Diagnostic Performance of Next-Generation Sequencing (NGS) in Indeterminate Thyroid Nodules: A Single Hospital Experience
Abstract
1. Introduction
2. Results
2.1. Nodule Characteristics
2.2. Molecular Test Performance
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. DNA and RNA Extraction
4.3. Myriapod NGS Cancer Panel DNA and RNA Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur. Thyroid. J. 2023, 12, e230067. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, L.; Poma, A.M.; Macerola, E.; Rago, T.; Vignali, P.; Romani, R.; Proietti, A.; Di Stefano, I.; Scuotri, G.; Ugolini, C.; et al. The Italian Consensus for the Classification and Reporting of Thyroid Cytology: Cytohistologic and molecular correlations on 37,371 nodules from a single institution. Cancer Cytopathol. 2022, 130, 899–912. [Google Scholar] [CrossRef]
- Biswas, S.; Shah, I.; Goswami, H.; Chaudhuri, A. Evaluation of concordance between the Bethesda System for Reporting Thyroid Cytopathology 2023 (TBSRTC) and ACR-TIRADS at a tertiary care center in Gujarat. Indian J. Pathol. Microbiol. 2025, 10–4103. [Google Scholar] [CrossRef] [PubMed]
- Belovarac, B.; Zhou, F.; Sharma, J.; Brandler, T.C. Indeterminate Thyroid Nodules and Advances in Molecular Pathology. Semin. Diagn. Pathol. 2023, 40, 349–352. [Google Scholar] [CrossRef]
- Muzza, M.; Colombo, C.; Pogliaghi, G.; Karapanou, O.; Fugazzola, L. Molecular markers for the classification of cytologically indeterminate thyroid nodules. J. Endocrinol. Investig. 2020, 43, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Nguyen, T.P.X.; Hassell, L.A.; Jung, C.K. Diagnostic performances of the Afirma Gene Sequencing Classifier in comparison with the Gene Expression Classifier: A meta-analysis. Cancer Cytopathol. 2021, 129, 182–189. [Google Scholar] [CrossRef]
