Antiviral Effects of Tecovirimat and Cellular Ultrastructural Changes in Human Bronchial Epithelial Cell Line Following Monkeypox Virus Infection
Abstract
1. Introduction
2. Results
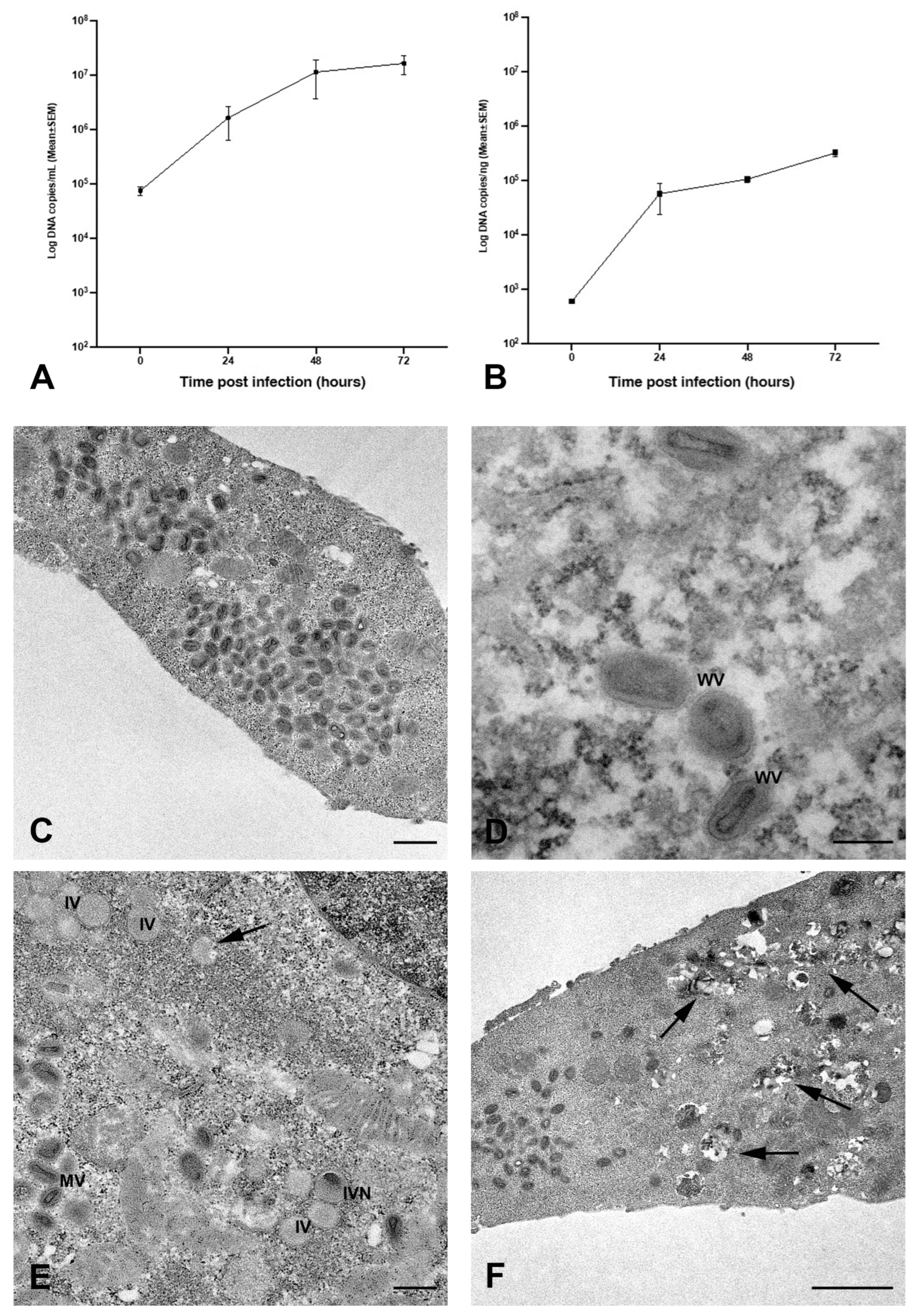
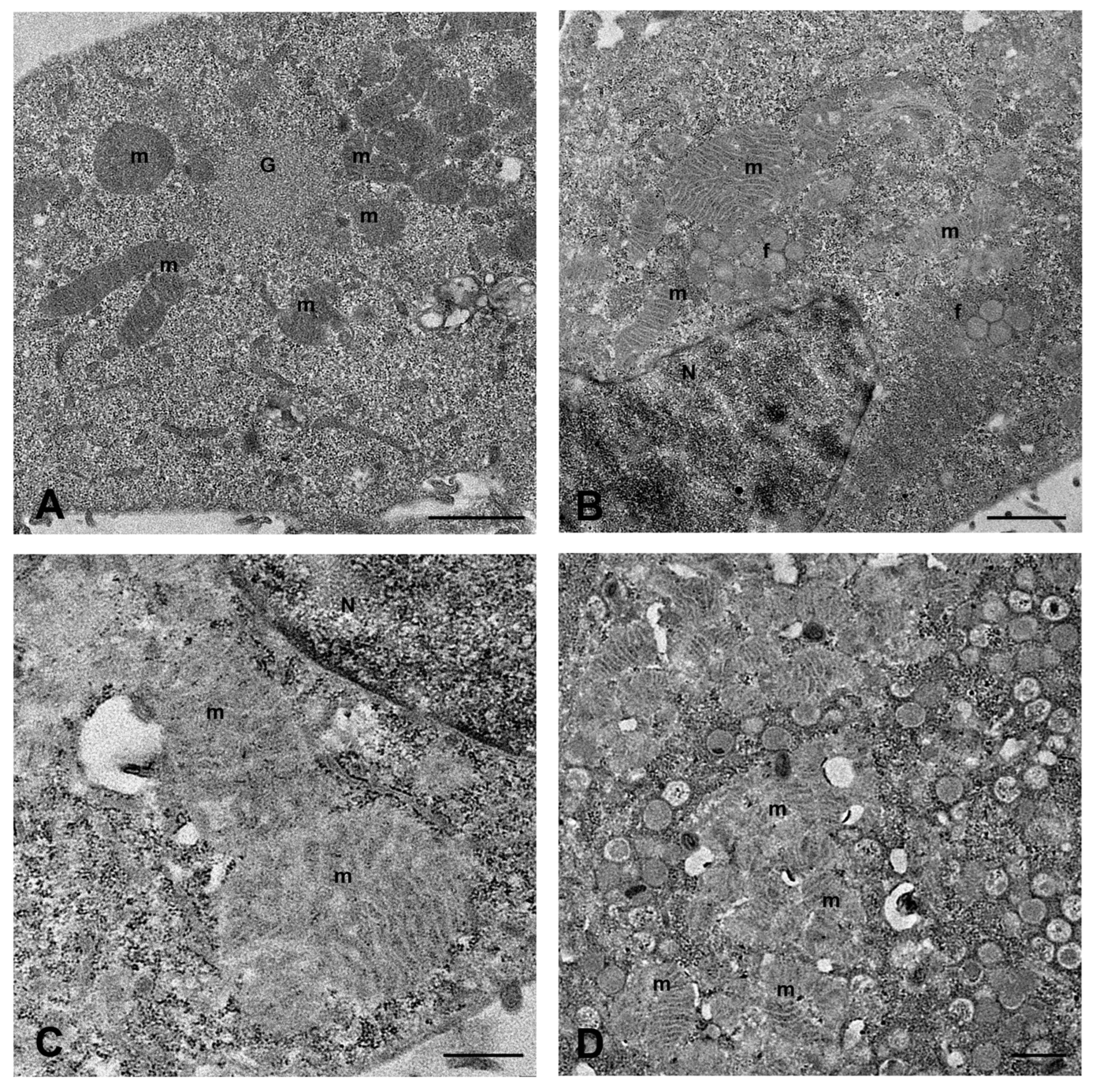
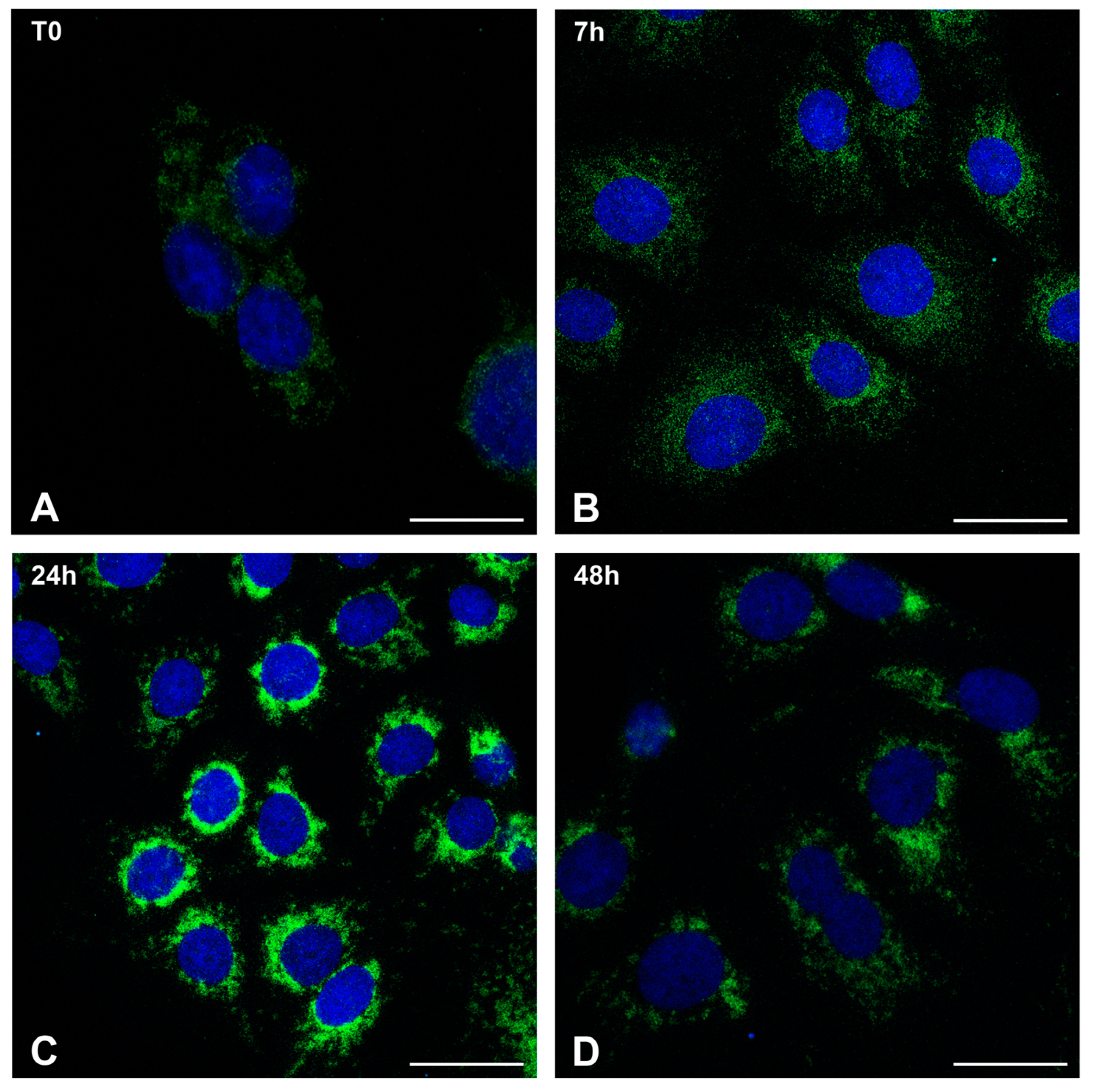
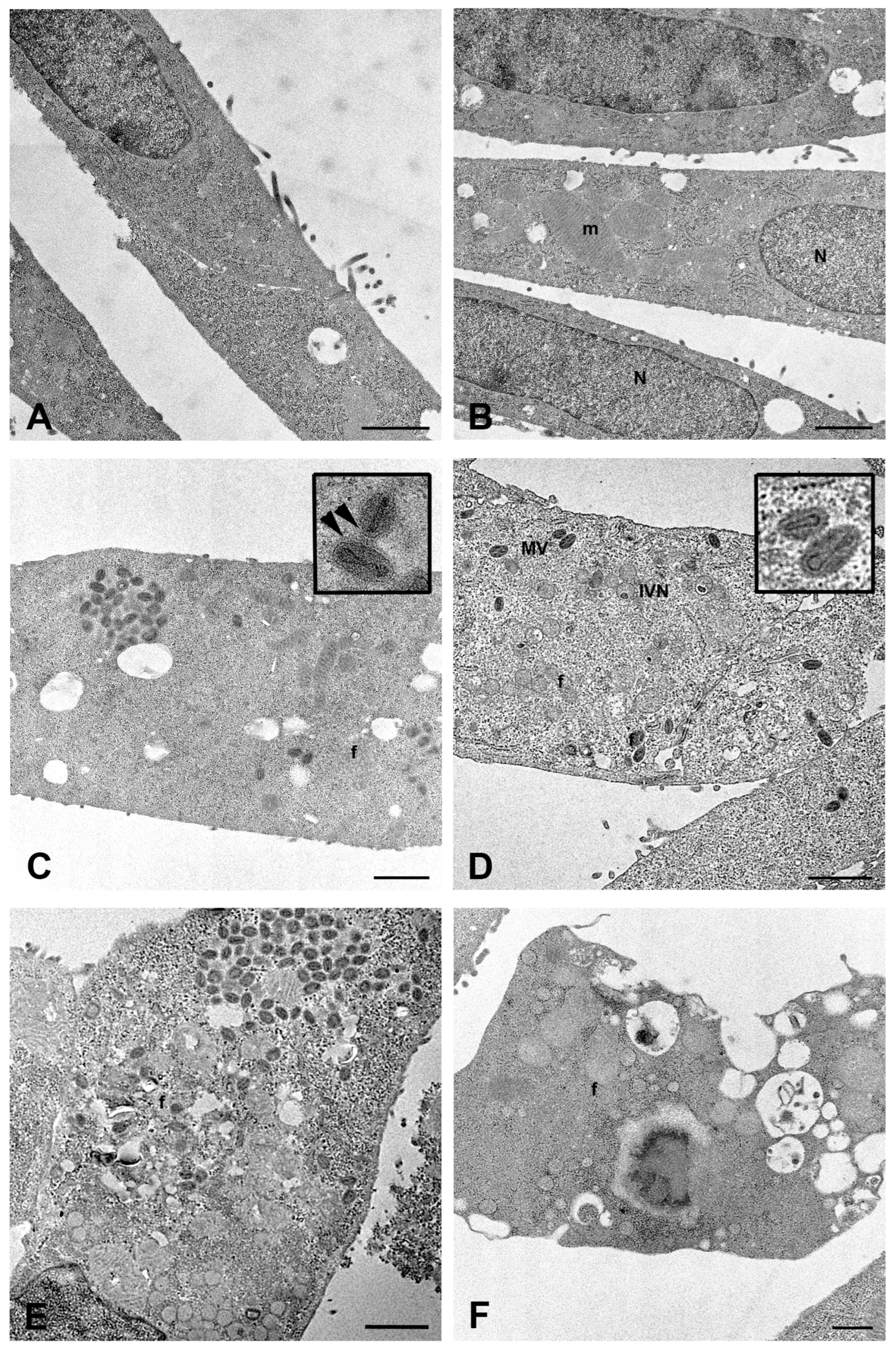
2.1. MPXV Infection of Calu-3 Cells and Analysis of Cell Ultrastructure
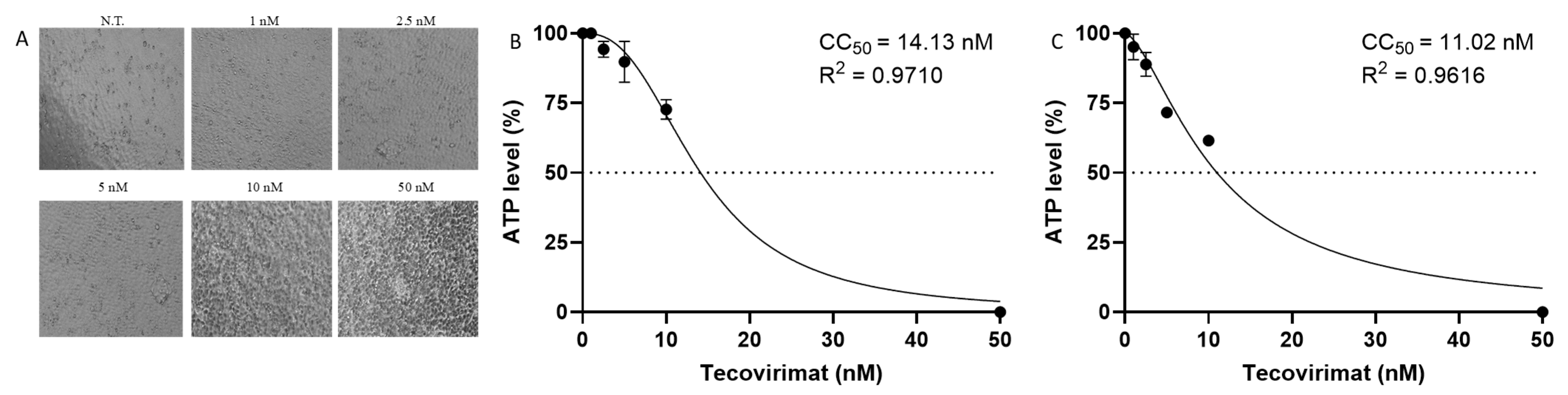
2.2. Tecovirimat Effect on Calu-3 Cells Metabolism
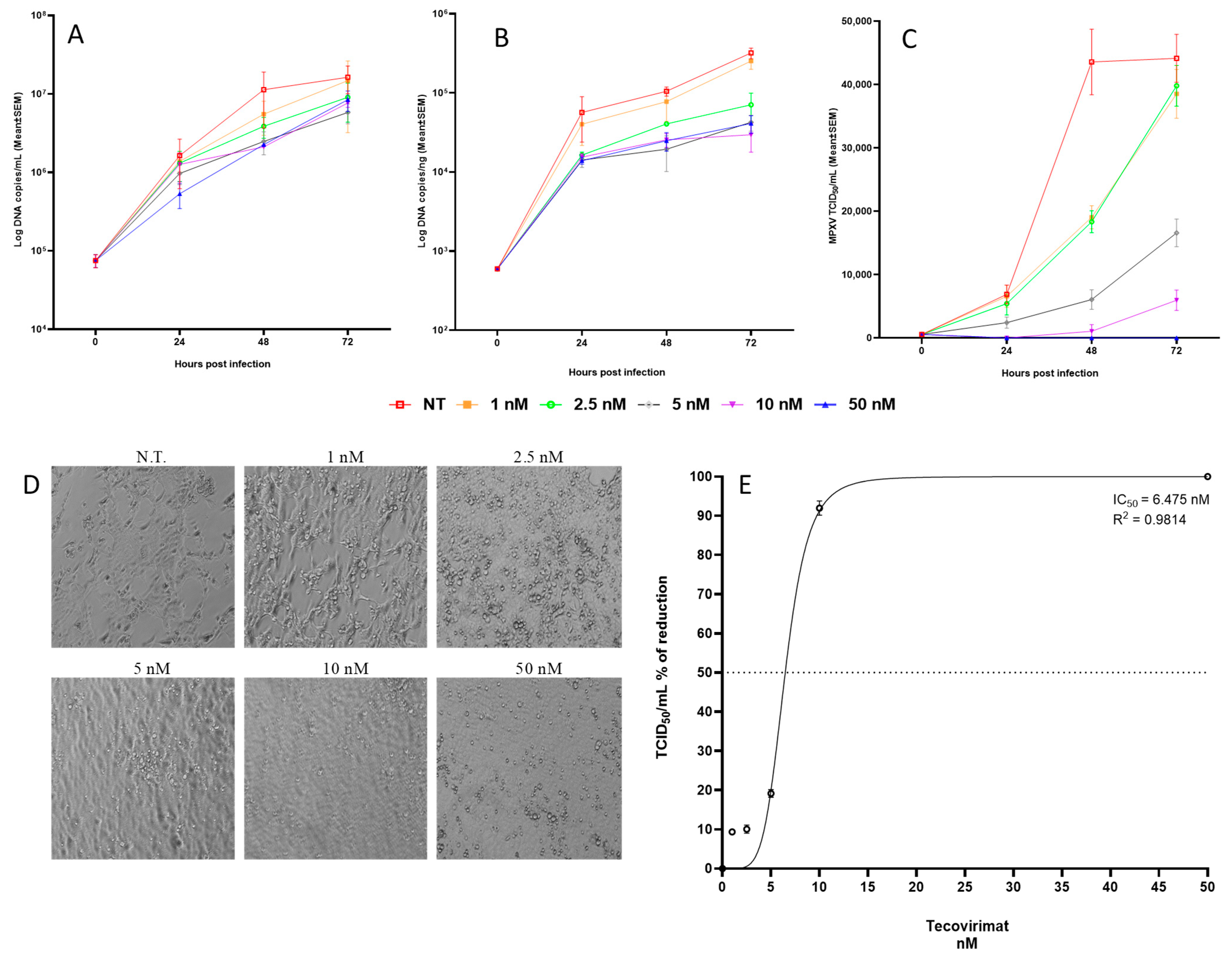
2.3. Tecovirimat Antiviral Activity on MPXV-Infected Calu-3 Cells
3. Discussion
4. Materials and Methods
4.1. Cell and Virus Stock Preparation
4.2. In Vitro Infection Experiments and Tecovirimat Antiviral Activity
4.3. Viral Infectivity
4.4. Detection of MPXV DNA by Real-Time PCR
4.5. Studies of Tecovirimat Effects on Cell Survival
4.6. Dose–Response Curve
4.7. Transmission Electron Microscopy Analysis
4.8. Confocal Microscopy Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ICoToV. Virus Taxonomy: 2023 Release. Available online: https://talk.ictvonline.org/taxonomy (accessed on 11 July 2023).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- World Health Organization. Mpox (Monkeypox) Outbreak 2022-Global. 2022. Available online: https://www.who.int/emergencies/situations/monkeypox-oubreak-2022 (accessed on 11 July 2023).
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro. Surveill. 2022, 27, 2200421. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef]
- Adepoju, P. Mpox declared a public health emergency. Lancet 2024, 404, e1–e2. [Google Scholar] [CrossRef]
- Russo, A.T.; Grosenbach, D.W.; Chinsangaram, J.; Honeychurch, K.M.; Long, P.G.; Lovejoy, C.; Maiti, B.; Meara, I.; Hruby, D.E. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert. Rev. Anti Infect. Ther. 2021, 19, 331–344. [Google Scholar] [CrossRef]
