Correlation of Anti-TULP1 Autoantibodies with Breast Cancer and Autoimmune Retinopathy
Abstract
1. Introduction
2. Results
2.1. Occurrence of Anti-TULP1 AAbs in Breast Cancer
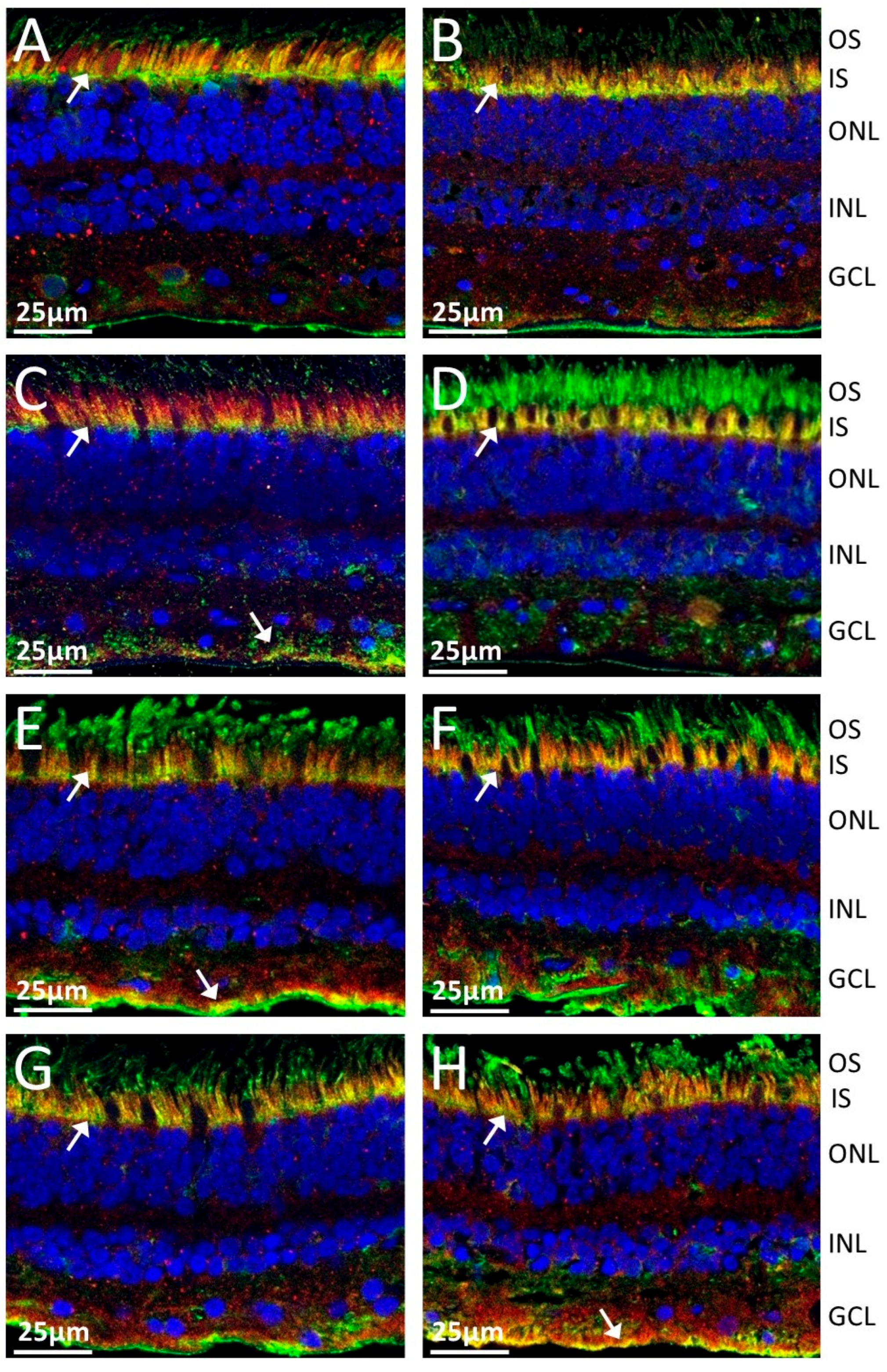
2.2. Anti-TULP1 AAbs Label Photoreceptor Inner Segments
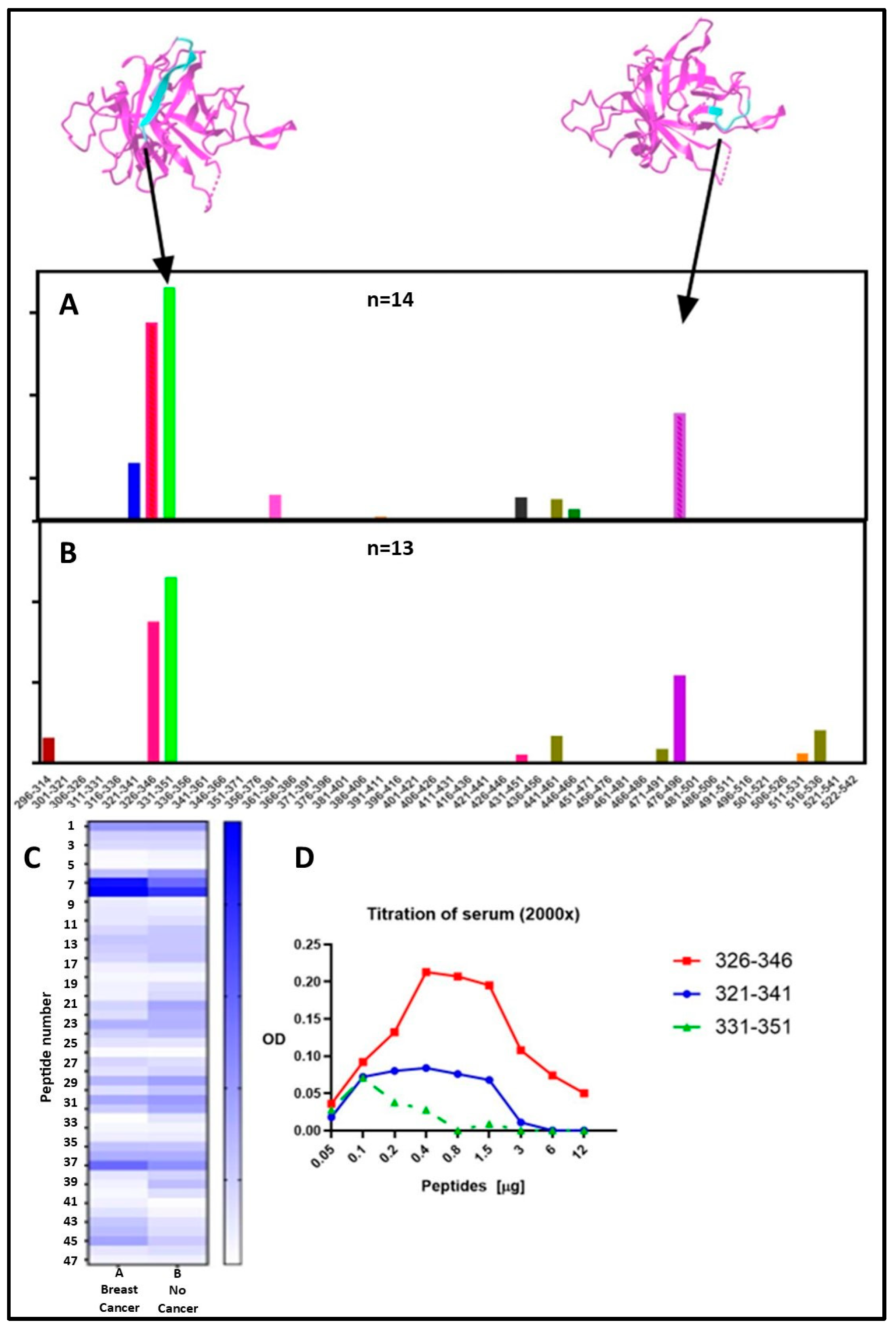
2.3. Epitope Mapping for Anti-TULP1 AAbs
2.4. Concurrence of Anti-Rab6 and Anti-Recoverin Autoantibodies
3. Discussion
4. Materials and Methods
4.1. Serum Samples
4.2. Immunoblotting
4.3. Double Fluorescent Immunolabeling
4.4. Epitope Mapping by ELISA
4.5. Screening Breast Cancer Tissue for TULP1
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamus, G.; Champaigne, R.; Yang, S. Occurrence of major anti-retinal autoantibodies associated with paraneoplastic autoimmune retinopathy. Clin. Immunol. 2020, 210, 108317. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Arai, J.; Shibuki, H.; Kawashima, H.; Yoshimura, N. Tubby-like protein 1 as an autoantigen in cancer-associated retinopathy. J. Neuroimmunol. 2000, 103, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.Z.; Mansour, H.A.; Zafar, M.K.; Uwaydat, S.H. Anti-Programmed Death Ligand-1 Induced Acute Vision Loss in a Patient With Cancer-Associated Retinopathy. Cureus 2022, 14, e21709. [Google Scholar] [CrossRef]
- Carroll, K.; Gomez, C.; Shapiro, L. Tubby proteins: The plot thickens. Nat. Rev. Mol. Cell Biol. 2004, 5, 55–64. [Google Scholar] [CrossRef]
- den Hollander, A.I.; Roepman, R.; Koenekoop, R.K.; Cremers, F.P. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008, 27, 391–419. [Google Scholar] [CrossRef]
- Majander, A.; Sankila, E.M.; Falck, A.; Vasara, L.K.; Seitsonen, S.; Kulmala, M.; Haavisto, A.K.; Avela, K.; Turunen, J.A. Natural history and biomarkers of retinal dystrophy caused by the biallelic TULP1 variant c.148delG. Acta Ophthalmol. 2023, 101, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Magupalli, V.G.; Dembla, M.; Katiyar, R.; Schwarz, K.; Köblitz, L.; Alpadi, K.; Krause, E.; Rettig, J.; Sung, C.-H.; et al. The Disease Protein Tulp1 Is Essential for Periactive Zone Endocytosis in Photoreceptor Ribbon Synapses. J. Neurosci. 2016, 36, 2473–2493. [Google Scholar] [CrossRef]
