Low Plasma Carnosinase-1 Activity in Patients with Left Ventricular Systolic Dysfunction: Implications for Carnosine Therapy in Heart Failure
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Variables
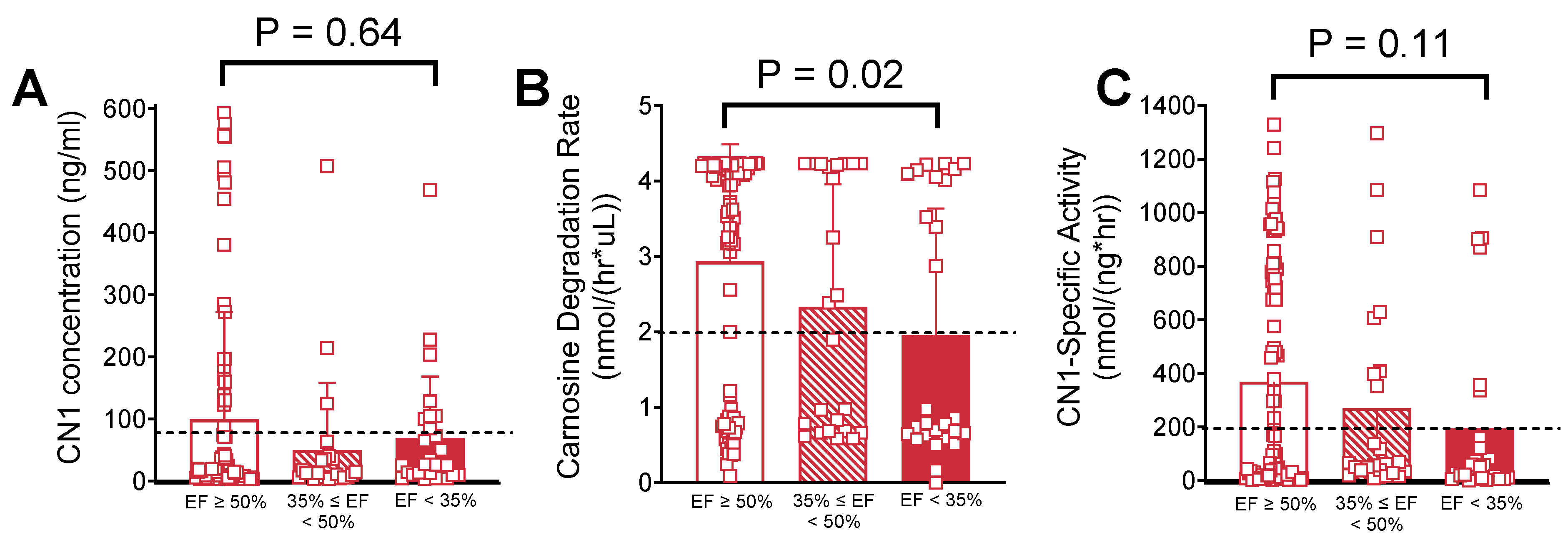
2.2. Cardiac Function
2.3. Univariable Analysis of Serum Carnosinase Content and Activity
2.4. Multivariable Analysis of Serum Carnosinase Content and Activity
3. Discussion
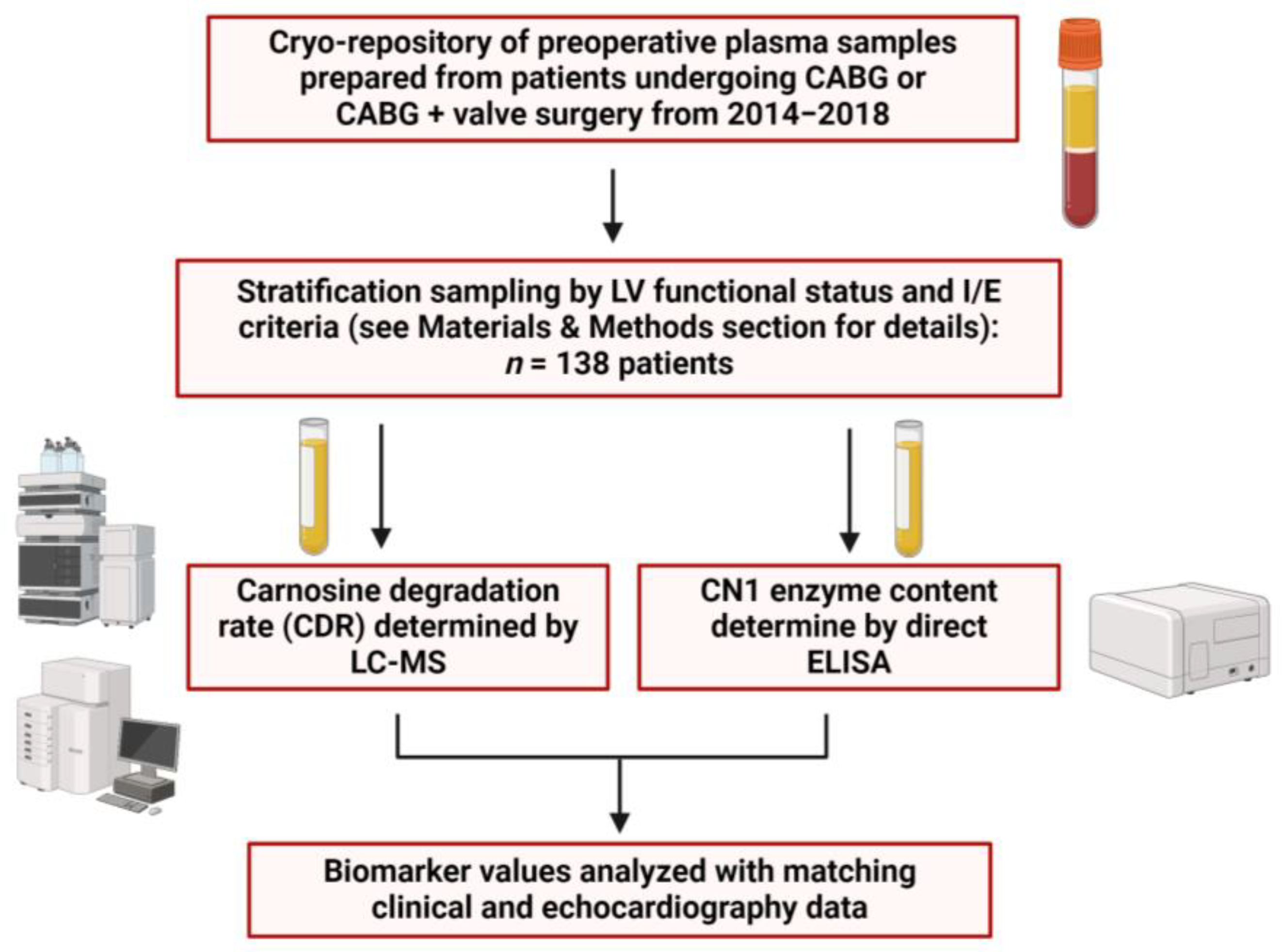
4. Materials and Methods
4.1. Study Design and Plasma Sample Collection
4.2. Plasma Carnosine Degradtion Rate (CDR) and CN1 Concentration Measurements
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usatyuk, P.V.; Parinandi, N.L.; Natarajan, V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J. Biol. Chem. 2006, 281, 35554–35566. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Grimsrud, P.A.; Picklo, M.J., Sr.; Griffin, T.J.; Bernlohr, D.A. Carbonylation of adipose proteins in obesity and insulin resistance: Identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol. Cell. Proteom. 2007, 6, 624–637. [Google Scholar] [CrossRef]
- Hwang, H.V.; Sandeep, N.; Paige, S.L.; Ranjbarvaziri, S.; Hu, D.-Q.; Zhao, M.; Lan, I.S.; Coronado, M.; Kooiker, K.B.; Wu, S.M.; et al. 4HNE Impairs Myocardial Bioenergetics in Congenital Heart Disease-Induced Right Ventricular Failure. Circulation 2020, 142, 1667–1683. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kusano, K.; Nakamura, Y.; Kakishita, M.; Ohta, K.; Nagase, S.; Yamamoto, M.; Miyaji, K.; Saito, H.; Morita, H.; et al. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation 2002, 105, 2867–2871. [Google Scholar] [CrossRef]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased adipose protein carbonylation in human obesity. Obesity 2011, 19, 1735–1741. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Dimitrijevic, Z.M.; Martinovic, S.S.S.; Nikolic, V.N.; Cvetkovic, T.P. Protein Carbonyl Content Is a Predictive Biomarker of Eccentric Left Ventricular Hypertrophy in Hemodialysis Patients. Diagnostics 2019, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Baye, E.; Ukropec, J.; de Courten, M.P.; Vallova, S.; Krumpolec, P.; Kurdiova, T.; Aldini, G.; Ukropcova, B.; de Courten, B. Effect of carnosine supplementation on the plasma lipidome in overweight and obese adults: A pilot randomised controlled trial. Sci. Rep. 2017, 7, 17458. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-α levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef]
