Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review
Abstract
1. Introduction
2. Tumor-Infiltrating Lymphocytes, Macrophages, and Fibroblasts: Key Players in the Cancer Immune Microenvironment
2.1. Tumor-Infiltrating Lymphocytes
2.2. Tumor-Associated Macrophages
2.3. Cancer-Associated Fibroblasts
3. Molecular Basis of Penile Cancer and the Inflammatory Process
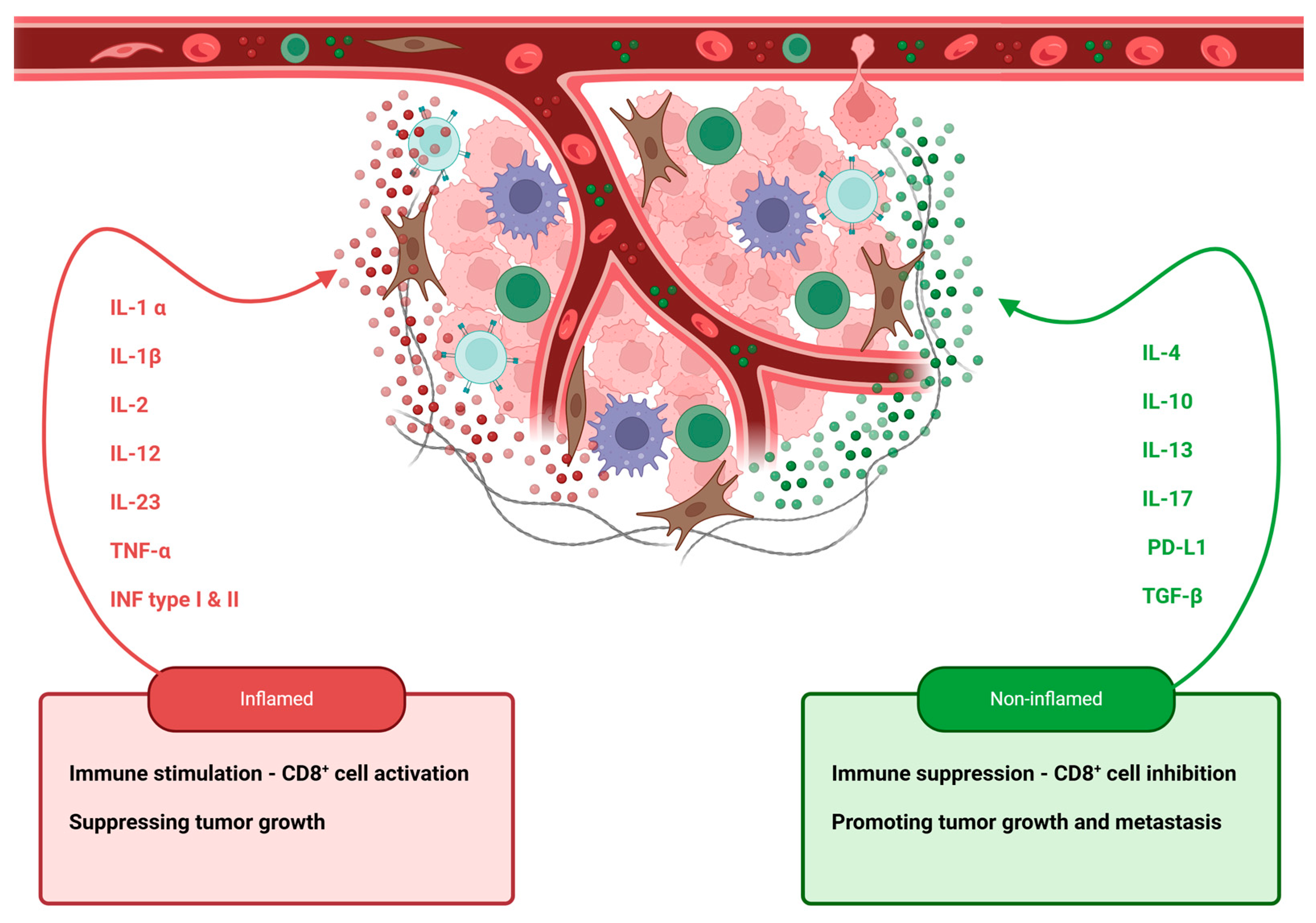
4. The Role of Pro-Inflammatory Cytokines and Chemokines in Penile Cancer Progression and Prognosis
4.1. IL-1 Family
4.2. IL-6
4.3. TGF-β
4.4. IFN-γ
4.5. Inflammasomes
4.6. Chemokines
5. Exploring NF-κB Pathway Activation and Its Implications
6. The Secreted Phosphoprotein 1 (SPP1) Gene: From Bone Mineralization to Penile Cancer Prognosis
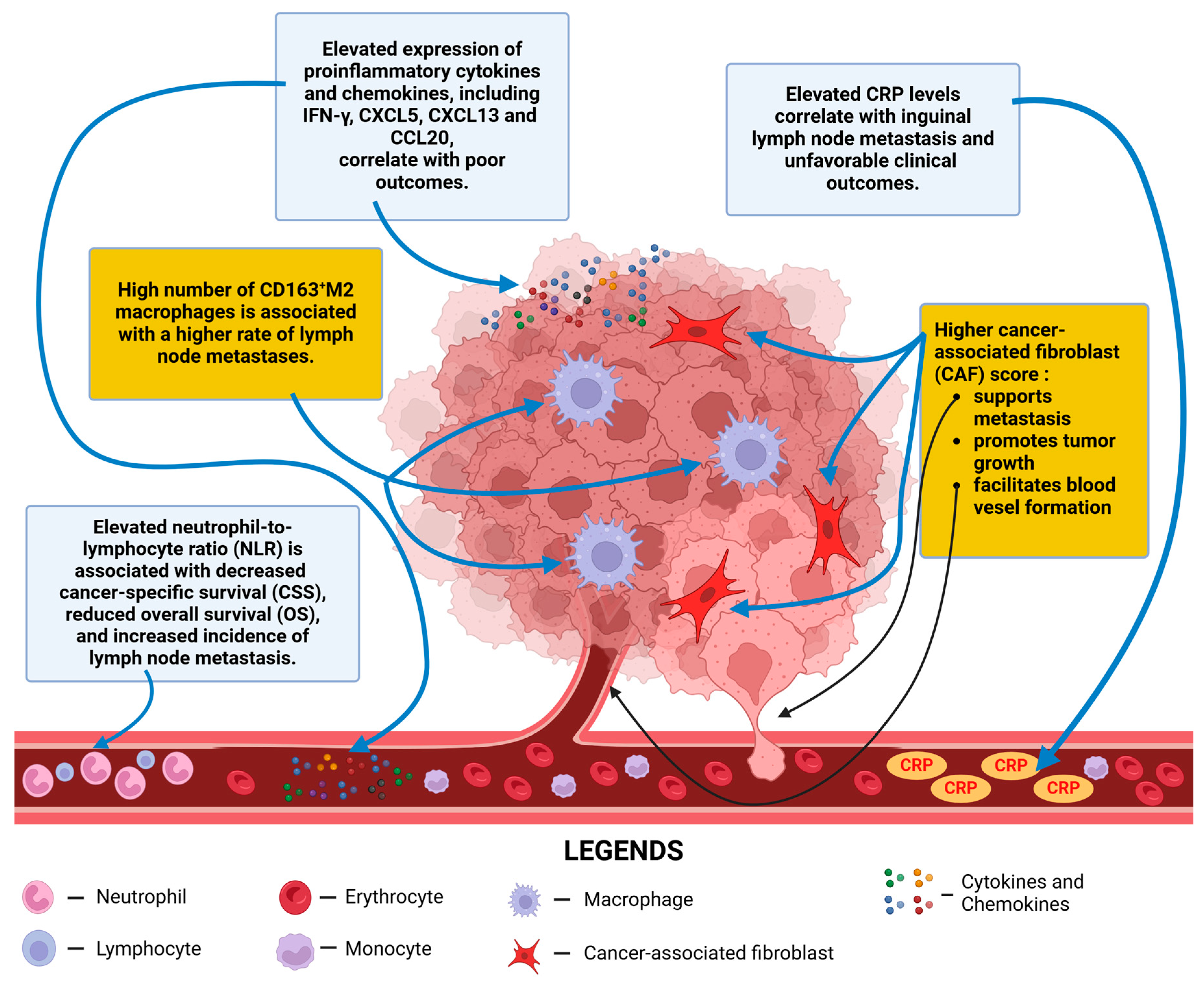
7. C-Reactive Protein as a Biomarker in Cancer: Implications for Penile Cancer Prognosis and Metastasis
8. The Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker in Penile Cancer
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIM2 | Absent in melanoma 2 |
| APCs | Antigen-presenting cells |
| BAFFR | B-cell-activating factor receptor |
| BCL2A1 | B-cell lymphoma-2-related protein A1 |
| BMI | Body mass index |
| C/EBP | CCAAT/enhancer-binding protein |
| CAFs | Cancer-associated fibroblasts |
| CCL | C-C motif chemokine ligand |
| CD | Cluster of differentiation |
| CI | Confidence interval |
| CR | Complete response |
| CRP | C-reactive protein |
| CSF | Colony-stimulating factor |
| CSS | Cancer-specific survival |
| CTLA-4 | Cytotoxic T-lymphocyte antigen-4 |
| CXCL | C-X-C motif chemokine ligand |
| DAMPs | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| DCR | Disease control rate |
| DFS | Disease-free survival |
| DKK1 | Dickkopf-related protein 1 |
| DNA | Deoxyribonucleic acid |
| DSS | Disease-specific survival |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| FAP | Fibroblast activation protein |
| FIGO | International Federation of Gynecology and Obstetrics |
| FOXP3 | Forkhead box P3 |
| FRCs | Fibroblastic reticular cells |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papillomavirus |
| ICI | Immune checkpoint inhibitors |
| IHC | Immunohistochemistry |
| IKK | Inhibitor of NF-κB kinase |
| IL | Interleukin |
| INF | Interferon |
| LTβR | Lymphotoxin-beta receptor |
| MHC | Major histocompatibility complex |
| MMP | Matrix metalloproteinase |
| NEMO | NF-kappa-B essential modulator |
| NF-κB | Nuclear factor-kappa B |
| NK | Natural killer |
| NLR | Neutrophil-to-lymphocyte ratio |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| NMIBC | Non-muscle-invasive bladder cancer |
| NO | Nitric oxide |
| NPR | Non-progression rate |
| ORR | Overall response rate |
| OS | Overall survival |
| PAMPs | Pathogen-associated molecular patterns |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death ligand 1 |
| PeCa | Penile cancer |
| PFS | Progression-free survival |
| PGE2 | Prostaglandin E2 |
| PSCC | Penile squamous cell carcinoma |
| qPCR | Quantitative polymerase chain reaction |
| RANK | Receptor activator of nuclear factor Kappa-B |
| RCC | Renal cell carcinoma |
| RFS | Recurrence-free survival |
| ROS | Reactive oxygen species |
| SIRS | Systemic inflammatory response |
| SPP1 | Secreted phosphoprotein 1 |
| TAMs | Tumor-associated macrophages |
| TCC | Transitional cell carcinoma |
| TGF | Transforming growth factor |
| TILs | Tumor-infiltrating lymphocytes |
| TIME | Tumor immune microenvironment |
| TMB | Tumor mutation burden |
| TNF | Tumor necrosis factor |
| TNFR | Tumor necrosis factor receptor |
| Tregs | Regulatory T lymphocytes |
| VEGF | Vascular endothelial growth factor |
References
- Wnętrzak, I.; Czajkowski, M.; Barańska, K.; Miklewska, M.; Wojciechowska, U.; Sosnowski, R.; Didkowska, J.A. Epidemiology of Penile Cancer in Poland Compared to Other European Countries. Cancer Med. 2024, 13, e70092. [Google Scholar] [CrossRef] [PubMed]
- Favorito, L.A.; Nardi, A.C.; Ronalsa, M.; Zequi, S.C.; Sampaio, F.J.; Glina, S. Epidemiologic Study on Penile Cancer in Brazil. Int. Braz. J. Urol. 2008, 34, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Necchi, A.; Muneer, A.; Tobias-Machado, M.; Tran, A.T.H.; Van Rompuy, A.-S.; Spiess, P.E.; Albersen, M. Penile Cancer. Nat. Rev. Dis. Primers 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Engelsgjerd, J.S.; Leslie, S.W.; LaGrange, C.A. Penile Cancer and Penile Intraepithelial Neoplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, J.; Pettaway, C.A.; Pagliaro, L.C. Treatment for Metastatic Penile Cancer after First-Line Chemotherapy Failure: Analysis of Response and Survival Outcomes. Urology 2015, 85, 1104–1110. [Google Scholar] [CrossRef]
- Chu, C.; Yao, K.; Lu, J.; Zhang, Y.; Chen, K.; Lu, J.; Zhang, C.Z.; Cao, Y. Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers 2020, 12, 1796. [Google Scholar] [CrossRef]
- Sachdeva, A.; McGuinness, L.; Zapala, L.; Greco, I.; Garcia-Perdomo, H.A.; Kailavasan, M.; Antunes-Lopes, T.; Ayres, B.; Barreto, L.; Campi, R. Management of Lymph Node–Positive Penile Cancer: A Systematic Review. Eur. Urol. 2024, 85, 257–273. [Google Scholar] [CrossRef]
- Leibovici, D.; Grossman, H.B.; Dinney, C.P.; Millikan, R.E.; Lerner, S.; Wang, Y.; Gu, J.; Dong, Q.; Wu, X. Polymorphisms in Inflammation Genes and Bladder Cancer: From Initiation to Recurrence, Progression, and Survival. J. Clin. Oncol. 2005, 23, 5746–5756. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.-J. Emerging Avenues Linking Inflammation and Cancer. Free Radic. Biol. Med. 2012, 52, 2013–2037. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Jóźwiak, K.; Heeren, A.M.; De Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front. Immunol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Catalano, M.; Roviello, G.; Santi, R.; Villari, D.; Spatafora, P.; Galli, I.C.; Sessa, F.; Conte, F.L.; Mini, E.; Cai, T. Inflammation in Urological Malignancies: The Silent Killer. Int. J. Mol. Sci. 2023, 24, 866. [Google Scholar] [CrossRef]
- Botelho, M.C.; Oliveira, P.A.; Lopes, C.; da Costa, J.M.C.; Machado, J.C. Urothelial Dysplasia and Inflammation Induced by Schistosoma Haematobium Total Antigen Instillation in Mice Normal Urothelium. In Proceedings of the Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2011; Volume 29, pp. 809–814. [Google Scholar]
