The Etiology of Moebius Syndrome—Making the Case for Animal Models
Abstract
1. Introduction
2. Results
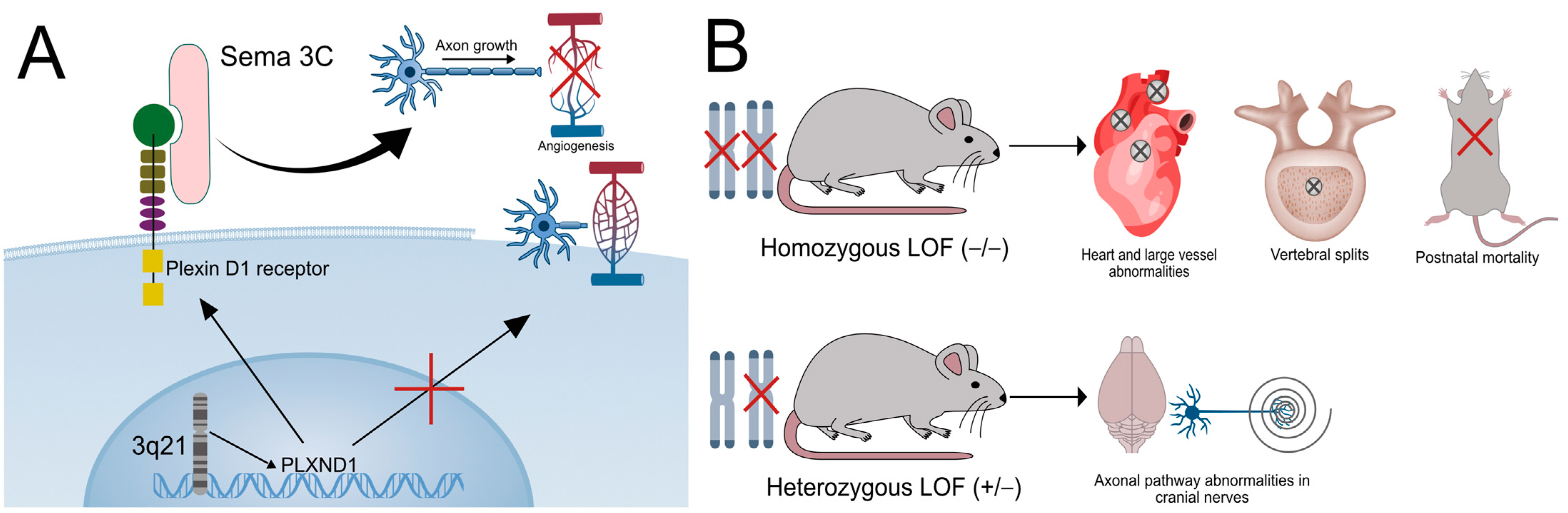
2.1. The Role of PLXND1 in Disease Pathogenesis in Human and Animal Models
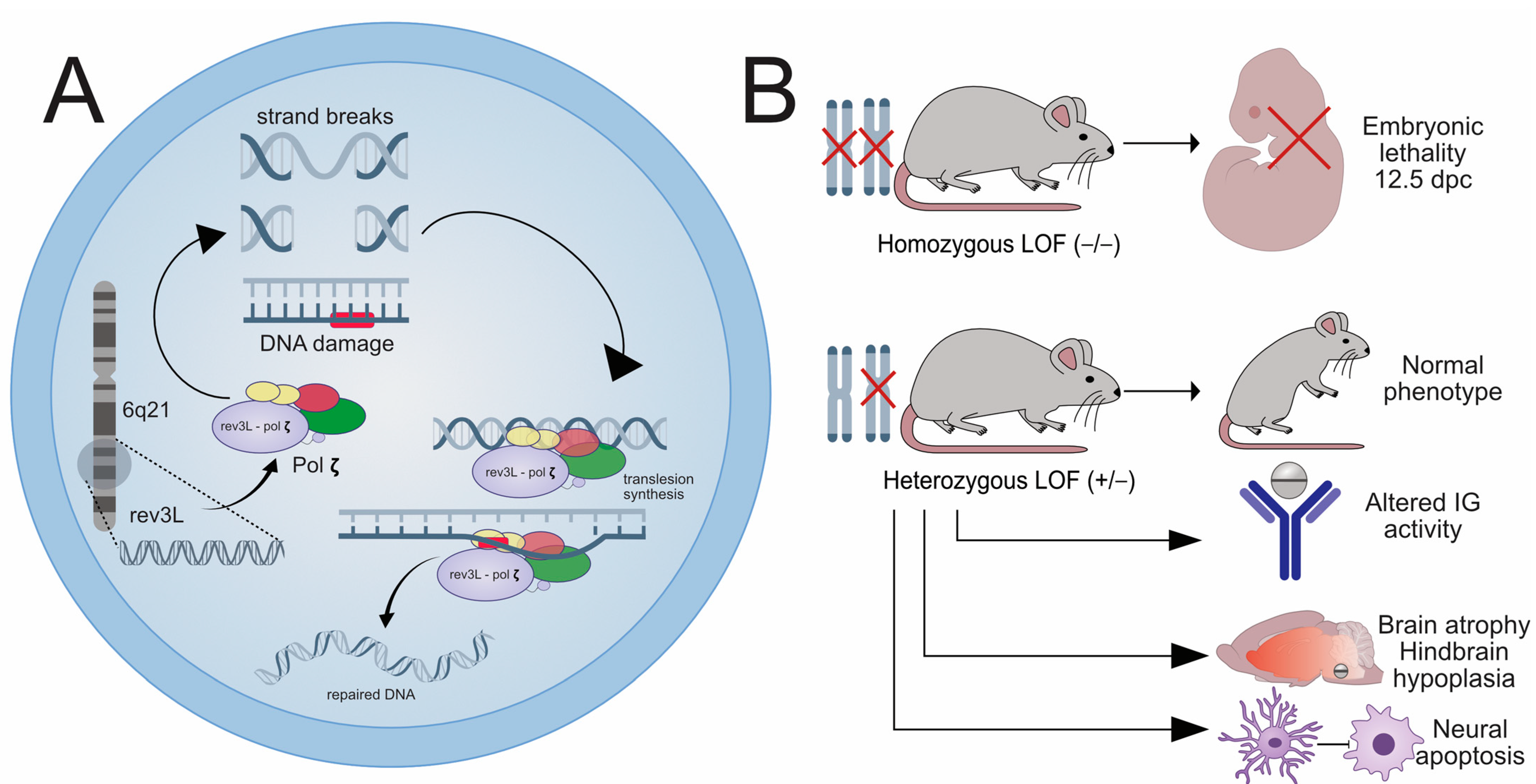
2.2. The Role of REV3L in Disease Pathogenesis in Human and Animal Models
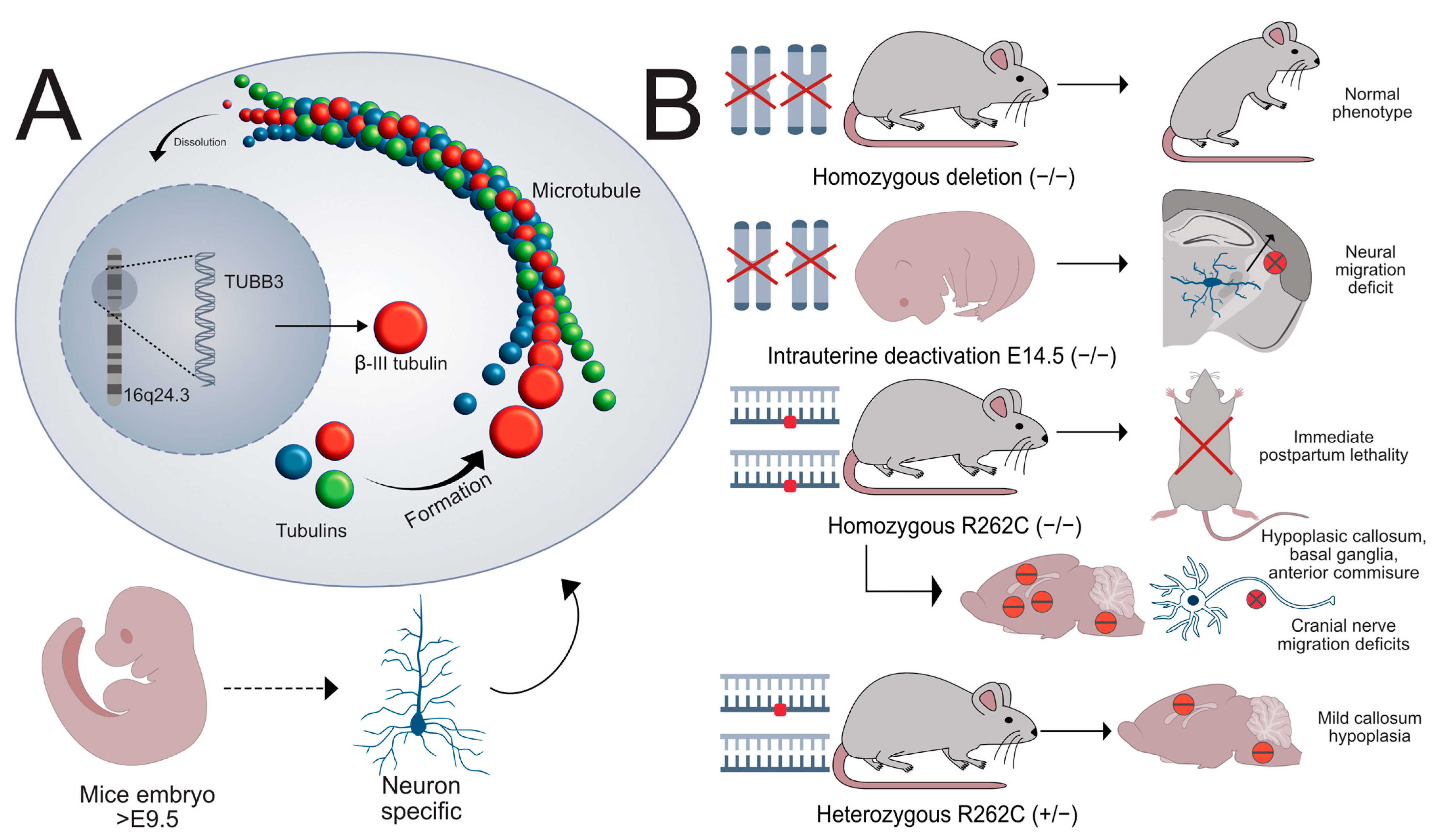
2.3. The Role of TUBB3 in Disease Pathogenesis in Human and Animal Models
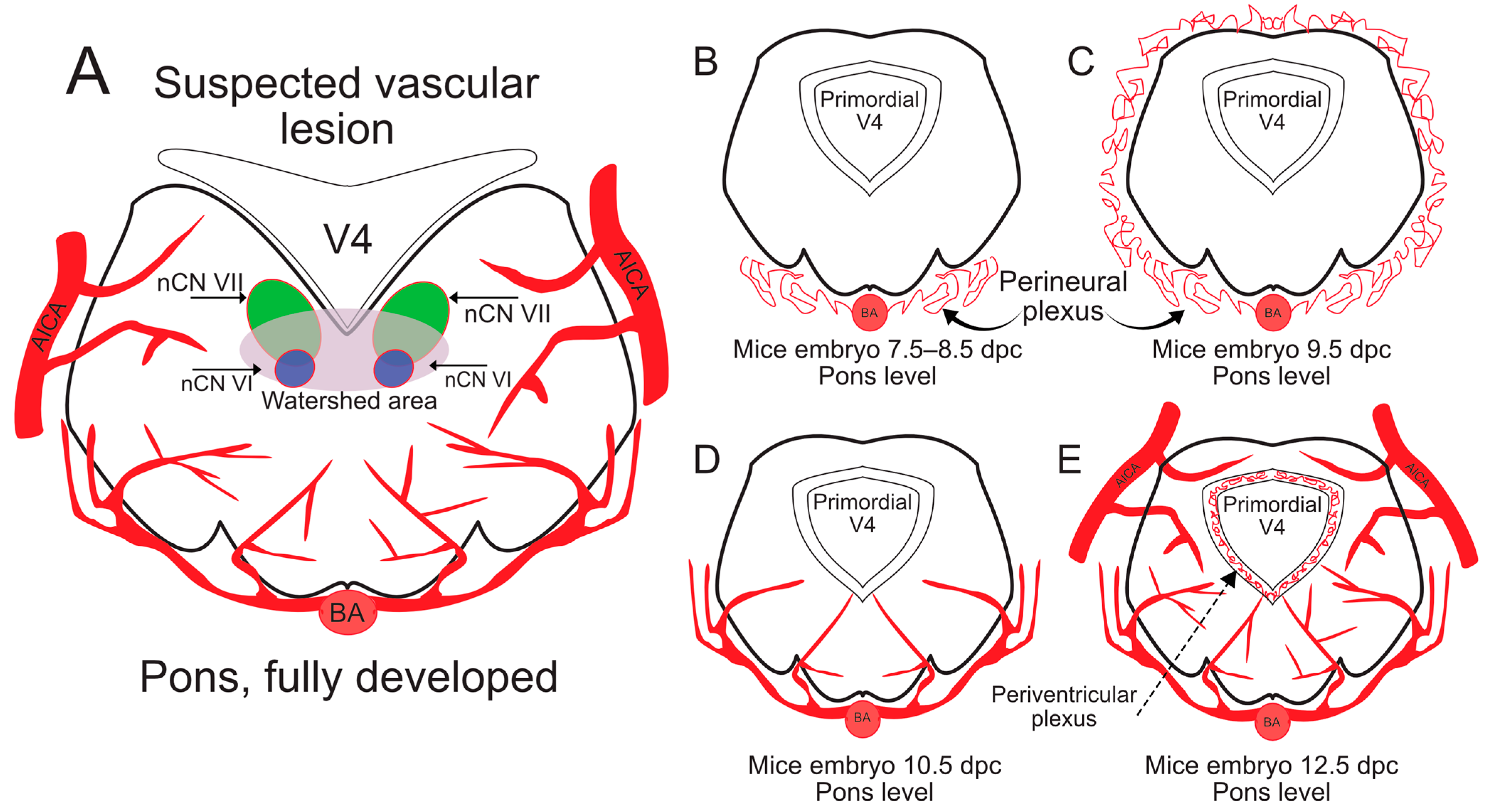
2.4. Animal Models for Vascular Congenital Disorders of the Cranial Nerves
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MBS | Moebius syndrome. |
| LOF | Loss of function. |
| DPC | Days post coitum. |
References
- Bell, C.; Nevitt, S.; McKay, V.H.; Fattah, A.Y. Will the real Moebius syndrome please stand up? A systematic review of the literature and statistical cluster analysis of clinical features. Am. J. Med. Genet. Part A 2019, 179, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.H.; Syed, I.N.; Tahir, U.; Noor, T.; Choudhry, M.S. Moebius Syndrome: What We Know So Far. Cureus 2023, 15, e35187. [Google Scholar] [CrossRef]
