Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice
Abstract
1. Introduction
2. Results
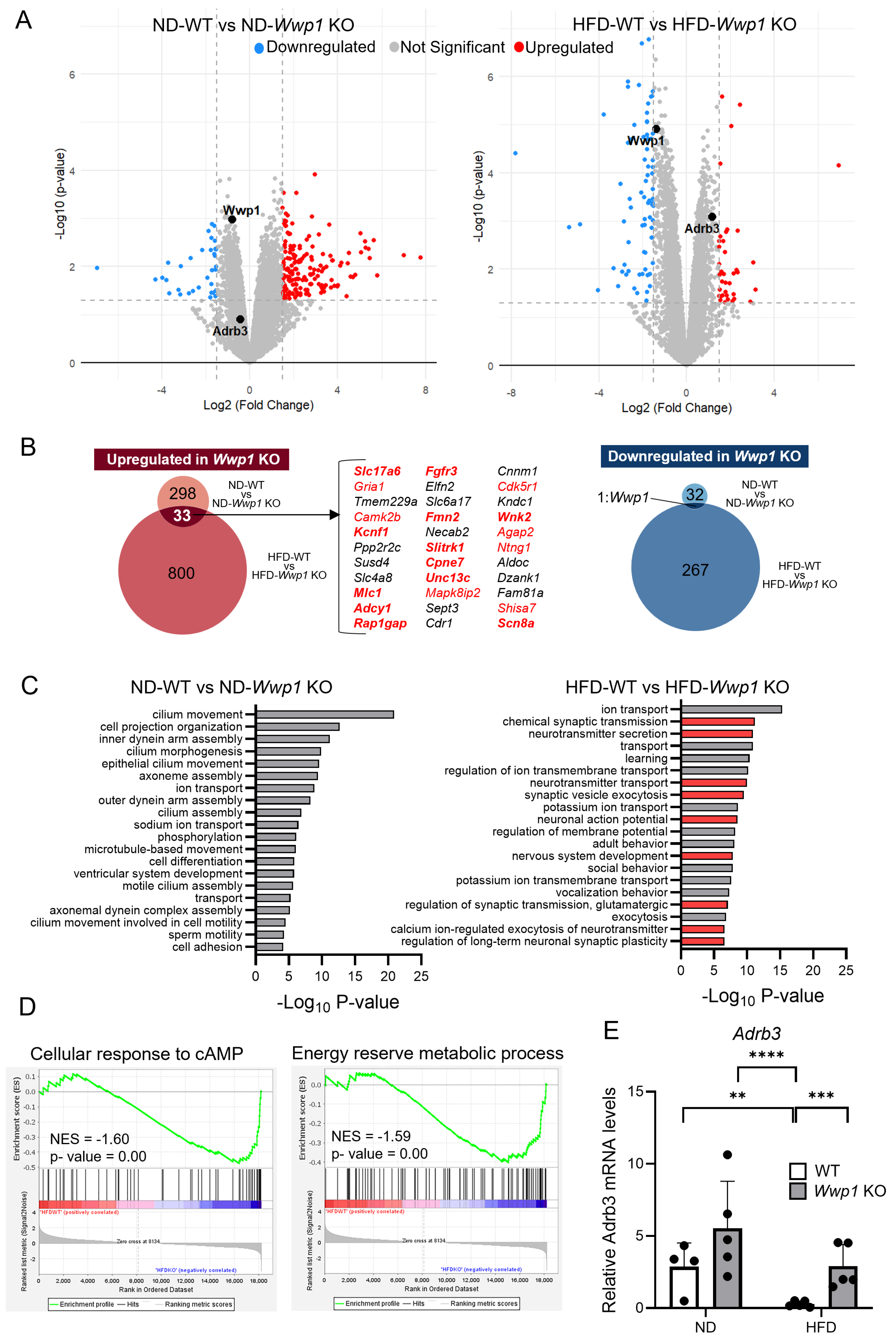
2.1. Depletion of WWP1 Increases Adrb3 mRNA Expression in Epididymal WAT
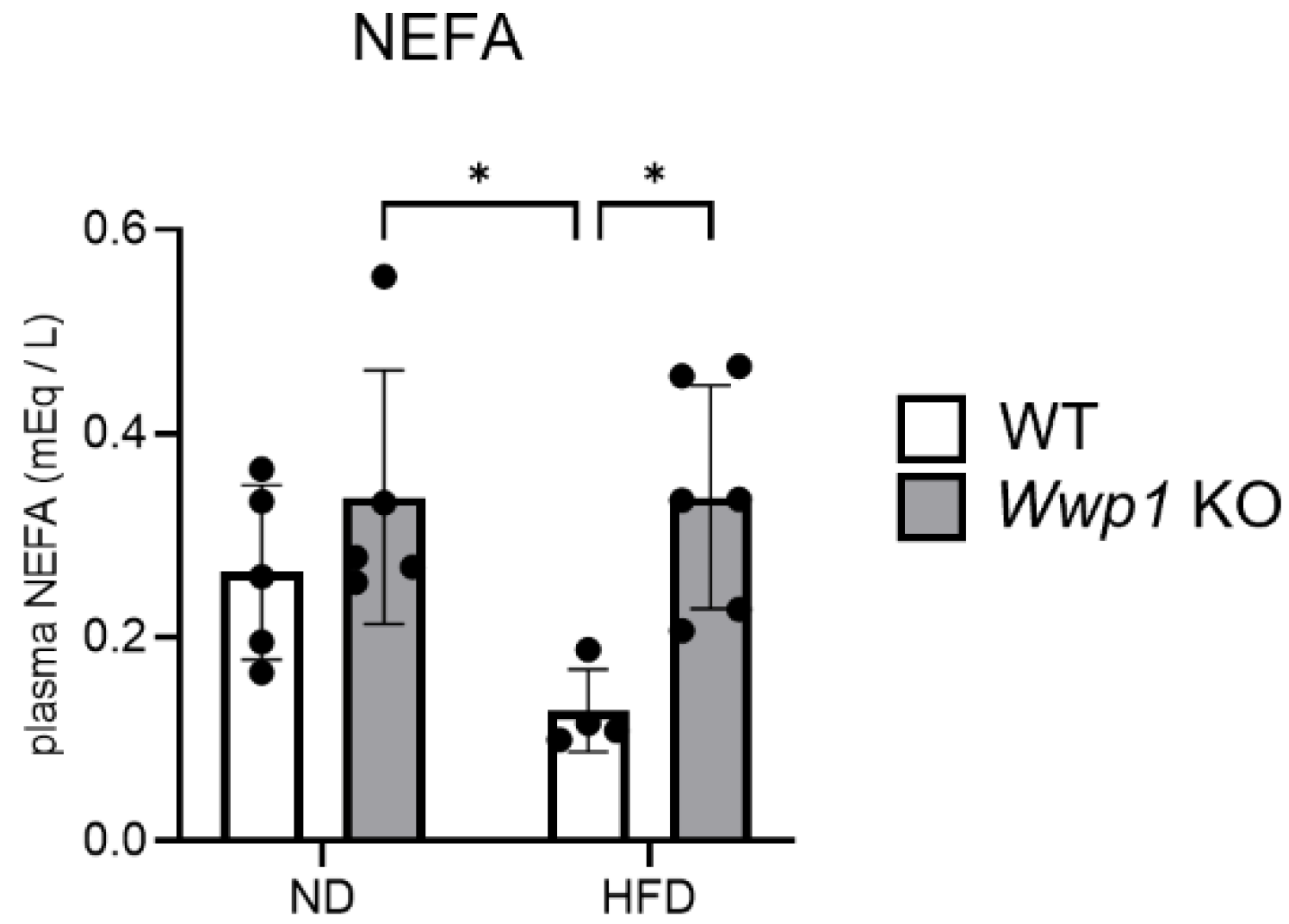
2.2. Depletion of WWP1 Increases Phosphorylation of HSL in eWAT and Plasma Non-Esterified Fatty Acid (NEFA) Concentrations
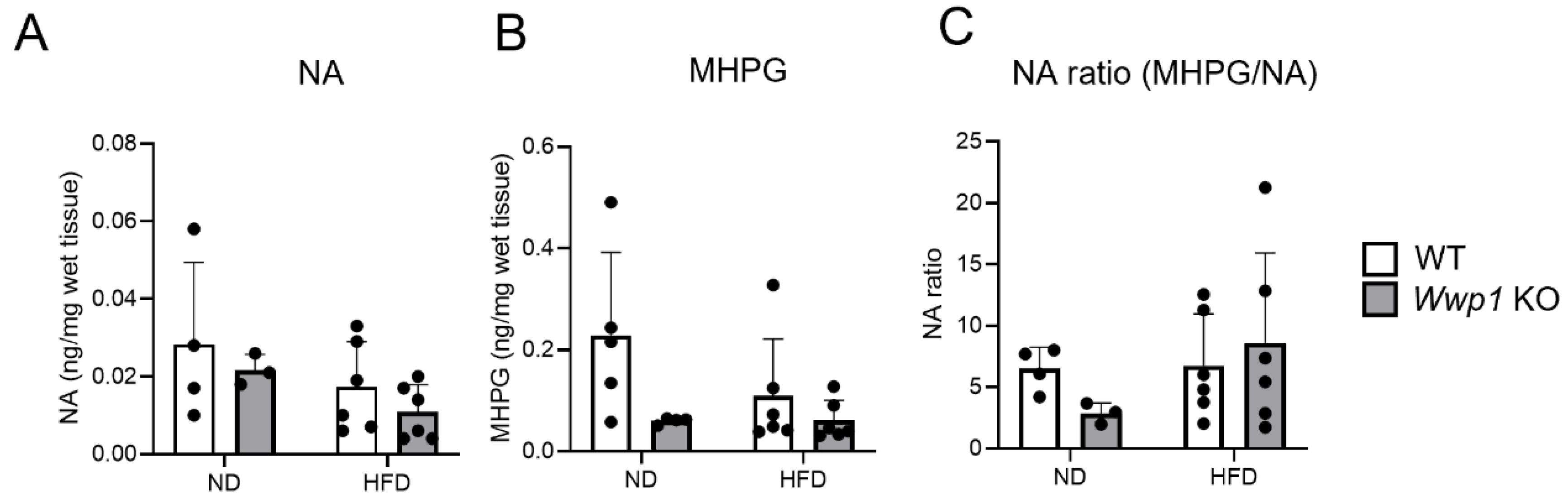
2.3. Noradrenaline Metabolism Is Not Changed by Wwp1 KO in eWAT
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. RNA-Seq and Gene Set Enrichment Analysis
4.3. Immunoblotting
4.4. Image Processing of Original Blots
4.5. Quantitative Real-Time PCR
4.6. Plasma Non-Esterified Fatty Acid Concentrations
4.7. Quantification of Monoamine Contents in the Brain
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | acetylcholine-gated chloride channel subunit |
| ACLY | ATP citrate synthase |
| AIP5 | atrophin-1-interacting protein 5 |
| eWAT | epididymal white adipose tissue |
| Adrb3 | beta-3 adrenergic receptor |
| cAMP | cyclic adenosine monophosphate |
| HECT | homologous to the E6-AP carboxyl terminus |
| HFD | high-fat diet |
| HSL | hormone-sensitive lipase |
| ME1 | malic enzyme 1 |
| MHPG | metabolite 3-methoxy-4-hydroxyphenilglycol |
| NA | noradrenaline |
| ND | normal diet |
| TIUL1 | TGIF-interacting ubiquitin ligase 1 |
| WWP1 | WW domain-containing E3 ubiquitin protein ligase 1 |
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 1, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Reynisdottir, S.; Langin, D.; Carlström, K.; Holm, C.; Rössner, S.; Arner, P. Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin. Sci. 1995, 89, 421–429. [Google Scholar] [CrossRef]