- Lombardi, C.; Carubelli, V.; Lazzarini, V.; Vizzardi, E.; Bordonali, T.; Ciccarese, C.; Castrini, A.I.; Cas, A.D.; Nodari, S.; Metra, M. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition 2015, 31, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P.; Roberts, P.R.; Black, K.W.; Lin, M.; Zapata-Sudo, G.; Sudo, R.T.; Nelson, T.E. Carnosine is a novel peptide modulator of intracellular calcium and contractility in cardiac cells. Am. J. Physiol. 1997, 272 Pt 2, H462–H468. [Google Scholar] [CrossRef] [PubMed]
- Berdaweel, I.A.; Monroe, T.B.; Alowaisi, A.A.; Mahoney, J.C.; Liang, I.-C.; Berns, K.A.; Gao, D.; McLendon, J.M.; Anderson, E.J. Iron scavenging and suppression of collagen cross-linking underlie antifibrotic effects of carnosine in the heart with obesity. Front. Pharmacol. 2023, 14, 1275388. [Google Scholar] [CrossRef]
- Stefani, G.P.; Capalonga, L.; da Silva, L.R.; Dal Lago, P. β-Alanine and l-histidine supplementation associated with combined training increased functional capacity and maximum strength in heart failure rats. Exp. Physiol. 2020, 105, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Hanson, H.T.; Smith, E.L. Carnosinase; an enzyme of swine kidney. J. Biol. Chem. 1949, 179, 789–801. [Google Scholar] [CrossRef]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef]
- Jackson, M.C.; Kucera, C.M.; Lenney, J.F. Purification and properties of human serum carnosinase. Clin. Chim. Acta 1991, 196, 193–205. [Google Scholar] [CrossRef]
- Chmielewska, K.; Vittorio, S.; Gervasoni, S.; Dzierzbicka, K.; Inkielewicz-Stepniak, I.; Vistoli, G. Human carnosinases: A brief history, medicinal relevance, and in silico analyses. Drug Discov. Today 2024, 29, 103860. [Google Scholar] [CrossRef]
- Peters, V.; Kebbewar, M.; Jansen, E.W.; Jakobs, C.; Riedl, E.; Koeppel, H.; Frey, D.; Adelmann, K.; Klingbeil, K.; Mack, M.; et al. Relevance of allosteric conformations and homocarnosine concentration on carnosinase activity. Amino Acids 2010, 38, 1607–1615. [Google Scholar] [CrossRef]
- Bando, K.; Shimotsuji, T.; Toyoshima, H.; Hayashi, C.; Miyai, K. Fluorometric assay of human serum carnosinase activity in normal children, adults and patients with myopathy. Ann. Clin. Biochem. 1984, 21 Pt 6, 510–514. [Google Scholar] [CrossRef]
- Vistoli, G.; Pedretti, A.; Cattaneo, M.; Aldini, G.; Testa, B. Homology modeling of human serum carnosinase, a potential medicinal target, and MD simulations of its allosteric activation by citrate. J. Med. Chem. 2006, 49, 3269–3277. [Google Scholar] [CrossRef]
- Bellia, F.; Calabrese, V.; Guarino, F.; Cavallaro, M.; Cornelius, C.; De Pinto, V.; Rizzarelli, E. Carnosinase levels in aging brain: Redox state induction and cellular stress response. Antioxid. Redox Signal. 2009, 11, 2759–2775. [Google Scholar] [CrossRef]