- Shi, J.; Wang, K.; Xiong, Z.; Yuan, C.; Wang, C.; Cao, Q.; Yu, H.; Meng, X.; Xie, K.; Cheng, Z.; et al. Impact of Inflammation and Immunotherapy in Renal Cell Carcinoma (Review). Oncol. Lett. 2020, 20, 272. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.P.; Nayak, B.; Shanmugasundaram, K.; Friedrichs, W.; Sudarshan, S.; Eid, A.A.; DeNapoli, T.; Parekh, D.J.; Gorin, Y.; Block, K. Nox4 Mediates Renal Cell Carcinoma Cell Invasion through Hypoxia-Induced Interleukin 6-and 8-Production. PLoS ONE 2012, 7, e30712. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, M.; Wierzbicki, P.; Kotulak-Chrząszcz, A.; Czajkowska, K.; Bolcewicz, M.; Kłącz, J.; Kreft, K.; Lewandowska, A.; Nedoszytko, B.; Sokołowska-Wojdyło, M.; et al. The Role of Occlusion and Micro-Incontinence in the Pathogenesis of Penile Lichen Sclerosus: An Observational Study of pro-Inflammatory Cytokines’ Gene Expression. Int. Urol. Nephrol. 2022, 54, 763–772. [Google Scholar] [CrossRef]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjær, S.K. Prevalence of Human Papillomavirus DNA and p16INK4a in Penile Cancer and Penile Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef]
- Wei, L.; Gravitt, P.E.; Song, H.; Maldonado, A.M.; Ozbun, M.A. Nitric Oxide Induces Early Viral Transcription Coincident with Increased DNA Damage and Mutation Rates in Human Papillomavirus–Infected Cells. Cancer Res. 2009, 69, 4878–4884. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Xu, D.; Zhuang, X.; Ma, H.; Li, Z.; Wei, L.; Luo, J.; Han, H. Altered Tumor Microenvironment Heterogeneity of Penile Cancer during Progression from Non-lymphatic to Lymphatic Metastasis. Cancer Med. 2024, 13, e70025. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Del Prete, A.; Schioppa, T.; Tiberio, L.; Stabile, H.; Sozzani, S. Leukocyte Trafficking in Tumor Microenvironment. Curr. Opin. Pharmacol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Gattinoni, L.; Restifo, N.P. CD8+ T-cell Memory in Tumor Immunology and Immunotherapy. Immunol. Rev. 2006, 211, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Niu, G.; Kortylewski, M.; Burdelya, L.; Shain, K.; Zhang, S.; Bhattacharya, R.; Gabrilovich, D.; Heller, R.; Coppola, D. Regulation of the Innate and Adaptive Immune Responses by Stat-3 Signaling in Tumor Cells. Nat. Med. 2004, 10, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When Worlds Collide: Th17 and Treg Cells in Cancer and Autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The Tumor Microenvironment at a Glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune Regulatory Function of B Cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef]
- Marcus, A.; Gowen, B.G.; Thompson, T.W.; Iannello, A.; Ardolino, M.; Deng, W.; Wang, L.; Shifrin, N.; Raulet, D.H. Recognition of Tumors by the Innate Immune System and Natural Killer Cells. Adv. Immunol. 2014, 122, 91–128. [Google Scholar]
- Pereira, M.A.; Ramos, M.F.K.P.; Cardili, L.; de Moraes, R.D.R.; Dias, A.R.; Szor, D.J.; Zilberstein, B.; Alves, V.A.F.; de Mello, E.S.; Ribeiro, U., Jr. Prognostic Implications of Tumor-Infiltrating Lymphocytes within the Tumor Microenvironment in Gastric Cancer. J. Gastrointest. Surg. 2024, 28, 151–157. [Google Scholar] [CrossRef]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-Infiltrating Lymphocytes: From Prognosis to Treatment Selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef]
- Sanders, C.; Hamad, A.S.M.; Ng, S.; Hosni, R.; Ellinger, J.; Klümper, N.; Ritter, M.; Stephan, C.; Jung, K.; Hölzel, M. CD103+ Tissue Resident T-Lymphocytes Accumulate in Lung Metastases and Are Correlated with Poor Prognosis in ccRCC. Cancers 2022, 14, 1541. [Google Scholar] [CrossRef]
- Lai, C.; Coltart, G.; Shapanis, A.; Healy, C.; Alabdulkareem, A.; Selvendran, S.; Theaker, J.; Sommerlad, M.; Rose-Zerilli, M.; Al-Shamkhani, A. CD8+ CD103+ Tissue-Resident Memory T Cells Convey Reduced Protective Immunity in Cutaneous Squamous Cell Carcinoma. J. Immunother. Cancer 2021, 9, e001807. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T Cells in Tumor Immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef]
- Vassallo, J.; Rodrigues, A.F.F.; Campos, A.H.J.; Rocha, R.M.; da Cunha, I.W.; Zequi, S.C.; Guimarães, G.C.; da Fonseca, F.P.; Lopes, A.; Cubilla, A. Pathologic and Imunohistochemical Characterization of Tumoral Inflammatory Cell Infiltrate in Invasive Penile Squamous Cell Carcinomas: Fox-P3 Expression Is an Independent Predictor of Recurrence. Tumor Biol. 2015, 36, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Hladek, L.; Bankov, K.; Von Der Grün, J.; Filmann, N.; Demes, M.; Vallo, S.; Wild, P.J.; Winkelmann, R. Tumor-Associated Immune Cell Infiltrate Density in Penile Squamous Cell Carcinomas. Virchows Arch. 2022, 480, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Zhao, C.; Zhang, S.; Zhu, J. Recent Advancement of PD-L1 Detection Technologies and Clinical Applications in the Era of Precision Cancer Therapy. J. Cancer 2023, 14, 850. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Hui, R.; Garon, E.B.; Goldman, J.W.; Leighl, N.B.; Hellmann, M.D.; Patnaik, A.; Gandhi, L.; Eder, J.P.; Ahn, M.-J.; Horn, L. Pembrolizumab as First-Line Therapy for Patients with PD-L1-Positive Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. Ann. Oncol. 2017, 28, 874–881. [Google Scholar] [CrossRef]
- De Vries, H.M.; Rafael, T.S.; Gil-Jimenez, A.; De Feijter, J.M.; Bekers, E.; Van Der Laan, E.; Lopez-Yurda, M.; Hooijberg, E.; Broeks, A.; Peters, D.; et al. Atezolizumab With or Without Radiotherapy for Advanced Squamous Cell Carcinoma of the Penis (The PERICLES Study): A Phase II Trial. J. Clin. Oncol. 2023, 41, 4872–4880. [Google Scholar] [CrossRef]
- Del Muro, X.G.; López-Bravo, D.P.; Cuéllar-Rivas, M.A.; Maroto, P.; Giannatempo, P.; Castellano, D.; Climent, M.A.; Valderrama, B.P.; de Liaño, A.G.; López-Montero, L. Retifanlimab in Advanced Penile Squamous Cell Carcinoma: The Phase 2 ORPHEUS Study. Eur. Urol. Oncol. 2024; in press. [Google Scholar]
- Apolo, A.B.; Girardi, D.M.; Niglio, S.A.; Nadal, R.; Kydd, A.R.; Simon, N.; Ley, L.; Cordes, L.M.; Chandran, E.; Steinberg, S.M.; et al. Final Results from a Phase I Trial and Expansion Cohorts of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced/Metastatic Genitourinary Tumors. J. Clin. Oncol. 2024, 42, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Rouvinov, K.; Mazor, G.; Kozlener, E.; Meirovitz, A.; Shrem, N.S.; Abu Saleh, O.; Shalata, S.; Yakobson, A.; Shalata, W. Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience. J. Pers. Med. 2023, 13, 1623. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Foulstone, E.; Ashurst, L.; Renninson, E.; White, P.; Bravo, A.; Afshar, M.; Alifrangis, C.; Venugopal, B.; Thomson, A.; et al. A Phase II Trial of Cemiplimab Alone or in Combination with Standard of Care Chemotherapy in Locally Advanced or Metastatic Penile Carcinoma (EPIC Trial). J. Clin. Oncol. 2024, 42, TPS14. [Google Scholar] [CrossRef]
- McGregor, B.A.; Campbell, M.T.; Xie, W.; Farah, S.; Bilen, M.A.; Schmidt, A.L.; Sonpavde, G.P.; Kilbridge, K.L.; Choudhury, A.D.; Mortazavi, A.; et al. Results of a Multicenter, Phase 2 Study of Nivolumab and Ipilimumab for Patients with Advanced Rare Genitourinary Malignancies. Cancer 2021, 127, 840–849. [Google Scholar] [CrossRef]
