Prometastatic Potential of Non-Functionalized Multiwalled Carbon Nanotubes in the MDA-MB-436 Breast Cancer Cell Line Model
Abstract
1. Introduction
2. Results
2.1. Effect of MWCNTs on Viability of MDA-MB-436 Cells
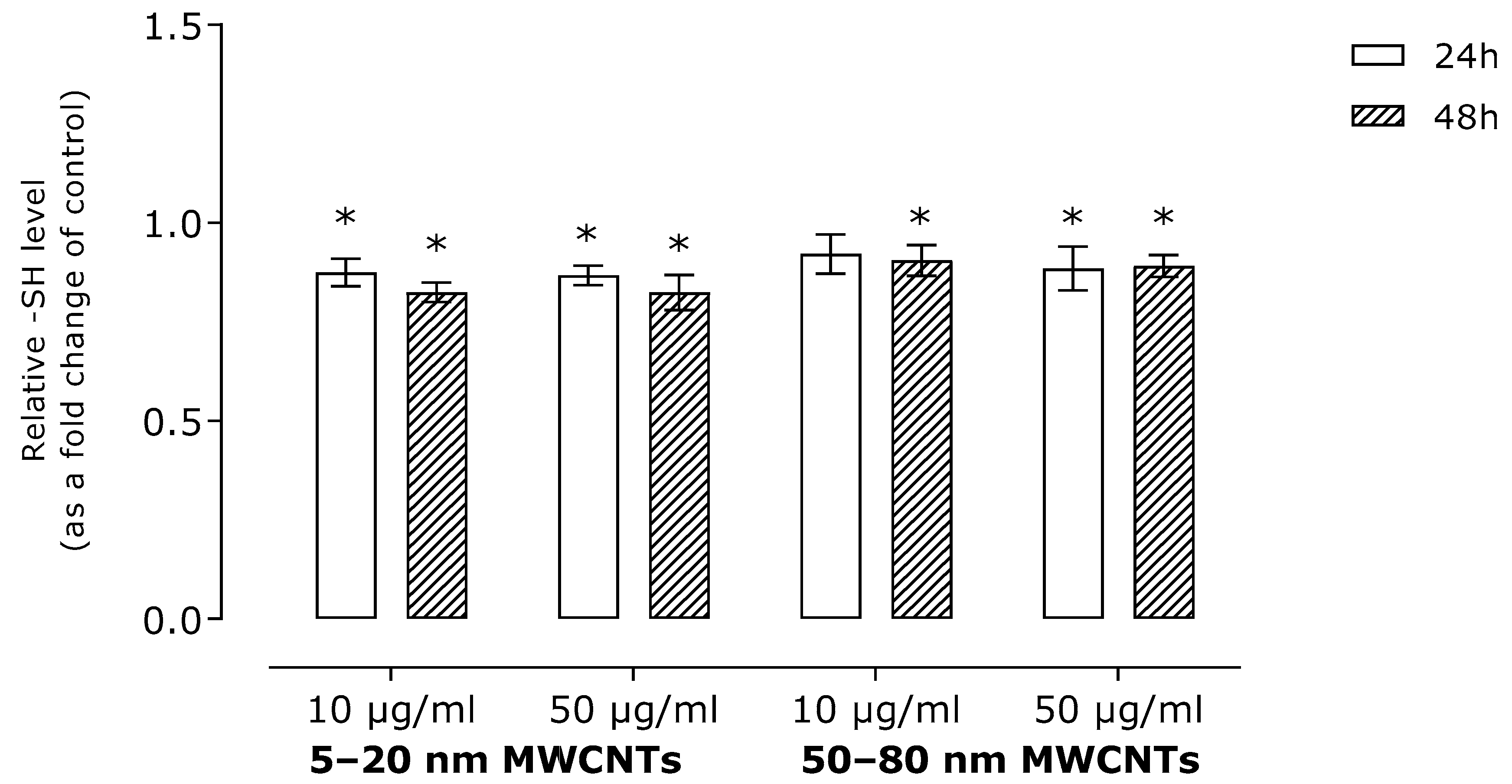
2.2. Effect of MWNCTs on Oxidative Stress Markers
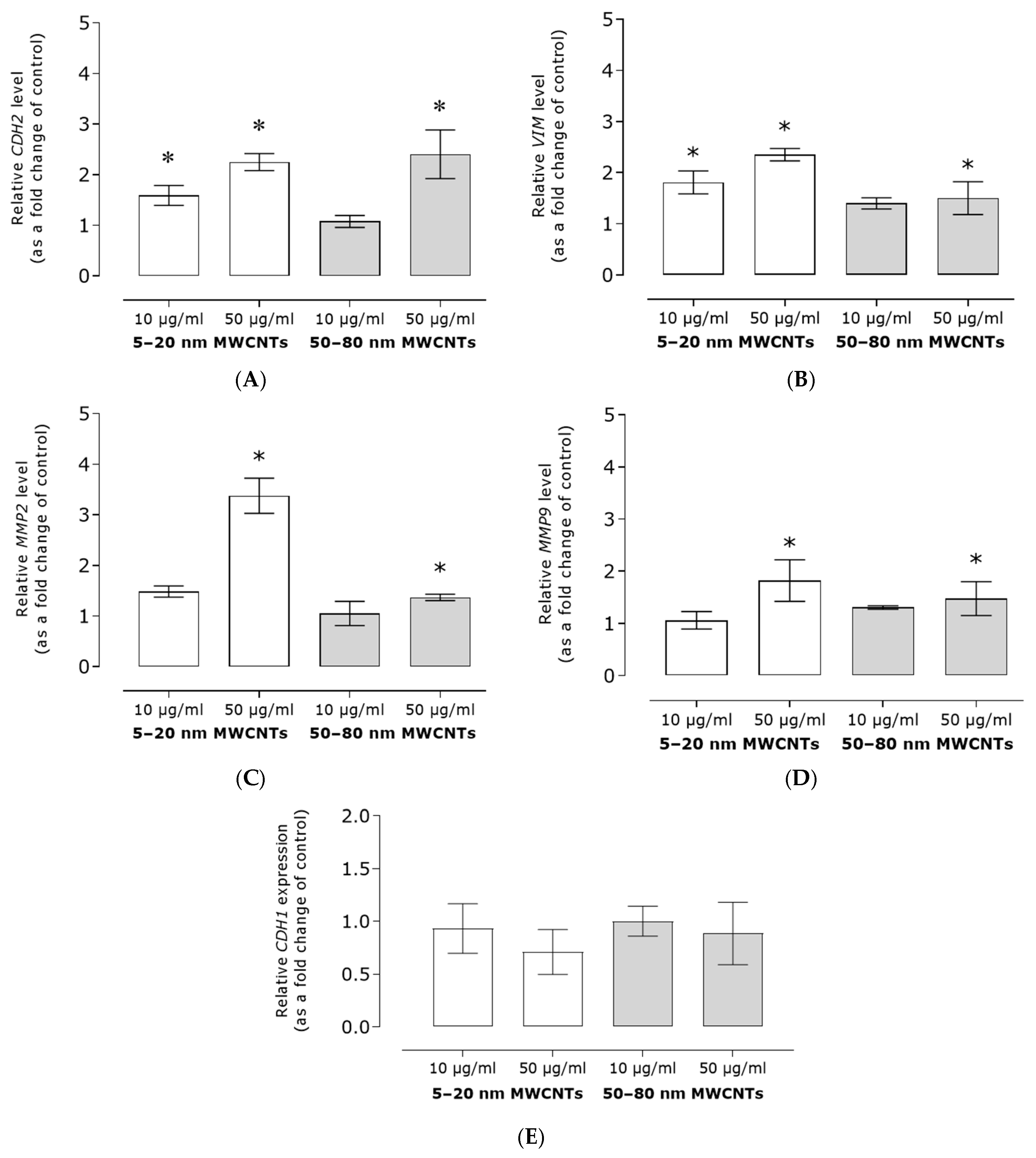
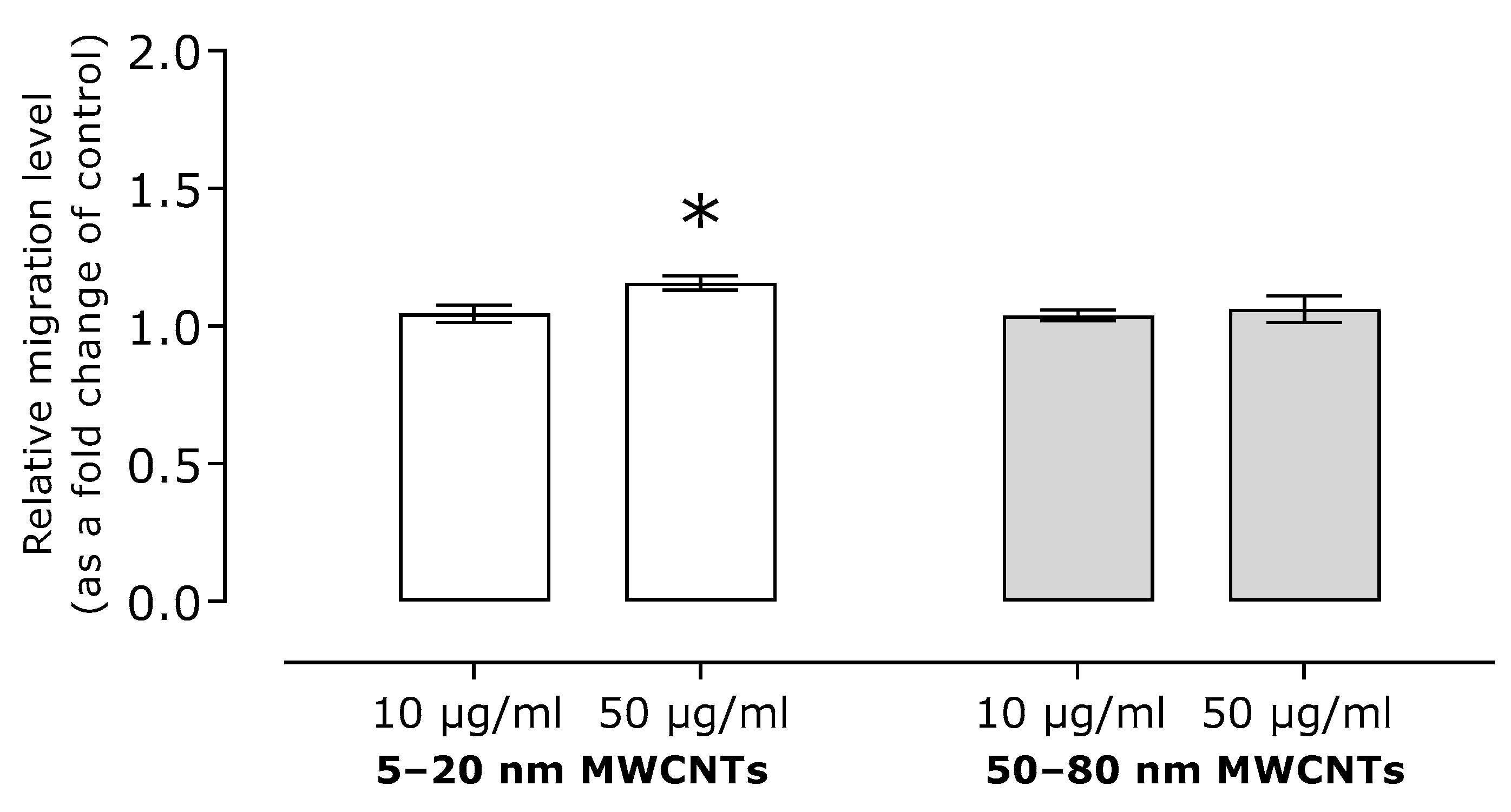
2.3. Effect of MWCNTs on Expression of Markers EMT, Migration, and TGFB1 Secretion
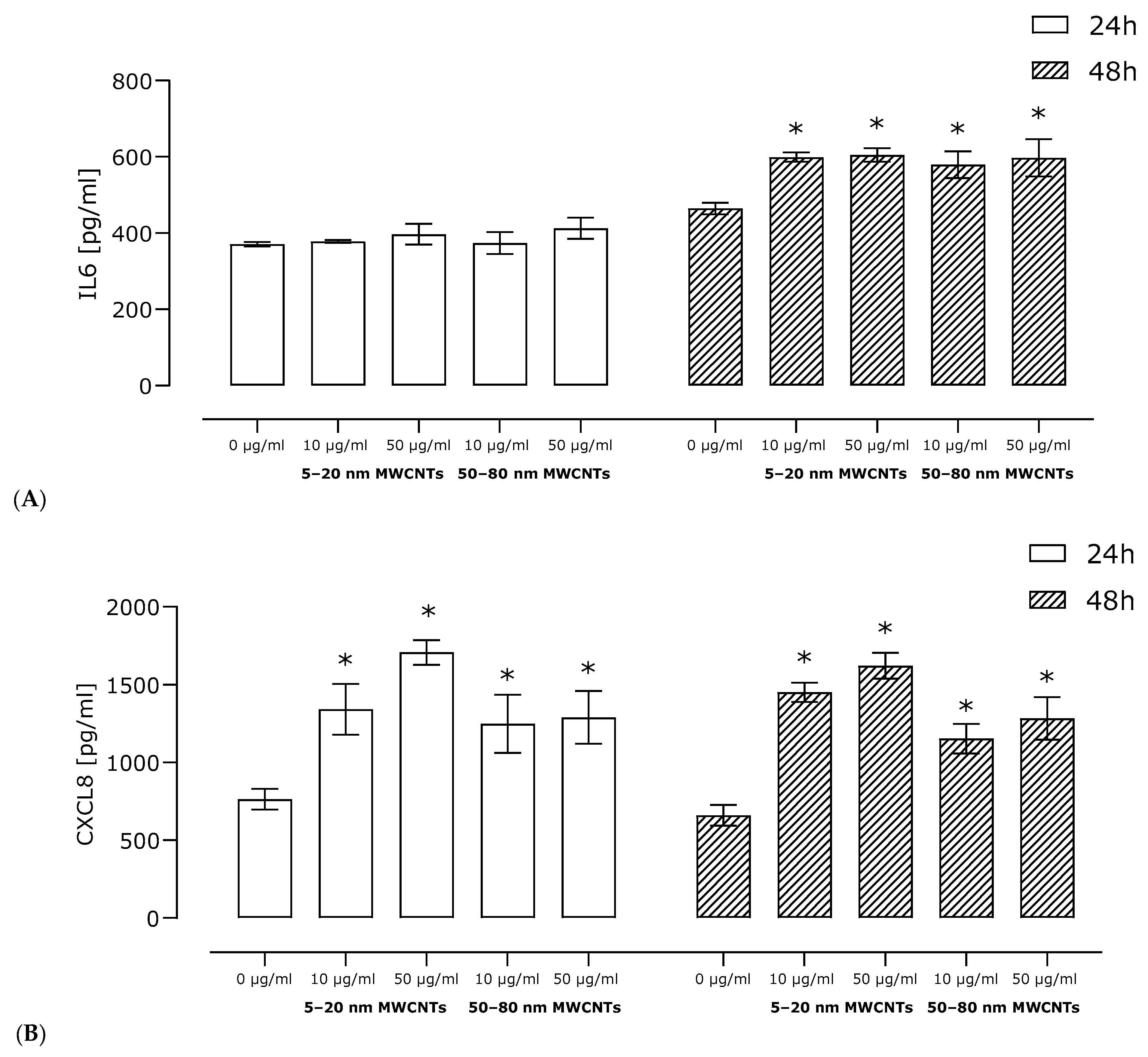
2.4. Effect of MWCNTs on Secretion of Pro-Inflammatory Proteins
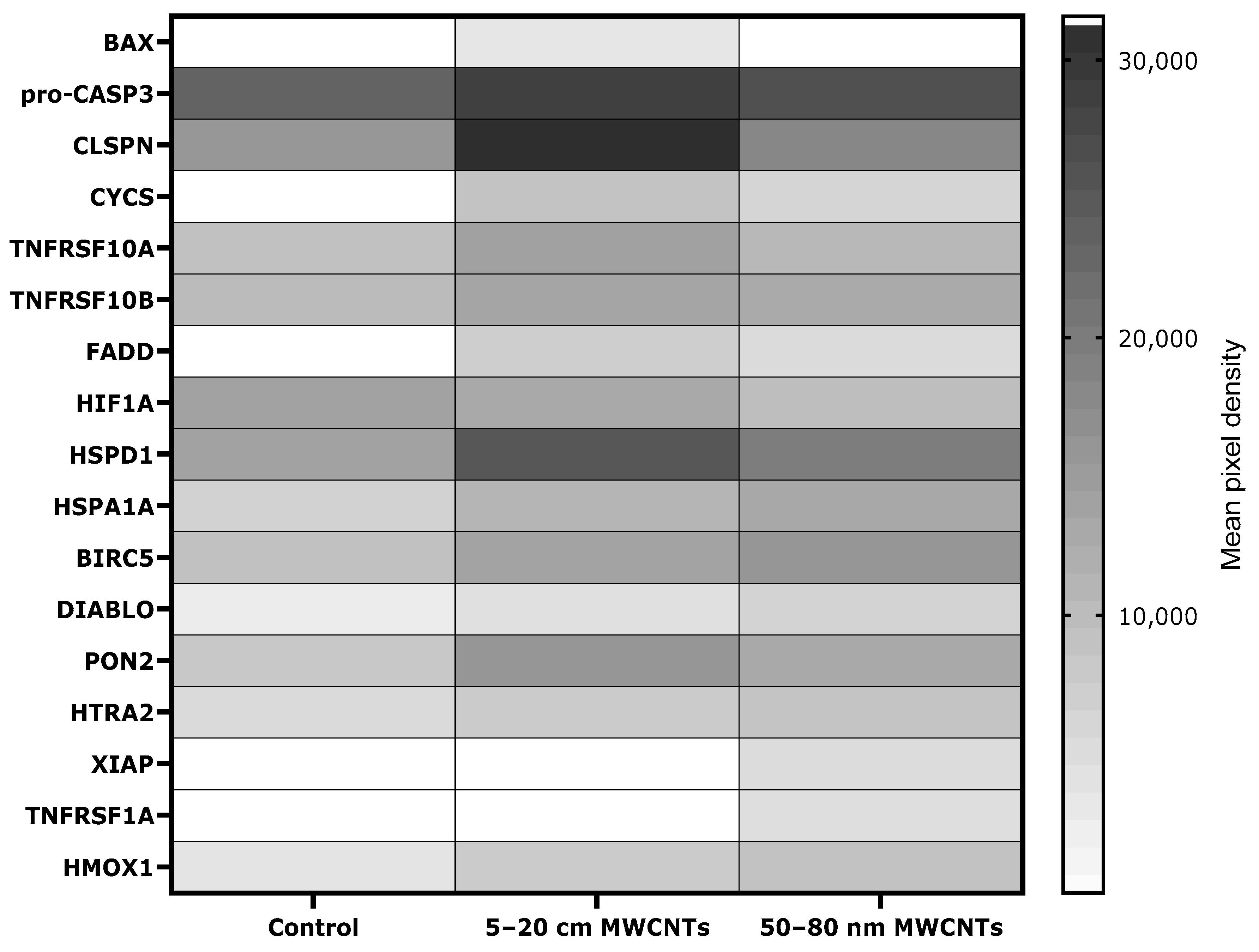
2.5. Effect of MWCNTs on Apoptosis Markers in MDA-MB-436-Treated Cells
2.6. Impact of MWCNTs on Actin Filaments
3. Discussion
4. Materials and Methods
4.1. MDA-MB-436 Cell Culture
4.2. MWCNT Preparation
4.3. MTT Assay
4.4. Neutral Red Assay
4.5. Sample Preparation for Analysis
4.6. QuantiChromTM TBARS Assay Kit
4.7. Fluorometric Thiol Assay Kit
4.8. Proteome Profiler Human Cytokine Array Kit and Proteome Profiler Human Apoptosis Array Kit
4.9. ELISA
4.10. Real-Time Reverse Transcription Polymerase Chain Reaction
4.11. Chemotaxis Cell Migration Assay
4.12. Phalloidin–TRITC Staining
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health. 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Srivastava, A.; Kumar, D.; Pandey, S.K.; Mubarak, N.M.; Dehghani, M.H.; Ansari, K. An insight into the toxicological impacts of carbon nanotubes (CNTs) on human health: A review. Environ. Adv. 2024, 18, 100601. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Tsai, M.L.; Huang, H.C.; Chen, C.Y.; Dai, S.X. Epithelial-mesenchymal transition contributes to SWCNT-induced pulmonary fibrosis. Nanotoxicology 2012, 6, 600–610. [Google Scholar] [CrossRef]
- Chen, T.; Nie, H.; Gao, X.; Yang, J.; Pu, J.; Chen, Z.; Cui, X.; Wang, Y.; Wang, H.; Jia, G. Epithelial–mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway. Toxicol. Lett. 2014, 226, 150–162. [Google Scholar] [CrossRef]
- Rakowski, M.; Porębski, S.; Grzelak, A. Silver Nanoparticles Modulate the Epithelial-to-Mesenchymal Transition in Estrogen-Dependent Breast Cancer Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 9203. [Google Scholar] [CrossRef]