- Carta, A.; Favilla, S.; Calzetti, G.; Casalini, M.C.; Ferrari, P.F.; Bianchi, B.; Simonelli, M.B.; Farci, R.; Gandolfi, S.; Mora, P. The epidemiology of Moebius syndrome in Italy. Orphanet J. Rare Dis. 2021, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Picciolini, O.; Porro, M.; Cattaneo, E.; Castelletti, S.; Masera, G.; Mosca, F.; Bedeschi, M.F. Moebius syndrome: Clinical features, diagnosis, management and early intervention. Ital. J. Pediatr. 2016, 42, 56. [Google Scholar] [CrossRef]
- MacKinnon, S.; Oystreck, D.T.; Andrews, C.; Chan, W.-M.; Hunter, D.G.; Engle, E.C. Diagnostic Distinctions and Genetic Analysis of Patients Diagnosed with Moebius Syndrome. Ophthalmology 2014, 121, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Munell, F.; Tormos, M.A.; Roig-Quilis, M. Brainstem dysgenesis: Beyond Moebius syndrome. Rev. Neurol. 2018, 66, 241–250. [Google Scholar]
- Glass, G.E.; Mohammedali, S.; Sivakumar, B.; Stotland, M.A.; Abdulkader, F.; Prosser, D.O.; Love, D.R. Poland-Möbius syndrome: A case report implicating a novel mutation of the PLXND1 gene and literature review. BMC Pediatr. 2022, 22, 745. [Google Scholar] [CrossRef]
- Bavinck, J.N.; Weaver, D.D. Subclavian artery supply disruption sequence: Hypothesis of a vascular etiology for Poland, Klippel-Feil, and Möbius anomalies. Am. J. Med. Genet. 1986, 23, 903–918. [Google Scholar] [CrossRef]
- Charles, S.S.; Dimario, F.J., Jr.; Grunnet, M.L. Möbius sequence: Further in vivo support for the subclavian artery supply disruption sequence. Am. J. Med. Genet. 1993, 47, 289–293. [Google Scholar] [CrossRef]
- López Gutierrez, D.; Luna López, I.; Medina Mata, B.A.; Moreno Castro, S.; García Rangel, F.Y. Physiopathologic Bases of Moebius Syndrome: Combining Genetic, Vascular, and Teratogenic Theories. Pediatr. Neurol. 2024, 153, 1–10. [Google Scholar] [CrossRef]
- Lammens, M.; Moerman, P.; Fryns, J.; Schröder, J.; Spinnewyn, D.; Casaer, P.; Dom, R. Neuropathological findings in Moebius syndrome. Clin. Genet. 1998, 54, 136–141. [Google Scholar] [CrossRef]
