Network Pharmacology Combined with Experimental Validation to Investigate the Effects and Mechanisms of Aucubin on Aging-Related Muscle Atrophy
Abstract
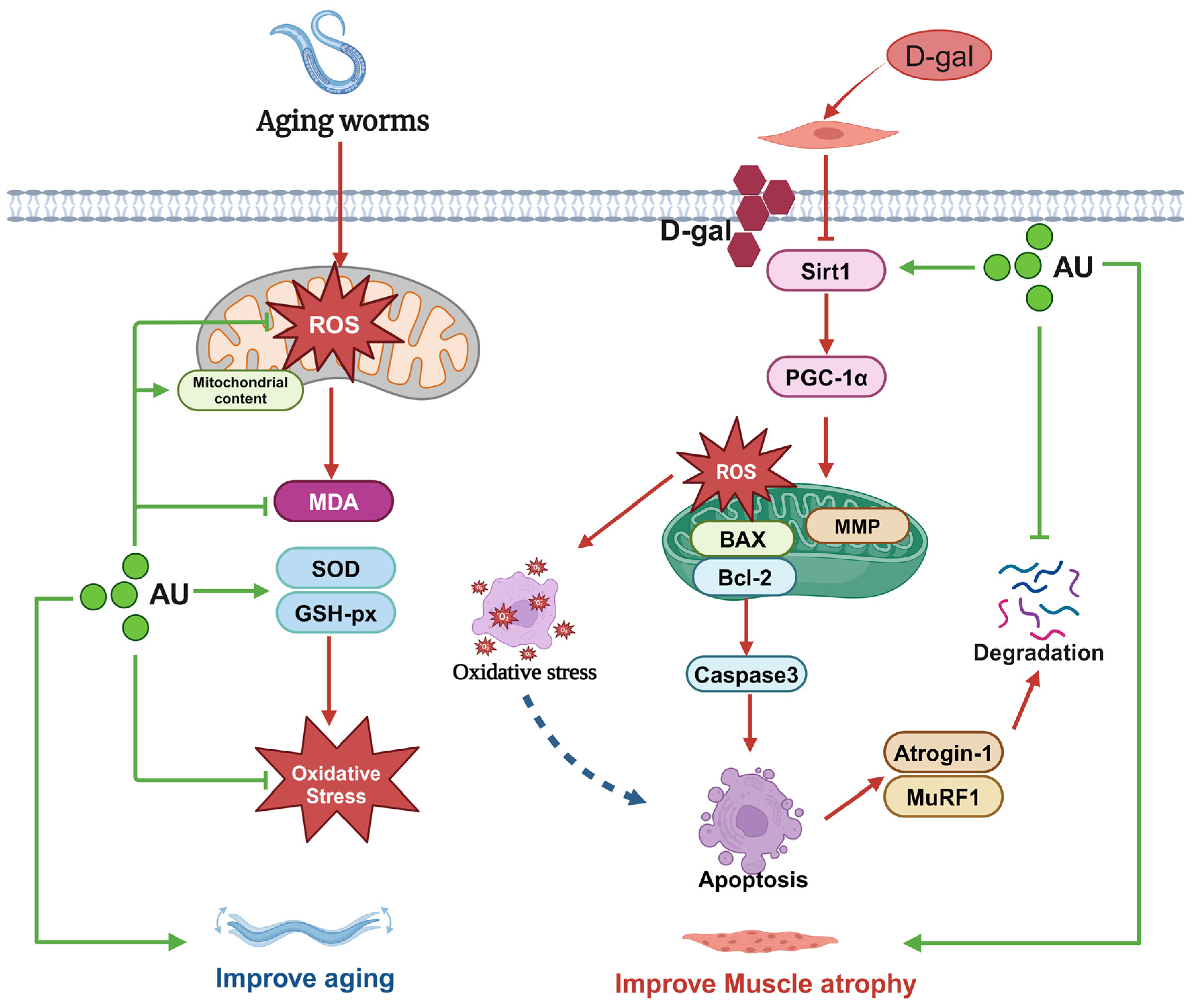
1. Introduction
2. Results
2.1. Network Pharmacology and Bioinformatics Analysis
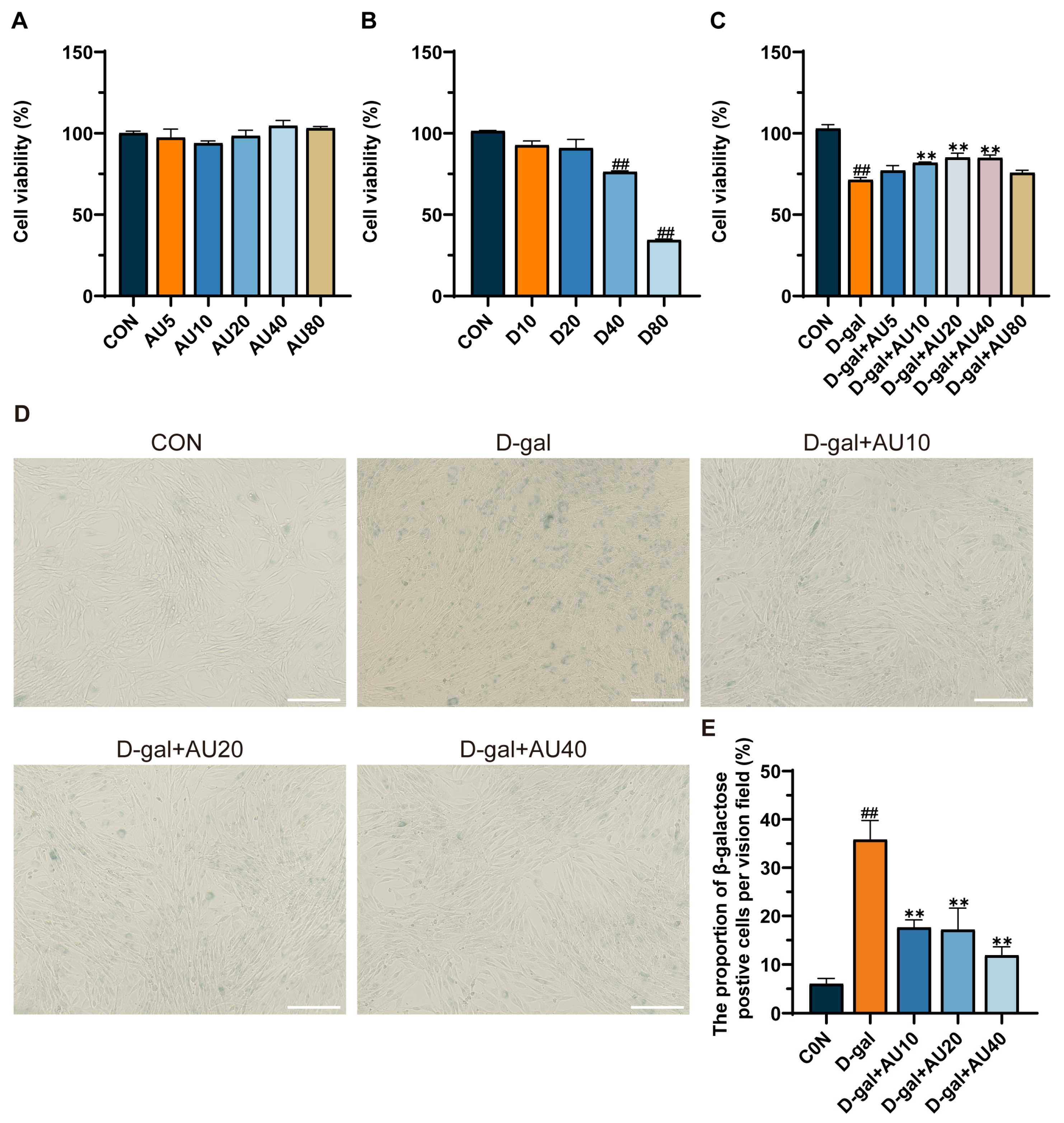
2.2. Effects of AU on D-Galactose (D-Gal)-Induced C2C12 Cell Viability and Senescence
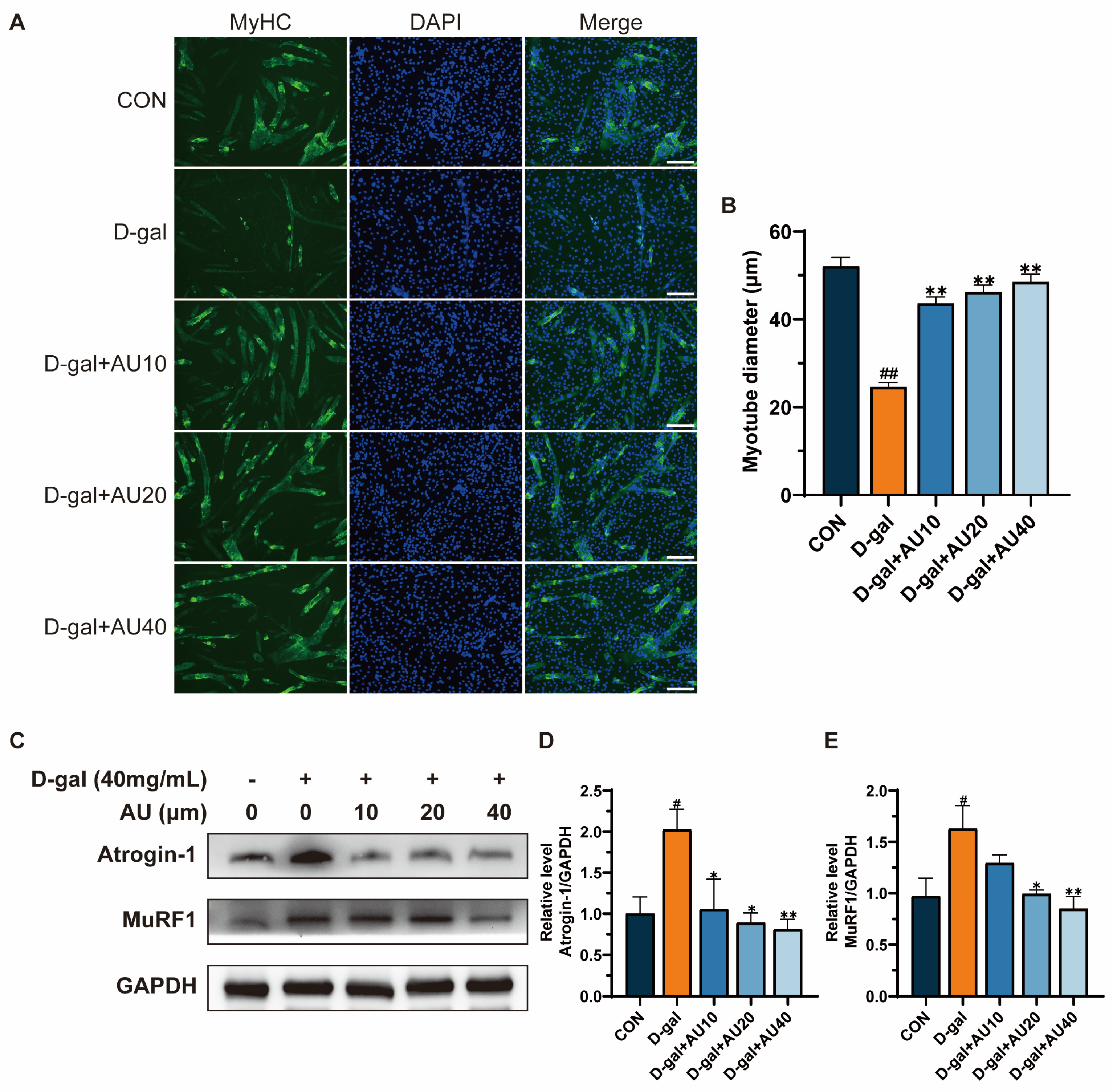
2.3. Effects of AU on D-Gal-Induced Myotube Diameter Atrophy
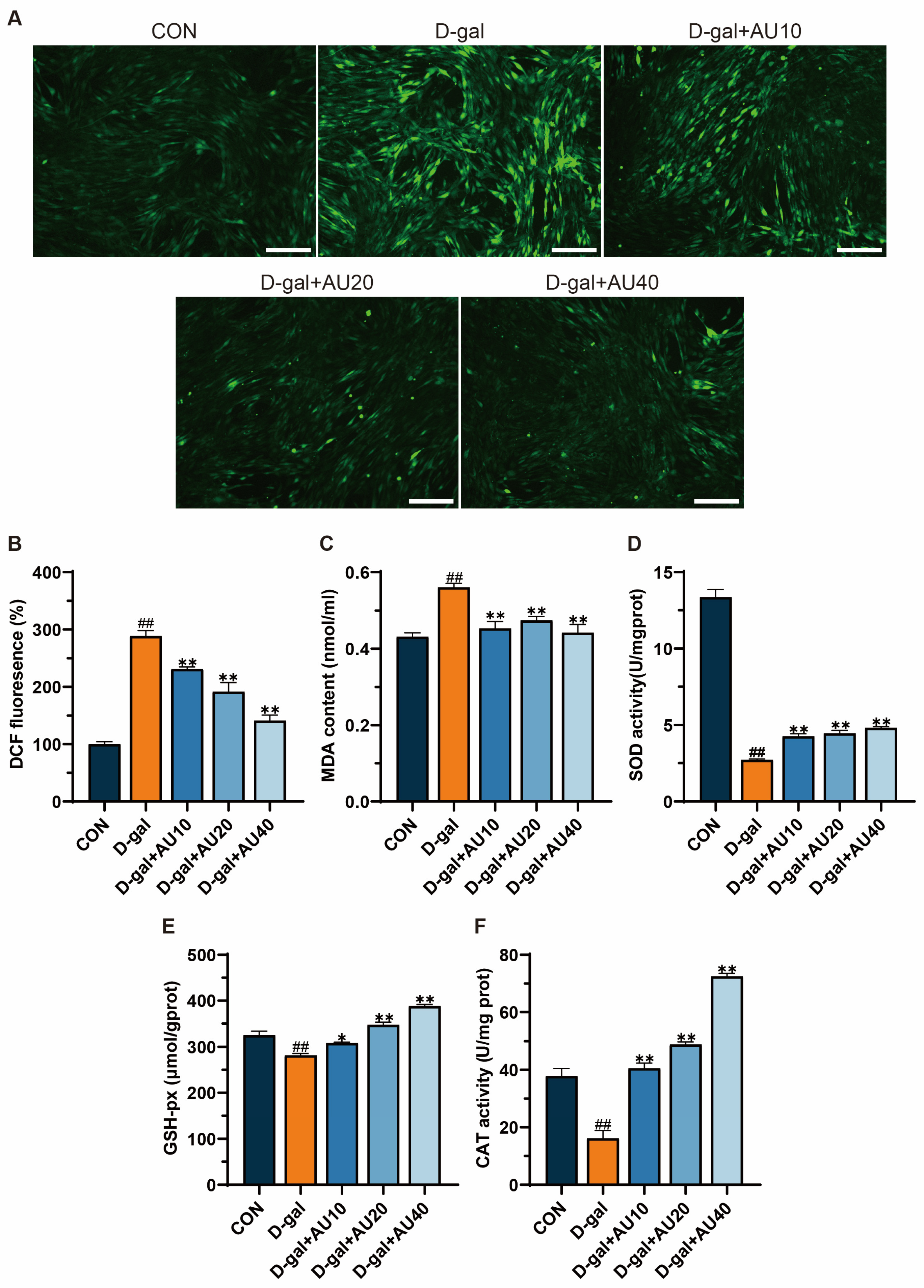
2.4. Effects of AU on Oxidative Stress in D-Gal-Induced C2C12 Cells
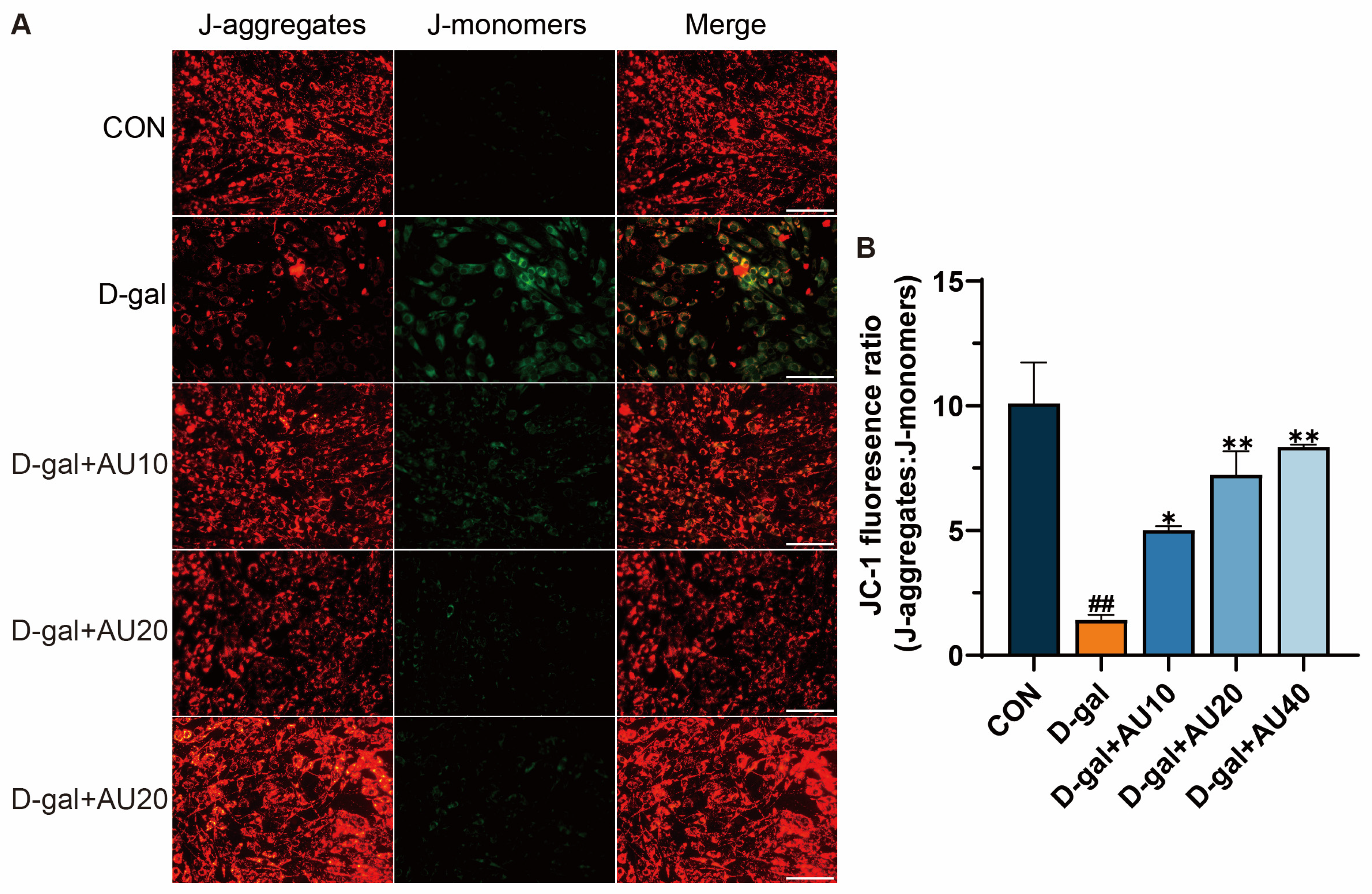
2.5. Effects of AU on the Mitochondrial Membrane Potential (MMP) in D-Gal-Induced C2C12 Cells
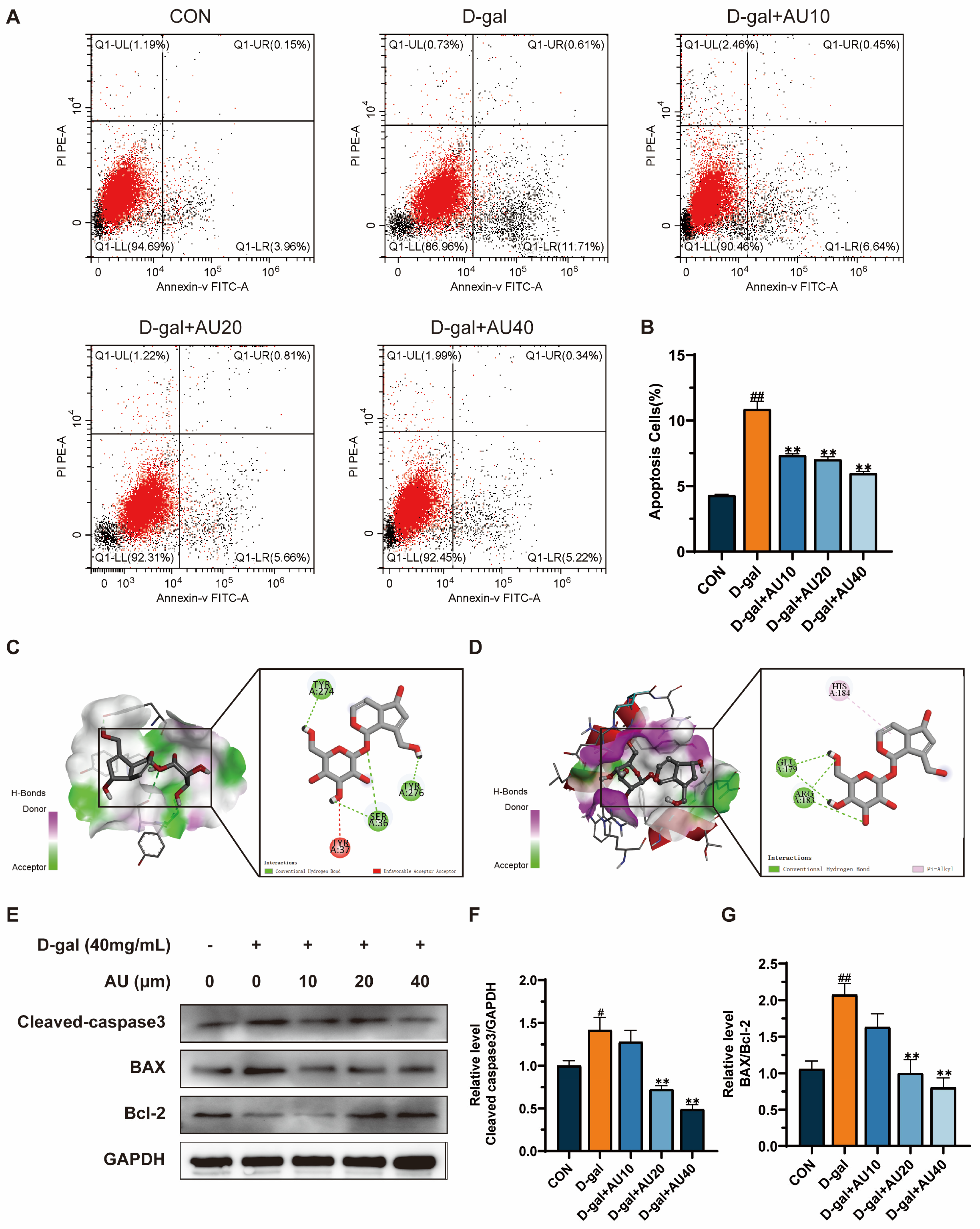
2.6. Effects of AU on D-Gal-Induced Apoptosis of C2C12 Cells
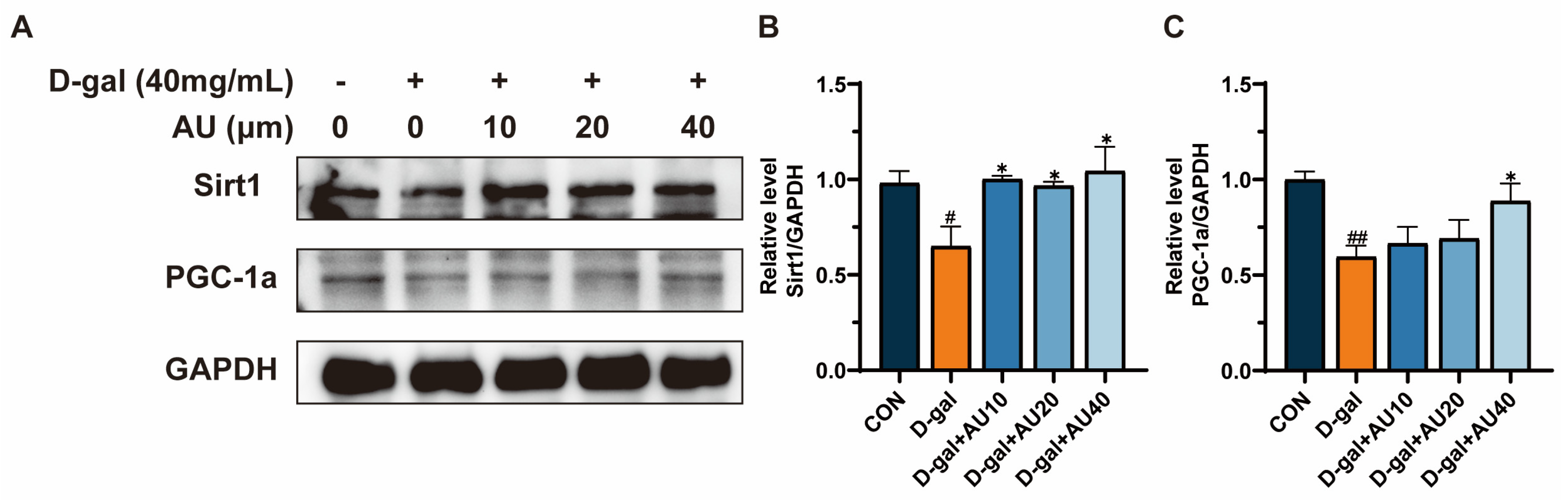
2.7. Effects of AU on D-Gal-Induced Sirt1/PGC1-α Signaling Pathway in C2C12 Cells
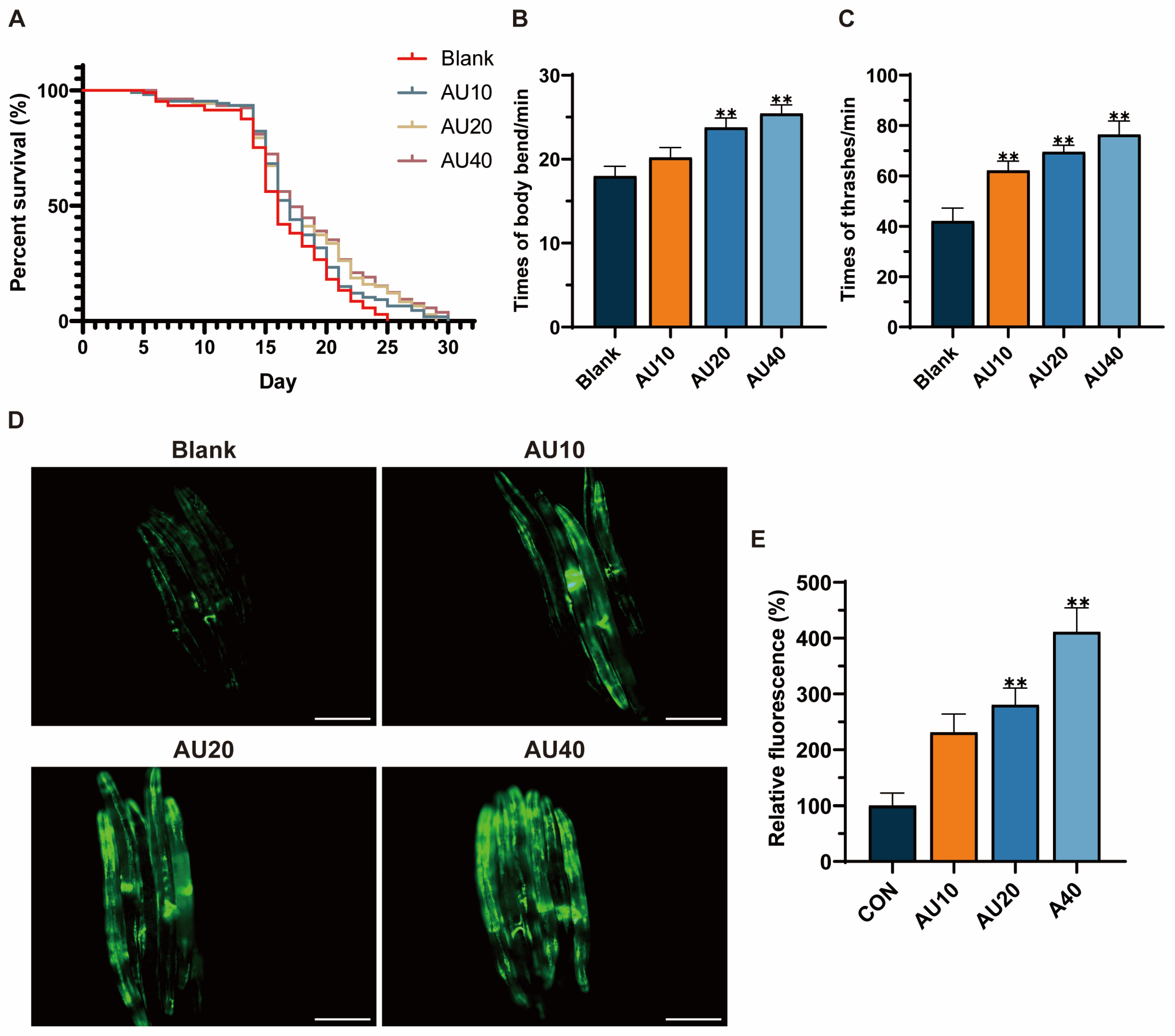
2.8. Effects of AU on the Lifespan and Behavior of the N2 Genotype of C. elegans
2.9. Effects of AU on Mitochondrial Content in the SJ4103 Genotype of C. elegans
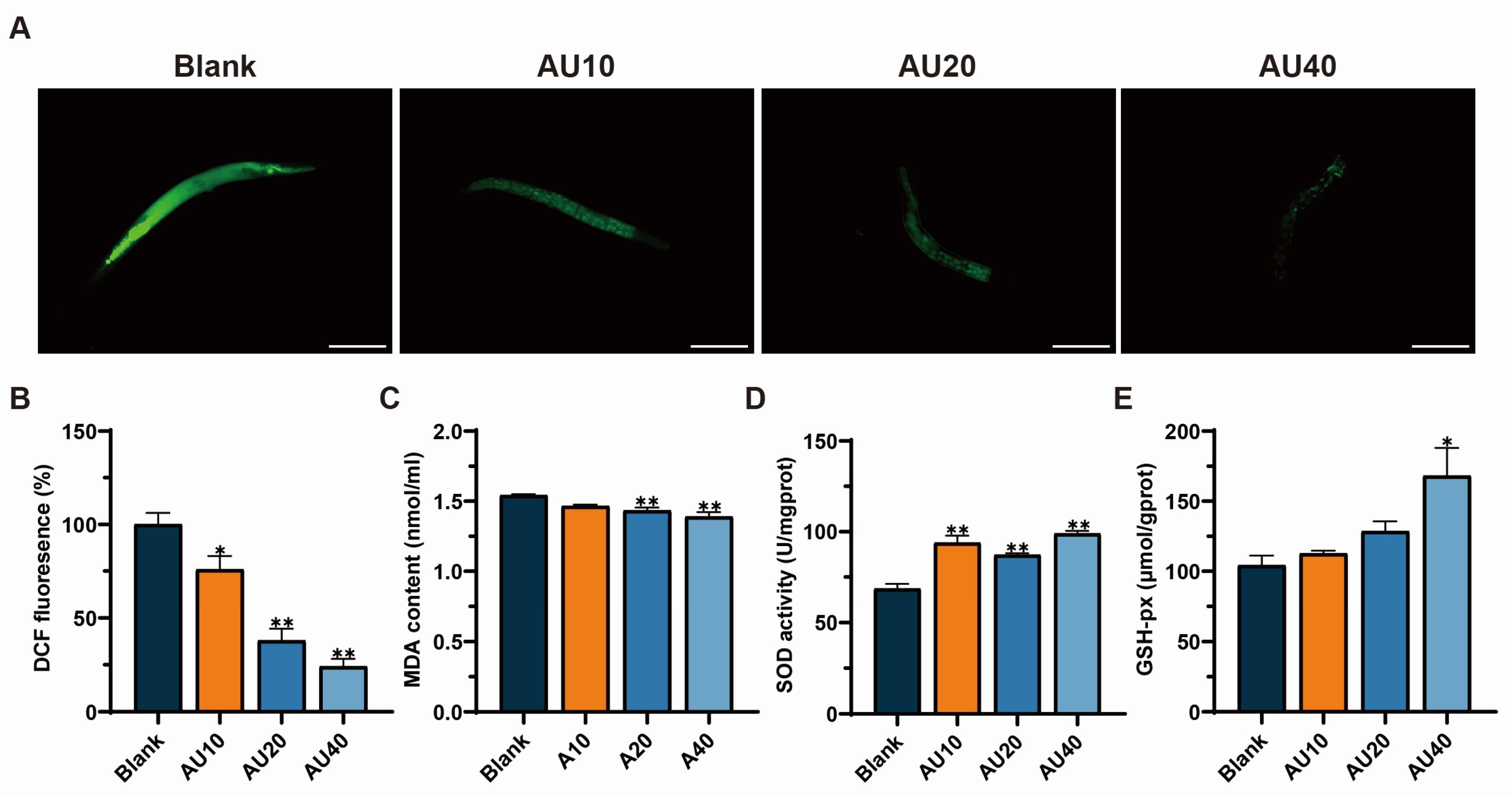
2.10. Effects of AU on Oxidative Stress in the N2 Genotype of C. elegans
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Identification of Targets
4.3. PPI Analysis
4.4. Establishment of GO and KEGG Enrichment Network Graphs
4.5. Molecular Docking
4.6. Cell Culture and Treatment
4.7. Cell Viability Assay
4.8. SA-β-Gal Staining
4.9. Measurement of Myotube Diameters
4.10. Determination of Antioxidant Activity in C2C12 Cells
4.11. MMP Assay
4.12. Apoptosis Analysis by Flow Cytometry
4.13. Western Blot
4.14. C. elegans Strains and Culture
4.15. Lifespan Assay
4.16. Motility
4.17. Fluorescence Measurements of Mitochondria
4.18. Oxidative Stress Levels of C. elegans
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Atrogin-1 | Muscle Atrophy F-box protein |
| AU | Aucubin |
| BAX | BCL2-associated X protein |