- Dhawan, U.; Sue, M.W.; Lam, K.C.; Buddhakosai, W.; Huang, P.H.; Chen, Y.C.; Chen, P.C.; Chen, W.L. Nanochip-Induced Epithelial-to-Mesenchymal Transition: Impact of Physical Microenvironment on Cancer Metastasis. ACS Appl. Mater. Interfaces 2018, 10, 11474–11485. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Sevencan, C.; Bay, B.H.; Xie, J.; Zhang, Y.; Demokritou, P.; Leong, D.T. Nano-TiO2 Drives Epithelial–Mesenchymal Transition in Intestinal Epithelial Cancer Cells. Small 2018, 14, 1800922. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Yourick, J.J.; Sprando, R.L.; Gao, X. Toxicogenomic responses of human liver HepG2 cells to silver nanoparticles. J. Appl. Toxicol. 2015, 35, 1160–1168. [Google Scholar] [CrossRef]
- Ventura, X.; Pereira, J.F.S.; Matos, P.; Marques, B.; Jordan, P.; Sousa-Uva, A.; Silva, M.J. Cytotoxicity and genotoxicity of MWCNT-7 and crocidolite: Assessment in alveolar epithelial cells versus their coculture with monocyte-derived macrophages. Nanotoxicology 2020, 4, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Shen, J.; Qin, C.Y.; Li, Y.M.; Gao, L.J.; Zheng, J.; Feng, Y.L.; Yan, Z.; Zhou, X.; Cao, J.M. Platinum nanoparticles promote breast cancer cell metastasis by disrupting endothelial barrier and inducing intravasation and extravasation. Nano Res. 2022, 15, 7366–7377. [Google Scholar] [CrossRef]
- Matysiak-Kucharek, M.; Sawicki, K.; Kurzepa, J.; Wojtyła-Buciora, P.; Kapka-Skrzypczak, L. The influence of silver nanoparticles on the proces of epithelial-mesenchymal transition in the context of cancer metastases. Med. Pr. Work. Health Saf. 2023, 74, 541–548. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, Y.; Yan, J.; Zhang, Y.; Mo, L.; Zhang, Q. MMP-3 activation is involved in copper oxide nanoparticle-induced epithelial-mesenchymal transition in human lung epithelial cells. Nanotoxicology 2021, 15, 1380–1402. [Google Scholar] [CrossRef]
- Yuan, J.; Mo, Y.; Zhang, Y.; Zhang, Y.; Zhang, Q. Nickel nanoparticles induce epithelial-mesenchymal transition in human bronchial epithelial cells via the HIF-1α/HDAC3 pathway. Nanotoxicology 2022, 16, 695–712. [Google Scholar] [CrossRef]
- Lüönd, F.; Sugiyama, N.; Bill, R.; Bornes, L.; Hager, C.; Tang, F.; Santacroce, N.; Beisel, C.; Ivanek, R.; Burglin, T.; et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 2021, 56, 3203-3221.e11. [Google Scholar] [CrossRef]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef]
- Yuan, J.; Mo, Y.; Zhang, Y.; Zhang, Y.; Zhang, Q. HMGB1 derived from lung epithelial cells after cobalt nanoparticle exposure promotes the activation of lung fibroblasts. Nanotoxicology 2024, 18, 565–581. [Google Scholar] [CrossRef]