- Byrareddy, S.N.; Sharma, K.; Sachdev, S.; Reddy, A.S.; Acharya, A.; Klaustermeier, K.M.; Lorson, C.L.; Singh, K. Potential therapeutic targets for Mpox: The evidence to date. Expert. Opin. Ther. Targets. 2023, 27, 419–431. [Google Scholar] [CrossRef]
- Warner, B.M.; Klassen, L.; Sloan, A.; Deschambault, Y.; Soule, G.; Banadyga, L.; Cao, J.; Strong, J.E.; Kobasa, D.; Safronetz, D. In vitro and in vivo efficacy of tecovirimat against a recently emerged 2022 monkeypox virus isolate. Sci. Transl. Med. 2022, 14, eade7646. [Google Scholar] [CrossRef]
- DeLaurentis, C.E.; Kiser, J.; Zucker, J. New Perspectives on Antimicrobial Agents: Tecovirimat for Treatment of Human Monkeypox Virus. Antimicrob. Agents Chemother. 2022, 66, e0122622. [Google Scholar] [CrossRef]
- Smith, S.K.; Olson, V.A.; Karem, K.L.; Jordan, R.; Hruby, D.E.; Damon, I.K. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob. Agents Chemother. 2009, 53, 1007–1012. [Google Scholar] [CrossRef]
- Smee, D.F. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir. Chem. Chemother. 2008, 19, 115–124. [Google Scholar] [CrossRef]
- Frenois-Veyrat, G.; Gallardo, F.; Gorgé, O.; Marcheteau, E.; Ferraris, O.; Baidaliuk, A.; Favier, A.L.; Enfroy, C.; Holy, X.; Lourenco, J.; et al. Tecovirimat is effective against human monkeypox virus in vitro at nanomolar concentrations. Nat. Microbiol. 2022, 7, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Alemany, A.; Marks, M.; Lezama Mora, J.I.; Rodríguez-Aldama, J.C.; Torres Silva, M.S.; Corral Herrera, E.A.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in people with advanced HIV infection: A global case series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef]