- Gu, S.; Lennon, A.; Li, Y.; Lorenz, B.; Fossarello, M.; North, M.; Gal, A.; Wright, A. Tubby-like protein-1 mutations in autosomal recessive retinitis pigmentosa. Lancet 1998, 351, 1103–1104. [Google Scholar] [CrossRef]
- Fairweather, D.; Rose, N.R. Women and autoimmune diseases. Emerg. Infect. Dis. 2004, 10, 2005–2011. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Zetterberg, M. Age-related eye disease and gender. Maturitas 2016, 83, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Yang, S.; Weleber, R.G. Unique epitopes for carbonic anhydrase II autoantibodies related to autoimmune retinopathy and cancer-associated retinopathy. Exp. Eye Res. 2016, 147, 161–168. [Google Scholar] [CrossRef]
- Fang, H.-S.; Yang, C.-S.; Cheng, C.-K.; Wang, Y.-S. Cancer-associated retinopathy as an initial presentation of gynecologic small-cell carcinoma. Taiwan J. Ophthalmol. 2024. [Google Scholar] [CrossRef]
- Deane, K.D. Preclinical Rheumatoid Arthritis (Autoantibodies): An Updated Review. Curr. Rheumatol. Rep. 2014, 16, 419. [Google Scholar] [CrossRef][Green Version]
- Adamus, G. Latest updates on antiretinal autoantibodies associated with vision loss and breast cancer. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Mangé, A.; Solassol, J. Use of autoantibodies to detect the onset of breast cancer. J. Immunol. Res. 2014, 2014, 574981. [Google Scholar] [CrossRef]
- Qiu, J.; Keyser, B.; Lin, Z.T.; Wu, T. Autoantibodies as Potential Biomarkers in Breast Cancer. Biosensors 2018, 8, 67. [Google Scholar] [CrossRef]
- Bazhin, A.V.; Savchenko, M.S.; Shifrina, O.N.; Demoura, S.A.; Chikina, S.Y.; Jaques, G.; Kogan, E.A.; Chuchalin, A.G.; Philippov, P.P. Recoverin as a paraneoplastic antigen in lung cancer: The occurrence of anti-recoverin autoantibodies in sera and recoverin in tumors. Lung Cancer 2004, 44, 193–198. [Google Scholar] [CrossRef]
- Polans, A.S.; Adamus, G. Recoverin is the tumor antigen in cancer-associated retinopathy. Behav. Brain Sci. 1995, 18, 483–485. [Google Scholar]
- Milam, A.H.; Hendrickson, A.E.; Xiao, M.; Smith, J.E.; Possin, D.E.; John, S.K.; Nishina, P.M. Localization of tubby-like protein 1 in developing and adult human retinas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2352–2356. [Google Scholar]
- Hagstrom, S.A.; Duyao, M.; North, M.A.; Li, T. Retinal degeneration in tulp1-/- mice: Vesicular accumulation in the interphotoreceptor matrix. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2795–2802. [Google Scholar]