- Bougnères, P.; Stunff, C.L.; Pecqueur, C.; Pinglier, E.; Adnot, P.; Ricquier, D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J. Clin. Investig. 1997, 99, 2568–2573. [Google Scholar] [CrossRef]
- Large, V.; Reynisdottir, S.; Langin, D.; Fredby, K.; Klannemark, M.; Holm, C.; Arner, P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J. Lipid Res. 1999, 40, 2059–2066. [Google Scholar] [CrossRef]
- Valentine, J.M.; Ahmadian, M.; Keinan, O.; Abu-Odeh, M.; Zhao, P.; Zhou, X.; Keller, M.P.; Gao, H.; Yu, R.T.; Liddle, C.; et al. β3-Adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J. Clin. Investig. 2022, 132, e153357. [Google Scholar] [CrossRef]
- Nagiri, C.; Kobayashi, K.; Tomita, A.; Kato, M.; Kobayashi, K.; Yamashita, K.; Nishizawa, T.; Inoue, A.; Shihoya, W.; Nureki, O. Cryo-EM structure of the β3-adrenergic receptor reveals the molecular basis of subtype selectivity. Mol. Cell 2021, 81, 3205–3215.e5. [Google Scholar] [CrossRef]
- Di Salvo, J.; Nagabukuro, H.; Wickham, L.A.; Abbadie, C.; DeMartino, J.A.; Fitzmaurice, A.; Gichuru, L.; Kulick, A.; Donnelly, M.J.; Jochnowitz, N.; et al. Pharmacological Characterization of a Novel Beta 3 Adrenergic Agonist, Vibegron: Evaluation of Antimuscarinic Receptor Selectivity for Combination Therapy for Overactive Bladder. J. Pharmacol. Exp. Ther. 2017, 360, 346–355. [Google Scholar] [CrossRef]
- Takasu, T.; Ukai, M.; Sato, S.; Matsui, T.; Nagase, I.; Maruyama, T.; Sasamata, M.; Miyata, K.; Uchida, H.; Yamaguchi, O. Effect of (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J. Pharmacol. Exp. Ther. 2007, 321, 642–647. [Google Scholar] [CrossRef]
- Sacco, E.; Bientinesi, R. Mirabegron: A review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 2012, 4, 315–324. [Google Scholar] [CrossRef]