| BCA | Bicinchoninic acid |
| Bcl-2 | B-cell lymphoma 2 |
| BP | Biological process |
| C. elegans | Caenorhabditis elegans |
| CAT | Catalase |
| CC | Cellular component |
| CGC | Caenorhabditis Genetics Center |
| D-gal | D-galactose |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| E. coli | Escherichia coli |
| EU | Eucommia ulmoides Oliv |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GFP | Green fluorescent protein |
| GO | Gene Ontology |
| GSH-Px | Glutathione peroxidase |
| HS | Horse serum |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MDA | Malondialdehyde |
| MF | Molecular function |
| MMP | Mitochondrial membrane potential |
| MuRF1 | Muscle RING-finger protein-1 |
| MyHC | Myosin heavy chain |
| PBS | Phosphate-Buffered Saline |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPI | Protein-protein interaction |
| ROS | Reactive oxygen species |
| SA-β-Gal | Senescence-associated β-galactosidase |
| SDF | Canonical Structure Data File |
| SEM | Standard Error of the Mean |
| Sirt1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
References
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global Prevalence of Sarcopenia and Severe Sarcopenia: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Zuo, X.; Li, X.; Tang, K.; Zhao, R.; Wu, M.; Wang, Y.; Li, T. Sarcopenia and Cardiovascular Diseases: A Systematic Review and Meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Mitochondrial Dysregulation and Muscle Disuse Atrophy. F1000Research 2019, 8, 1621. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal Muscle Atrophy: From Mechanisms to Treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K. Can Antioxidants Protect Against Disuse Muscle Atrophy? Sports Med. 2014, 44, 155–165. [Google Scholar] [CrossRef]
- Kobak, K.A.; Lawrence, M.M.; Pharaoh, G.; Borowik, A.K.; Peelor, F.F.; Shipman, P.D.; Griffin, T.M.; Van Remmen, H.; Miller, B.F. Determining the Contributions of Protein Synthesis and Breakdown to Muscle Atrophy Requires Non-steady-state Equations. J. Cachexia Sarcopenia Muscle 2021, 12, 1764–1775. [Google Scholar] [CrossRef]