- Matysiak-Kucharek, M.; Czajka, M.; Jodłowska-Jędrych, B.; Sawicki, K.; Wojtyła-Buciora, P.; Kruszewski, M.; Kapka-Skrzypczak, L. Two Sides to the Same Coin—Cytotoxicity vs. Potential Metastatic Activity of AgNPs Relative to Triple-Negative Human Breast Cancer MDA-MB-436 Cells. Molecules 2020, 25, 2375. [Google Scholar] [CrossRef]
- Zhou, L.; Forman, H.J.; Ge, Y.; Lunec, J. Multi-walled carbon nanotubes: A cytotoxicity study in relations to functionalization, dose and dispertion. Toxicol. Vitr. 2017, 42, 292–298. [Google Scholar] [CrossRef]
- Zhao, X.; Chang, S.; Long, J.; Li, J.; Li, X.; Cao, Y. The toxicity of multi-walled carbon nanotubes (MWCNTs) to human endothelial cells: The influence of diameters of MWCNTs. Food Chem. Toxicol. 2019, 126, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Meindl, C.; Höfler, A.; Leitinger, G.; Roblegg, E. Combination of small size and carboxyl functionalisation causes cytotoxicity of short carbon nanotubes. Nanotoxicology 2012, 7, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Fuijta, K.; Obara, S.; Maru, J.; Endoh, S. Cytotoxicity profiles of multi-walled carbon nanotubes with diffrent physico-chemical properties. Toxicol. Mech. Methods 2020, 30, 277–489. [Google Scholar]
- Fahrenholtz, C.D.; Ding, S.; Bernish, B.W.; Wright, M.L.; Zheng, Y.; Yang, M.; Yao, X.; Donati, G.L.; Gross, M.D.; Bierbach, U.; et al. Design and cellular studies of a carbon nanotube-based delivery system for a hybrid platinum-acridine anticancer agent. J. Inorg. Biochem. 2016, 165, 170–180. [Google Scholar] [CrossRef]
- Sharma, S.; Naskar, S.; Kuotsu, K. Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: Synthesis, characterization and in vitro toxicity in human breast cancer. Carbon Lett. 2020, 30, 435–447. [Google Scholar] [CrossRef]
- Badea, N.; Craciun, M.M.; Drogomir, A.S.; Balas, M.; Dinischiotu, A.; Nistor, C.; Gavan, C.; Ionita, D. Systems based on carbon nanotubes with potential in cancer therapy. Mater. Chem. Phys. 2020, 241, 122435. [Google Scholar] [CrossRef]
- Ozgen, P.S.O.; Atasoy, S.; Kurt, B.Z.; Durmus, Z.; Yigit, G.; Dag, A. Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy. J. Mater. Chem. B 2020, 8, 3123. [Google Scholar] [CrossRef]
- Badea, M.A.; Prodana, M.; Dinischiotu, A.; Crihana, C.; Ionita, D.; Balas, M. Cisplatin Loaded Multiwalled Carbon Nanotubes Induce Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2018, 10, 228. [Google Scholar] [CrossRef]
