, 347–355. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, J.; Wang, L.; Dong, Q.; Jin, D. CEBPB Regulates the Bile Acid Receptor FXR to Accelerate Colon Cancer Progression by Modulating Aerobic Glycolysis. J. Clin. Lab. Anal. 2022, 36, e24703. [Google Scholar] [CrossRef]
- Nenkov, M.; Shi, Y.; Ma, Y.; Gaßler, N.; Chen, Y. Targeting Farnesoid X Receptor in Tumor and the Tumor Microenvironment: Implication for Therapy. Int. J. Mol. Sci. 2023, 25, 6. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Liu, X.; Xue, S.; Chen, D.; Zou, J.; Jiang, H. TGR5 Deficiency Activates Antitumor Immunity in Non-Small Cell Lung Cancer via Restraining M2 Macrophage Polarization. Acta Pharm. Sin. B 2022, 12, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xie, W.; Li, X.; Liu, T.; Bai, J.; Yao, Y.; Ma, L.; Man, S. Inulin Enhanced Rifaximin-Inhibited Colon Cancer Pulmonary Metastasis by Flora-Regulated Bile Acid Pathway. Int. J. Biol. Macromol. 2024, 275, 133582. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Luo, L.; Liu, S.; Guan, Z.; Zhang, Q.; Wu, Z.; Tao, K. The Role of TGR5 as an Onco-Immunological Biomarker in Tumor Staging and Prognosis by Encompassing the Tumor Microenvironment. Front. Oncol. 2022, 12, 953091. [Google Scholar] [CrossRef] [PubMed]
- Burchat, N.; Vidola, J.; Pfreundschuh, S.; Sharma, P.; Rizzolo, D.; Guo, G.L.; Sampath, H. Intestinal Stearoyl-CoA Desaturase-1 Regulates Energy Balance via Alterations in Bile Acid Homeostasis. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101403. [Google Scholar] [CrossRef]
- Liu, J.; Tian, R.; Sun, C.; Guo, Y.; Dong, L.; Li, Y.; Song, X. Microbial Metabolites Are Involved in Tumorigenesis and Development by Regulating Immune Responses. Front. Immunol. 2023, 14, 1290414. [Google Scholar] [CrossRef]
- Jin, W.-B.; Li, T.-T.; Huo, D.; Qu, S.; Li, X.V.; Arifuzzaman, M.; Lima, S.F.; Shi, H.-Q.; Wang, A.; Putzel, G.G.; et al. Genetic Manipulation of Gut Microbes Enables Single-Gene Interrogation in a Complex Microbiome. Cell 2022, 185, 547–562.e22. [Google Scholar] [CrossRef]
- Bernardi, F.; D’Amico, F.; Bencardino, S.; Faggiani, I.; Fanizza, J.; Zilli, A.; Parigi, T.L.; Allocca, M.; Danese, S.; Furfaro, F. Gut Microbiota Metabolites: Unveiling Their Role in Inflammatory Bowel Diseases and Fibrosis. Pharmaceuticals 2024, 17, 347. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Cai, J.; Rimal, B.; Rocha, E.R.; Coleman, J.P.; Zhang, C.; Nichols, R.G.; Luo, Y.; Kim, B.; et al. Bile Salt Hydrolase in Non-Enterotoxigenic Bacteroides Potentiates Colorectal Cancer. Nat. Commun. 2023, 14, 755. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Wang, P.; Tang, C.; Wang, Z.; Chen, L.; Luo, G.; Chen, H.; Liu, Y.; Feng, B.; et al. Bile Acids and Their Receptors in Regulation of Gut Health and Diseases. Prog. Lipid Res. 2023, 89, 101210. [Google Scholar] [CrossRef]
- Lee, M.H.; Nuccio, S.-P.; Mohanty, I.; Hagey, L.R.; Dorrestein, P.C.; Chu, H.; Raffatellu, M. How Bile Acids and the Microbiota Interact to Shape Host Immunity. Nat. Rev. Immunol. 2024, 24, 798–809. [Google Scholar] [CrossRef]