- Monawwer, S.A.; Ali, S.; Naeem, R.; Ali, S.H.; Rabbani, A.; Khan, M.; Qazi, S.S.; Shah, S.M.I.; Farooqui, S.K. Moebius Syndrome: An Updated Review of Literature. Child Neurol. Open 2023, 10, 2329048X231205405. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.A.K.A.; Maher, H.; Kasem, M.A.; Helmy, E. Imaging of congenital cranial dysinnervation disorders: What radiologist wants to know? Clin. Imaging. 2021, 71, 106–116. [Google Scholar] [CrossRef]
- Tomas-Roca, L.; Tsaalbi-Shtylik, A.; Jansen, J.G.; Singh, M.K.; Epstein, J.A.; Altunoglu, U.; Verzijl, H.; Soria, L.; van Beusekom, E.; Roscioli, T.; et al. De novo mutations in PLXND1 and REV3L cause Möbius syndrome. Nat. Commun. 2015, 6, 7199. [Google Scholar] [CrossRef] [PubMed]
- Halas, A.; Fijak-Moskal, J.; Kuberska, R.; Murcia Pienkowski, V.; Kaniak-Golik, A.; Pollak, A.; Poznanski, J.; Rydzanicz, M.; Bik-Multanowski, M.; Sledziewska-Gojska, E.; et al. Developmental delay with hypotrophy associated with homozygous functionally relevant REV3L variant. J. Mol. Med. 2021, 99, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Moresco, G.; Bedeschi, M.F.; Venturin, M.; Villa, R.; Costanza, J.; Mauri, A.; Santaniello, C.; Picciolini, O.; Messina, L.; Triulzi, F.; et al. Exploring the Impact of Genetics in a Large Cohort of Moebius Patients by Trio Whole Exome Sequencing. Genes 2024, 15, 971. [Google Scholar] [CrossRef]
- Whitman, M.C.; Barry, B.J.; Robson, C.D.; Facio, F.M.; Van Ryzin, C.; Chan, W.-M.; Lehky, T.J.; Thurm, A.; Zalewski, C.; King, K.A.; et al. TUBB3 Arg262His causes a recognizable syndrome including CFEOM3, facial palsy, joint contractures, and early-onset peripheral neuropathy. Hum. Genet. 2021, 140, 1709–1731. [Google Scholar] [CrossRef]
- Whitman, M.C.; Andrews, C.; Chan, W.-M.; Tischfield, M.A.; Stasheff, S.F.; Brancati, F.; Ortiz-Gonzalez, X.; Nuovo, S.; Garaci, F.; MacKinnon, S.E.; et al. Two unique TUBB3 mutations cause both CFEOM3 and malformations of cortical development. Am. J. Med. Genet. Part A 2016, 170, 297–305. [Google Scholar] [CrossRef]
- Gene [Internet]; National Library of Medicine (US) National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 5 March 2025).
