Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation
Abstract
1. Introduction
2. Results
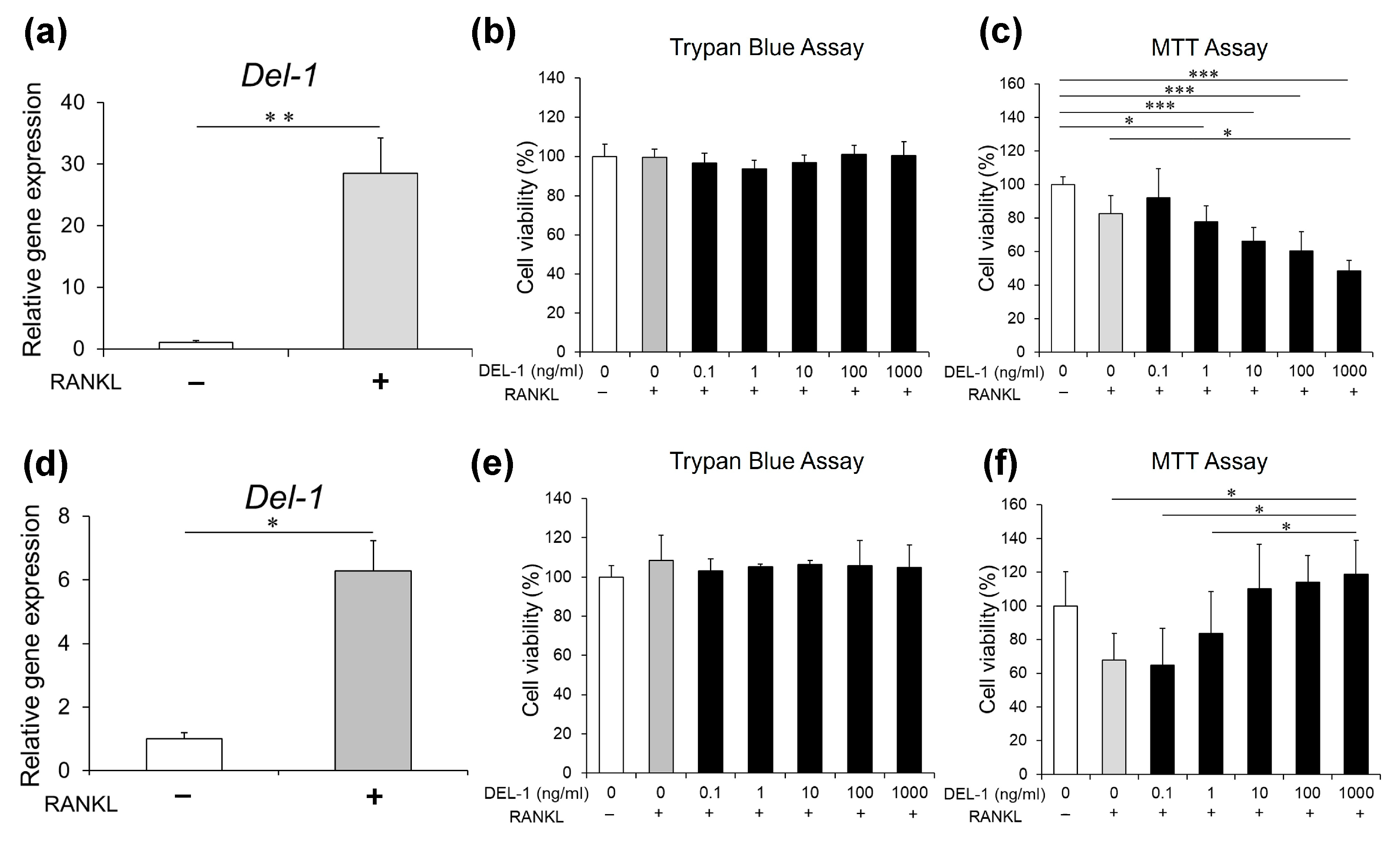
2.1. Del-1 mRNA Expression in Osteoclasts