- Pinnetti, C.; Cimini, E.; Mazzotta, V.; Matusali, G.; Vergori, A.; Mondi, A.; Rueca, M.; Batzella, S.; Tartaglia, E.; Bettini, A.; et al. Mpox as AIDS-defining event with a severe and protracted course: Clinical, immunological, and virological implications. Lancet Infect. Dis. 2024, 24, e127–e135. [Google Scholar] [CrossRef]
- Witt, A.S.A.; Trindade, G.S.; Souza, F.G.; Serafim, M.S.M.; da Costa, A.V.B.; Silva, M.V.F.; de Melo Iani, F.C.; Rodrigues, R.A.L.; Kroon, E.G.; Abrahão, J.S. Ultrastructural analysis of monkeypox virus replication in Vero cells. J. Med. Virol. 2023, 95, e28536. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Reidy, J.; Pagani, N.; Lednicky, J.A.; McGrail, J.P.; Kasminskaya, Y.; Patino, L.H.; Garcia-Sastre, A.; Palacios, G.; Gonzalez-Reiche, A.S.; et al. Genomic and ultrastructural analysis of monkeypox virus in skin lesions and in human/animal infected cells reveals further morphofunctional insights into viral pathogenicity. J. Med. Virol. 2023, 95, e28878. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S.; et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 2005, 79, 13139–13149. [Google Scholar] [CrossRef]
- Huggins, J.; Goff, A.; Hensley, L.; Mucker, E.; Shamblin, J.; Wlazlowski, C.; Johnson, W.; Chapman, J.; Larsen, T.; Twenhafel, N.; et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 2009, 53, 2620–2625. [Google Scholar] [CrossRef]
- Perry, M.R.; Warren, R.; Merchlinsky, M.; Houchens, C.; Rogers, J.V. Rabbitpox in New Zealand White Rabbits: A Therapeutic Model for Evaluation of Poxvirus Medical Countermeasures Under the FDA Animal Rule. Front. Cell Infect. Microbiol. 2018, 8, 356. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Buller, R.M.; Parker, S.; Keith, K.A.; Hruby, D.E.; Jordan, R.; Kern, E.R. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 2007, 51, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; King, D.S.; Mosier, S.; Jordan, R.; Jones, K.F.; Hruby, D.E.; Grosenbach, D.W. ST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation. Antimicrob. Agents Chemother. 