- Seo, S.; RLallemand, F.; Ferrand, N.; Pessah, M.; L’Hoste, S.; Camonis, J.; Atfi, A. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J. 2004, 23, 3780–3792. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Chen, C. WWP1: A versatile ubiquitin E3 ligase in signaling and diseases. Cell Mol. Life Sci. 2012, 69, 1425–1434. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hoshino, S.; Abe, T.; Okita, N.; Tagawa, R.; Nagai, W.; Konno, R.; Suzuki, Y.; Furuya, K.; Ishikawa, N.; et al. Identification of WWP1 as an obesity-associated E3 ubiquitin ligase with a protective role against oxidative stress in adipocytes. Biochem. Biophys. Res. Commun. 2019, 508, 117–122. [Google Scholar] [CrossRef]
- Hoshino, S.; Kobayashi, M.; Tagawa, R.; Konno, R.; Abe, T.; Furuya, K.; Miura, K.; Wakasawa, H.; Okita, N.; Sudo, Y.; et al. WWP1 knockout in mice exacerbates obesity-related phenotypes in white adipose tissue but improves whole-body glucose metabolism. FEBS Open Bio 2020, 10, 306–315. [Google Scholar] [CrossRef]
- Nozaki, Y.; Kobayashi, M.; Wakasawa, H.; Hoshino, S.; Suwa, F.; Ose, Y.; Tagawa, R.; Higami, Y. Systemic depletion of WWP1 improves insulin sensitivity and lowers triglyceride content in the liver of obese mice. FEBS Open Bio 2023, 13, 1086–1094. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Carmen, G.Y.; Víctor, S.M. Signalling mechanisms regulating lipolysis. Cell. Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef]
- Komai, A.M.; Musovic, S.; Peris, E.; Alrifaiy, A.; El Hachmane, M.F.; Johansson, M.; Asterholm, I.W.; Olofsson, C.S. White Adipocyte Adiponectin Exocytosis Is Stimulated via β3-Adrenergic Signaling and Activation of Epac1: Catecholamine Resistance in Obesity and Type 2 Diabetes. Diabetes 2016, 65, 3301–3313. [Google Scholar] [CrossRef]
- Cao, W.Y.; Liu, Z.; Guo, F.; Yu, J.; Li, H.; Yin, X. Adipocyte ADRB3 Down-Regulated in Chinese Overweight Individuals Adipocyte ADRB3 in Overweight. Obes. Facts 2018, 11, 524–533. [Google Scholar] [CrossRef]