2.2. Effects of DEL-1 on Osteoclast Viability
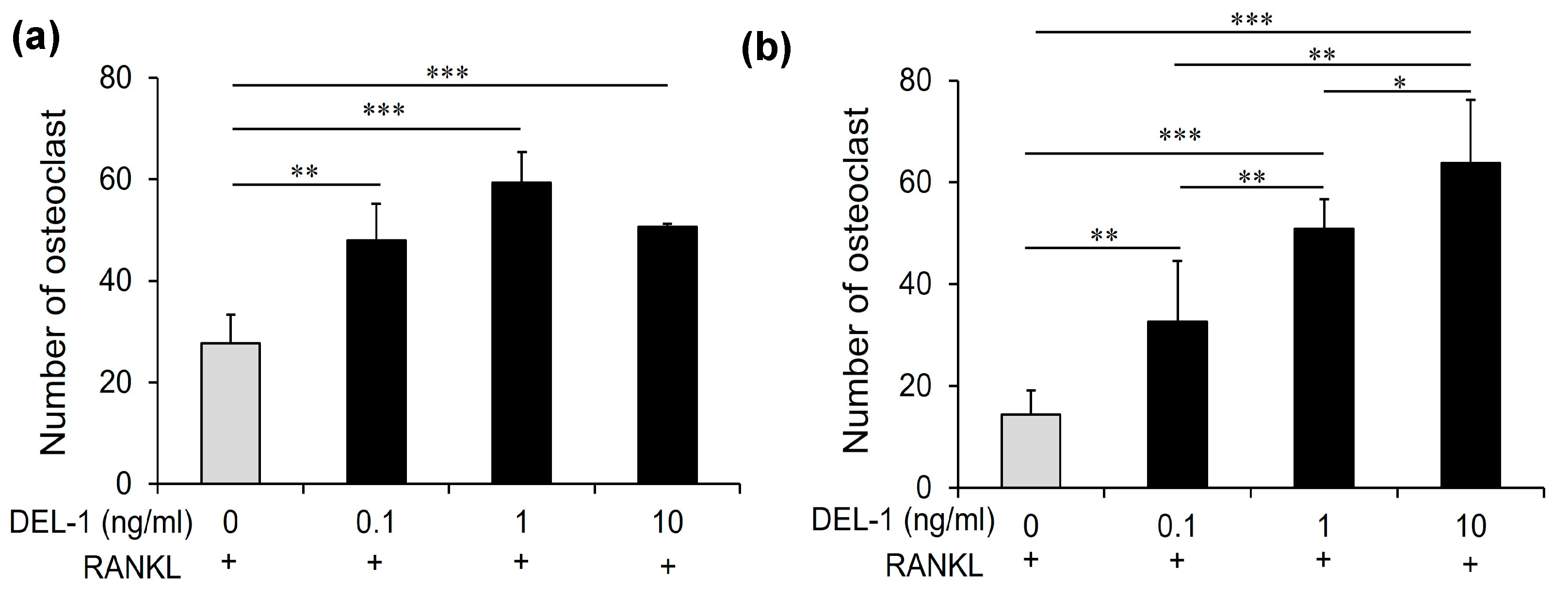
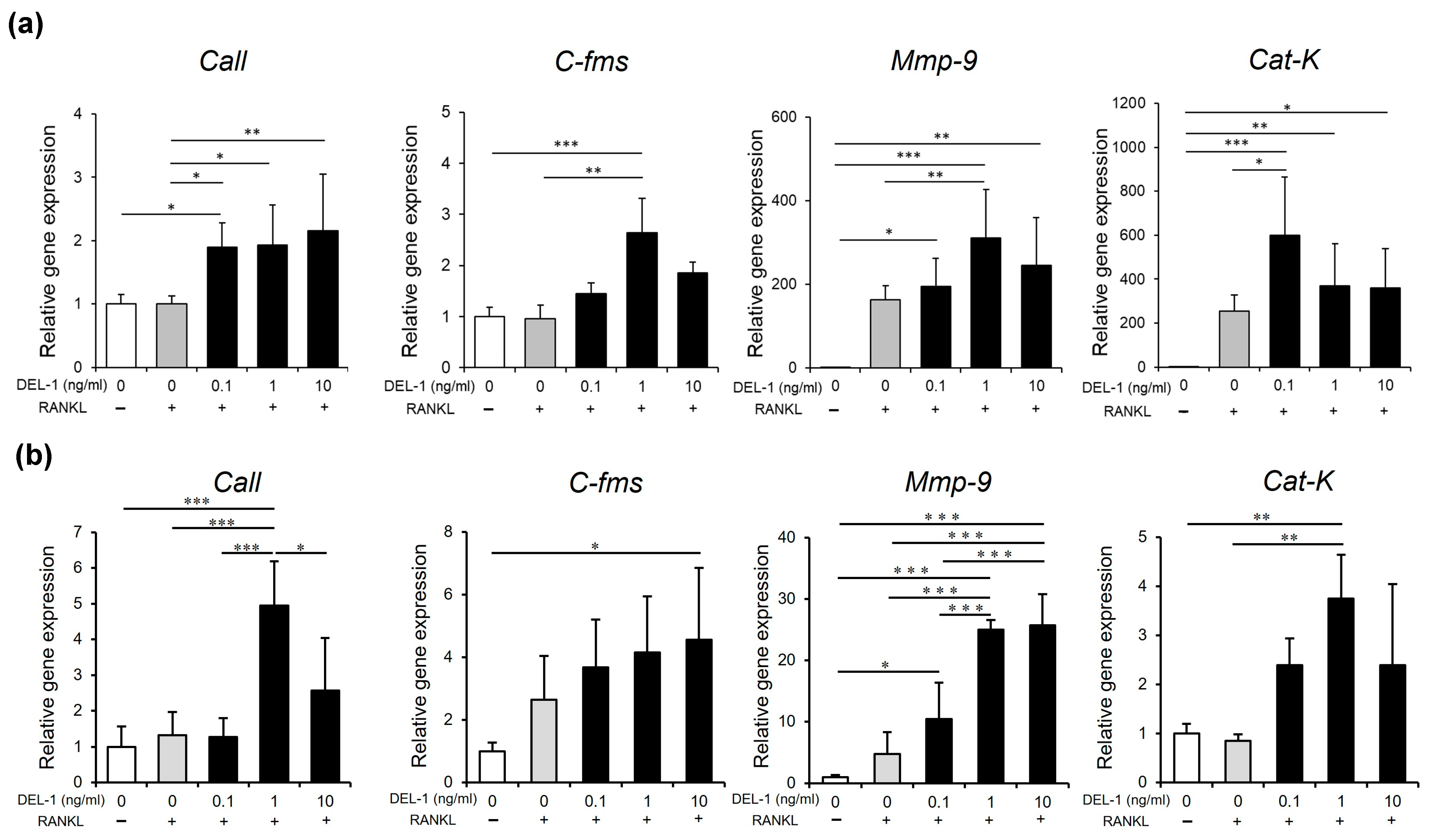
2.3. Effects of DEL-1 on