- Jose, S.; Devi, S.S.; Sajeev, A.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Alshammari, A.; Sethi, G.; Kunnumakkara, A.B. Repurposing FDA-Approved Drugs as FXR Agonists: A Structure Based in Silico Pharmacological Study. Biosci. Rep. 2023, 43, BSR20212791. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Liu, P.; Han, Z.; Ying, W.; Li, C.; Yang, Y.; Wang, S.; Yang, J.; Cao, F.; Shen, J.; et al. Bile Acids Modified by the Intestinal Microbiota Promote Colorectal Cancer Growth by Suppressing CD8+ T Cell Effector Functions. Immunity 2024, 57, 876–889.e11. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Duan, Z.; Deng, J.; Zhang, Z.; Fu, R.; Zhu, C.; Fan, D. Ginsenoside Rh4 Inhibits Colorectal Cancer via the Modulation of Gut Microbiota-Mediated Bile Acid Metabolism. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Keitel, V.; Häussinger, D. Role of TGR5 (GPBAR1) in Liver Disease. Semin. Liver Dis. 2018, 38, 333–339. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Cheng, G.; Zhu, T.; Zhang, Z.; Xiong, L.; Hu, H.; Liu, H. Berberine Improves DSS-Induced Colitis in Mice by Modulating the Fecal-Bacteria-Related Bile Acid Metabolism. Biomed. Pharmacother. 2023, 167, 115430. [Google Scholar] [CrossRef]
- Majait, S.; Nieuwdorp, M.; Kemper, M.; Soeters, M. The Black Box Orchestra of Gut Bacteria and Bile Acids: Who Is the Conductor? Int. J. Mol. Sci. 2023, 24, 1816. [Google Scholar] [CrossRef]
- Wang, X.J.; Camilleri, M. Personalized Medicine in Functional Gastrointestinal Disorders: Understanding Pathogenesis to Increase Diagnostic and Treatment Efficacy. World J. Gastroenterol. 2019, 25, 1185–1196. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Irritable Bowel Syndrome: Treatment Based on Pathophysiology and Biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Khalil, M.; Baffy, G.; Portincasa, P. Advances in the Pathophysiology, Diagnosis and Management of Chronic Diarrhoea from Bile Acid Malabsorption: A Systematic Review. Eur. J. Intern. Med. 2024, 128, 10–19. [Google Scholar] [CrossRef]
- Weaver, M.J.; McHenry, S.A.; Sayuk, G.S.; Gyawali, C.P.; Davidson, N.O. Bile Acid Diarrhea and NAFLD: Shared Pathways for Distinct Phenotypes. Hepatol. Commun. 2020, 4, 493–503. [Google Scholar] [CrossRef]
- Baumgartner, M.; Lang, M.; Holley, H.; Crepaz, D.; Hausmann, B.; Pjevac, P.; Moser, D.; Haller, F.; Hof, F.; Beer, A.; et al. Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis. Gastroenterology 2021, 161, 1245–1256.e20. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Liu, Z.; Cao, Y.; Chen, S.; Hou, R.; Zhou, Y.; Wang, Y. Bile Acid-Microbiota Crosstalk in Hepatitis B Virus Infection. J. Gastroenterol. Hepatol. 2024, 39, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Marcišauskas, S.; Ji, B.; Nielsen, J. Metagenomic Analysis of Bile Salt Biotransformation in the Human Gut Microbiome. BMC Genom. 2019, 20, 517. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.M.; Duboc, H.; Kay, G.L.; Alam, M.T.; Wicaksono, A.N.; Covington, J.A.; Quince, C.; Kokkorou, M.; Svolos, V.; Palmieri, L.J.; et al. The Pathophysiology of Bile Acid Diarrhoea: Differences in the Colonic Microbiome, Metabolome and Bile Acids. Sci. Rep. 2020, 10, 20436. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Cil, O.; Tse, C.M.; Sarker, R.; Lin, R.; Donowitz, M.; Verkman, A.S. Inhibition of CFTR-Mediated Intestinal Chloride Secretion as Potential Therapy for Bile Acid Diarrhea. FASEB J. 2019, 33, 10924–10934. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.-F.; Zhang, Y.; Zhang, Y.-L.; Niu, B.-Y.; Yao, S.-K. Altered Metabolism of Bile Acids Correlates with Clinical Parameters and the Gut Microbiota in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. World J. Gastroenterol. 2020, 26, 7153–7172. [Google Scholar] [CrossRef]
- Lembo, A.; Rao, S.S.C.; Heimanson, Z.; Pimentel, M. Abdominal Pain Response to Rifaximin in Patients With Irritable Bowel Syndrome With Diarrhea. Clin. Transl. Gastroenterol. 2020, 11, e00144. [Google Scholar] [CrossRef]