- Chiosea, S.; Hodak, S.P.; Yip, L.; Abraham, D.; Baldwin, C.; Baloch, Z.; Gulec, S.A.; Hannoush, Z.C.; Haugen, B.R.; Joseph, L.; et al. Molecular Profiling of 50 734 Bethesda III-VI Thyroid Nodules by ThyroSeq v3: Implications for Personalized Management. J. Clin. Endocrinol. Metab. 2023, 108, 2999–3008. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Trovato, M.; Drago, C.; Bartolazzi, A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 2017, 8, 49421–49442. [Google Scholar] [CrossRef]
- Vignali, P.; Macerola, E.; Poma, A.M.; Sparavelli, R.; Basolo, F. Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics 2023, 13, 3008. [Google Scholar] [CrossRef]
- Haddad, R.I.; Nasr, C.; Bischoff, L.; Busaidy, N.L.; Byrd, D.; Callender, G.; Dickson, P.; Duh, Q.Y.; Ehya, H.; Goldner, W.; et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Papini, E.; Frasoldati, A.; Lupo, M.A.; Harrell, R.M.; Parangi, S.; Patkar, V.; Baloch, Z.W.; Pessah-Pollack, R.; Hegedus, L.; et al. American Association of Clinical Endocrinology and Associazione Medici Endocrinologi Thyroid Nodule Algorithmic Tool. Endocr. Pract. 2021, 27, 649–660. [Google Scholar] [CrossRef]
- Pacini, F.; Basolo, F.; Bellantone, R.; Boni, G.; Cannizzaro, M.A.; De Palma, M.; Durante, C.; Elisei, R.; Fadda, G.; Frasoldati, A.; et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: Joint statements of six Italian societies. J. Endocrinol. Investig. 2018, 41, 849–876. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Next-generation sequencing in thyroid cancer. J. Transl. Med. 2016, 14, 322. [Google Scholar] [CrossRef]
- Potonnier, W.; Guillerm, E.; Bigorgne, C.; Ghander, C.; Roy, M.; Coulet, F.; Ansart, F.; Menegaux, F.; Leenhardt, L.; Brocheriou, I.; et al. Performance of the AmpliSeq NGS panel in thyroid nodules with indeterminate cytology. Eur. Thyroid. J. 2025, 14, e240160. [Google Scholar] [CrossRef]