Osteoclast Differentiation and Activation
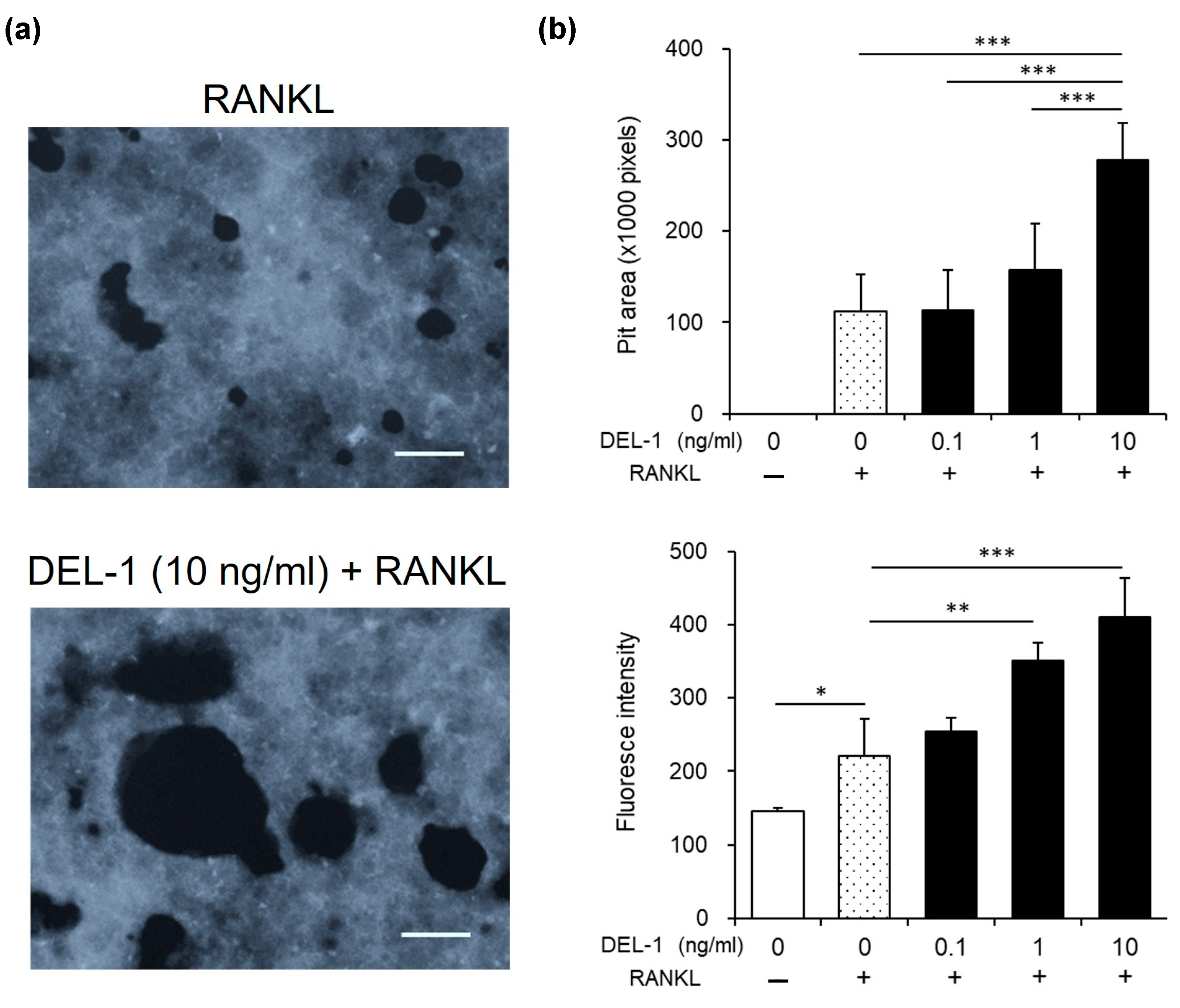
2.4. Effects of DEL-1 on Osteoclast Activity
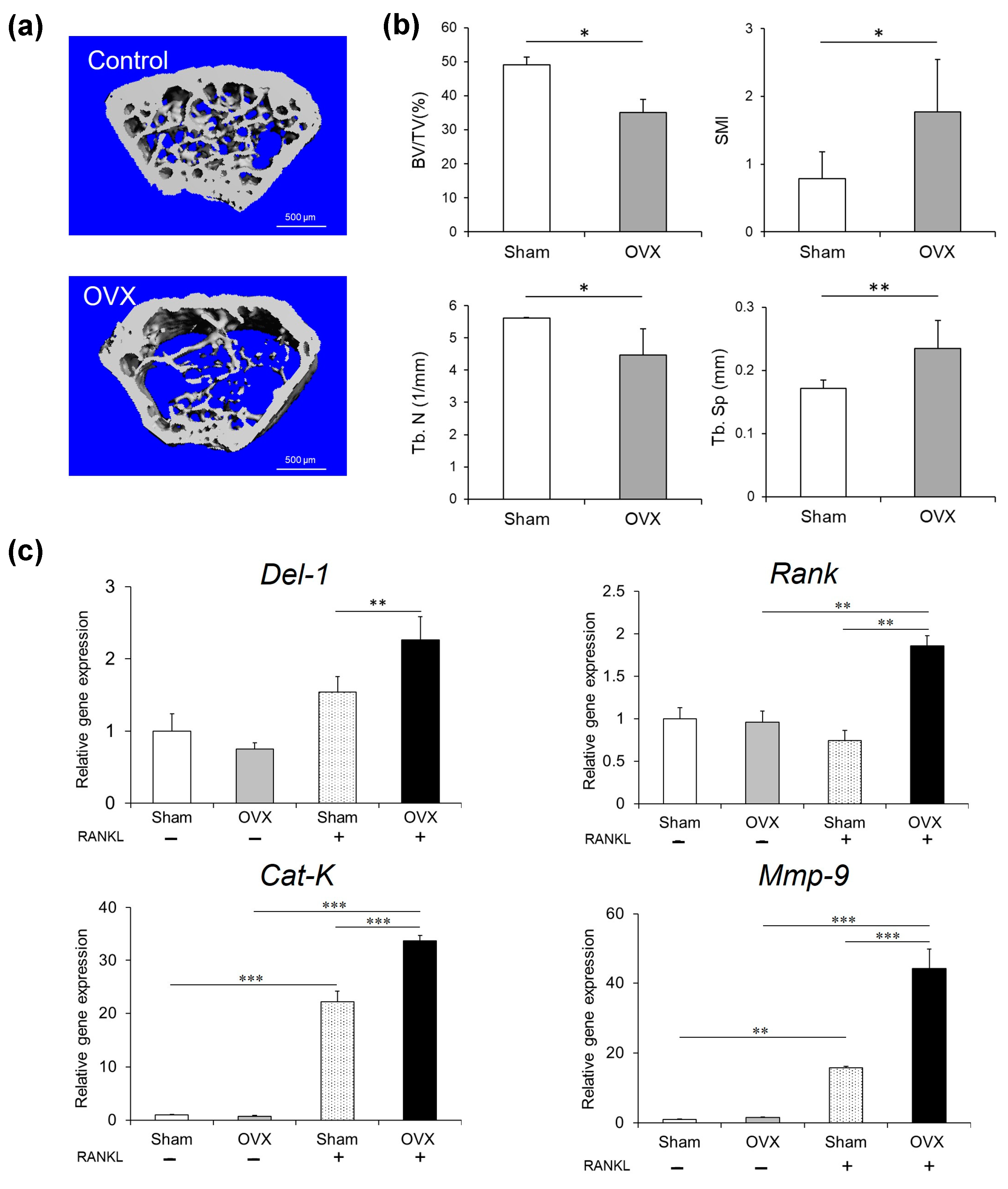