- Zaccolo, M. Spatial control of cAMP signalling in health and disease. Curr. Opin. Pharmacol. 2011, 11, 649–655. [Google Scholar] [CrossRef]
- Steinberg, S.F.; Brunton, L.L. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 751–773. [Google Scholar] [CrossRef] [PubMed]
- Ambrozkiewicz, M.C.; Schwark, M.; Kishimoto-Suga, M.; Borisova, E.; Hori, K.; Salazar-Lázaro, A.; Rusanova, A.; Altas, B.; Piepkorn, L.; Bessa, P.; et al. Polarity Acquisition in Cortical Neurons Is Driven by Synergistic Action of Sox9-Regulated Wwp1 and Wwp2 E3 Ubiquitin Ligases and Intronic miR-140. Neuron 2018, 100, 1097–1115.e1015. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Pu, H.X.; Li, M.; Ahmed, S.; Song, J. Identification and structural mechanism for a novel interaction between a ubiquitin ligase WWP1 and Nogo-A, a key inhibitor for central nervous system regeneration. Biochemistry 2008, 47, 13647–13658. [Google Scholar] [CrossRef]
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq Reads with STAR. Curr. Protoc. Bioinform. 2015, 51, 11–14. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Okita, N.; Hayashida, Y.; Kojima, Y.; Fukushima, M.; Yuguchi, K.; Mikami, K.; Yamauchi, A.; Watanabe, K.; Noguchi, M.; Nakamura, M.; et al. Differential responses of white adipose tissue and brown adipose tissue to caloric restriction in rats. Mech. Ageing Dev. 2012, 133, 255–266. [Google Scholar] [CrossRef]
- Saitoh, A.; Nagayama, Y.; Yamada, D.; Makino, K.; Yoshioka, T.; Yamanaka, N.; Nakatani, M.; Takahashi, Y.; Yamazaki, M.; Shigemoto, C.; et al. Disulfiram Produces Potent Anxiolytic-Like Effects Without Benzodiazepine Anxiolytics-Related Adverse Effects in Mice. Front. Pharmacol. 2022, 13, 826783. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef]