- Kavousi, M.; Chavoshi, M.S. Effect of coated carbon nanotubes with chitosan and cover of flaxseed in the induction of MDA-MB-231 apoptosis by analyzing the expression of Bax and Bcl-2. Meta Gene 2020, 26, 100807. [Google Scholar] [CrossRef]
- Salih, S.J.; Ghobadi, M.Z. Evaluating the cytotoxicity and pathogenicity of multi-walled carbon nanotube through weighted gene co-expression network analysis: A nanotoxicogenomics study. BMC Genom. Data 2022, 23, 12. [Google Scholar] [CrossRef]
- Sabido, O.; Figarol, A.; Klein, J.P.; Bin, V.; Forest, V.; Pourchez, J.; Fubini, B.; Cottier, M.; Tomatis, M.; Boudard, D. Quantitative Flow Cytometric Evaluation of Oxidative Stress and Mitochondrial Impairment in RAW 264.7 Macrophages after Exposure to Pristine, Acid Functionalized, or Annealed Carbon Nanotubes. Nanomaterials 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Akinoglu, E.M.; Ozbilgin, K.; Sonmez, P.K.; Ozkut, M.M.; Giersig, M.; Inan, S.; Gumustepe, E.; Kurtman, C. Biocompatibility of vertically aligned multi-walled carbon nanotube scaffolds for human breast cancer cell line MDA-MB-231. Prog. Biomater. 2017, 6, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.G.; Wailes, E.M.; Levi-Polyachenko, N.H. Multi-Walled Carbon Nanotubes Inhibit Breast Cancer Cell Migration. J. Biomed. Nanotechnol. 2016, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, B.; Zheng, J.; Yu, M.; Zhou, T.; Zhao, K.; Jia, Y.; Gao, X.; Chen, C.; Wei, T. The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials 2014, 35, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Qian, P.; Lu, X.; Sun, B.; Zhang, X.; Wang, L.; Gao, X.; Li, H.; Chen, Z.; et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat. Commun. 2015, 6, 5988. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Garcia-Hevia, L.; Valiente, R.; Fernandez-Luna, J.L.; Flahaut, E.; Rodriquez-Fernandez, L.; Villegas, J.C.; Gonzales, J.; Fanarraga, M.L. Inhibition of Cancer Cell Migration by Multiwalled Carbon Nanotubes. Adv. Health Mater. 2015, 4, 1640–1644. [Google Scholar] [CrossRef]
- Yao, H.J.; Zhang, Y.G.; Sun, L.; Liu, Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastrin cancer stem cells. Biomaterials 2014, 35, 9208–9223. [Google Scholar] [CrossRef]
- Polimeni, M.; Gulino, G.R.; Gazzano, E.; Kopecka, J.; Marucco, A.; Fenoglio, I.; Cesano, F.; Campagnolo, L.; Magrini, A.; Pietroiusti, A.; et al. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway. Part. Fibre Toxicol. 2016, 13, 27. [Google Scholar] [CrossRef]