2.5. Osteoporosis Model Investigation
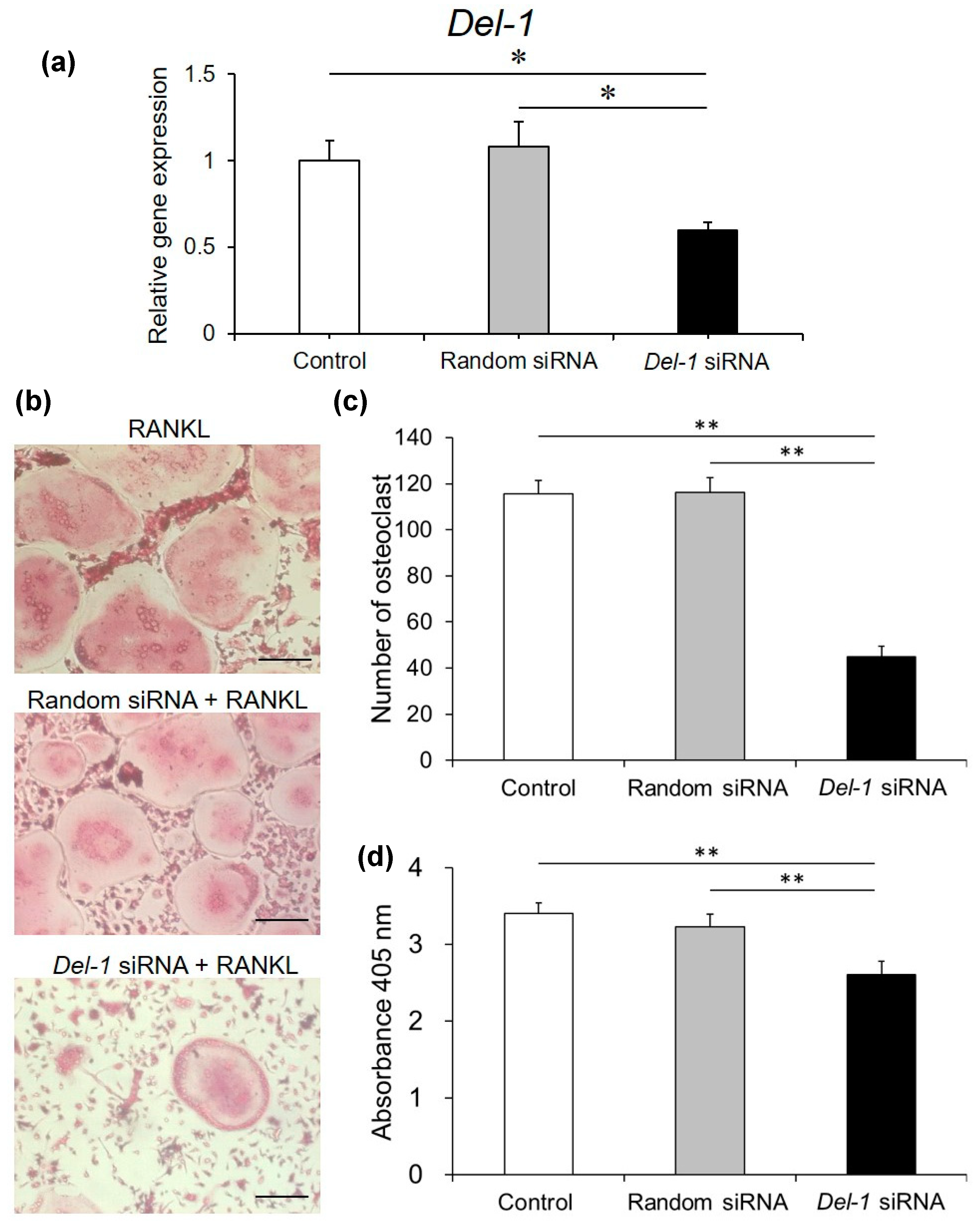
2.6. Effect of Silencing Del-1 mRNA Expression on Osteoclast Differentiation