- Rossi, E.D.; Locantore, P.; Bruno, C.; Dell’Aquila, M.; Tralongo, P.; Curatolo, M.; Revelli, L.; Raffaelli, M.; Larocca, L.M.; Pantanowitz, L.; et al. Molecular Characterization of Thyroid Follicular Lesions in the Era of “Next-Generation” Techniques. Front. Endocrinol. 2022, 13, 834456. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Klopper, J.; Cottrill, E.E. Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities. Front. Endocrinol. 2023, 14, 1101410. [Google Scholar] [CrossRef] [PubMed]
- Capezzone, M.; Cantara, S.; Di Santo, A.; Sagnella, A.; Pilli, T.; Brilli, L.; Ciuoli, C.; Maino, F.; Forleo, R.; Cartocci, A.; et al. The Combination of Sonographic Features and the Seven-Gene Panel May be Useful in the Management of Thyroid Nodules With Indeterminate Cytology. Front. Endocrinol. 2021, 12, 613727. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.H.; Oh, Y.L.; Hahn, S.Y.; Park, K.W. Approach to Bethesda system category III thyroid nodules according to US-risk stratification. Endocr. J. 2022, 69, 67–74. [Google Scholar] [CrossRef]
- Hong, H.S.; Lee, J.Y. Diagnostic Performance of Ultrasound Patterns by K-TIRADS and 2015 ATA Guidelines in Risk Stratification of Thyroid Nodules and Follicular Lesions of Undetermined Significance. Am. J. Roentgenol. 2019, 213, 444–450. [Google Scholar] [CrossRef]
- Słowińska-Klencka, D.; Klencki, M.; Duda-Szymańska, J.; Popowicz, B. Optimization of the Management of Category III Thyroid Nodules Using Repeat FNA and TIRADS. Cancers 2022, 14, 4889. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Song, K.H.; Kim, S.K.; Park, K.S.; Yoo, Y.B.; Yang, J.H.; Hwang, T.S.; Kim, D.L. RAS mutations in indeterminate thyroid nodules are predictive of the follicular variant of papillary thyroid carcinoma. Clin. Endocrinol. 2015, 82, 760–766. [Google Scholar] [CrossRef]