- Chen, P.; Tian, K.; Tu, W.; Zhang, Q.; Han, L.; Zhou, X. Sirtuin 6 inhibits MWCNTs-induced epithelial-mesenchymal transition in human bronchial epithelial cells via inactivating TGF-β1/Smad2 signaling pathway. Toxicol. Appl. Pharmacol. 2019, 374, 1–10. [Google Scholar] [CrossRef]
- Wang, P.; Voronkova, M.; Luanpitpong, S.; He, X.; Riedel, H.; Dinu, C.Z.; Wang, L.; Rojanasakul, Y. Induction of Slug by Chronic Exposure to Single-Walled Carbon Nanotubes Promotes Tumor Formation and Metastasis. Chem. Res. Toxicol. 2017, 30, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Zhang, G.; Zhang, X.; Jia, Z.; Gao, X.; Jiang, Y.; Yan, C.; Duerksen-Hughes, P.J.; Chen, F.F.; Li, H.; et al. Proteomic Analysis of Cellular Response Induced by Multi-Walled Carbon Nanotubes Exposure in A549 Cells. PLoS ONE 2014, 9, e84974. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Stueckle, T.A.; Park, J.; Tse, W.; Dinu, C.Z.; Rojanasakul, Y. Role of H-Ras/ERK signaling in carbon nanotube-induced neoplastic-like transformation of human mesothelial cells. Front. Physiol. 2014, 5, 222. [Google Scholar] [CrossRef]
- Turini, S.; Bergandi, L.; Gazzano, E.; Prado, M.; Alderi, E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int. J. Mol. Sci. 2019, 20, 150. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B.; Xu, M.; Liu, R.; Xia, T.; Zhang, Z.; Xu, Y.; Liu, S. Graphene Oxide Promotes Cancer Metastasis through Associating with Plasma Membrane to Promote TGF-β Signaling-Dependent Epithelial–Mesenchymal Transition. ACS Nano 2020, 14, 818–827. [Google Scholar] [CrossRef]
- Vetti, G.; Lison, D.; Van den Brule, S. Mechanisms of lung fibrosis induced by carbon nanotubes: Towards an Adverse Outcome Pathway (AOP). Part. Fibre Toxicol. 2016, 13, 11. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Myofibroblasts and lung fibrosis induced by carbon nanotube exposure. Part. Fibre Toxicol. 2016, 13, 60. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis. Front. Immunol. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Duke, K.S.; Bonner, J.C. Mechanisms of carbon nanotube-induced pulmonary fibrosis: A physicochemical characteristic perspective. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1498. [Google Scholar] [CrossRef]