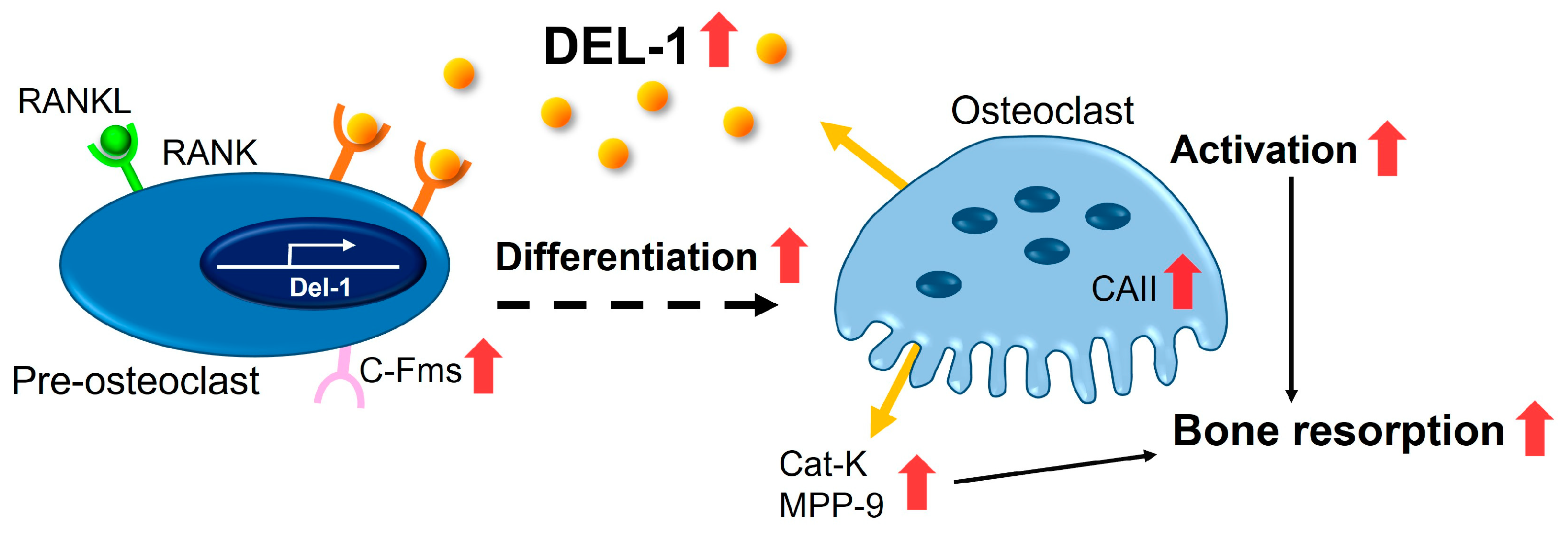
3. Discussion
- Elucidating the precise molecular mechanisms underlying DEL-1’s concentration-dependent effects;
- Investigating potential therapeutic interventions targeting DEL-1 in bone metabolic disorders;
- Exploring DEL-1’s broader roles in cellular differentiation and inflammatory processes.