- Everaert, I.; Taes, Y.; De Heer, E.; Baelde, H.; Zutinic, A.; Yard, B.; Sauerhöfer, S.; Vanhee, L.; Delanghe, J.; Aldini, G.; et al. Low plasma carnosinase activity promotes carnosinemia after carnosine ingestion in humans. Am. J. Physiol. Renal Physiol. 2012, 302, F1537–F1544. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef]
- Rodriguez-Niño, A.; Gant, C.M.; Braun, J.D.; Li, X.; Zhang, S.; Albrecht, T.; Qiu, J.; Bakker, S.J.L.; Laverman, G.D.; Krämer, B.K.; et al. Detection of carnosinase-1 in urine of healthy individuals and patients with type 2 diabetes: Correlation with albuminuria and renal function. Amino Acids 2019, 51, 17–25. [Google Scholar] [CrossRef]
- Qiu, J.; Yard, B.A.; Krämer, B.K.; van Goor, H.; van Dijk, P.; Kannt, A. Association Between Serum Carnosinase Concentration and Activity and Renal Function Impairment in a Type-2 Diabetes Cohort. Front. Pharmacol. 2022, 13, 899057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Albrecht, T.; Rodriguez-Niño, A.; Qiu, J.; Schnuelle, P.; Peters, V.; Schmitt, C.P.; Born, J.v.D.; Bakker, S.J.L.; Lammert, A.; et al. Carnosinase concentration, activity, and CNDP1 genotype in patients with type 2 diabetes with and without nephropathy. Amino Acids 2019, 51, 611–617. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.-Q.; Zhang, S.-Q.; Qi, X.-M.; Zhang, Q.; Yard, B.; Wu, Y.-G. Correlation between serum carnosinase concentration and renal damage in diabetic nephropathy patients. Amino Acids 2021, 53, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Terplan, K.L.; Cares, H.L. Histopathology of the nervous system in carnosinase enzyme deficiency with mental retardation. Neurology 1972, 22, 644–655. [Google Scholar] [CrossRef]
- Wisniewski, K.; Fleisher, L.; Rassin, D.; Lassmann, H. Neurological disease in a child with carnosinase deficiency. Neuropediatrics 1981, 12, 143–151. [Google Scholar] [CrossRef]
- Wassif, W.; Sherwood, R.; Amir, A.; Idowu, B.; Summers, B.; Leigh, N.; Peters, T. Serum carnosinase activities in central nervous system disorders. Clin. Chim. Acta 1994, 225, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bando, K.; Ichihara, K.; Toyoshima, H.; Shimotuji, T.; Koda, K.; Hayashi, C.; Miyai, K. Decreased activity of carnosinase in serum of patients with chronic liver disorders. Clin. Chem. 1986, 32, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- CDC. United States, National Center for Health Statistics. Percentage of coronary heart disease for adults aged 18 and over. In CDC National Health Interview Survey: CDC; United States, National Center for Health Statistics: Atlanta, GA, USA, 2019–2021. [Google Scholar]