- LaMantia, A.-S. The changing Brain. In Neuroscience, 6th ed.; Purves, D., Augustine, G.J., Fitzpatrick, D., Hall, W.C., LaMantia, A.-S., Mooney, R.D., Platt, M.L., White, L.E., Hayden, B., Wang, F., Eds.; Oxford University Press: Sunderland, MA, USA, 2018; pp. 521–550. [Google Scholar]
- Wang, F.; Eagleson, K.L.; Levitt, P. Positive regulation of neocortical synapse formation by the Plexin-D1 receptor. Brain Res. 2015, 1616, 157–165. [Google Scholar] [CrossRef][Green Version]
- Fukuhara, K.; Imai, F.; Ladle, D.R.; Katayama, K.I.; Leslie, J.R.; Arber, S.; Jessell, T.M.; Yoshida, Y. Specificity of Monosynaptic Sensory-Motor Connections Imposed by Repellent Sema3E-PlexinD1 Signaling. Cell Rep. 2013, 5, 748–758. [Google Scholar] [CrossRef]
- Martins, L.F.; Brambilla, I.; Motta, A.; de Pretis, S.; Bhat, G.P.; Badaloni, A.; Malpighi, C.; Amin, N.D.; Imai, F.; Almeida, R.D.; et al. Motor neurons use push-pull signals to direct vascular remodeling critical for their connectivity. Neuron 2022, 110, 4090–4107. [Google Scholar] [CrossRef] [PubMed]
- Wälchli, T.; Bisschop, J.; Carmeliet, P.; Zadeh, G.; Monnier, P.P.; De Bock, K.; Radovanovic, I. Shaping the brain vasculature in development and disease in the single-cell era. Nat. Rev. Neurosci. 2023, 24, 271–298. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Pang, K.-L.; Rozbesky, D.; Nather, K.; Keen, A.; Lachowski, D.; Kong, Y.; Karia, D.; Ameismeier, M.; Huang, J.; et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 2020, 578, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Lu, M.M.; Epstein, J.A. PlexinD1 and Semaphorin Signaling Are Required in Endothelial Cells for Cardiovascular Development. Dev. Cell 2004, 7, 107–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Singh, M.K.; Degenhardt, K.R.; Lu, M.M.; Bennett, J.; Yoshida, Y.; Epstein, J.A. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev. Biol. 2009, 325, 82–93. [Google Scholar] [CrossRef]
- Kanda, T.; Yoshida, Y.; Izu, Y.; Nifuji, A.; Ezura, Y.; Nakashima, K.; Noda, M. PlexinD1 deficiency induces defects in axial skeletal morphogenesis. J. Cell. Biochem. 2007, 101, 1329–1337. [Google Scholar] [CrossRef]
- Menezes, M.R.; Sweasy, J.B. Mouse models of DNA polymerases. Environ. Mol. Mutagen. 2012, 53, 645–665. [Google Scholar] [CrossRef]
- Martin, S.K.; Wood, R.D. DNA polymerase ζ in DNA replication and repair. Nucleic Acids Res. 2019, 47, 8348–8361. [Google Scholar] [CrossRef]
- Wittschieben, J.; Shivji, M.K.K.; Lalani, E.; Jacobs, M.A.; Marini, F.; Gearhart, P.J.; Rosewell, I.; Stamp, G.; Wood, R.D. Disruption of the developmentally regulated Rev3l gene causes embryonic lethality. Curr. Biol. 2000, 10, 1217–1220. [Google Scholar] [CrossRef]
- Martin, S.K.; Tomida, J.; Wood, R.D. Disruption of DNA polymerase ζ engages an innate immune response. Cell Rep. 2021, 34, 108775. [Google Scholar] [CrossRef]
- Fritzen, R.; Delbos, F.; De Smet, A.; Palancade, B.; Canman, C.E.; Aoufouchi, S.; Weill, J.C.; Reynaud, C.A.; Storck, S. A single aspartate mutation in the conserved catalytic site of Rev3L generates a hypomorphic phenotype in vivo and in vitro. DNA Repair 2016, 46, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Tanaka, H.; Knudsen, B.S.; Rutgers, J.K. Mutant POLQ and POLZ/REV3L DNA polymerases may contribute to the favorable survival of patients with tumors with POLE mutations outside the exonuclease domain. BMC Med. Genet. 2020, 21, 167. [Google Scholar] [CrossRef]
- Latremoliere, A.; Cheng, L.; DeLisle, M.; Wu, C.; Chew, S.; Hutchinson, E.B.; Sheridan, A.; Alexandre, C.; Latremoliere, F.; Sheu, S.-H.; et al. Neuronal-Specific TUBB3 Is Not Required for Normal Neuronal Function but Is Essential for Timely Axon Regeneration. Cell Rep. 2018, 24, 1865–1879.e1869. [Google Scholar] [CrossRef]