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Conditions
4.3. OVX Mice
4.4. Real-Time RT-PCR
4.5. Trypan Blue Assay
4.6. MTT Assay
4.7. Osteoclast Forming Assay
4.8. Osteoclast Activity Assay
4.9. Gene Silencing Using an siRNA
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vernal, R.; Dutzan, N.; Chaparro, A.; Puente, J.; Valenzuela, M.A.; Gamonal, J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. 2005, 32, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Lu, L.; Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Inflammatory bone destruction and osteoimmunology. J. Periodontal Res. 2005, 40, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Yang, T.L.; Shen, H.; Liu, A.; Dong, S.S.; Zhang, L.; Deng, F.Y.; Zhao, Q.; Deng, H.W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2020, 16, 91–103. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Gamsjaeger, S.; Hassler, N.; Fahrleitner-Pammer, A.; Dobnig, H.; Stepan, J.J.; Pavo, I.; Eriksen, E.F.; Klaushofer, K. Vitamin D and calcium supplementation for three years in postmenopausal osteoporosis significantly alters bone mineral and organic matrix quality. Bone 2017, 95, 41–46. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J.; Riggs, B.L. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: Is a revision needed? J. Bone Miner. Res. 2011, 26, 441–451. [Google Scholar] [CrossRef]
- Kanczkowski, W.; Chatzigeorgiou, A.; Grossklaus, S.; Sprott, D.; Bornstein, S.R.; Chavakis, T. Role of the endothelial-derived endogenous anti-inflammatory factor Del-1 in inflammation-mediated adrenal gland dysfunction. Endocrinology 2013, 154, 1181–1189. [Google Scholar] [CrossRef]
- Hidai, C.; Kawana, M.; Kitano, H.; Kokubun, S. Discoidin domain of Del1 protein contributes to its deposition in the extracellular matrix. Cell Tissue Res. 2007, 330, 83–95. [Google Scholar] [CrossRef]
- Penta, K.; Varner, J.A.; Liaw, L.; Hidai, C.; Schatzman, R.; Quertermous, T. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J. Biol. Chem. 1999, 274, 11101–11109. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, A.; Matsuo-Takasaki, M.; Takai, A.; Inomata, H.; Matsumura, M.; Ikeya, M.; Takahashi, K.; Miyachi, Y.; Sasai, N.; Sasai, Y. The secreted EGF-Discoidin factor xDel1 is essential for dorsal development of the Xenopus embryo. Dev. Biol. 2007, 306, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Eskan, M.A.; Jotwani, R.; Abe, T.; Chmelar, J.; Lim, J.H.; Liang, S.; Ciero, P.A.; Krauss, J.L.; Li, F.; Rauner, M.; et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012, 13, 465–473. [Google Scholar] [CrossRef]
- Shin, J.; Maekawa, T.; Abe, T.; Hajishengallis, E.; Hosur, K.; Pyaram, K.; Mitroulis, I.; Chavakis, T.; Hajishengallis, G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 2015, 7, 307ra155. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Li, X.; Mitroulis, I.; Grosser, D.; Kajikawa, T.; Wang, B.; Grzybek, M.; Von Renesse, J.; Czogalla, A.; Troullinaki, M.; et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 2019, 20, 40–49. [Google Scholar] [CrossRef]
- Kim, K.; Punj, V.; Kim, J.M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev. 2016, 30, 208–219. [Google Scholar] [CrossRef]
- Ishibashi, O.; Niwa, S.; Kadoyama, K.; Inui, T. MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sci. 2006, 79, 1657–1660. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J. Bone Miner. Res. 2003, 18, 1404–1418. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An overview of the effects of estrogen deficiency and glucocorticoid overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ponte, F.; Nookaew, I.; Ucer Ozgurel, S.; Marques-Carvalho, A.; Iyer, S.; Warren, A.; Aykin-Burns, N.; Krager, K.; Sardao, V.A.; et al. Estrogens decrease osteoclast number by attenuating mitochondria oxidative phosphorylation and ATP production in early osteoclast precursors. Sci. Rep. 2020, 10, 11933. [Google Scholar] [CrossRef] [PubMed]