- Ebke, L.A.; Sinha, S.; Pauer, G.J.T.; Hagstrom, S.A. Photoreceptor Compartment-Specific TULP1 Interactomes. Int. J. Mol. Sci. 2021, 22, 8066. [Google Scholar] [CrossRef]
- Remez, L.; Cohen, B.; Nevet, M.J.; Rizel, L.; Ben-Yosef, T. TULP1 and TUB Are Required for Specific Localization of PRCD to Photoreceptor Outer Segments. Int. J. Mol. Sci. 2020, 21, 8677. [Google Scholar] [CrossRef]
- Ebke, L.A.; Pauer, G.J.T.; Willard, B.; Hagstrom, S.A. A novel approach to identify photoreceptor compartment-specific tulp1 binding partners. Adv. Exp. Med. Biol. 2016, 854, 605–611. [Google Scholar] [PubMed]
- Grossman, G.H.; Watson, R.F.; Pauer, G.J.T.; Bollinger, K.; Hagstrom, S.A. Immunocytochemical evidence of Tulp1-dependent outer segment protein transport pathways in photoreceptor cells. Exp. Eye Res. 2011, 93, 658–668. [Google Scholar] [CrossRef]
- Bodenbender, J.P.; Marino, V.; Bethge, L.; Stingl, K.; Haack, T.B.; Biskup, S.; Kohl, S.; Kühlewein, L.; Dell’Orco, D.; Weisschuh, N. Biallelic Variants in TULP1 Are Associated with Heterogeneous Phenotypes of Retinal Dystrophy. Int. J. Mol. Sci. 2023, 24, 2709. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Rayborn, M.E.; Bell, B.A.; Marino, M.J.; Beight, C.D.; Pauer, G.J.; Traboulsi, E.I.; Hollyfield, J.G.; Hagstrom, S.A. Retinal histopathology in eyes from patients with autosomal dominant retinitis pigmentosa caused by rhodopsin mutations. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2161–2169. [Google Scholar] [CrossRef]
- Paloma, E.; Hjelmqvist, L.; Bayés, M.; García–Sandoval, B.; Ayuso, C.; Balcells, S.; Gonzàlez–Duarte, R. Novel Mutations in the TULP1 Gene Causing Autosomal Recessive Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2000, 41, 656–659. [Google Scholar]
- Wu, X.; Liu, X.; Koul, S.; Lee, C.Y.; Zhang, Z.; Halmos, B. AXL kinase as a novel target for cancer therapy. Oncotarget 2014, 5, 9546–9563. [Google Scholar] [CrossRef]
- Sartor, I.T.S.; Recamonde-Mendoza, M.; Ashton-Prolla, P. TULP3: A potential biomarker in colorectal cancer? PLoS ONE 2019, 14, e0210762. [Google Scholar] [CrossRef]