- Heart Disease and African Americans. U.S. Department of Health and Human Services Office of Minority Health: U.S. Department of Health and Human Services Office of Minority Health. Available online: https://minorityhealth.hhs.gov/heart-disease-and-blackafrican-americans (accessed on 9 March 2025).
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; van der Woude, F.J. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef]
- Yadav, A.K.; Sinha, N.; Kumar, V.; Bhansali, A.; Dutta, P.; Jha, V. Association of CTG repeat polymorphism in carnosine dipeptidase 1 (CNDP1) gene with diabetic nephropathy in north Indians. Indian J. Med. Res. 2016, 144, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.A.; Hanson, R.L.; Kobes, S.; Millis, M.P.; Nelson, R.G.; Knowler, W.C.; DiStefano, J.K. Association of variants in the carnosine peptidase 1 gene (CNDP1) with diabetic nephropathy in American Indians. Mol. Genet. Metab. 2011, 103, 185–190. [Google Scholar] [CrossRef]
- McDonough, C.W.; Hicks, P.J.; Lu, L.; Langefeld, C.D.; Freedman, B.I.; Bowden, D.W. The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum. Genet. 2009, 126, 265–275. [Google Scholar] [CrossRef]
- McDonough, C.W.; Bostrom, M.A.; Lu, L.; Hicks, P.J.; Langefeld, C.D.; Divers, J.; Mychaleckyj, J.C.; Freedman, B.I.; Bowden, D.W. Genetic analysis of diabetic nephropathy on chromosome 18 in African Americans: Linkage analysis and dense SNP mapping. Hum. Genet. 2009, 126, 805–817. [Google Scholar] [CrossRef]
- Pavlin, M.; Rossetti, G.; De Vivo, M.; Carloni, P. Carnosine and Homocarnosine Degradation Mechanisms by the Human Carnosinase Enzyme CN1: Insights from Multiscale Simulations. Biochemistry 2016, 55, 2772–2784. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, their substrates and diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef]
- Hecht, E.M.; Arheart, K.L.; Lee, D.J.; Hennekens, C.H.; Hlaing, W.M. Interrelation of Cadmium, Smoking, and Cardiovascular Disease (from the National Health and Nutrition Examination Survey). Am. J. Cardiol. 2016, 118, 204–209. [Google Scholar] [CrossRef]
- Fontana, S.A.; Boulos, B.M. Blood cadmium level as affected by hypertension, smoking, occupation, and body mass. Am. J. Hypertens. 1988, 1 Pt 3, 158S–160S. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.J.; Wassif, W.S.; Sherwood, R.A.; Gerges, A.; Poyser, K.H.; Garthwaite, J.; Bath PM, W. Serum neuron-specific enolase, carnosinase, and their ratio in acute stroke: An enzymatic test for predicting outcome? Stroke 1996, 27, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Schoen, P.; Everts, H.; de Boer, T.; van Oeveren, W. Serum carnosinase activity in plasma and serum: Validation of a method and values in cardiopulmonary bypass surgery. Clin. Chem. 2003, 49, 1930–1932. [Google Scholar] [CrossRef] [PubMed]