- Loven, M.A.; Wood, J.R.; Nardulli, A.M. Interaction of estrogen receptors alpha and beta with estrogen response elements. Mol. Cell Endocrinol. 2001, 181, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Pakdel, F. Role of estrogen receptors in health and disease. Int. J. Mol. Sci. 2023, 24, 11354. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, F.M.; Wang, A.H.; Chen, Y.J.; Lv, X.; Wu, S.; Zhao, R.N. Estrogen and its receptor enhance mechanobiological effects in compressed bone mesenchymal stem cells. Cells Tissues Organs 2012, 195, 400–413. [Google Scholar] [CrossRef]
- Kim, S.S.; Jeong, S.P.; Park, B.S.; Kim, I.R. Melatonin attenuates RANKL-induced osteoclastogenesis via inhibition of Atp6v0d2 and DC-STAMP through MAPK and NFATc1 signaling pathways. Molecules 2022, 27, 501. [Google Scholar] [CrossRef]
- Tzeng, H.E.; Huang, P.H.; Tsai, C.H.; Tsay, G.J.; Lee, Y.J.; Huang, T.J.; Lin, T.H.; Chiu, Y.M.; Wu, Y.Y. Isosteviol derivative inhibits osteoclast differentiation and ameliorates ovariectomy-induced osteoporosis. Sci. Rep. 2018, 8, 11190. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Tumor necrosis factor superfamily member RANKL mediates the effects of glucocorticoids on bone resorption. J. Clin. Investig. 2012, 122, 592–602. [Google Scholar]
- Robinson, J.A.; Huang, Y.; Kazakia, G.J.; Ding, X.Z.; Crisco, J.J.; Glimcher, M.J. Osteocyte apoptosis and bone turnover in the proximal femoral neck of patients with femoral neck fractures. Calcif. Tissue Int. 2008, 83, 317–325. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Wen, X.; Liu, J.; Xu, B. siRNA targeting of NF-κB inhibits inflammatory response in RAW264.7 macrophages. Mol. Med. Rep. 2016, 14, 3543–3549. [Google Scholar]
- Li, X.; Tai, R.; Zheng, Y.; Liu, Y. Role of toll-like receptor signaling in the progression of osteoarthritis. Mol. Med. Rep. 2017, 16, 4037–4043. [Google Scholar]
- Zhang, J.; Wu, B.; Liu, Y.; Jiang, L.; Zhu, J. Long non-coding RNA H19 regulated lipopolysaccharide-induced inflammatory response in human cardiac microvascular endothelial cells via targeting NF-κB. J. Cell. Biochem. 2018, 119, 10022–10031. [Google Scholar]
- Silver, I. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef]
- Elbaz, A.; Rivas, D.; Duque, G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 2009, 10, 747–755. [Google Scholar] [CrossRef]
- Suda, T.; Jimi, E.; Nakamura, I.; Takahashi, N. Role of 1 alpha,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol. 1997, 282, 223–235. [Google Scholar]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
| Del-1 | Forward | ACAGGCATCATTACACAAGGAG |
| Reverse | GGGAGGGTCGATGACATTTTT | |
| C-fms | Forward | GGTTGTAGAGCCGGGTGAAA |
| Reverse | AAGAGTGGGCCGGATCTTTG | |
| Mmp-9 | Forward | GGGTCTAGGCCCAGAGGTAA |
| Reverse | TAACGCCCAGTAGAGAGCCT | |
| CaII | Forward | GACCCAGGTGTCTCATGTGG |
| Reverse | GACGCCAGTTGTCCACCATC | |
| Cat-k | Forward | CTCAACAGCAGGATGTGGGT |
| Reverse | TTCAGGGCTTTCTCGTTCCC | |
| Gapdh | Forward | AACGACCCCTTCATTGAC |
| Reverse | TCCACGACATACTCAGCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, K.; Tachi, K.; Takayama, T.; Kasai, H.; Shohara, R.; Inoue, K.; Taguchi, Y.; Nakane-Koyachi, S.; Saito, A.; Yamano, S. Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation. Int. J. Mol. Sci. 2025, 26, 2673. https://doi.org/10.3390/ijms26062673
Imamura K, Tachi K, Takayama T, Kasai H, Shohara R, Inoue K, Taguchi Y, Nakane-Koyachi S, Saito A, Yamano S. Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation. International Journal of Molecular Sciences. 2025; 26(6):2673. https://doi.org/10.3390/ijms26062673
Chicago/Turabian StyleImamura, Kentaro, Keita Tachi, Tadahiro Takayama, Hironori Kasai, Ryutaro Shohara, Kenji Inoue, Yoichiro Taguchi, Saki Nakane-Koyachi, Atsushi Saito, and Seiichi Yamano. 2025. "Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation" International Journal of Molecular Sciences 26, no. 6: 2673. https://doi.org/10.3390/ijms26062673
APA StyleImamura, K., Tachi, K., Takayama, T., Kasai, H., Shohara, R., Inoue, K., Taguchi, Y., Nakane-Koyachi, S., Saito, A., & Yamano, S. (2025). Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation. International Journal of Molecular Sciences, 26(6), 2673. https://doi.org/10.3390/ijms26062673