2009, 53, 4999–5009. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Berhanu, A.; King, D.S.; Mosier, S.; Jones, K.F.; Jordan, R.A.; Bolken, T.C.; Hruby, D.E. Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice. Proc. Natl. Acad. Sci. USA 2010, 107, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Chen, N.G.; Foster, S.; Hartzler, H.; Hembrador, E.; Hruby, D.; Jordan, R.; Lanier, R.; Painter, G.; Painter, W.; et al. Evaluation of disease and viral biomarkers as triggers for therapeutic intervention in respiratory mousepox-an animal model of smallpox. Antiviral Res. 2012, 94, 44–53. [Google Scholar] [CrossRef]
- Sherwat, A.; Brooks, J.T.; Birnkrant, D.; Kim, P. Tecovirimat and the Treatment of Monkeypox-Past, Present, and Future Considerations. N. Engl. J. Med. 2022, 387, 579–581. [Google Scholar] [CrossRef]
- Barreto-Vieira, D.F.; Miranda, M.D.; da Silva, M.A.N.; de Almeida, A.S.; de Almeida, A.L.T.; Bandeira, D.M.; Ferreira, V.N.S.; Rosa, A.S.; Girard-Dias, W.; Archanjo, B.S.; et al. MPXV: Update on Morphological and Morphogenesis Aspects Through Transmission and Scanning Electron Microscopies and 3D Reconstruction. J. Med. Virol. 2025, 97, e70180. [Google Scholar] [CrossRef]
- Gould, S.; Atkinson, B.; Onianwa, O.; Spencer, A.; Furneaux, J.; Grieves, J.; Taylor, C.; Milligan, I.; Bennett, A.; Fletcher, T.; et al. Air and surface sampling for monkeypox virus in a UK hospital: An observational study. Lancet Microbe. 2022, 3, e904–e911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, B.U. Airborne transmission of MPXV and its aerosol dynamics under different viral load conditions. Lancet Microbe. 2023, 4, e288–e289. [Google Scholar] [CrossRef]
