miRNAs-Set of Plasmatic Extracellular Vesicles as Novel Biomarkers for Hepatocellular Carcinoma Diagnosis Across Tumor Stage and Etiologies
Abstract
1. Introduction
2. Results
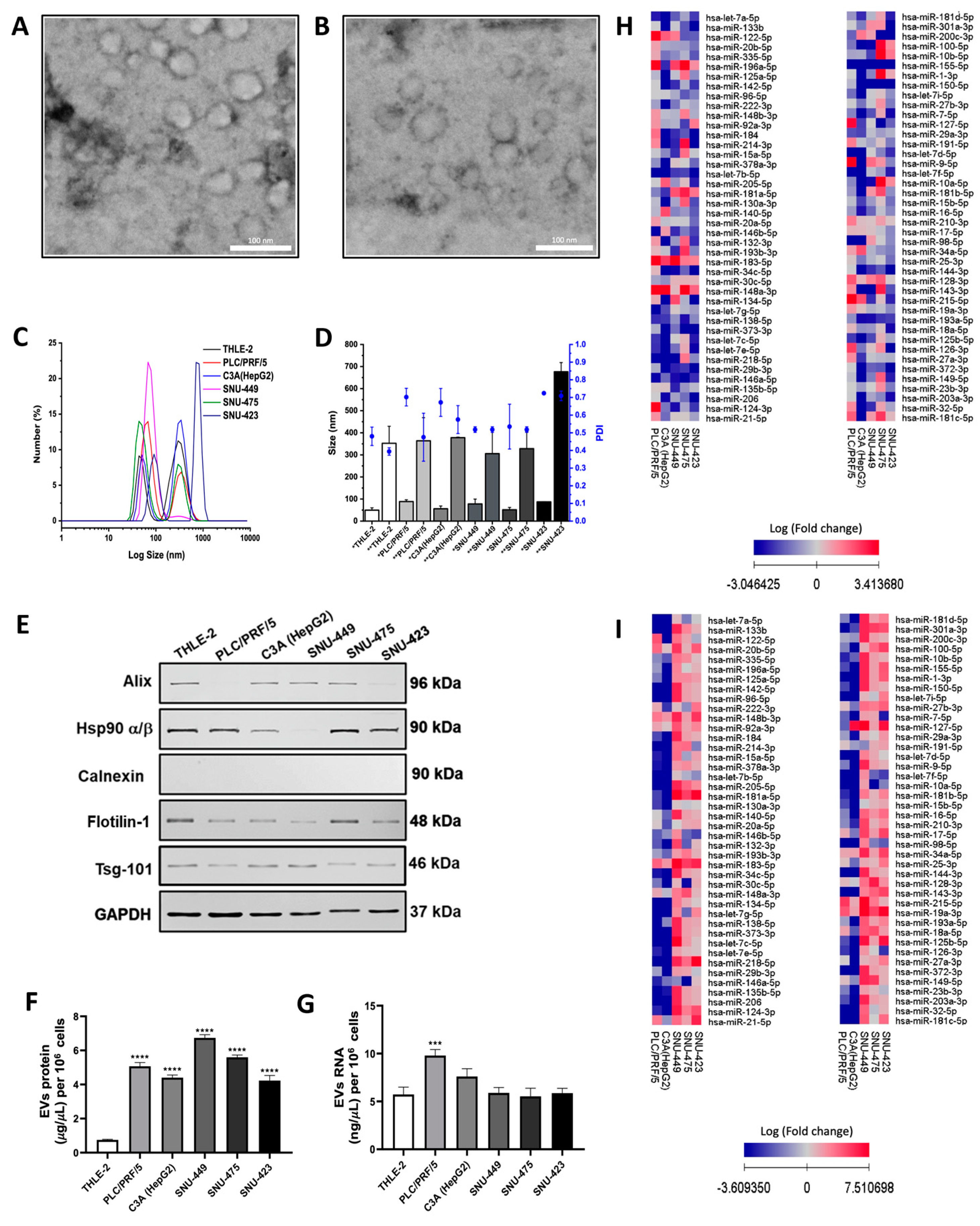
2.1. Isolation and Characterization of EVs Secreted by HCC Cell Lines
2.2. miRNA Expression Profiles of EVs Derived from Hepatoma Cell Lines
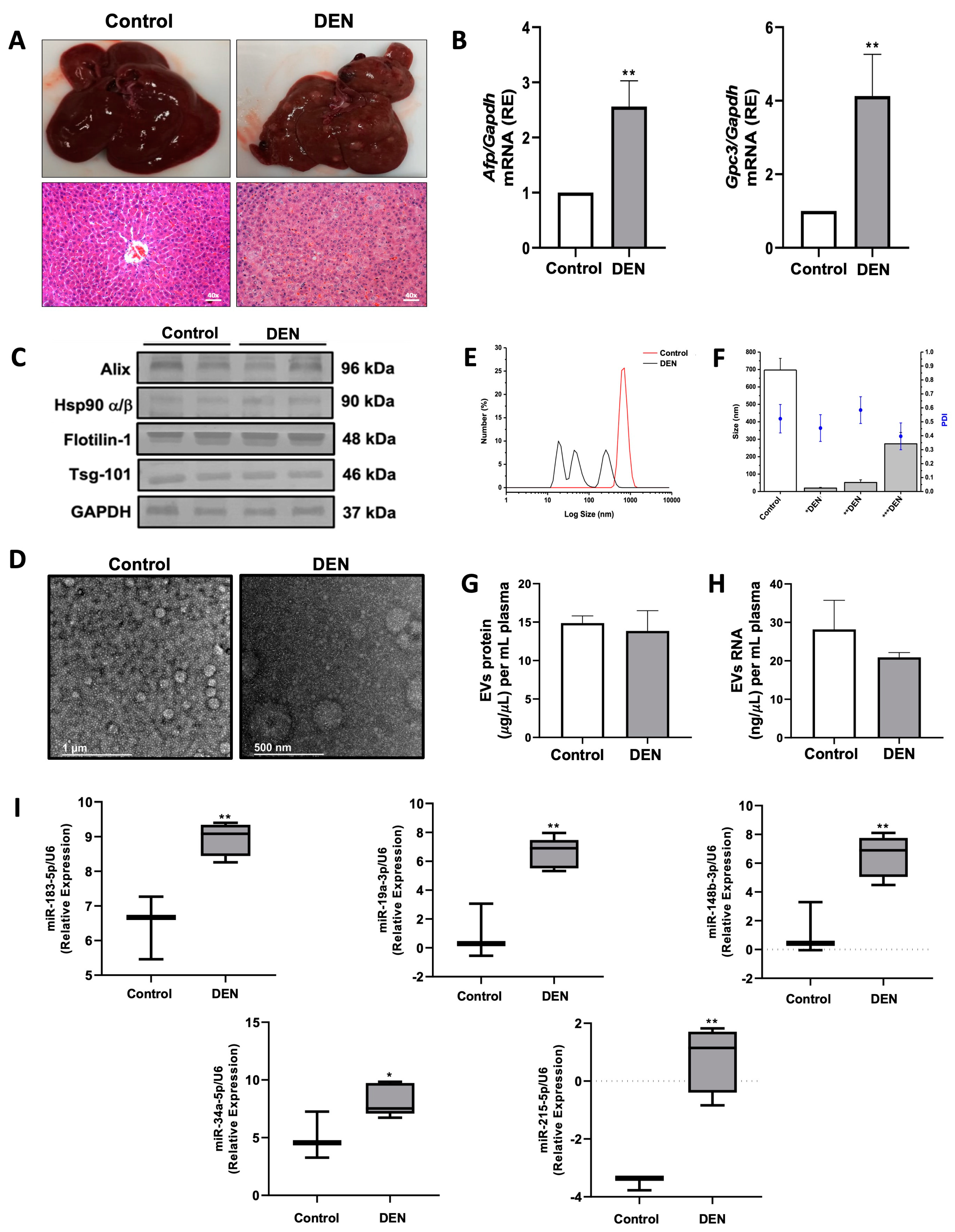