- Lee, S.R.; Jung, C.K.; Kim, T.E.; Bae, J.S.; Jung, S.L.; Choi, Y.J.; Kang, C.S. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fine-needle aspiration cytology: Values of RAS mutation testing. Thyroid 2013, 23, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Sfreddo, H.J.; Koh, E.S.; Zhao, K.; Swartzwelder, C.E.; Untch, B.R.; Marti, J.L.; Roman, B.R.; Dublin, J.; Wang, R.S.; Xia, R.; et al. RAS-Mutated Cytologically Indeterminate Thyroid Nodules: Prevalence of Malignancy and Behavior Under Active Surveillance. Thyroid 2024, 34, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Toraldo, G.; Cerda, S.; Godley, F.A.; Rao, S.R.; McAneny, D.; Doherty, G.; Braverman, L.; Lee, S.L. Utilities of RAS Mutations in Preoperative Fine Needle Biopsies for Decision Making for Thyroid Nodule Management: Results from a Single-Center Prospective Cohort. Thyroid 2020, 30, 536–547. [Google Scholar] [CrossRef]
- Riccio, I.; Laforteza, A.; Landau, M.B.; Hussein, M.H.; Linhuber, J.; Staav, J.; Issa, P.P.; Toraih, E.A.; Kandil, E. Decoding RAS mutations in thyroid cancer: A meta-analysis unveils specific links to distant metastasis and increased mortality. Am. J. Otolaryngol. 2025, 46, 104570. [Google Scholar] [CrossRef]
- Pruneri, G.; De Braud, F.; Sapino, A.; Aglietta, M.; Vecchione, A.; Giusti, R.; Marchiò, C.; Scarpino, S.; Baggi, A.; Bonetti, G.; et al. Next-Generation Sequencing in Clinical Practice: Is It a Cost-Saving Alternative to a Single-Gene Testing Approach? Pharmacoecon. Open 2021, 5, 285–298. [Google Scholar] [CrossRef]