- Lacy, B.E.; Rosenbaum, D.; Edelstein, S.; Kozuka, K.; Williams, L.A.; Kunkel, D.C. Intestinal Permeability, Irritable Bowel Syndrome with Constipation, and the Role of Sodium-Hydrogen Exchanger Isoform 3 (NHE3). Clin. Exp. Gastroenterol. 2024, 17, 173–183. [Google Scholar] [CrossRef]
- Horikawa, T.; Oshima, T.; Li, M.; Kitayama, Y.; Eda, H.; Nakamura, K.; Tamura, A.; Ogawa, T.; Yamasaki, T.; Okugawa, T.; et al. Chenodeoxycholic Acid Releases Proinflammatory Cytokines from Small Intestinal Epithelial Cells Through the Farnesoid X Receptor. Digestion 2019, 100, 286–294. [Google Scholar] [CrossRef]
- Wei, Y.; Fan, Y.; Huang, S.; Lv, J.; Zhang, Y.; Hao, Z. Baizhu Shaoyao Decoction Restores the Intestinal Barrier and Brain-Gut Axis Balance to Alleviate Diarrhea-Predominant Irritable Bowel Syndrome via FoxO1/FoxO3a. Phytomedicine 2024, 122, 155163. [Google Scholar] [CrossRef]
- Anderson, K.M.; Gayer, C.P. The Pathophysiology of Farnesoid X Receptor (FXR) in the GI Tract: Inflammation, Barrier Function and Innate Immunity. Cells 2021, 10, 3206. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Tan, H.; Huang, W.; You, Y.; Zhan, J. Blueberry Extract Improves Obesity through Regulation of the Gut Microbiota and Bile Acids via Pathways Involving FXR and TGR5. iScience 2019, 19, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xiao, L.; Luo, M.; Xie, P.; Xiong, L. Intestinal Crosstalk between Bile Acids and Microbiota in Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2023, 38, 1072–1082. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local Immune Response to Food Antigens Drives Meal-Induced Abdominal Pain. Nature 2021, 590, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Ishioh, M.; Nozu, T. Central Regulatory Mechanisms of Visceral Sensation in Response to Colonic Distension with Special Reference to Brain Orexin. Neuropeptides 2021, 86, 102129. [Google Scholar] [CrossRef]
- Alemi, F.; Poole, D.P.; Chiu, J.; Schoonjans, K.; Cattaruzza, F.; Grider, J.R.; Bunnett, N.W.; Corvera, C.U. The Receptor TGR5 Mediates the Prokinetic Actions of Intestinal Bile Acids and Is Required for Normal Defecation in Mice. Gastroenterology 2013, 144, 145–154. [Google Scholar] [CrossRef]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable Bowel Syndrome: A Clinical Review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef]
- Fan, F.; Tang, Y.; Dai, H.; Cao, Y.; Sun, P.; Chen, Y.; Chen, A.; Lin, C. Blockade of BDNF Signalling Attenuates Chronic Visceral Hypersensitivity in an IBS-like Rat Model. Eur. J. Pain 2020, 24, 839–850. [Google Scholar] [CrossRef]
- Chen, M.-X.; Chen, Y.; Fu, R.; Liu, S.-Y.; Yang, Q.-Q.; Shen, T.-B. Activation of 5-HT and NR2B Contributes to Visceral Hypersensitivity in Irritable Bowel Syndrome in Rats. Am. J. Transl. Res. 2016, 8, 5580–5590. [Google Scholar]
- Barbaro, M.R.; Di Sabatino, A.; Cremon, C.; Giuffrida, P.; Fiorentino, M.; Altimari, A.; Bellacosa, L.; Stanghellini, V.; Barbara, G. Interferon-γ Is Increased in the Gut of Patients with Irritable Bowel Syndrome and Modulates Serotonin Metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G439–G447. [Google Scholar] [CrossRef]
- Liu, C.; Du, M.-X.; Xie, L.-S.; Wang, W.-Z.; Chen, B.-S.; Yun, C.-Y.; Sun, X.-W.; Luo, X.; Jiang, Y.; Wang, K.; et al. Gut Commensal Christensenella Minuta Modulates Host Metabolism via Acylated Secondary Bile Acids. Nat. Microbiol. 2024, 9, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging Roles of Bile Acids in Mucosal Immunity and Inflammation. Mucosal Immunol. 2019, 12, 851–861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).