- Mazzotta, V.; Nozza, S.; Lanini, S.; Moschese, D.; Tavelli, A.; Rossotti, R.; Fusco, F.M.; Biasioli, L.; Matusali, G.; Raccagni, A.R.; et al. Clinical and laboratory predictors of mpox severity and duration: An Italian multicentre cohort study (mpox-Icona). EBioMedicine 2024, 107, 105289. [Google Scholar] [CrossRef]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Hughes, C.M.; Kabamba, J.; Malekani, J.; et al. Extended Human-to-Human Transmission during a Monkeypox Outbreak in the Democratic Republic of Congo. Emerg. Infect. Dis. 2016, 22, 1014–1021. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Mohamed, M.G.; Dabou, E.A.; Abuijlan, I.; Chandran, D.; El-Shall, N.A.; Chopra, H.; Dhama, K. Monkeypox (mpox) in immunosuppressed patients. F1000Research 2023, 12, 127. [Google Scholar] [CrossRef]
- Beeson, A.; Styczynski, A.; Hutson, C.L.; Whitehill, F.; Angelo, K.M.; Minhaj, F.S.; Morgan, C.; Ciampaglio, K.; Reynolds, M.G.; McCollum, A.M.; et al. Mpox respiratory transmission: The state of the evidence. Lancet Microbe. 2023, 4, e277–e283. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Götz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef]
- Ma, A.; Langer, J.; Hanson, K.E.; Bradley, B.T. Characterization of the Cytopathic Effects of Monkeypox Virus Isolated from Clinical Specimens and Differentiation from Common Viral Exanthems. J. Clin. Microbiol. 2022, 60, e0133622. [Google Scholar] [CrossRef]
- Moltrasio, C.; Boggio, F.L.; Romagnuolo, M.; Cagliani, R.; Sironi, M.; Di Benedetto, A.; Marzano, A.V.; Leone, B.E.; Vergani, B. Monkeypox: A Histopathological and Transmission Electron Microscopy Study. Microorganisms 2023, 11, 1781. [Google Scholar] [CrossRef]
- Müller, M.; Ingold-Heppner, B.; Stocker, H.; Heppner, F.L.; Dittmayer, C.; Laue, M. Electron microscopy images of monkeypox virus infection in 24-year-old man. Lancet 2022, 400, 1618. [Google Scholar] [CrossRef]