- Phillips, K.A.; Deverka, P.A.; Marshall, D.A.; Wordsworth, S.; Regier, D.A.; Christensen, K.D.; Buchanan, J. Methodological Issues in Assessing the Economic Value of Next-Generation Sequencing Tests: Many Challenges and Not Enough Solutions. Value Health 2018, 21, 1033–1042. [Google Scholar] [CrossRef]
- Persani, L.; de Filippis, T.; Colombo, C.; Gentilini, D. Genetics in Endocrinology: Genetic diagnosis of endocrine diseases by NGS: Novel scenarios and unpredictable results and risks. Eur. J. Endocrinol. 2018, 179, R111–R123. [Google Scholar] [CrossRef]
- Aydemirli, M.D.; Snel, M.; van Wezel, T.; Ruano, D.; Obbink, C.M.H.; van den Hout, W.B.; Schepers, A.; Morreau, H. Yield and costs of molecular diagnostics on thyroid cytology slides in the Netherlands, adapting the Bethesda classification. Endocrinol. Diabetes Metab. 2021, 4, e00293. [Google Scholar] [CrossRef]
- Capezzone, M.; Rossi, M.; Macerola, E.; Cantara, S.; Pepe, F.; Morabito, E.M.; Dalmazio, G.; Bardi, S.; Ognibene, A.; Alessandri, M.; et al. Identification of a Novel Non-V600E BRAF Mutation in Papillary Thyroid Cancer. Case Rep. Endocrinol. 2024, 2024, 6621510. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]

| Parameters | TIR3A (N = 73) | TIR3B (N = 32) | p |
|---|---|---|---|
| Age at Diagnosis (Years) | 0.5 | ||
| Mean ± SD | 57.5 ± 12.1 | 58.9 ± 13.2 | |
| Sex | 0.2 | ||
| Males | 20 (27.4) | 13 (40.6) | |
| Females | 53 (72.6) | 19 (59.4) | |
| EU-TIRADS: N (%) | 0.9 | ||
| 2 | 23 (31.5) | 10 (31.2) | |
| 3 | 33 (45.2) | 15 (46.9) | |
| 4 | 15 (20.5) | 6 (18.7) | |
| 5 | 2 (2.8) | 1 (3.2) | |
| Size of Thyroid Nodule (mm) | 0.1 | ||
| Mean ± SD | 22.3 ± 9.1 | 25.6 ± 10.1 | |
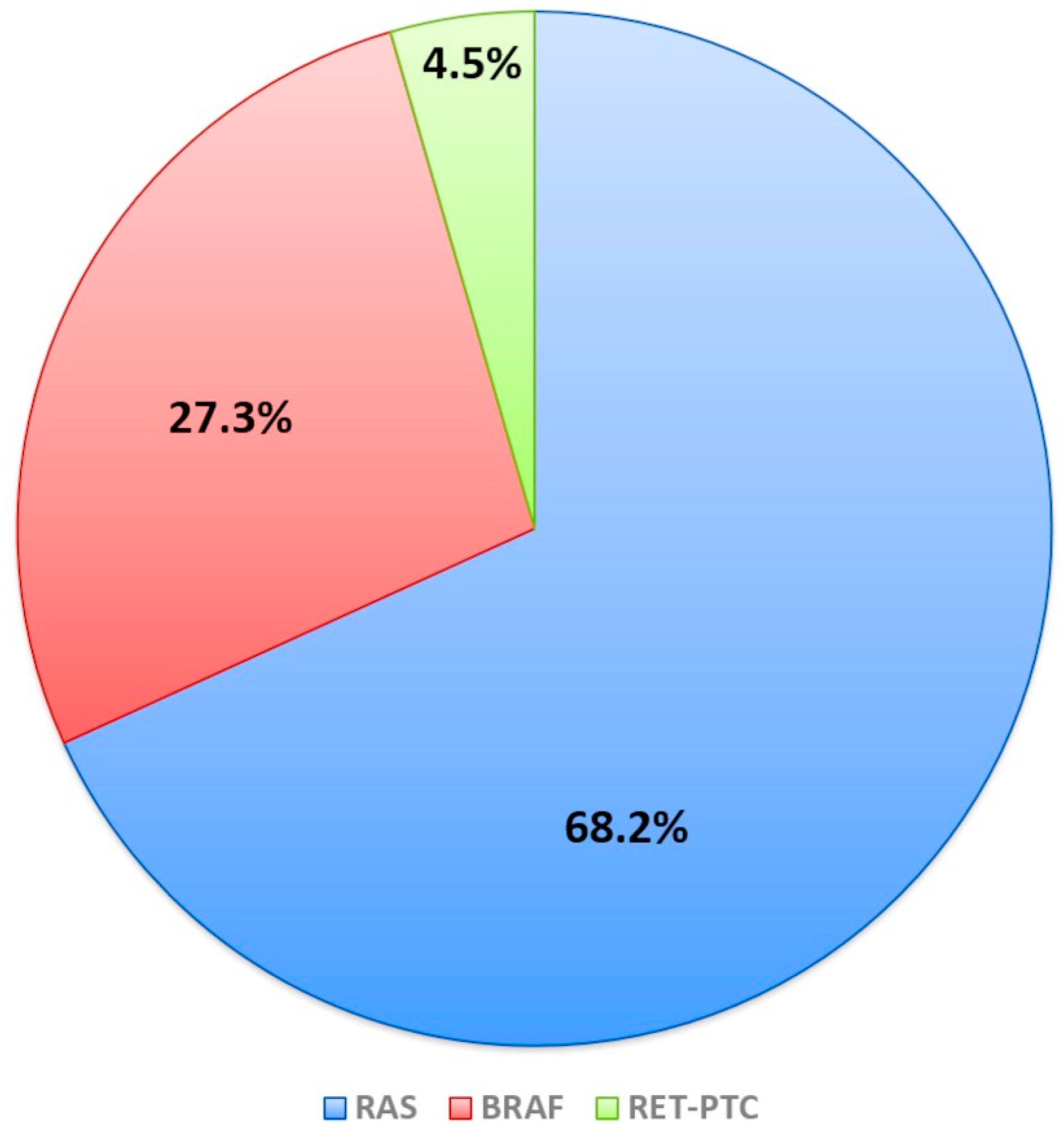
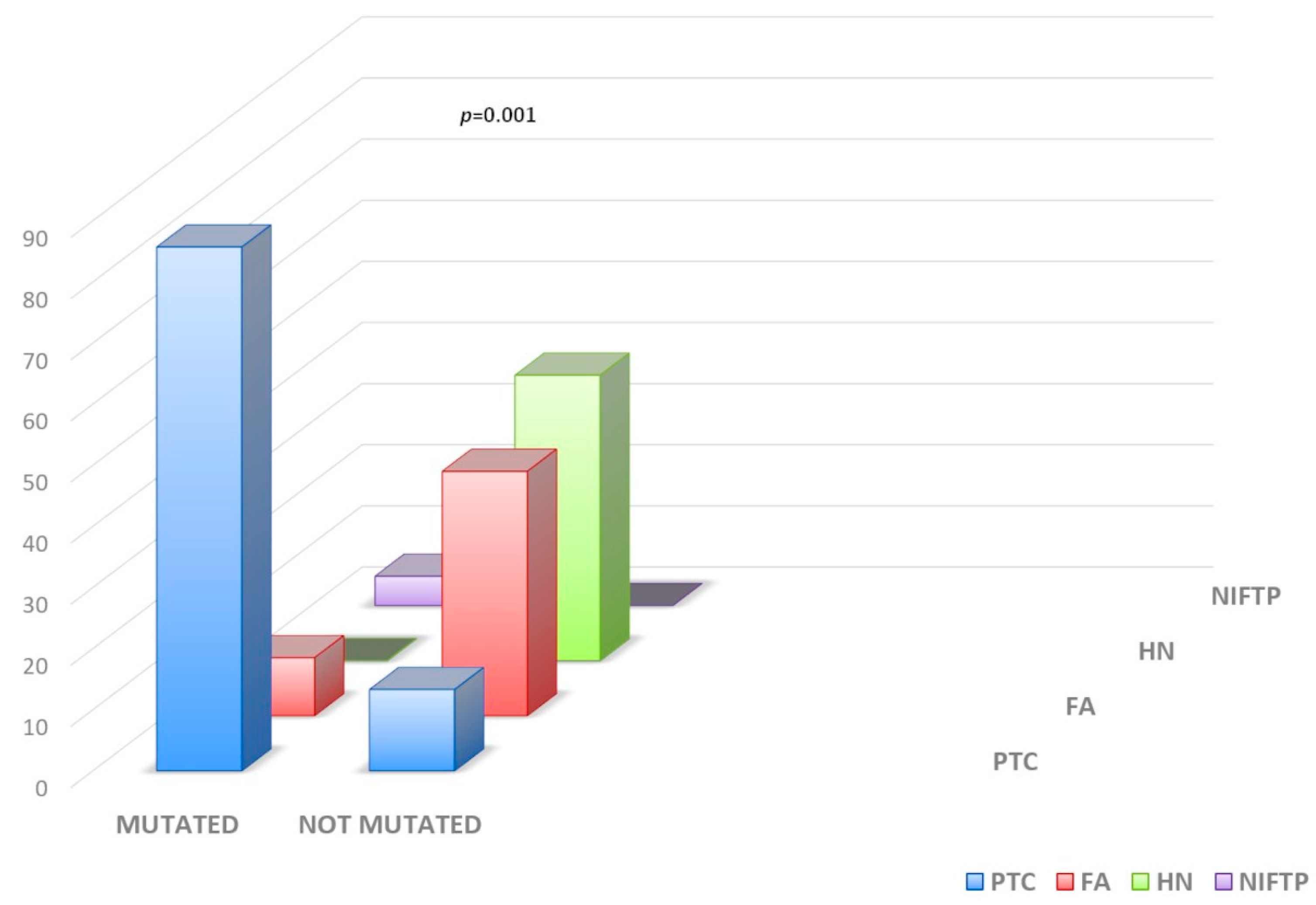
| Mutations: N (%) | 0.0001 | ||
| BRAF | 1 (1.4) | 5 (15.7) | |
| NRAS | 5 (6.9) | 6 (18.7) | |
| KRAS | 2 (2.7) | 0 | |