- Graham, Z.A.; Lavin, K.M.; O’Bryan, S.M.; Thalacker-Mercer, A.E.; Buford, T.W.; Ford, K.M.; Broderick, T.J.; Bamman, M.M. Mechanisms of Exercise as a Preventative Measure to Muscle Wasting. Am. J. Physiol.-Cell Physiol. 2021, 321, C40–C57. [Google Scholar] [CrossRef]
- Ebner, N.; Sliziuk, V.; Scherbakov, N.; Sandek, A. Muscle Wasting in Ageing and Chronic Illness. ESC Heart Fail. 2015, 2, 58–68. [Google Scholar] [CrossRef]
- Huang, L.; Lyu, Q.; Zheng, W.; Yang, Q.; Cao, G. Traditional Application and Modern Pharmacological Research of Eucommia Ulmoides Oliv. Chin. Med. 2021, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hong, R.; Yim, P.; Yeom, M.; Lee, B.; Yang, W.M.; Hong, J.; Lee, H.S.; Hahm, D. An Herbal Formula Consisting of Schisandra Chinensis (Turcz.) Baill, Lycium Chinense Mill and Eucommia Ulmoides Oliv Alleviates Disuse Muscle Atrophy in Rats. J. Ethnopharmacol. 2018, 213, 328–339. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, F.; Ouyang, D. A Review of the Pharmacology and Toxicology of Aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Duan, J.-X.; Liu, T.; Guan, X.-X.; Mei, W.-X.; Liu, Y.-P.; Sun, G.-Y.; Wan, L.; Zhong, W.-J.; et al. Aucubin Alleviates Bleomycin-Induced Pulmonary Fibrosis in a Mouse Model. Inflammation 2017, 40, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jin, H.; Zhang, X.; Li, W.; Wang, D.; Tong, P.; Liu, Y.; Tan, Z. Aucubin Prevents Steroid-induced Osteoblast Apoptosis by Enhancing Autophagy via AMPK Activation. J. Cell Mol. Med. 2021, 25, 10175–10184. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, X.; Lu, W.; Liu, X.; Hu, M.; Wang, D. Aucubin Exerts Anti-Osteoporotic Effects by Promoting Osteoblast Differentiation. Aging 2020, 12, 2226–2245. [Google Scholar] [CrossRef]