- Duly, A.M.P.; Kao, F.C.L.; Teo, W.S.; Kavallaris, M. βIII-Tubulin Gene Regulation in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 851542. [Google Scholar] [CrossRef] [PubMed]
- Tischfield, M.A.; Baris, H.N.; Wu, C.; Rudolph, G.; Van Maldergem, L.; He, W.; Chan, W.-M.; Andrews, C.; Demer, J.L.; Robertson, R.L.; et al. Human TUBB3 Mutations Perturb Microtubule Dynamics, Kinesin Interactions, and Axon Guidance. Cell 2010, 140, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Puri, D.; Barry, B.J.; Engle, E.C. TUBB3 and KIF21A in neurodevelopment and disease. Front. Neurosci. 2023, 17, 1226181. [Google Scholar] [CrossRef]
- Huang, H.; Yang, T.; Shao, Q.; Majumder, T.; Mell, K.; Liu, G. Human TUBB3 Mutations Disrupt Netrin Attractive Signaling. Neuroscience 2018, 374, 155–171. [Google Scholar] [CrossRef]
- Saillour, Y.; Broix, L.; Bruel-Jungerman, E.; Lebrun, N.; Muraca, G.; Rucci, J.; Poirier, K.; Belvindrah, R.; Francis, F.; Chelly, J. Beta tubulin isoforms are not interchangeable for rescuing impaired radial migration due to Tubb3 knockdown. Hum. Mol. Genet. 2013, 23, 1516–1526. [Google Scholar] [CrossRef]
- Poirier, K.; Saillour, Y.; Bahi-Buisson, N.; Jaglin, X.H.; Fallet-Bianco, C.; Nabbout, R.; Castelnau-Ptakhine, L.; Roubertie, A.; Attie-Bitach, T.; Desguerre, I.; et al. Mutations in the neuronal β-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 2010, 19, 4462–4473. [Google Scholar] [CrossRef]
- Jin, S.; Park, S.-E.; Won, D.; Lee, S.-T.; Han, S.-H.; Han, J. TUBB3 M323V Syndrome Presents with Infantile Nystagmus. Genes 2021, 12, 575. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, Y.; Sun, G.; Wang, Z.; Chen, J.; Zhang, X.; Zhao, J.; Zhu, X.; Dai, J.; Liu, Z.; et al. Exosomal TUBB3 mRNA expression of metastatic castration-resistant prostate cancer patients: Association with patient outcome under abiraterone. Cancer Med. 2021, 10, 6282–6290. [Google Scholar] [CrossRef]
- Levallet, G.; Bergot, E.; Antoine, M.; Creveuil, C.; Santos, A.O.; Beau-Faller, M.; de Fraipont, F.; Brambilla, E.; Levallet, J.; Morin, F.; et al. High TUBB3 Expression, an Independent Prognostic Marker in Patients with Early Non–Small Cell Lung Cancer Treated by Preoperative Chemotherapy, Is Regulated by K-Ras Signaling Pathway. Mol. Cancer Ther. 2012, 11, 1203–1213. [Google Scholar] [CrossRef]
- Sekino, Y.; Han, X.; Babasaki, T.; Miyamoto, S.; Kitano, H.; Kobayashi, G.; Goto, K.; Inoue, S.; Hayashi, T.; Teishima, J.; et al. TUBB3 Is Associated with High-Grade Histology, Poor Prognosis, p53 Expression, and Cancer Stem Cell Markers in Clear Cell Renal Cell Carcinoma. Oncology 2020, 98, 689–698. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Dong, J.; Miao, Y. TUBB3 Promotes Growth and Invasion of Gallbladder Cancer Cells by Akt/mTOR Signal Pathway. 2021; 40, 23–33. [Google Scholar] [CrossRef]
- Kanojia, D.; Morshed, R.A.; Zhang, L.; Miska, J.M.; Qiao, J.; Kim, J.W.; Pytel, P.; Balyasnikova, I.V.; Lesniak, M.S.; Ahmed, A.U. βIII-Tubulin Regulates Breast Cancer Metastases to the Brain. Mol. Cancer Ther. 2015, 14, 1152–1161. [Google Scholar] [CrossRef]
- Lyulcheva-Bennett, E.; Blumenow, W.; O’Connor, A.; Kelly, M.; Bennett, D.; Fattah, A. Towards an understanding of the aetiology, genomic landscape and management of Moebius syndrome. J. Transl. Genet. Genom. 2023, 7, 259–273. [Google Scholar] [CrossRef]
- Sarnat, H.B. Watershed infarcts in the fetal and neonatal brainstem. An aetiology of central hypoventilation, dysphagia, Möbius syndrome and micrognathia. Eur. J. Paediatr. Neurol. 2004, 8, 71–87. [Google Scholar] [CrossRef]