| HRAS | 0 | 2 (6.3) | |
| RET-PTC1 | 0 | 1 (3.1) | |
| No Mutations | 65 (89) | 18 (56.2) |
| All Mutations | Ras-like Mutations | Braf-like Mutations | |
|---|---|---|---|
| Total Cases | |||
| AUC | 0.89 (0.76–0.98) | 0.72 (0.58–0.86) | 0.67 (0.57–0.76) |
| Specificity | 0.87 (0.67–1) | 0.87 (0.67–1) | 1 (1–1) |
| Sensitivity | 0.90 (0.76–1) | 0.57 (0.38–0.81) | 0.33 (0.14–0.52) |
| Accuracy | 0.89 (0.78–0.97) | 0.69 (0.56–0.83) | 0.61 (0.50–0.72) |
| NPV | 0.87 (0.72–1) | 0.59 (0.48–0.75) | 0.52 (0.45–0.60) |
| PPV | 0.91 (0.79–1) | 0.87 (0.70–1) | 1 (1–1) |
| TIR3A Cases | |||
| AUC | 0.86 (0.64–1) | 0.79 (0.57–1) | 0.57 (0.50–0.71) |
| Specificity | 0.86 (0.57–1) | 0.86 (0.57–1) | 1 (1–1) |
| Sensitivity | 0.86 (0.57–1) | 0.71 (0.43–1) | 0.14 (0–1) |
| Accuracy | 0.86 (0.64–1) | 0.79 (0.57–1) | 0.57 (0.50–0.71) |
| NPV | 0.86 (0.63–1) | 0.75 (0.56–1) | 0.54 (0.50–0.64) |
| PPV | 0.86 (0.64–1) | 0.86 (0.60–1) | 1 (1–1) |
| TIR3B Cases | |||
| AUC | 0.90 (0.75–1) | 0.69 (0.50–0.86) | 0.71 (0.57–0.82) |
| Specificity | 0.87 (0.62–1) | 0.87 (0.62–1) | 1 (1–1) |
| Sensitivity | 0.93 (0.79–1) | 0.50 (0.51–0.79) | 0.43 (0.14–0.71) |
| Accuracy | 0.91 (0.77–1) | 0.63 (0.45–0.82) | 0.64 (0.45–0.82) |
| NPV | 0.87 (0.67–1) | 0.50 (0.37–0.67) | 0.50 (0.40–0.67) |
| PPV | 0.93 (0.81–1) | 0.89 (0.67–1) | 1 (1–1) |
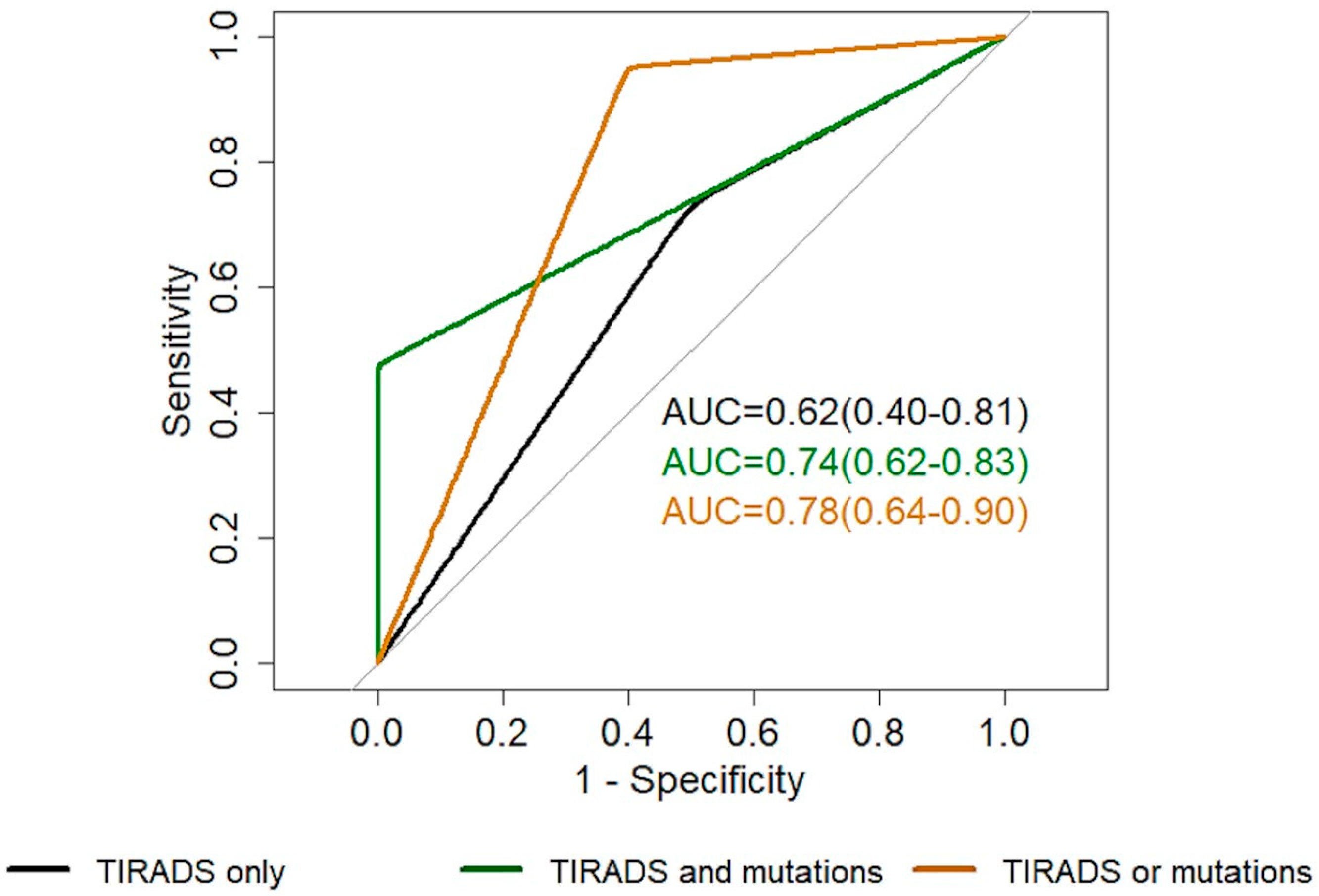
| EU-TIRADS Alone (2–3 vs. 4–5) | EU-TIRADS 4–5 and Presence of Mutations | EU-TIRADS 4–5 or Presence of Mutations | |
|---|---|---|---|
| AUC | 0.62 (0.40–0.81) | 0.74 (0.62–0.83) | 0.78 (0.64–0.90) |
| Specificity | 0.50 (0–1) | 1 (1–1) | 0.60 (0.33–0.80) |
| Sensitivity | 0.73 (0–1) | 0.48 (0.29–0.67) | 0.95 (0.86–1) |
| Accuracy | 0.65 (0.35–0.83) | 0.69 (0.58–0.81) | 0.81 (0.69–0.92) |