- Arnoldussen, Y.J.; Skaug, V.; Aleksandersen, M.; Ropstad, E.; Anmarkrud, K.H.; Einarsdottir, E.; Chin-Lin, F.; Bjorklund, C.G.; Kasem, M.; Eilertsen, E.; et al. Inflammation in the pleural cavity following injection of multi-walled carbon nanotubes is dependent on their characteristics and the presence of IL-1 genes. Nanotoxicology 2018, 12, 522–538. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, S.; Lee, A.Y.; Seo, H.W.; Chae, C.; Cho, M.H. Intratracheal exposure to multi-walled carbon nanotubes induces a nonalcoholic steatohepatitis-like phenotype in C57BL/6J mice. Nanotoxicology 2015, 9, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Wang, X.; Li, X.; Tian, P.; Xu, H.; Li, Z.; Wang, L. Co-exposure to multi-walled carbon nanotube and lead ions aggravates hepatotoxicity of nonalcoholic fatty liver via inhibiting AMPK/PPARγ pathway. Aging 2020, 12, 14189–14204. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, Y.; Bai, R.; Wu, Z.; Qian, W.; Yang, L.; Cai, R.; Yan, H.; Li, T.; Pandey, V.; et al. Long-term pulmonary exposure to multi-walled carbon nanotubes promotes breast cancer metastatic cascades. Nat. Nanotechnol. 2019, 14, 719–727. [Google Scholar] [CrossRef]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Asl, F.S.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The impact of epithelial-mesenchymal transition (EMT) induced by metabolic processes and intracellular signaling pathways on chemo-resistance, metastasis, and recurrence in solid tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef]
- Deng, F.; Weng, Y.; Li, X.; Wang, T.; Fan, M.; Shi, Q. Overexpression of IL-8 promotes cell migration via PI3K-Akt signaling pathway and EMT in triple-negative breast cancer. Pathol. Res. Pract. 2021, 223, 152824. [Google Scholar] [CrossRef]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef]
- Keeley, T.; Costanzo-Garvey, D.L.; Cook, L.M. Unmasking the Many Faces of Tumor-Associated Neutrophils and Macrophages: Considerations for Targeting Innate Immune Cells in Cancer. Trends Cancer 2019, 5, 789–798. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef]
- Cai, J.; Cui, Y.; Yang, J.; Wang, S. Epithelial-mesenchymal transition: When tumor cells meet myeloid-derived supressor cells. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1876, 188564. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J. The impact of multi-walled carbon nanotubes (MWCNTs) on macrophages: Contribution of MWCNT characteristics. Sci. China Life Sci. 2018, 61, 1333–1351. [Google Scholar] [CrossRef]