- Pahari, D.R.; Gu, Y.J.; van Oeveren, W.; El-Essawi, A.; Harringer, W.; Brouwer, R.M. Effect of minimized perfusion circuit on brain injury markers carnosinase and brain-type fatty binding protein in coronary artery bypass grafting patients. Artif. Organs 2013, 37, 128–135. [Google Scholar] [CrossRef]
- Regazzoni, L. State of the Art in the Development of Human Serum Carnosinase Inhibitors. Molecules 2024, 29, 2488. [Google Scholar] [CrossRef]
- Gilardoni, E.; Gervasoni, S.; Maspero, M.; Dallanoce, C.; Vistoli, G.; Carini, M.; Aldini, G.; Regazzoni, L. Development of a direct LC-ESI-MS method for the measurement of human serum carnosinase activity. J. Pharm. Biomed. Anal. 2020, 189, 113440. [Google Scholar] [CrossRef]
| Variables n (%) | EF ≥ 50% n = 87 | EF ≥ 35 to <50% n = 24 | EF < 35% n = 27 | p-Value (Chi-Square) |
|---|---|---|---|---|
| Overall (Total n = 138) | 87 (63.0%) | 24 (17.4%) | 27 (19.6%) | |
| Demographic | ||||
| Age—yr (Mean ± SD) | 65 ± 6.3 | 64.5 ± 5.9 | 63.9 ± 6.8 | 0.80 † |
| Caucasian Race (%) | 73 (83.9%) | 20 (83.3%) | 16 (59.3%) | 0.02 |
| African American | 14 (16.1%) | 4 (16.7%) | 11 (40.7%) | |
| Obese (BMI ≥ 30 kg/m2) | 31 (35.6%) | 6 (25.0%) | 8 (29.6%) | 0.58 |
| Male | 51 (58.6%) | 22 (91.7%) | 21 (77.8%) | <0.01 |
| Female | 36 (41.4%) | 2 (8.3%) | 6 (22.2%) | |
| Comorbidities | ||||
| DM | 44 (50.6%) | 11 (45.8%) | 15 (55.6%) | 0.79 |
| HF | 6 (6.9%) | 6 (25.0%) | 14 (51.9%) | <0.01 |
| History of Afib | 7 (8.2%) | 3 (12.5%) | 2 (7.4%) | 0.83 * |
| COPD | 8 (9.3%) | 4 (16.7%) | 2 (7.4%) | 0.56 * |
| Prior MI | 34 (39.1%) | 14 (58.3%) | 17 (63.0%) | 0.045 |
| HTN | 72 (82.8%) | 17 (70.8%) | 24 (88.9%) | 0.256 * |
| Medication Use | ||||
| BB | 69 (79.3%) | 20 (83.3%) | 19 (70.4%) | 0.49 |
| ACEI/ARB | 36 (41.4%) | 10 (41.7%) | 10 (37.0%) | 0.92 |
| Diuretic | 30 (34.5%) | 4 (16.7%) | 11 (40.7%) | 0.16 |
| CCB | 19 (21.8%) | 5 (20.8%) | 2 (7.4%) | 0.24 |
| Nitrates | 57 (65.5%) | 18 (75.0%) | 14 (51.9%) | 0.21 |
| Statin | 70 (80.5%) | 21 (87.5%) | 22 (81.5%) | 0.77 * |
| Antihyperglycemic Medications | ||||
| DM medications | 39 (44.8%) | 11 (45.8%) | 14 (51.9%) | 0.81 |
| Insulin | 27 (31.0%) | 8 (33.3%) | 13 (48.2%) | 0.26 |
| Metformin | 18 (20.7%) | 3 (12.5%) | 1 (3.7%) | 0.08 * |
| Sulfonylurea | 4 (4.6%) | 1 (4.2%) | 1 (3.7%) | 1.00 * |
| Thiazolidinediones | 1 (1.2%) | 0 (0%) | 0 (0%) | 1.00 * |
| GLP-1 agonist | 2 (2.3%) | 0 (0%) | 0 (0%) | 1.00 * |