- Naing, A.; Meric-Bernstam, F.; Stephen, B.; Karp, D.D.; Hajjar, J.; Ahnert, J.R.; Piha-Paul, S.A.; Colen, R.R.; Jimenez, C.; Raghav, K.P. Phase 2 Study of Pembrolizumab in Patients with Advanced Rare Cancers. J. Immunother. Cancer 2020, 8, e000347. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and Macrophages: Developmental Pathways and Tissue Homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Wu, L.; Zhang, T.; Wu, J.; Li, L.; Tai, Z.; Chen, Z.; Zhu, Q. Targeting Tumor-Associated Macrophages: Novel Insights into Immunotherapy of Skin Cancer. J. Adv. Res. 2025, 67, 231–252. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Allison, E.; Edirimanne, S.; Matthews, J.; Fuller, S.J. Breast Cancer Survival Outcomes and Tumor-Associated Macrophage Markers: A Systematic Review and Meta-Analysis. Oncol. Ther. 2023, 11, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhan, J.; Guan, Z.; Zhang, J.; Li, T.; Zhong, L.; Zhang, C.; Li, M. Clinicopathologic and Prognostic Significance of Tumor-Associated Macrophages in Cervical Cancer: A Systematic Review and Meta-Analysis. Clin. Transl. Oncol. 2025, 27, 351–362. [Google Scholar] [CrossRef]
- Bisheshar, S.K.; van der Kamp, M.F.; de Ruiter, E.J.; Ruiter, L.N.; van der Vegt, B.; Breimer, G.E.; Willems, S.M. The Prognostic Role of Tumor Associated Macrophages in Squamous Cell Carcinoma of the Head and Neck: A Systematic Review and Meta-Analysis. Oral. Oncol. 2022, 135, 106227. [Google Scholar] [CrossRef]
- De Palma, M.; Murdoch, C.; Venneri, M.A.; Naldini, L.; Lewis, C.E. Tie2-Expressing Monocytes: Regulation of Tumor Angiogenesis and Therapeutic Implications. Trends Immunol. 2007, 28, 519–524. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose Receptor (CD206) Activation in Tumor-Associated Macrophages Enhances Adaptive and Innate Antitumor Immune Responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Hay, E.D. An Overview of Epithelio-Mesenchymal Transformation. Cells Tissues Organs 1995, 154, 8–20. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Avery, D.; Govindaraju, P.; Jacob, M.; Todd, L.; Monslow, J.; Puré, E. Extracellular Matrix Directs Phenotypic Heterogeneity of Activated Fibroblasts. Matrix Biol. 2018, 67, 90–106. [Google Scholar] [CrossRef]
- Knops, A.M.; South, A.; Rodeck, U.; Martinez-Outschoorn, U.; Harshyne, L.A.; Johnson, J.; Luginbuhl, A.J.; Curry, J.M. Cancer-Associated Fibroblast Density, Prognostic Characteristics, and Recurrence in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2020, 10, 565306. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-Associated Fibroblasts Induce Epithelial–Mesenchymal Transition of Bladder Cancer Cells through Paracrine IL-6 Signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- López, J.I.; Errarte, P.; Erramuzpe, A.; Guarch, R.; Cortés, J.M.; Angulo, J.C.; Pulido, R.; Irazusta, J.; Llarena, R.; Larrinaga, G. Fibroblast Activation Protein Predicts Prognosis in Clear Cell Renal Cell Carcinoma. Hum. Pathol. 2016, 54, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Errarte, P.; Guarch, R.; Pulido, R.; Blanco, L.; Nunes-Xavier, C.E.; Beitia, M.; Gil, J.; Angulo, J.C.; Lopez, J.I.; Larrinaga, G. The Expression of Fibroblast Activation Protein in Clear Cell Renal Cell Carcinomas Is Associated with Synchronous Lymph Node Metastases. PLoS ONE 2016, 11, e0169105. [Google Scholar] [CrossRef] [PubMed]
- Cury, S.S.; Kuasne, H.; Souza, J.D.S.; Muñoz, J.J.M.; da Silva, J.P.; Lopes, A.; Scapulatempo-Neto, C.; Faria, E.F.; Delaissé, J.-M.; Marchi, F.A. Interplay Between Immune and Cancer-Associated Fibroblasts: A Path to Target Metalloproteinases in Penile Cancer. Front. Oncol. 2022, 12, 935093. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Miralles-Guri, C.; Bruni, L.; Cubilla, A.L.; Castellsague, X.; Bosch, F.X.; De Sanjose, S. Human Papillomavirus Prevalence and Type Distribution in Penile Carcinoma. J. Clin. Pathol. 2009, 62, 870–878. [Google Scholar] [CrossRef]
- Vanthoor, J.; Vos, G.; Albersen, M. Penile Cancer: Potential Target for Immunotherapy? World J. Urol. 2021, 39, 1405–1411. [Google Scholar] [CrossRef]
- Eich, M.-L.; Pena, M.D.C.R.; Schwartz, L.; Granada, C.P.; Rais-Bahrami, S.; Giannico, G.; Amador, B.M.; Matoso, A.; Gordetsky, J.B. Morphology, P16, HPV, and Outcomes in Squamous Cell Carcinoma of the Penis: A Multi-Institutional Study. Hum. Pathol. 2020, 96, 79–86. [Google Scholar] [CrossRef]
- Kayes, O.; Ahmed, H.U.; Arya, M.; Minhas, S. Molecular and Genetic Pathways in Penile Cancer. Lancet Oncol. 2007, 8, 420–429. [Google Scholar] [CrossRef]
- Stankiewicz, E.; Prowse, D.M.; Ng, M.; Cuzick, J.; Mesher, D.; Hiscock, F.; Lu, Y.-J.; Watkin, N.; Corbishley, C.; Lam, W. Alternative HER/PTEN/Akt Pathway Activation in HPV Positive and Negative Penile Carcinomas. PLoS ONE 2011, 6, e17517. [Google Scholar] [CrossRef]
- Ferrandiz-Pulido, C.; Masferrer, E.; Toll, A.; Hernandez-Losa, J.; Mojal, S.; Pujol, R.M.; Ramon y Cajal, S.; De Torres, I.; Garcia-Patos, V. mTOR Signaling Pathway in Penile Squamous Cell Carcinoma: pmTOR and peIF4E Over Expression Correlate with Aggressive Tumor Behavior. J. Urol. 2013, 190, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- De Paula, A.A.P.; Motta, E.D.; Alencar, R.D.C.; Saddi, V.A.; Da Silva, R.C.; Caixeta, G.N.; Almeida Netto, J.C.; Carneiro, M.A.D.S. The Impact of Cyclooxygenase-2 and Vascular Endothelial Growth Factor C Immunoexpression on the Prognosis of Penile Carcinoma. J. Urol. 2012, 187, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.; Nettleton, J.; Watkin, N.; Berney, D.M. The Molecular Pathogenesis of Penile Carcinoma—Current Developments and Understanding. Virchows Arch. 2019, 475, 397–405. [Google Scholar] [CrossRef]
- Tsikouras, P.; Zervoudis, S.; Manav, B.; Tomara, E.; Iatrakis, G.; Romanidis, C.; Bothou, A.; Galazios, G. Cervical Cancer: Screening, Diagnosis and Staging. J. Buon 2016, 21, 320–325. [Google Scholar]
- Rasmussen, C.L.; Sand, F.L.; Hoffmann Frederiksen, M.; Kaae Andersen, K.; Kjær, S.K. Does HPV Status Influence Survival after Vulvar Cancer? Int. J. Cancer 2018, 142, 1158–1165. [Google Scholar] [CrossRef]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between Human Papillomavirus and Penile Cancer—Implications for Prevention and Treatment. Transl. Androl. Urol. 2017, 6, 791. [Google Scholar] [CrossRef]
- Sand, F.L.; Rasmussen, C.L.; Frederiksen, M.H.; Andersen, K.K.; Kjaer, S.K. Prognostic Significance of HPV and P16 Status in Men Diagnosed with Penile Cancer: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1123–1132. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Moen, C.A.; Falkenthal, T.E.; Thorkelsen, T.K.; Hopland, A.; Rio, O.E.; Honoré, A.; Juliebø-Jones, P.; Dongre, H.N.; Costea, D.E.; Bostad, L. Penile Cancers Attributed to Human Papillomavirus Are Associated with Improved Survival for Node-Positive Patients. Findings from a Norwegian Cohort Study Spanning 50 Years. Eur. Urol. Oncol. 2024, 7, 778–785. [Google Scholar] [CrossRef]