- Fanous, I.; Dillon, P. Paraneoplastic neurological complications of breast cancer. Exp. Hematol. Oncol. 2015, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Sartor, I.T.; Zeidán-Chuliá, F.; Albanus, R.D.; Dalmolin, R.J.; Moreira, J.C. Computational analyses reveal a prognostic impact of TULP3 as a transcriptional master regulator in pancreatic ductal adenocarcinoma. Mol. Biosyst. 2014, 10, 1461–1468. [Google Scholar] [CrossRef]
- Yang, S.; Dizhoor, A.; Wilson, D.J.; Adamus, G. GCAP1, Rab6, and HSP27: Novel Autoantibody Targets in Cancer-Associated Retinopathy and Autoimmune Retinopathy. Trans. Vis. Sci. Technol. 2016, 5, 1. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Storrie, B. Are Rab Proteins the Link Between Golgi Organization and Membrane Trafficking? Cell Mol. Life Sci. 2012, 69, 4093–4106. [Google Scholar] [CrossRef] [PubMed]
- Cayre, S.; Faraldo, M.M.; Bardin, S.; Miserey-Lenkei, S.; Deugnier, M.A.; Goud, B. RAB6 GTPase regulates mammary secretory function by controlling the activation of STAT5. Development 2020, 147, dev190744. [Google Scholar] [CrossRef]
- Young, J.; Menetrey, J.; Goud, B. RAB6C is a retrogene that encodes a centrosomal protein involved in cell cycle progression. J. Mol. Biol. 2010, 397, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Erol, Ö.D.; Şenocak, Ş.; Aerts-Kaya, F. The Role of Rab GTPases in the development of genetic and malignant diseases. Mol. Cell Biochem. 2024, 479, 255–281. [Google Scholar] [CrossRef]
- Anand, S.; Khan, M.A.; Khushman, M.; Dasgupta, S.; Singh, S.; Singh, A.P. Comprehensive Analysis of Expression, Clinicopathological Association and Potential Prognostic Significance of RABs in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 5580. [Google Scholar] [CrossRef]
- Adamus, G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun. Rev. 2003, 2, 63–69. [Google Scholar] [CrossRef]
- Xi, Q.; Pauer, G.J.T.; Marmorstein, A.D.; Crabb, J.W.; Hagstrom, S.A. Tubby-like Protein 1 (TULP1) Interacts with F-actin in Photoreceptor Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4754–4761. [Google Scholar] [CrossRef]