- Bruno, S.R.; Anathy, V. Lung epithelial endoplasmic reticulum and mitochondrial 3D ultrastructure: A new frontier in lung diseases. Histochem. Cell Biol. 2021, 155, 291–300. [Google Scholar] [CrossRef]
- Rowlands, D.J. Mitochondria dysfunction: A novel therapeutic target in pathological lung remodeling or bystander? Pharmacol. Ther. 2016, 166, 96–105. [Google Scholar] [CrossRef]
- Zhan, B.; Shen, J. Mitochondria and their potential role in acute lung injury (Review). Exp. Ther. Med. 2022, 24, 479. [Google Scholar] [CrossRef]
- Bian, Q.; Lu, J.; Zhang, L.; Chi, Y.; Li, Y.; Guo, H. Highly pathogenic avian influenza A virus H5N1 non-structural protein 1 is associated with apoptotic activation of the intrinsic mitochondrial pathway. Exp. Ther. Med. 2017, 14, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Yeung-Luk, B.H.; Narayanan, G.A.; Ghosh, B.; Wally, A.; Lee, E.; Mokaya, M.; Wankhade, E.; Zhang, R.; Lee, B.; Park, B.; et al. SARS-CoV-2 infection alters mitochondrial and cytoskeletal function in human respiratory epithelial cells mediated by expression of spike protein. mBio 2023, 14, e0082023. [Google Scholar] [CrossRef]
- Hu, M.; Schulze, K.E.; Ghildyal, R.; Henstridge, D.C.; Kolanowski, J.L.; New, E.J.; Hong, Y.; Hsu, A.C.; Hansbro, P.M.; Wark, P.A.; et al. Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production. eLife 2019, 8, e42448. [Google Scholar] [CrossRef] [PubMed]
- Gregorczyk, K.P.; Wyżewski, Z.; Szczepanowska, J.; Toka, F.N.; Mielcarska, M.B.; Bossowska-Nowicka, M.; Gieryńska, M.; Boratyńska-Jasińska, A.; Struzik, J.; Niemiałtowski, M.G.; et al. Ectromelia Virus Affects Mitochondrial Network Morphology, Distribution, and Physiology in Murine Fibroblasts and Macrophage Cell Line. Viruses 2018, 10, 266. [Google Scholar] [CrossRef]
- Pandey, P.; Al Rumaih, Z.; Kels, M.J.T.; Ng, E.; Kc, R.; Chaudhri, G.; Karupiah, G. Targeting ectromelia virus and TNF/NF-κB or STAT3 signaling for effective treatment of viral pneumonia. Proc. Natl. Acad. Sci. USA 2022, 119, e2112725119. [Google Scholar] [CrossRef]