- Powell, S.F.; Vu, L.; Spanos, W.C.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and-Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef]
- Necchi, A.; Spiess, P.E.; de Padua, T.C.; Li, R.; Grivas, P.; Huang, R.S.; Lin, D.I.; Danziger, N.; Ross, J.S.; Jacob, J.M. Genomic Profiles and Clinical Outcomes of Penile Squamous Cell Carcinoma with Elevated Tumor Mutational Burden. JAMA Netw. Open 2023, 6, e2348002. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T. Prognostic Significance of PD-L1+ and CD8+ Immune Cells in HPV+ Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef]
- Welters, M.J.; Ma, W.; Santegoets, S.J.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; Van Ham, V.J.; Van Unen, V.; Koning, F.; Van Egmond, S.I. Intratumoral HPV16-Specific T Cells Constitute a Type I–Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, L.; Olive, D. Tumor Associated Macrophage in HPV+ Tumors: Between Immunosuppression and Inflammation. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 65, p. 101671. [Google Scholar]
- Lohneis, P.; Boral, S.; Kaufmann, A.M.; Lehmann, A.; Schewe, C.; Dietel, M.; Anagnostopoulos, I.; Jöhrens, K. Human Papilloma Virus Status of Penile Squamous Cell Carcinoma Is Associated with Differences in Tumour-Infiltrating T Lymphocytes. Virchows Arch. 2015, 466, 323–331. [Google Scholar] [CrossRef]
- Lohse, S.; Mink, J.N.; Eckhart, L.; Hans, M.C.; Jusufi, L.; Zwick, A.; Mohr, T.; Bley, I.A.; Khalmurzaev, O.; Matveev, V.B. The Impact of the Tumor Microenvironment on the Survival of Penile Cancer Patients. Sci. Rep. 2024, 14, 22050. [Google Scholar] [CrossRef]
- Guimarães, S.J.A.; Vale, A.A.M.; Rocha, M.C.B.; Butarelli, A.L.D.A.; da Silva, J.M.; de Deus, A.J.S.; Nogueira, L.; Coelho, R.W.P.; Pereira, S.R.; Azevedo-Santos, A.P.S. Human Papillomavirus Infection Affects the Immune Microenvironment and Antigen Presentation in Penile Cancer. Front. Oncol. 2024, 14, 1463445. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Cillo, A.R.; Tabib, T.; Liu, A.; Onkar, S.; Kunning, S.R.; Lampenfeld, C.; Atiya, H.I.; Abecassis, I.; Kürten, C.H. B Cell Signatures and Tertiary Lymphoid Structures Contribute to Outcome in Head and Neck Squamous Cell Carcinoma. Nat. Commun. 2021, 12, 3349. [Google Scholar] [CrossRef]
- Hladíková, K.; Koucký, V.; Bouček, J.; Laco, J.; Grega, M.; Hodek, M.; Zábrodský, M.; Vošmik, M.; Rozkošová, K.; Vošmiková, H.; et al. Tumor-Infiltrating B Cells Affect the Progression of Oropharyngeal Squamous Cell Carcinoma via Cell-to-Cell Interactions with CD8+ T Cells. J. Immunother. Cancer 2019, 7, 261. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and Chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic Balance of Pro- and Anti-inflammatory Signals Controls Disease and Limits Pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Zaporowska-Stachowiak, I.; Springer, M.; Stachowiak, K.; Oduah, M.; Sopata, M.; Wieczorowska-Tobis, K.; Bryl, W. Interleukin-6 Family of Cytokines in Cancers. J. Interferon Cytokine Res. 2024, 44, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer–Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Su, H.; Li, H. PD-L1 Is Associated with the Prognosis of Penile Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 1013806. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Xu, J.; Yin, Z.; Cao, S.; Gao, W.; Liu, L.; Yin, Y.; Liu, P.; Shu, Y. Systematic Review and Meta-Analysis on the Association between IL-1B Polymorphisms and Cancer Risk. PLoS ONE 2013, 8, e63654. [Google Scholar] [CrossRef]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of Interleukin-1β. Cytokine Growth Factor. Rev. 2002, 13, 483–502. [Google Scholar] [CrossRef]
- Song, X.; Voronov, E.; Dvorkin, T.; Fima, E.; Cagnano, E.; Benharroch, D.; Shendler, Y.; Bjorkdahl, O.; Segal, S.; Dinarello, C.A. Differential Effects of IL-1α and IL-1β on Tumorigenicity Patterns and Invasiveness. J. Immunol. 2003, 171, 6448–6456. [Google Scholar] [CrossRef]
- Saijo, Y.; Tanaka, M.; Miki, M.; Usui, K.; Suzuki, T.; Maemondo, M.; Hong, X.; Tazawa, R.; Kikuchi, T.; Matsushima, K. Proinflammatory Cytokine IL-1β Promotes Tumor Growth of Lewis Lung Carcinoma by Induction of Angiogenic Factors: In Vivo Analysis of Tumor-Stromal Interaction. J. Immunol. 2002, 169, 469–475. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- León, X.; Bothe, C.; García, J.; Parreño, M.; Alcolea, S.; Quer, M.; Vila, L.; Camacho, M. Expression of IL-1α Correlates with Distant Metastasis in Patients with Head and Neck Squamous Cell Carcinoma. Oncotarget 2015, 6, 37398. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.; Lu, M.-S.; Chen, P.-T.; Chen, W.-C.; Lin, P.-Y.; Lee, K.-D. Role of Interleukin 1 Beta in Esophageal Squamous Cell Carcinoma. J. Mol. Med. 2012, 90, 89–100. [Google Scholar] [CrossRef]
- Elaraj, D.M.; Weinreich, D.M.; Varghese, S.; Puhlmann, M.; Hewitt, S.M.; Carroll, N.M.; Feldman, E.D.; Turner, E.M.; Alexander, H.R. The Role of Interleukin 1 in Growth and Metastasis of Human Cancer Xenografts. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1088–1096. [Google Scholar] [CrossRef]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical Overview of the Interleukin-6 Family Cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef]
- Gilabert, M.; Calvo, E.; Airoldi, A.; Hamidi, T.; Moutardier, V.; Turrini, O.; Iovanna, J. Pancreatic Cancer-Induced Cachexia Is Jak2-Dependent in Mice. J. Cell. Physiol. 2014, 229, 1437–1443. [Google Scholar] [CrossRef]
- Nagasaki, T.; Hara, M.; Nakanishi, H.; Takahashi, H.; Sato, M.; Takeyama, H. Interleukin-6 Released by Colon Cancer-Associated Fibroblasts Is Critical for Tumour Angiogenesis: Anti-Interleukin-6 Receptor Antibody Suppressed Angiogenesis and Inhibited Tumour–Stroma Interaction. Br. J. Cancer 2014, 110, 469–478. [Google Scholar] [CrossRef]
- Chen, M.-F.; Lin, P.-Y.; Wu, C.-F.; Chen, W.-C.; Wu, C.-T. IL-6 Expression Regulates Tumorigenicity and Correlates with Prognosis in Bladder Cancer. PLoS ONE 2013, 8, e61901. [Google Scholar] [CrossRef]
- Rahimi, R.A.; Leof, E.B. TGF-β Signaling: A Tale of Two Responses. J. Cell. Biochem. 2007, 102, 593–608. [Google Scholar] [CrossRef]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-β Signaling: A Recap of SMAD-independent and SMAD-dependent Pathways. J. Cell. Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar]
- Syed, V. TGF-β Signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Boguslawska, J.; Kryst, P.; Poletajew, S.; Piekielko-Witkowska, A. TGF-β and microRNA Interplay in Genitourinary Cancers. Cells 2019, 8, 1619. [Google Scholar] [CrossRef] [PubMed]