| NPV | 0.50 (0.35–0.80) | 0.58 (0.50–0.68) | 0.90 (0.70–1) |
| PPV | 0.74 (0.65–0.91) | 1 (1–1) | 0.77 (0.67–0.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capezzone, M.; Rossi, M.; Bardi, S.; Morabito, E.M.; Dalmazio, G.; Iapichino, G.; Galassi, S.; Seralessandri, S.; Torregrossa, L.; Tosti Balducci, M.; et al. Diagnostic Performance of Next-Generation Sequencing (NGS) in Indeterminate Thyroid Nodules: A Single Hospital Experience. Int. J. Mol. Sci. 2025, 26, 4225. https://doi.org/10.3390/ijms26094225
Capezzone M, Rossi M, Bardi S, Morabito EM, Dalmazio G, Iapichino G, Galassi S, Seralessandri S, Torregrossa L, Tosti Balducci M, et al. Diagnostic Performance of Next-Generation Sequencing (NGS) in Indeterminate Thyroid Nodules: A Single Hospital Experience. International Journal of Molecular Sciences. 2025; 26(9):4225. https://doi.org/10.3390/ijms26094225
Chicago/Turabian StyleCapezzone, Marco, Maja Rossi, Sara Bardi, Eugenia Maria Morabito, Gilda Dalmazio, Giuseppe Iapichino, Simona Galassi, Serena Seralessandri, Liborio Torregrossa, Massimo Tosti Balducci, and et al. 2025. "Diagnostic Performance of Next-Generation Sequencing (NGS) in Indeterminate Thyroid Nodules: A Single Hospital Experience" International Journal of Molecular Sciences 26, no. 9: 4225. https://doi.org/10.3390/ijms26094225
APA StyleCapezzone, M., Rossi, M., Bardi, S., Morabito, E. M., Dalmazio, G., Iapichino, G., Galassi, S., Seralessandri, S., Torregrossa, L., Tosti Balducci, M., Marchetti, E., Alessandri, M., Ognibene, A., De Napoli, L., Materazzi, G., Cantara, S., & Poma, A. M. (2025). Diagnostic Performance of Next-Generation Sequencing (NGS) in Indeterminate Thyroid Nodules: A Single Hospital Experience. International Journal of Molecular Sciences, 26(9), 4225. https://doi.org/10.3390/ijms26094225