2.3. miRNA Expression Profiles in Circulating Plasma EVs of DEN-Induced Liver Tumor Rat Model
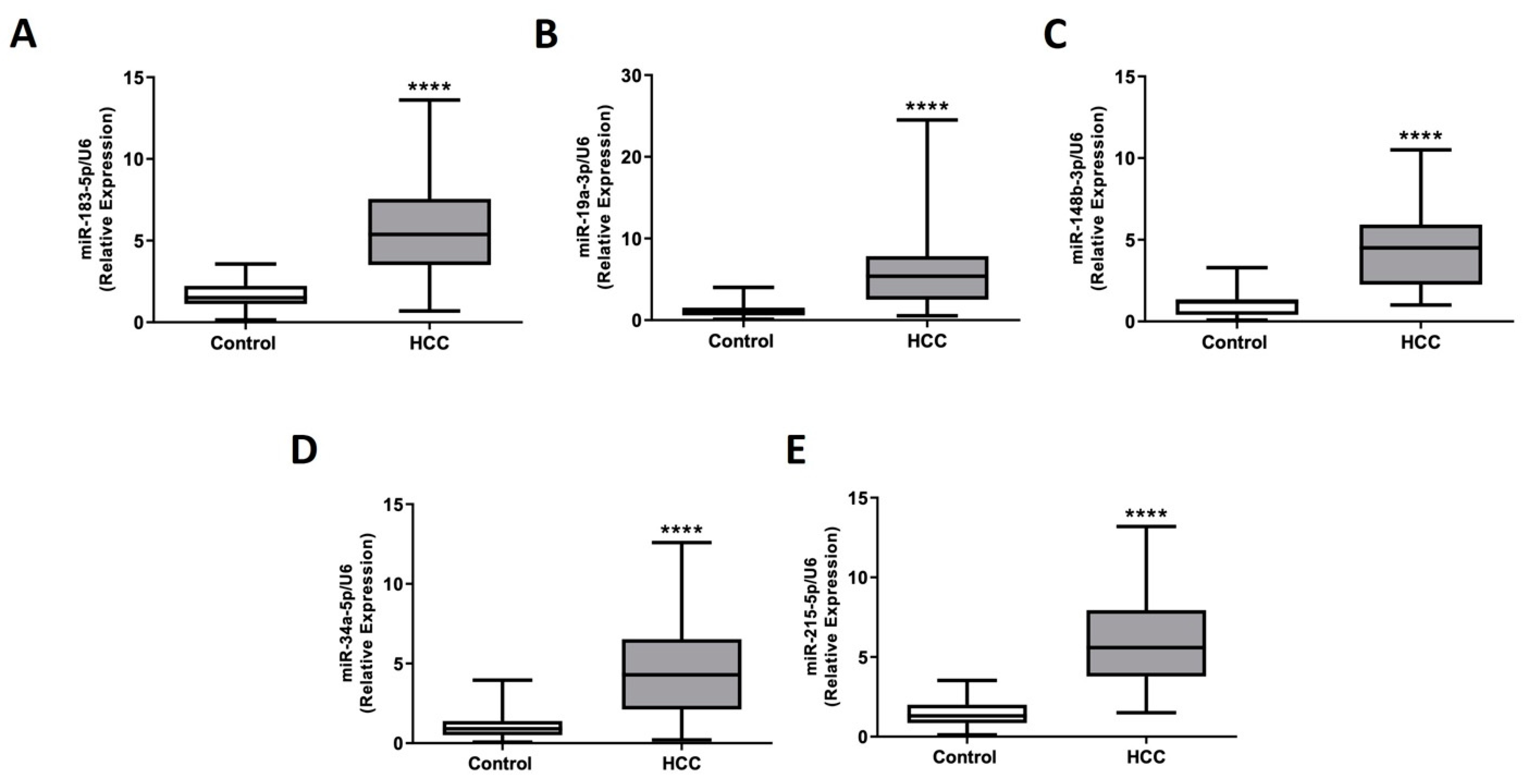
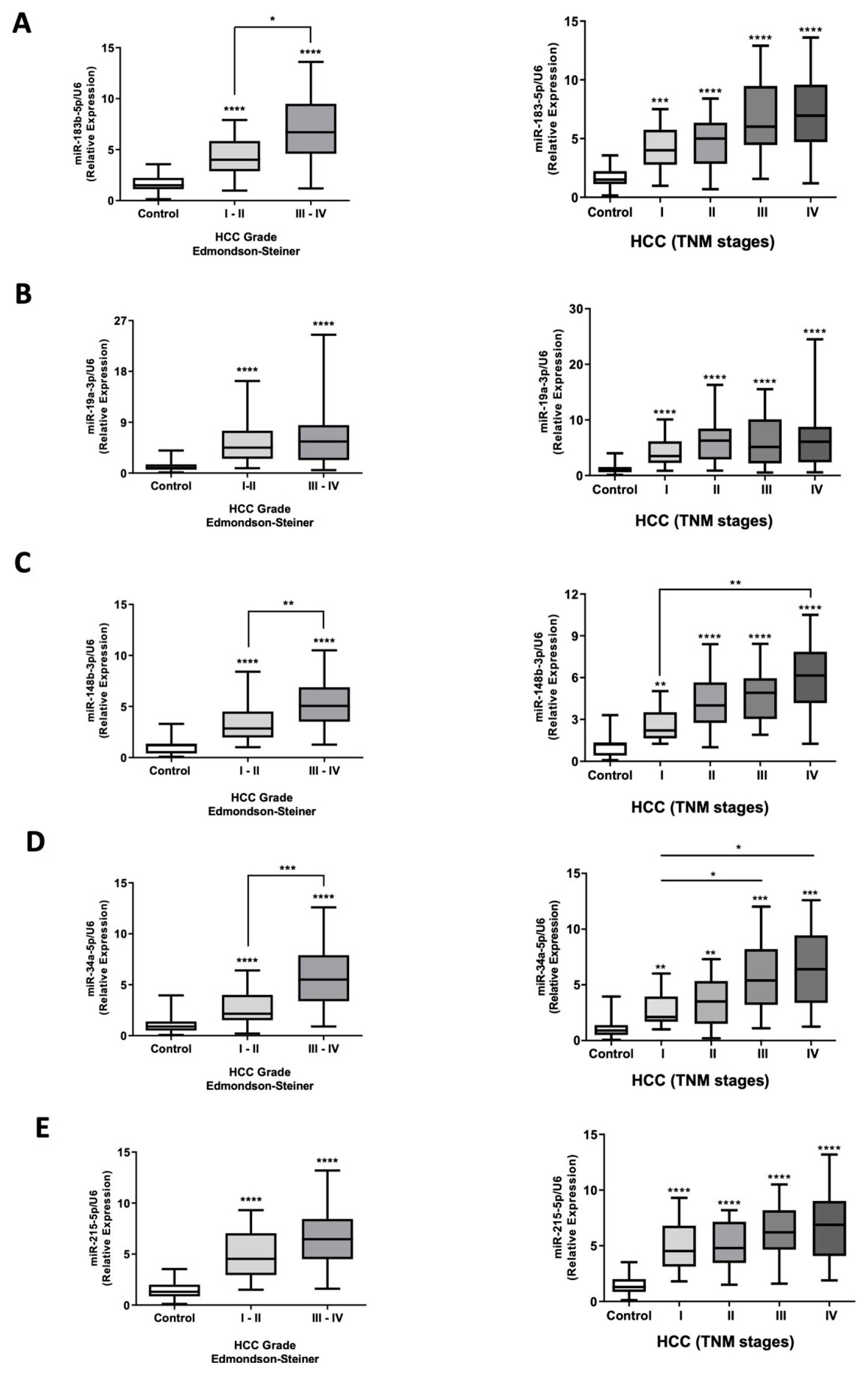
2.4. miRNA Expression Assessment in Plasma EVs Obtained from HCC Patients
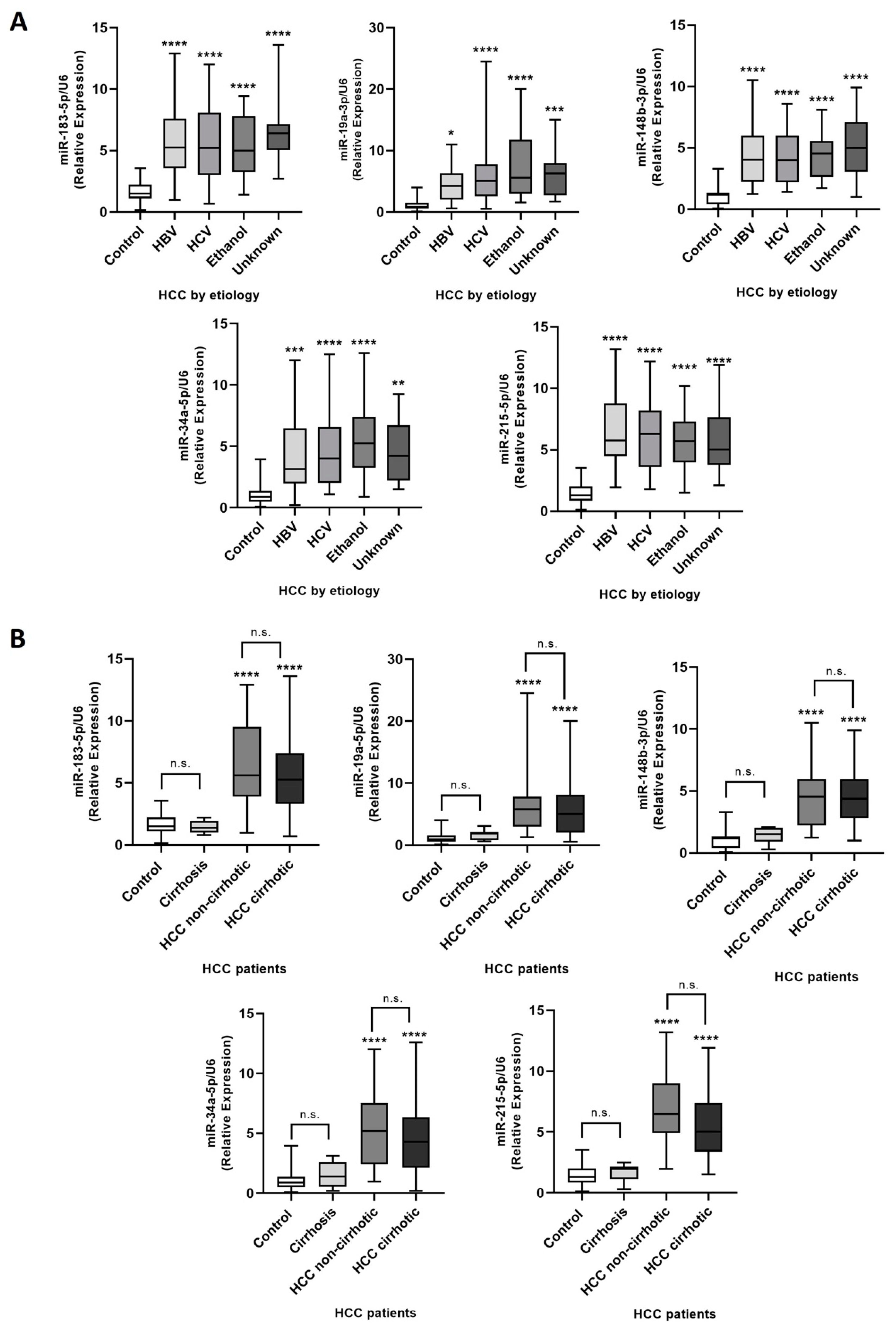
2.5. Efficacy Validation of miRNA Contained in Plasma-Circulating EVs as Diagnostic Biomarkers of HCC