- Hegele, A.; Varga, Z.; Von Knobloch, R.; Heidenreich, A.; Kropf, J.; Hofmann, R. TGF-Β1 in Patients with Renal Cell Carcinoma. Urol. Res. 2002, 30, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Jackson, C.; Costello, B.; Singer, N.; Colligan, B.; Douglass, L.; Pemberton, J.; Deddens, J.; Graff, J.R.; Carter, J.H. An Intronic Variant of the TGFBR1 Gene Is Associated with Carcinomas of the Kidney and Bladder. Int. J. Cancer 2004, 112, 420–425. [Google Scholar] [CrossRef]
- Fang, L.; Sun, B.; Huang, L.; Yuan, H.; Zhang, S.; Chen, J.; Yu, Z.; Luo, H. Potent Inhibition of miR-34b on Migration and Invasion in Metastatic Prostate Cancer Cells by Regulating the TGF-β Pathway. Int. J. Mol. Sci. 2017, 18, 2762. [Google Scholar] [CrossRef]
- Shtrichman, R.; Samuel, C.E. The Role of Gamma Interferon in Antimicrobial Immunity. Curr. Opin. Microbiol. 2001, 4, 251–259. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Fonseca, B.P.; Barboza, B.A.; Viola, J.P. The Role of Interferon-Gamma on Immune and Allergic Responses. Memórias Inst. Oswaldo Cruz 2005, 100, 137–144. [Google Scholar] [CrossRef]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef]
- Street, S.E.; Cretney, E.; Smyth, M.J. Perforin and Interferon-γ Activities Independently Control Tumor Initiation, Growth, and Metastasis. Blood J. Am. Soc. Hematol. 2001, 97, 192–197. [Google Scholar] [CrossRef]
- Al Sharie, A.H.; Zahra, A.M.A.; El-Elimat, T.; Darweesh, R.F.; Al-Khaldi, A.K.; Mousa, B.M.A.; Amer, M.S.B.; Al Zu’bi, Y.O.; Al-Kammash, K.; Lil, A.A. Cyclin Dependent Kinase Inhibitor 3 (CDKN3) Upregulation Is Associated with Unfavorable Prognosis in Clear Cell Renal Cell Carcinoma and Shapes Tumor Immune Microenvironment: A Bioinformatics Analysis. Medicine 2023, 102, e35004. [Google Scholar] [CrossRef]
- Ottesen, S.S.; Ahrenkiel, V.; Kieler, J. Recombinant Human Interferon γ Exerts an Anti-Proliferative Effect and Modulates the Expression of Human Leukocyte Antigens A, B, C and DR in Human Urothelial Cell Lines. Cancer Immunol. Immunother. 1990, 31, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, M.; Wierzbicki, P.M.; Kotulak-Chrząszcz, A.; Ma\lkiewicz, B.; Sosnowski, R.; Kmieć, Z.; Matuszewski, M. Pro-Inflammatory Cytokine Gene Expression in Penile Cancer: Preliminary Studies. Medicina 2023, 59, 1623. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, C.; Li, Z.; Chen, J.; Yao, K.; Huang, K.; Liu, T.; Liu, Z.; Qin, Z.; Zhou, F. Molecular Characterization and Integrative Genomic Analysis of a Panel of Newly Established Penile Cancer Cell Lines. Cell Death Dis. 2018, 9, 684. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of Inflammasome Activation: Recent Advances and Novel Insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and Their Roles in Health and Disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in Health and Disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Casanova-Martín, C.; Boaru, D.L.; Fraile-Martinez, O.; Garcia-Montero, C.; De Leon-Oliva, D.; De Castro-Martinez, P.; Gimeno-Longas, M.J.; Bujan, J.; García-Honduvilla, N.; Guijarro, L.G. Identification of New Tissue Markers for the Monitoring and Standardization of Penile Cancer According to the Degree of Differentiation. Histol. Histopathol. 2024, 18846. [Google Scholar] [CrossRef]
- Tan, X.; Chen, D.; Guo, S.; Wang, Y.; Zou, Y.; Wu, Z.; Zhou, F.; Qin, Z.; Liu, Z.; Cao, Y. Molecular Stratification by BCL2A1 and AIM2 Provides Additional Prognostic Value in Penile Squamous Cell Carcinoma. Theranostics 2021, 11, 1364. [Google Scholar] [CrossRef]
- Shadab, A.; Mahjoor, M.; Abbasi-Kolli, M.; Afkhami, H.; Moeinian, P.; Safdarian, A.-R. Divergent Functions of NLRP3 Inflammasomes in Cancer: A Review. Cell Commun. Signal 2023, 21, 232. [Google Scholar] [CrossRef]
- Saponaro, C.; Scarpi, E.; Sonnessa, M.; Cioffi, A.; Buccino, F.; Giotta, F.; Pastena, M.I.; Zito, F.A.; Mangia, A. Prognostic Value of NLRP3 Inflammasome and TLR4 Expression in Breast Cancer Patients. Front. Oncol. 2021, 11, 705331. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, H.; Qin, Z.; Zhao, F.; Zhou, L.; Xu, L.; Jia, R. NLRP3 Inflammasome Promoted the Malignant Progression of Prostate Cancer via the Activation of Caspase-1. Cell Death Discov. 2021, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mu, K.; Li, T.; Zhang, Y.; Yang, Z.; Jia, X.; Zhao, W.; Huai, W.; Guo, P.; Han, L. Deregulation of the NLRP3 Inflammasome in Hepatic Parenchymal Cells during Liver Cancer Progression. Lab. Investig. 2014, 94, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Farshchian, M.; Nissinen, L.; Siljamäki, E.; Riihilä, P.; Piipponen, M.; Kivisaari, A.; Kallajoki, M.; Grénman, R.; Peltonen, J.; Peltonen, S. Tumor Cell-Specific AIM2 Regulates Growth and Invasion of Cutaneous Squamous Cell Carcinoma. Oncotarget 2017, 8, 45825. [Google Scholar] [CrossRef]
- Dihlmann, S.; Tao, S.; Echterdiek, F.; Herpel, E.; Jansen, L.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M.; Kloor, M. Lack of Absent in Melanoma 2 (AIM2) Expression in Tumor Cells Is Closely Associated with Poor Survival in Colorectal Cancer Patients. Int. J. Cancer 2014, 135, 2387–2396. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the Chemokine Network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Kazanietz, M.G.; Durando, M.; Cooke, M. CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond. Front. Endocrinol. 2019, 10, 471. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543. [Google Scholar] [CrossRef]
- Mo, M.; Li, Y.; Hu, X. Serum CXCL5 Level Is Associated with Tumor Progression in Penile Cancer. Biosci. Rep. 2021, 41, BSR20202133. [Google Scholar] [CrossRef]
- Mo, M.; Tong, S.; Li, T.; Zu, X.; Hu, X. Serum CXCL13 Level Is Associated with Tumor Progression and Unfavorable Prognosis in Penile Cancer. OncoTargets Ther. 2020, 13, 8757–8769. [Google Scholar] [CrossRef]
- Mo, M.; Tong, S.; Huang, W.; Cai, Y.; Zu, X.; Hu, X. High Serum CCL20 Is Associated with Tumor Progression in Penile Cancer. J. Cancer 2020, 11, 6812. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating Cell-Signalling Pathways with NF-κB and IKK Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB Signaling Pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Cartwright, T.; Perkins, N.D.; Wilson, C.L. NFKB1: A Suppressor of Inflammation, Ageing and Cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef]
- Pan, W.; Deng, L.; Wang, H.; Wang, V.Y.-F. Atypical IκB Bcl3 Enhances the Generation of the NF-κB P52 Homodimer. Front. Cell Dev. Biol. 2022, 10, 930619. [Google Scholar] [CrossRef]