- Yang, X.; Xue, P.; Chen, H.; Yuan, M.; Kang, Y.; Duscher, D.; Machens, H.-G.; Chen, Z. Denervation Drives Skeletal Muscle Atrophy and Induces Mitochondrial Dysfunction, Mitophagy and Apoptosis via MiR-142a-5p/MFN1 Axis. Theranostics 2020, 10, 1415–1432. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Ran, L.; Jiang, Y.; Chen, M.; Du, H.; Zhang, Y.; Wu, D.; Xiang, X.; Zhang, J.; et al. Tetrahedral Framework Nucleic Acids Inhibit Muscular Mitochondria-Mediated Apoptosis and Ameliorate Muscle Atrophy in Sarcopenia. Nano Lett. 2023, 23, 8816–8826. [Google Scholar] [CrossRef]
- Dupont-Versteegden, E.E. Apoptosis in Skeletal Muscle and Its Relevance to Atrophy. World J. Gastroenterol. 2006, 12, 7463. [Google Scholar] [CrossRef]
- Nie, C.; Wang, B.; Fan, M.; Wang, Y.; Sun, Y.; Qian, H.; Li, Y.; Wang, L. Highland Barley Tea Polyphenols Extract Alleviates Skeletal Muscle Fibrosis in Mice by Reducing Oxidative Stress, Inflammation, and Cell Senescence. J. Agric. Food Chem. 2023, 71, 739–748. [Google Scholar] [CrossRef]
- Seok, Y.M.; Yoo, J.-M.; Nam, Y.; Kim, J.; Kim, J.S.; Son, J.-H.; Kim, H.J. Mountain Ginseng Inhibits Skeletal Muscle Atrophy by Decreasing Muscle RING FInger Protein-1 and Atrogin1 through Forkhead Box O3 in L6 Myotubes. J. Ethnopharmacol. 2021, 270, 113557. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase Prevents Oxidative Stress-Related Muscle Atrophy and Weakness. Redox Biol. 2019, 20, 68–74. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Huang, J.; Hu, R.; You, H.; Liu, L.; Wang, D.; Wei, L. Paeoniflorin Ameliorates Skeletal Muscle Atrophy in Chronic Kidney Disease via AMPK/SIRT1/PGC-1α-Mediated Oxidative Stress and Mitochondrial Dysfunction. Front. Pharmacol. 2022, 13, 859723. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, S.; Yue, H.; Wang, M.; Zeng, J.; Wu, W.; Wang, J.; Zheng, H.; Xue, C.; Zhao, Y.-T. Isolation, Identification and in Silico Analysis of Two Novel Cytoprotective Peptides from Tilapia Skin against Oxidative Stress-Induced Ovarian Granulosa Cell Damage. J. Funct. Foods 2023, 107, 105629. [Google Scholar] [CrossRef]