- Nunes, D.D.S.; Higa, L.M.; Oliveira, R.L.; da Costa, L.C.; Bomfim, L.M.; Gonçalves, C.C.A.; Mariani, D.; Hruby, D.E.; Voloch, C.M.; Castiñeiras, T.M.P.P.; et al. In vitro susceptibility of eighteen clinical isolates of human monkeypox virus to tecovirimat. Mem. Inst. Oswaldo Cruz. 2023, 118, e230056. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods. 2010, 169, 223–227. [Google Scholar] [CrossRef]
- Colavita, F.; Mazzotta, V.; Rozera, G.; Abbate, I.; Carletti, F.; Pinnetti, C.; Matusali, G.; Meschi, S.; Mondi, A.; Lapa, D.; et al. Kinetics of viral DNA in body fluids and antibody response in patients with acute Monkeypox virus infection. iScience 2023, 26, 106102. [Google Scholar] [CrossRef]
- Yu, P.A.; Elmor, R.; Muhammad, K.; Yu, Y.C.; Rao, A.K. Tecovirimat Use under Expanded Access to Treat Mpox in the United States, 2022–2023. NEJM Evid. 2024, 3, EVIDoa2400189. [Google Scholar] [CrossRef]
- Mondi, A.; Gagliardini, R.; Mazzotta, V.; Vita, S.; Carletti, F.; Pinnetti, C.; Giancola, M.L.; Specchiarello, E.; Lanini, S.; Faraglia, F.; et al. Clinical experience with use of oral Tecovirimat or Intravenous Cidofovir for the treatment of Monkeypox in an Italian reference hospital. J. Infect. 2023, 86, 66–117. [Google Scholar] [CrossRef]
- Mazzotta, V.; Cozzi-Lepri, A.; Lanini, S.; Mondi, A.; Carletti, F.; Tavelli, A.; Gagliardini, R.; Vita, S.; Pinnetti, C.; Aguglia, C.; et al. Effect of tecovirimat on healing time and viral clearance by emulation of a target trial in patients hospitalized for mpox. J. Med. Virol. 2023, 95, e28868. [Google Scholar] [CrossRef]
- NIH. The Antiviral Tecovirimat Is Safe But Did Not Improve Clade I Mpox Resolution in Democratic Republic of Congo. 2024. Available online: https://www.nih.gov/news-events/news-releases/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic-congo (accessed on 23 August 2024).