- Xi, Q.; Pauer, G.J.; Ball, S.L.; Rayborn, M.; Hollyfield, J.G.; Peachey, N.S.; Crabb, J.W.; Hagstrom, S.A. Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest. Ophthalmol. Vis. Sci. 2007, 48, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G. Current techniques to accurately measure anti-retinal autoantibodies. Expert. Rev. Ophthalmol. 2020, 15, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Amundson, D. Epitope recognition of recoverin in cancer associated retinopathy: Evidence for calcium-dependent conformational epitopes. J. Neurosci. Res. 1996, 45, 863–872. [Google Scholar] [CrossRef]



| Cancer |
Total Cases (n = 430) |
TULP1 Positivity (n = 76) | Positivity Rate (%) | p Value |
|---|---|---|---|---|
| BCC | 32 | 6 | 19% | 0.715 |
| Bladder | 17 | 4 | 24% | 0.422 |
| Breast | 90 | 36 | 40% | 0.015 |
| Colon | 16 | 1 | 6% | 0.638 |
| Kidney | 21 | 4 | 19% | 0.689 |
| Lung | 59 | 11 | 19% | 0.748 |
| Melanoma | 103 | 5 | 5% | 0.173 |
| Ovarian | 11 | 2 | 18% | 0.640 |
| Prostate | 36 | 5 | 14% | 1.000 |
| Lymphoma | 45 | 2 | 4% | 0.333 |
| Ocular Findings | Number of Patients (n = 90) | Frequency (%) |
|---|---|---|
| Uveitis | 7 | 8 |
| Central vision loss | 8 | 9 |
| Peripheral vision loss | 12 | 13 |
| Color vision loss | 11 | 12 |
| Decreased visual acuity | 27 | 30 |
| Visual field defect (scotomas) | 32 | 36 |
| ERG defects | 16 | 18 |
| Retinal pigmentary changes | 15 | 17 |
| Retinal atrophy | 13 | 14 |
| Attenuated vessels | 8 | 9 |
| Optic disc pallor | 13 | 14 |
| Asymmetric loss | 17 | 19 |
| Sudden loss | 4 | 4 |
| Gradual loss | 4 | 4 |
| Progressive course | 17 | 19 |
| Blurry vision | 21 | 23 |
| Photopsia/photophobia | 11 | 12 |
| Nyctalopia (dark vision) | 11 | 12 |
| Peptide | Frequency Breast Cancer Sera (n = 14) |
Frequency No-Cancer Sera (n = 13) |
|---|---|---|
| 326–346 | 93% | 92% |
| 331–351 (epitope 1) | 100% (p = 0.00061) | 85% (p = 0.0112) |
| 471–491 | 93% | 62% |
| 476–496 (epitope 2) | 93% (p = 0.000915) | 92% (0.00172) |
| Peptide Number | Peptide Position | Sequence |
|---|---|---|
| 1 | 296–314 | RPAPQGRTVRCRLTRDKKGM |
| 2 | 301–321 | GRTVRCRLTRDKKGMDRGMY |
| 3 | 306–326 | CRLTRDKKGMDRGMYPSYFL |
| 4 | 311–331 | DKKGMDRGMYPSYFLHLDTE |
| 5 | 316–336 | DRGMYPSYFLHLDTEKKVFL |
| 6 | 321–341 | PSYFLHLDTEKKVFLLAGRK |
| 7 | 326–346 | HLDTEKKVFLLAGRKRKRSK |
| 8 | 331–351 | KKVFLLAGRKRKRSKTANYL |
| 9 | 336–356 | LAGRKRKRSKTANYLISIDP |
| 10 | 341–361 | RKRSKTANYLISIDPTNLSR |
| 11 | 346–366 | TANYLISIDPTNLSRGGENF |
| 12 | 351–371 | ISIDPTNLSRGGENFIGKLR |
| 13 | 356–376 | TNLSRGGENFIGKLRSNLLG |
| 14 | 361–381 | GGENFIGKLRSNLLGNRFTV |