- Kou, X.; Li, J.; Liu, X.; Yang, X.; Fan, J.; Chen, N. Ampelopsin Attenuates the Atrophy of Skeletal Muscle from D-Gal-Induced Aging Rats through Activating AMPK/SIRT1/PGC-1α Signaling Cascade. Biomed. Pharmacother. 2017, 90, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Zhang, T.; Guo, Y.; Pei, W.; Liu, R.; Chang, M.; Wang, X. Linolenic Acid Ameliorates Sarcopenia in C. elegans by Promoting Mitophagy and Fighting Oxidative Stress. Food Funct. 2023, 14, 1498–1509. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone Β-hydroxybutyrylation Is Critical in Reversal of Sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Pohl, F.; Egan, B.M.; Kocsisova, Z.; Kornfeld, K. Reproductive Aging in Caenorhabditis Elegans: From Molecules to Ecology. Front. Cell Dev. Biol. 2021, 9, 718522. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-Proteasome Pathway in Skeletal Muscle Atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Zhu, N.; Xu, M.; Li, Y. Beneficial Effects of Whey Protein Peptides on Muscle Loss in Aging Mice Models. Front. Nutr. 2022, 9, 897821. [Google Scholar] [CrossRef]
- Zhang, H.; Chi, M.; Wang, Y.; Chen, L.; Sun, X.; Wan, L.; Yang, Q.; Guo, C. Naringenin Alleviates Cisplatin Induced Muscle Atrophy by Regulating RIPK1/AMPK/NF-ΚB Pathway. J. Funct. Foods 2021, 86, 104714. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative Stress: Roles in Skeletal Muscle Atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Agrawal, S.; Chakole, S.; Shetty, N.; Prasad, R.; Lohakare, T.; Wanjari, M. Exploring the Role of Oxidative Stress in Skeletal Muscle Atrophy: Mechanisms and Implications. Cureus 2023, 15, e42178. [Google Scholar] [CrossRef]
- Wang, H.-H.; Sun, Y.-N.; Qu, T.-Q.; Sang, X.-Q.; Zhou, L.-M.; Li, Y.-X.; Ren, F.-Z. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 11963. [Google Scholar] [CrossRef] [PubMed]