- Jeannin, P.; Magistrelli, G.; Goetsch, L.; Haeuw, J.-F.; Thieblemont, N.; Bonnefoy, J.-Y.; Delneste, Y. Outer Membrane Protein A (OmpA): A New Pathogen-Associated Molecular Pattern That Interacts with Antigen Presenting Cells—Impact on Vaccine Strategies. Vaccine 2002, 20, A23–A27. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB System. WIREs Mech. Dis. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and Target Genes of Rel/NF-κB Transcription Factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Pennacchiotti, G.; Valdés-Gutiérrez, F.; González-Arriagada, W.A.; Montes, H.F.; Parra, J.M.R.; Guida, V.A.; Gómez, S.E.; Guerrero-Gimenez, M.E.; Fernandez-Muñoz, J.M.; Zoppino, F.C.M. SPINK7 Expression Changes Accompanied by HER2, P53 and RB1 Can Be Relevant in Predicting Oral Squamous Cell Carcinoma at a Molecular Level. Sci. Rep. 2021, 11, 6939. [Google Scholar] [CrossRef]
- Fonseca, A.; Perez, M.; Veiga, G.; Prosdócimi, F.; Nunes, F.; Bianco, B.; Fonseca, F.; Alves, B. Expression of MMR System Genes Is Correlated to NF-kB in Patients with Oral Squamous Cell Carcinoma. J. Clin. Pathol. 2020, 73, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lehman, H.L.; Kidacki, M.; Warrick, J.I.; Stairs, D.B. NFkB Hyperactivation Causes Invasion of Esophageal Squamous Cell Carcinoma with EGFR Overexpression and P120-Catenin down-Regulation. Oncotarget 2018, 9, 11180. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, P.M.; Czajkowski, M.; Kotulak-Chrząszcz, A.; Bukowicz, J.; Dzieciuch, K.; Sokołowska-Wojdyło, M.; Kmieć, Z.; Matuszewski, M. Altered mRNA Expression of NFKB1 and NFKB2 Genes in Penile Lichen Sclerosus, Penile Cancer and Zoon Balanitis. J. Clin. Med. 2022, 11, 7254. [Google Scholar] [CrossRef]
- Senba, M.; Mori, N.; Fujita, S.; Jutavijittum, P.; Yousukh, A.; Toriyama, K.; Wada, A. Relationship among Human Papillomavirus Infection, p16INK4a, P53 and NF-κB Activation in Penile Cancer from Northern Thailand. Oncol. Lett. 2010, 1, 599–603. [Google Scholar] [CrossRef]
- Yang, E.S.; Willey, C.D.; Mehta, A.; Crowley, M.R.; Crossman, D.K.; Chen, D.; Anderson, J.C.; Naik, G.; Della Manna, D.L.; Cooper, T.S. Kinase Analysis of Penile Squamous Cell Carcinoma on Multiple Platforms to Identify Potential Therapeutic Targets. Oncotarget 2017, 8, 21710. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Karmakar, S.; Leon, F.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Mallya, K.; Zhang, C.; Ly, Q.P. Pancreatic Tumor Microenvironment Factor Promotes Cancer Stemness via SPP1–CD44 Axis. Gastroenterology 2021, 161, 1998–2013. [Google Scholar] [CrossRef]
- Qi, J.; Sun, H.; Zhang, Y.; Wang, Z.; Xun, Z.; Li, Z.; Ding, X.; Bao, R.; Hong, L.; Jia, W. Single-Cell and Spatial Analysis Reveal Interaction of FAP+ Fibroblasts and SPP1+ Macrophages in Colorectal Cancer. Nat. Commun. 2022, 13, 1742. [Google Scholar] [CrossRef]
- Deng, G.; Zeng, F.; Su, J.; Zhao, S.; Hu, R.; Zhu, W.; Hu, S.; Chen, X.; Yin, M. BET Inhibitor Suppresses Melanoma Progression via the Noncanonical NF-κB/SPP1 Pathway. Theranostics 2020, 10, 11428. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, X.; Yuan, G.; Tang, Y.; Wang, Y.; Yang, C.; Luo, S.; Wu, Z.; Yao, K. SPP1 Is Associated with Adverse Prognosis and Predicts Immunotherapy Efficacy in Penile Cancer. Hum. Genom. 2023, 17, 116. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-Reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Pletnikoff, P.P.; Laukkanen, J.A.; Tuomainen, T.-P.; Kauhanen, J.; Rauramaa, R.; Ronkainen, K.; Kurl, S. Cardiorespiratory Fitness, C-Reactive Protein and Lung Cancer Risk: A Prospective Population-Based Cohort Study. Eur. J. Cancer 2015, 51, 1365–1370. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Branchini, C.; Guallar, E.; Helzlsouer, K.J.; Erlinger, T.P.; Platz, E.A. C-reactive Protein and Colorectal Cancer Risk: A Systematic Review of Prospective Studies. Int. J. Cancer 2008, 123, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Wang, T.; Cushman, M.; Xue, X.; Wassertheil-Smoller, S.; Strickler, H.D.; Rohan, T.E.; Manson, J.E.; McTiernan, A.; Kaplan, R.C. Circulating Adipokines and Inflammatory Markers and Postmenopausal Breast Cancer Risk. J. Natl. Cancer Inst. 2015, 107, djv169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ma, Z.; Zhang, X.; Hang, D.; Yin, R.; Feng, J.; Xu, L.; Shen, H. C-Reactive Protein and Cancer Risk: A Pan-Cancer Study of Prospective Cohort and Mendelian Randomization Analysis. BMC Med. 2022, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Chen, I.-H.; Liao, C.-T.; Wei, F.-C.; Lee, L.-Y.; Huang, S.-F. Preoperative Circulating C-reactive Protein Levels Predict Pathological Aggressiveness in Oral Squamous Cell Carcinoma: A Retrospective Clinical Study. Clin. Otolaryngol. 2011, 36, 147–153. [Google Scholar] [CrossRef]
- Nakatsu, T.; Motoyama, S.; Maruyama, K.; Usami, S.; Sato, Y.; Miura, M.; Hinai, Y.; Saito, H.; Minamiya, Y.; Murata, K. Tumoral CRP Expression in Thoracic Esophageal Squamous Cell Cancers Is Associated with Poor Outcomes. Surg. Today 2012, 42, 652–658. [Google Scholar] [CrossRef]
- Neuss, H.; Koplin, G.; Raue, W.; Reetz, C.; Mall, J.W. Analysing the Serum Levels of Tumour Markers and Primary Tumour Data in Stage III Melanoma Patients in Correlation to the Extent of Lymph Node Metastases—A Prospective Study in 231 Patients. Acta Chir. Belg. 2011, 111, 214–218. [Google Scholar] [CrossRef]
- Al Ghazal, A.; Steffens, S.; Steinestel, J.; Lehmann, R.; Schnoeller, T.J.; Schulte-Hostede, A.; Wegener, G.; Jentzmik, F.; Schrader, M.; Kuczyk, M.A.; et al. Elevated C-Reactive Protein Values Predict Nodal Metastasis in Patients with Penile Cancer. BMC Urol. 2013, 13, 53. [Google Scholar] [CrossRef]
- Steffens, S.; Al Ghazal, A.; Steinestel, J.; Lehmann, R.; Wegener, G.; Schnoeller, T.J.; Cronauer, M.V.; Jentzmik, F.; Schrader, M.; Kuczyk, M.A.; et al. High CRP Values Predict Poor Survival in Patients with Penile Cancer. BMC Cancer 2013, 13, 223. [Google Scholar] [CrossRef]
- Li, Z.; Yao, K.; Li, Y.; Chen, J.; Deng, C.; Zhao, Q.; Chen, P.; Wang, B.; Mi, Q.; Liu, Z.; et al. Clinical Significance of Preoperative C-reactive Protein and Squamous Cell Carcinoma Antigen Levels in Patients with Penile Squamous Cell Carcinoma. BJU Int. 2016, 118, 272–278. [Google Scholar] [CrossRef]
- Li, Z.-S.; Chen, P.; Yao, K.; Wang, B.; Li, J.; Mi, Q.-W.; Chen, X.-F.; Zhao, Q.; Li, Y.-H.; Chen, J.-P. Development of a New Outcome Prediction Model for Chinese Patients with Penile Squamous Cell Carcinoma Based on Preoperative Serum C-Reactive Protein, Body Mass Index, and Standard Pathological Risk Factors: The TNCB Score Group System. Oncotarget 2016, 7, 21023. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Takagi, K.; Kawada, K.; Ishida, T.; Tomioka, M.; Enomoto, T.; Fujimoto, S.; Taniguchi, T.; Ito, H.; Kameyama, K. Clinical Lymph Node Involvement as a Predictor for Cancer-Specific Survival in Patients with Penile Squamous Cell Cancer. Curr. Oncol. 2022, 29, 5466–5474. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.; Garmo, H.; Arthur, R.; Hammar, N.; Jungner, I.; Malmström, H.; Lambe, M.; Walldius, G.; Van Hemelrijck, M. Serum Biomarkers to Predict Risk of Testicular and Penile Cancer in AMORIS. ecancermedicalscience 2017, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Corbeau, I.; Jacot, W.; Guiu, S. Neutrophil to Lymphocyte Ratio as Prognostic and Predictive Factor in Breast Cancer Patients: A Systematic Review. Cancers 2020, 12, 958. [Google Scholar] [CrossRef]