- Lipson, A.H.; Webster, W.S.; Brown-Woodman, P.D.C.; Osborn, R.A. Moebius syndrome: Animal model—Human correlations and evidence for a brainstem vascular etiology. Teratology 1989, 40, 339–350. [Google Scholar] [CrossRef]
- Paulsen, F. Brainsteam. In Sobotta Anatomy Textbook English Edition with Latin Nomenclature; Paulsen, F., Waschke, J., Böckers, T.M., Eds.; Elsevier: Munich, Germany, 2019; pp. 664–673. [Google Scholar]
- Schröder, H.; Moser, N.; Huggenberger, S. The Mouse Circle of Willis. In Neuroanatomy of the Mouse: An Introduction; Springer International Publishing: Cham, Switzerland, 2020; pp. 333–340. [Google Scholar]
- Vallon, M.; Chang, J.; Zhang, H.; Kuo, C.J. Developmental and pathological angiogenesis in the central nervous system. Cell. Mol. Life Sci. 2014, 71, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Tata, M.; Ruhrberg, C.; Fantin, A. Vascularisation of the central nervous system. Mech. Dev. 2015, 138, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Berrios-Otero, C.A.; Wadghiri, Y.Z.; Nieman, B.J.; Joyner, A.L.; Turnbull, D.H. Three-dimensional micro-MRI analysis of cerebral artery development in mouse embryos. Magn. Reson. Med. 2009, 62, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Maeda, R.; Shoji, W.; Wada, H.; Masai, I.; Shiraki, T.; Kobayashi, M.; Nakayama, R.; Okamoto, H. Novel mutations affecting axon guidance in zebrafish and a role for plexin signalling in the guidance of trigeminal and facial nerve axons. Development 2007, 134, 3259–3269. [Google Scholar] [CrossRef]
- Peterson, R.G. Vascularization and basement membranes of the chick brain in light microscopy. Anat. Rec. 1968, 161, 37–43. [Google Scholar] [CrossRef]
- Roy, S.; Hirano, A.; Kochen, J.A.; Zimmerman, H.M. The fine structure of cerebral blood vessels in chick embryo. Acta Neuropathol. 1974, 30, 277–285. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Yang, W.; Rubel, E.W. Normal and abnormal pathfinding of facial nerve fibers in the chick embryo. J. Neurobiol. 1992, 23, 1021–1036. [Google Scholar] [CrossRef]
- Molla, K.A.; Karmakar, S.; Islam, M.T. Wide Horizons of CRISPR-Cas-Derived Technologies for Basic Biology, Agriculture, and Medicine. In CRISPR-Cas Methods; Islam, M.T., Bhowmik, P.K., Molla, K.A., Eds.; Springer: New York, NY, USA, 2020; pp. 1–23. [Google Scholar]
- Kurz, H.; Gärtner, T.; Eggli, P.S.; Christ, B. First Blood Vessels in the Avian Neural Tube Are Formed by a Combination of Dorsal Angioblast Immigration and Ventral Sprouting of Endothelial Cells. Dev. Biol. 1996, 173, 133–147. [Google Scholar] [CrossRef]
- Kuratani, S.; Tanaka, S.; Ishikawa, Y.; Zukeran, C. Early development of the facial nerve in the chick embryo with special reference to the development of the chorda tympani. Am. J. Anat. 1988, 182, 169–182. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tracicaru, M.-P.; Tracicaru, R.-V.; Hînganu, D.; Hînganu, M.V. The Etiology of Moebius Syndrome—Making the Case for Animal Models. Int. J. Mol. Sci. 2025, 26, 4217. https://doi.org/10.3390/ijms26094217
Tracicaru M-P, Tracicaru R-V, Hînganu D, Hînganu MV. The Etiology of Moebius Syndrome—Making the Case for Animal Models. International Journal of Molecular Sciences. 2025; 26(9):4217. https://doi.org/10.3390/ijms26094217
Chicago/Turabian StyleTracicaru, Manuela-Petronela, Rareș-Vasile Tracicaru, Delia Hînganu, and Marius Valeriu Hînganu. 2025. "The Etiology of Moebius Syndrome—Making the Case for Animal Models" International Journal of Molecular Sciences 26, no. 9: 4217. https://doi.org/10.3390/ijms26094217
APA StyleTracicaru, M.-P., Tracicaru, R.-V., Hînganu, D., & Hînganu, M. V. (2025). The Etiology of Moebius Syndrome—Making the Case for Animal Models. International Journal of Molecular Sciences, 26(9), 4217. https://doi.org/10.3390/ijms26094217