- U.S. Centers for Disease Control and Prevention (CDC). Tecovirimat (TPOXX) for Treatment of Mpox. 10 December 2024. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/tecovirimat.html (accessed on 23 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falasca, L.; Mija, C.; Sberna, G.; Francalancia, M.; Meschi, S.; Mazzotta, V.; Girardi, E.; Antinori, A.; Maggi, F.; Bordi, L. Antiviral Effects of Tecovirimat and Cellular Ultrastructural Changes in Human Bronchial Epithelial Cell Line Following Monkeypox Virus Infection. Int. J. Mol. Sci. 2025, 26, 2718. https://doi.org/10.3390/ijms26062718
Falasca L, Mija C, Sberna G, Francalancia M, Meschi S, Mazzotta V, Girardi E, Antinori A, Maggi F, Bordi L. Antiviral Effects of Tecovirimat and Cellular Ultrastructural Changes in Human Bronchial Epithelial Cell Line Following Monkeypox Virus Infection. International Journal of Molecular Sciences. 2025; 26(6):2718. https://doi.org/10.3390/ijms26062718
Chicago/Turabian StyleFalasca, Laura, Cosmina Mija, Giuseppe Sberna, Massimo Francalancia, Silvia Meschi, Valentina Mazzotta, Enrico Girardi, Andrea Antinori, Fabrizio Maggi, and Licia Bordi. 2025. "Antiviral Effects of Tecovirimat and Cellular Ultrastructural Changes in Human Bronchial Epithelial Cell Line Following Monkeypox Virus Infection" International Journal of Molecular Sciences 26, no. 6: 2718. https://doi.org/10.3390/ijms26062718
APA StyleFalasca, L., Mija, C., Sberna, G., Francalancia, M., Meschi, S., Mazzotta, V., Girardi, E., Antinori, A., Maggi, F., & Bordi, L. (2025). Antiviral Effects of Tecovirimat and Cellular Ultrastructural Changes in Human Bronchial Epithelial Cell Line Following Monkeypox Virus Infection. International Journal of Molecular Sciences, 26(6), 2718. https://doi.org/10.3390/ijms26062718