- Faria, S.S.; Fernandes Jr, P.C.; Silva, M.J.B.; Lima, V.C.; Fontes, W.; Freitas-Junior, R.; Eterovic, A.K.; Forget, P. The Neutrophil-to-Lymphocyte Ratio: A Narrative Review. ecancermedicalscience 2016, 10, 702. [Google Scholar]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in Cancer: Neutral No More. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Larosa, D.F.; Orange, J.S. 1. Lymphocytes. J. Allergy Clin. Immunol. 2008, 121, S364–S369; quiz S412. [Google Scholar] [CrossRef]
- Park, J.M. Neutrophil-to-Lymphocyte Ratio in Trauma Patients. J. Trauma. Acute Care Surg. 2017, 82, 225–226. [Google Scholar] [CrossRef]
- Niu, D.; Huang, Q.; Yang, F.; Tian, W.; Li, C.; Ding, L.; Fang, H.-C.; Zhao, Y. Serum Biomarkers to Differentiate Gram-Negative, Gram-Positive and Fungal Infection in Febrile Patients. J. Med. Microbiol. 2021, 70, 001360. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 686983. [Google Scholar] [CrossRef] [PubMed]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The Neutrophil–Lymphocyte Ratio and Incident Atherosclerotic Events: Analyses from Five Contemporary Randomized Trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, T.R.; Groot Koerkamp, B.; Rizopoulos, D.; Ikram, M.A.; Van Eijck, C.H.J.; Stricker, B.H. The Neutrophil-to-Lymphocyte Ratio Is Associated with Mortality in the General Population: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 463–470. [Google Scholar] [CrossRef]
- Lowsby, R.; Gomes, C.; Jarman, I.; Lisboa, P.; Nee, P.A.; Vardhan, M.; Eckersley, T.; Saleh, R.; Mills, H. Neutrophil to Lymphocyte Count Ratio as an Early Indicator of Blood Stream Infection in the Emergency Department. Emerg. Med. J. 2015, 32, 531–534. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Kao, S.C.; Pavlakis, N.; Harvie, R.; Vardy, J.L.; Boyer, M.J.; van Zandwijk, N.; Clarke, S.J. High Blood Neutrophil-to-Lymphocyte Ratio Is an Indicator of Poor Prognosis in Malignant Mesothelioma Patients Undergoing Systemic Therapy. Clin. Cancer Res. 2010, 16, 5805–5813. [Google Scholar] [CrossRef]
- An, X.; Ding, P.-R.; Li, Y.-H.; Wang, F.-H.; Shi, Y.-X.; Wang, Z.-Q.; He, Y.-J.; Xu, R.-H.; Jiang, W.-Q. Elevated Neutrophil to Lymphocyte Ratio Predicts Survival in Advanced Pancreatic Cancer. Biomarkers 2010, 15, 516–522. [Google Scholar] [CrossRef]
- Cedres, S.; Torrejon, D.; Martinez, A.; Martinez, P.; Navarro, A.; Zamora, E.; Mulet-Margalef, N.; Felip, E. Neutrophil to Lymphocyte Ratio (NLR) as an Indicator of Poor Prognosis in Stage IV Non-Small Cell Lung Cancer. Clin. Transl. Oncol. 2012, 14, 864–869. [Google Scholar] [CrossRef]
- Cordeiro, M.D.; Ilario, E.N.; Abe, D.K.; de Carvalho, P.A.; Muniz, D.Q.B.; Sarkis, A.S.; Coelho, R.F.; Guimarães, R.M.; Haddad, M.V.; Nahas, W.C. Neutrophil-to-Lymphocyte Ratio Predicts Cancer Outcome in Locally Advanced Clear Renal Cell Carcinoma. Clin. Genitourin. Cancer 2022, 20, 102–106. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhang, X.; Chen, P.; Wang, B.; Chen, X.; Han, H.; Zhou, F. Prognostic Significance of Common Preoperative Laboratory Variables in Penile Squamous Cell Carcinoma. Int. J. Urol. 2020, 27, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, Y.; Li, J.; Zhang, G.; Yang, L.; Bi, C.; Zhao, B.; Yang, Y.; Li, R.; Wu, H.; et al. Prognostic Value of Systemic Inflammatory Factors NLR, LMR, PLR and LDH in Penile Cancer. BMC Urol. 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Peyton, C.C.; Boulware, D.C.; Chipollini, J.; Juwono, T.; Pow-Sang, J.M.; Spiess, P.E. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Penile Squamous Cell Carcinoma Patients Undergoing Inguinal Lymph Node Dissection. Eur. Urol. Focus. 2019, 5, 1085–1090. [Google Scholar] [CrossRef]
- Jindal, T.; Pawar, P.; Agarwal, S.; Jain, P.; Meena, M.; Sarwal, A.; Dhanalakshmi, M. The Use of Preoperative Neutrophil–Lymphocyte Ratio and Lymphocyte–Monocyte Ratio in Predicting Survival and Groin Node Involvement of Patients with Squamous Cell Carcinoma of Penis. Urol. Ann. 2021, 13, 391–396. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; He, T.; Deng, H.; Gong, G.; Cui, Y.; Liu, P.; Ren, W.; Li, C.; Chen, J. A Nomogram Incorporating PD-L1, NLR, and Clinicopathologic Features to Predict Inguinal Lymph Node Metastasis in Penile Squamous Cell Carcinoma. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2020; Volume 38, p. 641-e19. [Google Scholar]
- Zhou, Q.; Han, H.; Lu, J.; Liu, T.; Huang, K.; Deng, C.; Li, Z.; Chen, J.; Yao, K.; Qin, Z.; et al. Up-regulation of Indoleamine 2,3-dioxygenase 1 (IDO1) Expression and Catalytic Activity Is Associated with Immunosuppression and Poor Prognosis in Penile Squamous Cell Carcinoma Patients. Cancer Commun. 2020, 40, 3–15. [Google Scholar] [CrossRef]
- Kasuga, J.; Kawahara, T.; Takamoto, D.; Fukui, S.; Tokita, T.; Tadenuma, T.; Narahara, M.; Fusayasu, S.; Terao, H.; Izumi, K.; et al. Increased Neutrophil-to-Lymphocyte Ratio Is Associated with Disease-Specific Mortality in Patients with Penile Cancer. BMC Cancer 2016, 16, 396. [Google Scholar] [CrossRef]
- Tan, T.W.; Chia, S.J.; Chong, K.T. Management of Penile Cancer in a Singapore Tertiary Hospital. Arab. J. Urol. 2017, 15, 123–130. [Google Scholar] [CrossRef]
- Pond, G.R.; Di Lorenzo, G.; Necchi, A.; Eigl, B.J.; Kolinsky, M.P.; Chacko, R.T.; Dorff, T.B.; Harshman, L.C.; Milowsky, M.I.; Lee, R.J. Prognostic Risk Stratification Derived from Individual Patient Level Data for Men with Advanced Penile Squamous Cell Carcinoma Receiving First-Line Systemic Therapy. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2014; Volume 32, pp. 501–508. [Google Scholar]
- Buonerba, C.; Di Lorenzo, G.; Pond, G.; Cartenì, G.; Scagliarini, S.; Rozzi, A.; Quevedo, F.J.; Dorff, T.; Nappi, L.; Lanzetta, G. Prognostic and Predictive Factors in Patients with Advanced Penile Cancer Receiving Salvage (2nd or Later Line) Systemic Treatment: A Retrospective, Multi-Center Study. Front. Pharmacol. 2016, 7, 487. [Google Scholar] [CrossRef]

| Author | Particle | Article Type | No. of PeCa | Key Findings | Ref. |
|---|---|---|---|---|---|
| Xu et al. | TIME | Prospective study | n = 6 | Provides insights into the mechanisms driving PeCa progression, premetastatic niche formation, and lymphatic metastasis. | [20] |