- Son, R.H.; Kim, M.I.; Kim, H.M.; Guo, S.; Lee, D.H.; Lim, G.M.; Kim, S.-M.; Kim, J.-Y.; Kim, C.Y. Potential of Lycii Radicis Cortex as an Ameliorative Agent for Skeletal Muscle Atrophy. Pharmaceuticals 2024, 17, 462. [Google Scholar] [CrossRef]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell Death, Clearance and Immunity in the Skeletal Muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Siu, P.M.; Pistilli, E.E.; Butler, D.C.; Alway, S.E. Aging Influences Cellular and Molecular Responses of Apoptosis to Skeletal Muscle Unloading. Am. J. Physiol.-Cell Physiol. 2005, 288, C338–C349. [Google Scholar] [CrossRef]
- Vilchinskaya, N.A.; Rozhkov, S.V.; Turtikova, O.V.; Mirzoev, T.M.; Shenkman, B.S. AMPK Phosphorylation Impacts Apoptosis in Differentiating Myoblasts Isolated from Atrophied Rat Soleus Muscle. Cells 2023, 12, 920. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, H.; Guo, H.; Feng, W.; Jiang, C.; Jiang, Y. Propolis Ethanolic Extract Attenuates D-Gal-Induced C2C12 Cell Injury by Modulating Nrf2/HO-1 and P38/P53 Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 6408. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Ellis, R.E.; Yuan, J.; Horvitz, H.R. Mechanisms and Functions of Cell Death. Annu. Rev. Cell Biol. 1991, 7, 663–698. [Google Scholar] [CrossRef] [PubMed]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax Directly Induces Release of Cytochrome c from Isolated Mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef] [PubMed]
- Ghose, P.; Shaham, S. Cell Death in Animal Development. Development 2020, 147, dev191882. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging Roles of Caspase-3 in Apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Lee, H.; Eo, Y.; Kim, S.Y.; Lim, Y. Guava Leaf Extract Attenuated Muscle Proteolysis in Dexamethasone Induced Muscle Atrophic Mice via Ubiquitin Proteasome System, MTOR-Autophagy, and Apoptosis Pathway. Nutr. Res. 2024, 127, 97–107. [Google Scholar] [CrossRef]
- Bak, D.; Na, J.; Im, S.I.; Oh, C.T.; Kim, J.; Park, S.; Han, H.J.; Seok, J.; Choi, S.Y.; Ko, E.J.; et al. Antioxidant Effect of Human Placenta Hydrolysate against Oxidative Stress on Muscle Atrophy. J. Cell Physiol. 2019, 234, 1643–1658. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, G.; Peng, Y.; Wu, Y.; Han, X.; Xie, L.; Xu, H.; Chen, G.; Liu, B.; Xu, T.; et al. Danggui Shaoyao San Protects Cyclophosphamide-Induced Premature Ovarian Failure by Inhibiting Apoptosis and Oxidative Stress through the Regulation of the SIRT1/P53 Signaling Pathway. J. Ethnopharmacol. 2024, 323, 117718. [Google Scholar] [CrossRef]