| 15 | 366–386 | IGKLRSNLLGNRFTVFDNGQ |
| 16 | 371–391 | SNLLGNRFTVFDNGQNPQRG |
| 17 | 376–396 | NRFTVFDNGQNPQRGYSTNV |
| 18 | 381–401 | FDNGQNPQRGYSTNVASLRQ |
| 19 | 386–406 | NPQRGYSTNVASLRQELAAV |
| 20 | 391–411 | YSTNVASLRQELAAVIYETN |
| 21 | 396–416 | ASLRQELAAVIYETNVLGFR |
| 22 | 401–421 | ELAAVIYETNVLGFRGPRRM |
| 23 | 406–426 | IYETNVLGFRGPRRMTVIIP |
| 24 | 411–431 | VLGFRGPRRMTVIIPGMSAE |
| 25 | 416–436 | GPRRMTVIIPGMSAENERVP |
| 26 | 421–441 | TVIIPGMSAENERVPIRPRN |
| 27 | 426–446 | GMSAENERVPIRPRNASDGL |
| 28 | 431–451 | NERVPIRPRNASDGLLVRWQ |
| 29 | 436–456 | IRPRNASDGLLVRWQNKTLE |
| 30 | 441–461 | ASDGLLVRWQNKTLESLIEL |
| 31 | 446–466 | LVRWQNKTLESLIELHNKPP |
| 32 | 451–471 | NKTLESLIELHNKPPVWNDD |
| 33 | 456–476 | SLIELHNKPPVWNDDSGSYT |
| 34 | 461–481 | HNKPPVWNDDSGSYTLNFQG |
| 35 | 466–486 | VWNDDSGSYTLNFQGRVTQA |
| 36 | 471–491 | SGSYTLNFQGRVTQASVKNF |
| 37 | 476–496 | LNFQGRVTQASVKNFQIVHA |
| 38 | 481–501 | RVTQASVKNFQIVHADDPDY |
| 39 | 486–506 | SVKNFQIVHADDPDYIVLQF |
| 40 | 491–511 | QIVHADDPDYIVLQFGRVAE |
| 41 | 496–516 | DDPDYIVLQFGRVAEDAFTL |
| 42 | 501–521 | IVLQFGRVAEDAFTLDYRYP |
| 43 | 506–526 | GRVAEDAFTLDYRYPLCALQ |
| 44 | 511–531 | DAFTLDYRYPLCALQAFAIA |
| 45 | 516–536 | DYRYPLCALQAFAIALSSFD |
| 46 | 521–541 | LCALQAFAIALSSFDGKLAC |
| 47 | 522–542 | CALQAFAIALSSFDGKLACE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaster, C.; Yang, S.; Adamus, G. Correlation of Anti-TULP1 Autoantibodies with Breast Cancer and Autoimmune Retinopathy. Int. J. Mol. Sci. 2025, 26, 2569. https://doi.org/10.3390/ijms26062569
Kaster C, Yang S, Adamus G. Correlation of Anti-TULP1 Autoantibodies with Breast Cancer and Autoimmune Retinopathy. International Journal of Molecular Sciences. 2025; 26(6):2569. https://doi.org/10.3390/ijms26062569
Chicago/Turabian StyleKaster, Collin, Sufang Yang, and Grazyna Adamus. 2025. "Correlation of Anti-TULP1 Autoantibodies with Breast Cancer and Autoimmune Retinopathy" International Journal of Molecular Sciences 26, no. 6: 2569. https://doi.org/10.3390/ijms26062569
APA StyleKaster, C., Yang, S., & Adamus, G. (2025). Correlation of Anti-TULP1 Autoantibodies with Breast Cancer and Autoimmune Retinopathy. International Journal of Molecular Sciences, 26(6), 2569. https://doi.org/10.3390/ijms26062569