| DPP-4 inhibitors | 1 (1.2%) | 0 (0%) | 0 (0%) | 1.00 * |
| Variables Total n = 138 Mean ± SD | EF ≥ 50% n = 87 | EF ≥ 35 to <50% n = 24 | EF < 35% n = 27 | p-Value (Kruskal–Wallis Test) |
|---|---|---|---|---|
| MV E/A | 0.95 ± 0.36 | 0.94 ± 0.45 | 1.38 ± 0.73 | 0.02 |
| FS (%) | 39.2 ± 38.5 | 24.9 ± 8.9 | 16.2 ± 7.2 | <0.01 |
| LV mass DI (g) | 86.8 ± 24 | 100.7 ± 37.5 | 109.7 ± 31.5 | <0.01 |
| LA dimension (cm) | 3.67 ± 0.5 | 3.53 ± 0.6 | 3.73 ± 0.7 | 0.60 |
| EDV (Teich) (mL) | 98.8 ± 31 | 114.5 ± 25.6 | 143.3 ± 39.6 | <0.01 |
| ESV (Teich) (mL) | 37.2 ± 17.8 | 61.3 ± 26.8 | 96.9 ± 36 | <0.01 |
| SV (Teich) (mL) | 61.8 ± 21.6 | 51.8 ± 15.9 | 46.4 ± 22.6 | <0.01 |
| Odds Ratio Estimates | |||
|---|---|---|---|
| Variable | Ref | Point Estimate | 95% Wald Confidence Limits |
| Odds of CDR > 2 nmol/(h*μL) | |||
| DM | No DM | 0.46 | (0.21, 0.99) |
| EF ≥ 35 to <50% | EF < 35% | 1.10 | (0.34, 3.56) |
| EF ≥ 50% | EF < 35% | 5.93 | (2.10, 16.79) |
| Male | Female | 5.89 | (2.34, 14.81) |
| Odds Ratio Estimates | |||
|---|---|---|---|
| Variable | Ref | Point Estimate | 95% Wald Confidence Limits |
| Odds of CN1-Specific Activity > 200 nmol/(ng*h) | |||
| EF ≥ 35 to <50% | EF < 35% | 1.75 | (0.51, 6.06) |
| EF ≥ 50% | EF < 35% | 3.12 | (1.15, 8.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, I.-C.; Gilardoni, E.; Berdaweel, I.A.; Carter, K.D.; Anderson, E.J. Low Plasma Carnosinase-1 Activity in Patients with Left Ventricular Systolic Dysfunction: Implications for Carnosine Therapy in Heart Failure. Int. J. Mol. Sci. 2025, 26, 2608. https://doi.org/10.3390/ijms26062608
Liang I-C, Gilardoni E, Berdaweel IA, Carter KD, Anderson EJ. Low Plasma Carnosinase-1 Activity in Patients with Left Ventricular Systolic Dysfunction: Implications for Carnosine Therapy in Heart Failure. International Journal of Molecular Sciences. 2025; 26(6):2608. https://doi.org/10.3390/ijms26062608
Chicago/Turabian StyleLiang, I-Chau, Ettore Gilardoni, Islam A. Berdaweel, Knute D. Carter, and Ethan J. Anderson. 2025. "Low Plasma Carnosinase-1 Activity in Patients with Left Ventricular Systolic Dysfunction: Implications for Carnosine Therapy in Heart Failure" International Journal of Molecular Sciences 26, no. 6: 2608. https://doi.org/10.3390/ijms26062608
APA StyleLiang, I.-C., Gilardoni, E., Berdaweel, I. A., Carter, K. D., & Anderson, E. J. (2025). Low Plasma Carnosinase-1 Activity in Patients with Left Ventricular Systolic Dysfunction: Implications for Carnosine Therapy in Heart Failure. International Journal of Molecular Sciences, 26(6), 2608. https://doi.org/10.3390/ijms26062608