- Kim, A.; Park, S.-M.; Kim, N.S.; Lee, H. Ginsenoside Rc, an Active Component of Panax Ginseng, Alleviates Oxidative Stress-Induced Muscle Atrophy via Improvement of Mitochondrial Biogenesis. Antioxidants 2023, 12, 1576. [Google Scholar] [CrossRef]
- Menconi, M.J.; Arany, Z.P.; Alamdari, N.; Aversa, Z.; Gonnella, P.; O’Neal, P.; Smith, I.J.; Tizio, S.; Hasselgren, P.-O. Sepsis and Glucocorticoids Downregulate the Expression of the Nuclear Cofactor PGC-1β in Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E533–E543. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Zhang, P.; Liu, H.; He, J.; Zhang, C.; Fan, M.; Chen, X. PGC-1α over-Expression Suppresses the Skeletal Muscle Atrophy and Myofiber-Type Composition during Hindlimb Unloading. Biosci. Biotechnol. Biochem. 2017, 81, 500–513. [Google Scholar] [CrossRef]
- Song, X.; Yang, B.; Qiu, F.; Jia, M.; Fu, G. High Glucose and Free Fatty Acids Induce Endothelial Progenitor Cell Senescence via PGC-1α/SIRT1 Signaling Pathway. Cell Biol. Int. 2017, 41, 1146–1159. [Google Scholar] [CrossRef]
- Ducreux, S.; Gregory, P.; Schwaller, B. Inverse Regulation of the Cytosolic Ca2+ Buffer Parvalbumin and Mitochondrial Volume in Muscle Cells via SIRT1/PGC-1α Axis. PLoS ONE 2012, 7, e44837. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform 2014, 6, 13. [Google Scholar] [CrossRef]
- Stelzer, G.; Dalah, I.; Stein, T.; Satanower, Y.; Rosen, N.; Nativ, N.; Oz-Levi, D.; Olender, T.; Belinky, F.; Bahir, I.; et al. In-Silico Human Genomics with GeneCards. Hum. Genom. 2011, 5, 709. [Google Scholar] [CrossRef]
- Collier, N.; Groza, T.; Smedley, D.; Robinson, P.N.; Oellrich, A.; Rebholz-Schuhmann, D. PhenoMiner: From Text to a Database of Phenotypes Associated with OMIM Diseases. Database 2015, 2015, bav104. [Google Scholar] [CrossRef]