| Lohse et al. | TIME | Retrospective study | n = 94 | PeCas exhibiting an HPV+/p63+/CD15+/DKK1+/CD147+ profile are associated with increased aggressiveness and metastasis. | [88] |
| Guimarães et al. | TIME | Prospective study | n = 30 | HPV infection may trigger an inadequate immune response, potentially facilitating the development of PeCa. | [89] |
| Ottenhof et al. | TILs | Retrospective study | n = 213 | Higher infiltration of CD8+ TILs in tumor-associated stroma is associated with lymph node metastasis in PeCa. | [11] |
| Vassallo et al. | TILs | Retrospective study | n = 122 | Abundant Fox-P3+ cells and pronounced inflammation are significant predictors of poor prognosis in PeCa. | [35] |
| Lohneis et al. | TILs | Retrospective study | n = 28 | HPV-associated PeCa exhibits elevated levels of TILs, characterized predominantly by Th1 and cytotoxic profiles. Nevertheless, the increased presence of regulatory T cells (Tregs) in these neoplasms may contribute to immune evasion mechanisms. | [87] |
| Hladek et al. | TILs | Retrospective study | n = 55 | PeCa tissues exhibit increased immune cell infiltration, especially CD3+, CD8+, and CD20+. | [36] |
| Ottenhof et al. | TAMs | Retrospective study | n = 213 | A high number of intramural CD163+ M2 macrophages is significantly associated with a higher incidence of lymph node metastasis. Nevertheless, CD4+ T cells could reprogram them into M1. | [11] |
| Chu et al. | TAMs | Retrospective study | n = 178 | Elevated levels of CD68+ and CD206+ TAMs are correlated with a more favorable prognosis. | [6] |
| Cury et al. | CAFs | Retrospective study | n = 63 | Patients with elevated CAF scores exhibited reduced survival rates. | [66] |
| Czajkowski et al. | IL-1A, IL-1B, IL-6, INF-γ, and TGF-β | Prospective study | n = 6 | Elevated expression of proinflammatory cytokines (IL-1A, IL-1B, IL-6, INF-γ, and TGF-β) in PeCa was observed. A positive correlation was found between higher INF-γ levels and clinical advancement. | [125] |
| Zhou et al. | INF-γ | Retrospective study | n = 114 | The IFNγ-mediated induction of IDO1 contributes significantly to the formation of an immunosuppressive tumor microenvironment in PeCa. | [199] |
| Casanova-Martín et al. | NLRP3 inflammasome, AIF-1 | Retrospective study | n = 34 | Elevated levels of NLRP3 and AIF-1 contribute to the development of more aggressive phenotypes in PeCa. | [131] |
| Tan et al. | AIM2 inflammasome | Retrospective study | n = 220 | AIM2 is a reliable oncogene in PeCa, with its overexpression correlated with CSS. | [132] |
| Mo et al. | CXCL5 | Retrospective study | n = 81 | Elevated preoperative CXCL5 levels predict PeCa progression and may serve as a prognostic biomarker. | [142] |
| Mo et al. | CXCL13 | Retrospective study | n = 76 | Elevated serum CXCL13 levels correlate with PeCa progression. | [143] |
| Mo et al. | CCL20 | Retrospective study | n = 76 | Elevated serum CCL20 levels correlate with PeCa progression. | [144] |
| Wierzbicki et al. | NF-κB | Retrospective study | n = 6 | Both canonical and non-canonical NF-κB pathways can be activated in PeCa. | [156] |
| Senba et al. | NF-κB | Retrospective study | n = 51 | NF-κB was more frequently detected in HPV-positive PeCa. | [157] |
| Zou et al. | SPP1 | Retrospective study | n = 183 | Elevated SPP1 expression was associated with favorable prognosis in PeCa patients, suggesting that SPP1 may augment antitumor immunity mediated by T cells and regulatory T cells. | [162] |
| Al Ghazal et al. | CRP | Retrospective study | n = 51 | CRP could help identify PeCa patients needing inguinal lymph node dissection. | [171] |
| Steffens et al. | CRP | Retrospective study | n = 79 | Elevated preoperative CRP levels predicted poor survival in PeCa. | [172] |
| Li et al. | CRP | Retrospective study | n = 124 | Combined CRP and SCC-Ag levels predict lymph node metastasis, advanced stage, and survival in PeCa. | [173] |
| Li et al. | CRP | Retrospective study | n = 172 | Elevated CRP levels and lower BMI were identified as independent risk factors for poor CSS in PeCa. | [174] |
| Kawase et al. | CRP | Retrospective study | n = 64 | High CRP levels were significantly associated with poorer cancer-specific survival (CSS). | [175] |
| Ghoshal et al. | CRP | Retrospective study | n = 50 | No association was observed between elevated serum CRP levels and the development of PeCa. | [176] |
| Li et al. | NLR | Retrospective study | n = 228 | The preoperative NLR was an independent prognostic factor for both DFS and CSS in PeCa patients. | [194] |
| Hu et al. | NLR | Retrospective study | n = 225 | An elevated NLR was associated with decreased OS and PFS. Furthermore, elevated NLR has been correlated with nodal involvement. | [195] |
| Azizi et al. | NLR | Retrospective study | n = 68 | An elevated NLR was associated with advanced-stage disease, lymph node involvement, extranodal extension, and significantly reduced OS. | [196] |
| Jindal et al. | NLR | Retrospective study | n = 69 | An elevated NLR was correlated with lymph node metastasis, a higher T stage, and inferior CSS. | [197] |
| Hu et al. | NLR | Retrospective study | n = 79 | An elevated NLR was associated with advanced tumor grades and increased incidence of lymph node metastasis. | [198] |
| Zhou et al. | NLR | Retrospective study | n = 114 | An elevated NLR was correlated with inferior CSS. | [199] |
| Kasuga et al. | NLR | Retrospective study | n = 41 | An elevated NLR was associated with a higher incidence of lymph node metastasis and inferior CSS and OS. | [200] |
| Tan et al. | NLR | Prospective study | n = 39 | An elevated NLR was associated with higher T stages and significantly worse CSS. | [201] |
| Pond et al. | NLR | Retrospective study | n = 140 | An elevated NLR was associated with poorer OS. | [202] |
| Buonerba et al. | NLR | Retrospective study | n = 65 | An elevated NLR was not correlated with OS or responses to systemic treatment. | [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czajkowski, M.; Wierzbicki, P.M.; Dolny, M.; Matuszewski, M.; Hakenberg, O.W. Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 2785. https://doi.org/10.3390/ijms26062785
Czajkowski M, Wierzbicki PM, Dolny M, Matuszewski M, Hakenberg OW. Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(6):2785. https://doi.org/10.3390/ijms26062785
Chicago/Turabian StyleCzajkowski, Mateusz, Piotr M. Wierzbicki, Maciej Dolny, Marcin Matuszewski, and Oliver W. Hakenberg. 2025. "Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 6: 2785. https://doi.org/10.3390/ijms26062785
APA StyleCzajkowski, M., Wierzbicki, P. M., Dolny, M., Matuszewski, M., & Hakenberg, O. W. (2025). Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review. International Journal of Molecular Sciences, 26(6), 2785. https://doi.org/10.3390/ijms26062785