- Ma, J.; Li, R.; Qu, G.; Liu, H.; Yan, B.; Xia, T.; Liu, Y.; Liu, S. Carbon nanotubes stimulate synovial inflammation by inducing systemic pro-inflammatory cytokines. Nanoscale 2016, 8, 18070–18086. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, L.; Zhu, D.; Zhang, H.; Li, Y.; Leng, X. Effects of Carboxylated Multiwalled Carbon Nanotubes on the Function of Macrophages. J. Nanomater. 2015, 2015, 638760. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, D.; Zhang, Z.; Li, X.; Shi, J.; Sun, J.; Liu, H.Z.; Li, X.; Zhou, M.; Zheng, T. The pan-cancer landscape of crosstalk between epithelial-mesenchymal transition and immune evasion relevant to prognosis and immunotherapy response. npj Precis. Oncol. 2021, 5, 56. [Google Scholar] [CrossRef]
- Brzóska, K.; Męczyńska-Wielgosz, S.; Stępkowski, T.M.; Kruszewski, M. Adaptation of HepG2 cells to silver nanoparticles-induced stress is based on the pro-proliferative and anti-apoptotic changes in gene expression. Mutagenesis 2015, 30, 431–439. [Google Scholar] [CrossRef]
- Hosseini, A.; Baeeri, M.; Rahimifard, M.; Navaei-Nigjeh, M.; Mohammadirad, A.; Pourkhalili, N.; Hassani, S.; Kamali, M.; Abdollahi, M. Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum. Exp. Toxicol. 2013, 32, 544. [Google Scholar] [CrossRef]
- Goncalves, D.M.; Girard, D.; Goncalves, D. Zinc oxide nanoparticles delay human neutrophilapoptosis by a de novo protein synthesis-dependent and reactive oxygen species-independent mechanism. Toxicol. Vitr. 2014, 28, 926–931. [Google Scholar] [CrossRef]
- Cao, Z.; Livas, T.; Kyprianou, N. Anoiks and EMT: Lethal “Liaisons” during Cancer Progression. Crit. Rev. Oncog. 2016, 21, 155–168. [Google Scholar] [CrossRef]
- Assani, G.; Zhou, Y. Effect of modulation of epithelial mesenchymal transition regulators Snail1 and Snail2 on cancer cell radiosensitivity by targeting of the cell cycle, cell apoptosis and cell migration/invasion. Oncol. Lett. 2018, 17, 23–30. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mir, K.B.; Seligson, N.D.; Nayak, D.; Kumar, R.; Goswami, A. Integration of EMT and cellular survival instincts in reprogramming of programmed cell death to anastasis. Cancer Metastasis Rev. 2020, 39, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Guzman, E.; Balasanyan, V.; Conner, C.M.; Wong, K.; Zhou, H.R.; Kosik, K.S.; Montell, D.J. A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J. Cell Biol. 2017, 216, 3355–3368. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.D.; Zerbi, G.; Lucotti, A.; Catelani, T.; Mantecca, P. Carbon nanotubes: Structural defects as stressors inducing lung cell toxicity. Chem. Biol. Interact. 2023, 382, 110613. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I.; et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2009, 21, 1698–1705. [Google Scholar] [CrossRef]

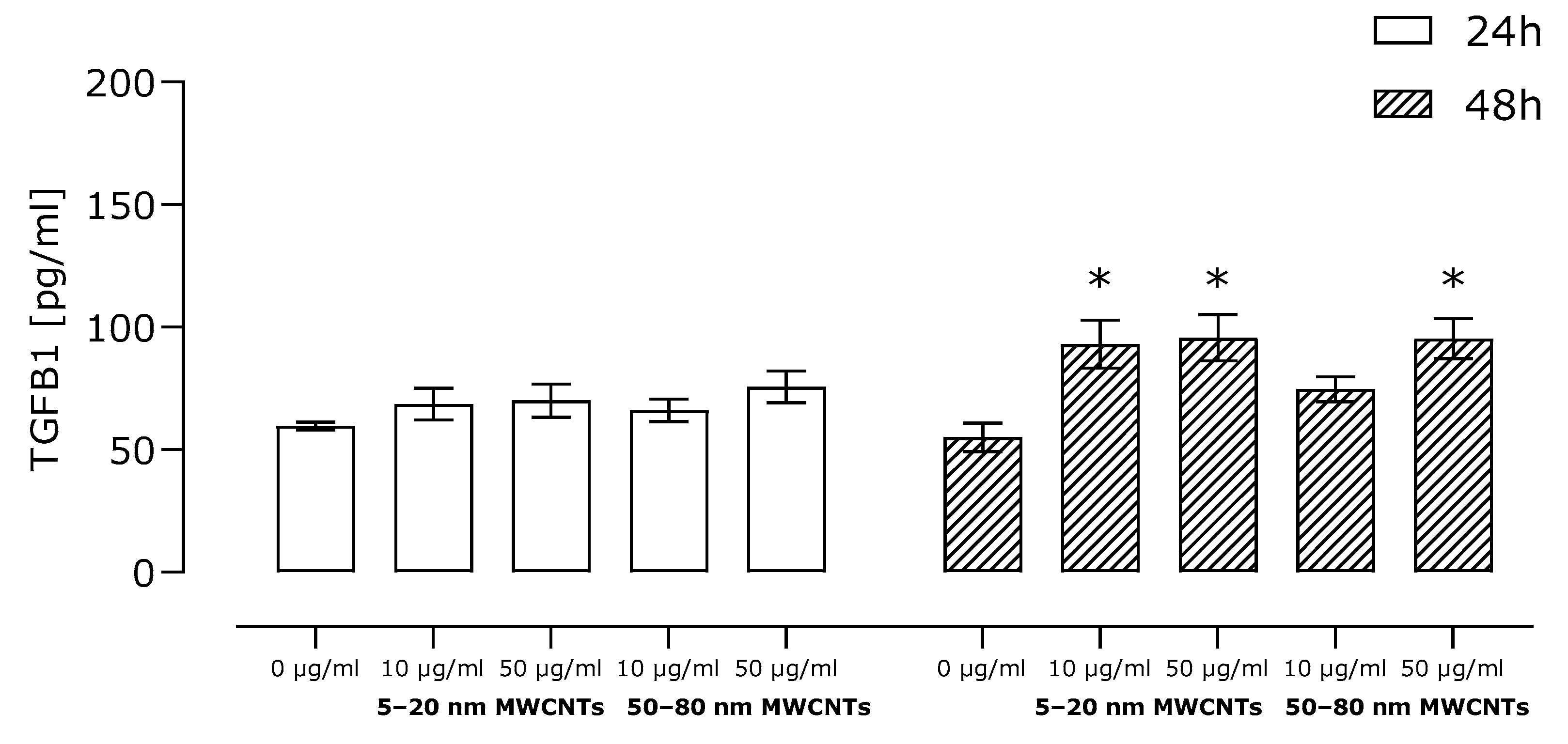

| MTT | ||||
|---|---|---|---|---|
| 5–20 nm MWCNTs | 50–80 nm MWCNTs | |||
| 24 h | 48 h | 24 h | 48 h | |
| 10 µg/mL | 81 ± 6 * | 73 ± 4 * | 90 ± 3 | 70 ± 4 * |
| 25 µg/mL | 67 ± 4 * | 67 ± 5 * | 78 ± 6 * | 60 ± 5 * |
| 50 µg/mL | 67 ± 6 * | 60 ± 6 * | 69 ± 7 * | 56 ± 6 * |
| 100 µg/mL | 69 ± 5 * | 57 ± 5 * | 63 ± 8 * | 48 ± 3 * |
| NR | ||||
|---|---|---|---|---|
| 5–20 nm MWCNTs | 50–80 nm MWCNTs | |||
| 24 h | 48 h | 24 h | 48 h | |
| 10 µg/mL | 83 ± 1 * | 83 ± 3 * | 96 ± 2 | 94 ± 3 |
| 25 µg/mL | 80 ± 4 * | 80 ± 5 * | 88 ± 7 | 85 ± 4 |
| 50 µg/mL | 80 ± 6 * | 81 ± 1 * | 88 ± 4 | 74 ± 4 * |
| 100 µg/mL | 72 ± 5 * | 67 ± 4 * | 83 ± 7 * | 67 ± 11 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matysiak-Kucharek, M.; Sawicki, K.; Kruszewski, M.; Kurzepa, J.; Kapka-Skrzypczak, L. Prometastatic Potential of Non-Functionalized Multiwalled Carbon Nanotubes in the MDA-MB-436 Breast Cancer Cell Line Model. Int. J. Mol. Sci. 2025, 26, 2777. https://doi.org/10.3390/ijms26062777
Matysiak-Kucharek M, Sawicki K, Kruszewski M, Kurzepa J, Kapka-Skrzypczak L. Prometastatic Potential of Non-Functionalized Multiwalled Carbon Nanotubes in the MDA-MB-436 Breast Cancer Cell Line Model. International Journal of Molecular Sciences. 2025; 26(6):2777. https://doi.org/10.3390/ijms26062777
Chicago/Turabian StyleMatysiak-Kucharek, Magdalena, Krzysztof Sawicki, Marcin Kruszewski, Jacek Kurzepa, and Lucyna Kapka-Skrzypczak. 2025. "Prometastatic Potential of Non-Functionalized Multiwalled Carbon Nanotubes in the MDA-MB-436 Breast Cancer Cell Line Model" International Journal of Molecular Sciences 26, no. 6: 2777. https://doi.org/10.3390/ijms26062777
APA StyleMatysiak-Kucharek, M., Sawicki, K., Kruszewski, M., Kurzepa, J., & Kapka-Skrzypczak, L. (2025). Prometastatic Potential of Non-Functionalized Multiwalled Carbon Nanotubes in the MDA-MB-436 Breast Cancer Cell Line Model. International Journal of Molecular Sciences, 26(6), 2777. https://doi.org/10.3390/ijms26062777