- UniProt: The Universal Protein Knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Lin, C.; Zeng, J.; Zhang, S.; Xu, X.; Chen, L.; Yang, Z.; Wu, W.; Hu, C.; Zhao, Y.-T. Remedial Effects of Tilapia Skin Peptides against Dexamethasone-Induced Muscle Atrophy in Mice by Modulation of AKT/FOXO3a and Sirt1/PGC-1α Signaling Pathways. J. Funct. Foods 2024, 113, 105954. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, Y.; Wang, W.; Liang, G.; Qi, J. Mulberry Leaves Attenuate D-Galactose-Induced Aging in Vivo and in Vitro. J. Ethnopharmacol. 2023, 311, 116286. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Deng, K.; Zhang, M.; Xu, Y.; Zhang, J.; Liang, Q.; Yang, Z.; Jin, L.; Hu, C.; Zhao, Y.-T. Network Pharmacology Combined with Experimental Validation to Investigate the Effects and Mechanisms of Aucubin on Aging-Related Muscle Atrophy. Int. J. Mol. Sci. 2025, 26, 2626. https://doi.org/10.3390/ijms26062626
Li W, Deng K, Zhang M, Xu Y, Zhang J, Liang Q, Yang Z, Jin L, Hu C, Zhao Y-T. Network Pharmacology Combined with Experimental Validation to Investigate the Effects and Mechanisms of Aucubin on Aging-Related Muscle Atrophy. International Journal of Molecular Sciences. 2025; 26(6):2626. https://doi.org/10.3390/ijms26062626
Chicago/Turabian StyleLi, Wenan, Kaishu Deng, Mengyue Zhang, Yan Xu, Jingxi Zhang, Qingsheng Liang, Zhiyou Yang, Leigang Jin, Chuanyin Hu, and Yun-Tao Zhao. 2025. "Network Pharmacology Combined with Experimental Validation to Investigate the Effects and Mechanisms of Aucubin on Aging-Related Muscle Atrophy" International Journal of Molecular Sciences 26, no. 6: 2626. https://doi.org/10.3390/ijms26062626
APA StyleLi, W., Deng, K., Zhang, M., Xu, Y., Zhang, J., Liang, Q., Yang, Z., Jin, L., Hu, C., & Zhao, Y.-T. (2025). Network Pharmacology Combined with Experimental Validation to Investigate the Effects and Mechanisms of Aucubin on Aging-Related Muscle Atrophy. International Journal of Molecular Sciences, 26(6), 2626. https://doi.org/10.3390/ijms26062626