- Norman, G. Likert scales, levels of measurement and the “laws” of statistics. Adv. Health Sci. Educ. Theory Pract. 2010, 15, 625–632. [Google Scholar] [CrossRef]


| ND | HFD | |||
|---|---|---|---|---|
| WT | Wwp1 KO | WT | Wwp1 KO | |
| Body Weight (g) | 29.30 ± 0.97 | 27.38 ± 1.01 | 41.67 ± 2.45 #### | 37.11 ± 3.23 * #### |
| sWAT (g) | 0.37 ± 0.04 | 0.33 ± 0.16 | 2.04 ± 0.28 #### | 2.32 ± 0.62 #### |
| eWAT (g) | 0.62 ± 0.16 | 0.46 ± 0.14 | 1.64 ± 0.28 #### | 1.92 ± 0.52 #### |
| Liver (g) | 1.00 ± 0.03 | 1.02 ± 0.04 | 1.56 ± 0.32 ### | 1.24 ± 0.17 * |
| QFM (g) | 0.12 ± 0.03 | 0.13 ± 0.04 | 0.12 ± 0.04 | 0.13 ± 0.03 |
| Heart (g) | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 |
| Brain (g) | 0.39 ± 0.04 | 0.39 ± 0.03 | 0.37 ± 0.03 | 0.39 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozaki, Y.; Ose, Y.; Ohmori, C.; Mizunoe, Y.; Kobayashi, M.; Saitoh, A.; Higami, Y. Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice. Int. J. Mol. Sci. 2025, 26, 4219. https://doi.org/10.3390/ijms26094219
Nozaki Y, Ose Y, Ohmori C, Mizunoe Y, Kobayashi M, Saitoh A, Higami Y. Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice. International Journal of Molecular Sciences. 2025; 26(9):4219. https://doi.org/10.3390/ijms26094219
Chicago/Turabian StyleNozaki, Yuka, Yuko Ose, Chinatsu Ohmori, Yuhei Mizunoe, Masaki Kobayashi, Akiyoshi Saitoh, and Yoshikazu Higami. 2025. "Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice" International Journal of Molecular Sciences 26, no. 9: 4219. https://doi.org/10.3390/ijms26094219
APA StyleNozaki, Y., Ose, Y., Ohmori, C., Mizunoe, Y., Kobayashi, M., Saitoh, A., & Higami, Y. (2025). Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice. International Journal of Molecular Sciences, 26(9), 4219. https://doi.org/10.3390/ijms26094219