2.6. miRNA Contained in Plasma-Circulating EVs as a Diagnostic Panel for HCC Detection
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Isolation of Extracellular Vesicles
4.3. Hepatocellular Carcinoma-Induced Rat Model
4.4. Ethical Approval, Informed Consent for Participation, and Sample Collection from Human Subjects
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group | miRNA | Cut-Off Value | Sensitivity (%) | Specificity (%) | Accuracy (%) | Youden’s Index (J) |
|---|---|---|---|---|---|---|
| Control vs. HCC | miR-183-5p | 2.614 | 84.44 | 92.68 | 88.56 | 0.7712 |
| miR-19a-3p | 1.801 | 88.89 | 82.93 | 85.91 | 0.7182 | |
| miR-148b-3p | 1.515 | 94.44 | 85.37 | 89.90 | 0.7981 | |
| miR-34a-5p | 1.810 | 84.44 | 82.93 | 83.68 | 0.6737 | |
| miR-215-5p | 3.163 | 84.44 | 95.12 | 89.78 | 0.7956 | |
| Control vs. Stage I | miR-183-5p | 2.495 | 80.95 | 90.24 | 85.59 | 0.7119 |
| miR-19a-3p | 1.801 | 90.48 | 82.93 | 86.70 | 0.7341 | |
| miR-148b-3p | 1.515 | 90.48 | 85.37 | 87.92 | 0.7585 | |
| miR-34a-5p | 1.510 | 90.48 | 78.05 | 84.24 | 0.6853 | |
| miR-215-5p | 2.833 | 85.71 | 90.24 | 87.97 | 0.7595 | |
| Control vs. Stage II | miR-183-5p | 2.480 | 85.71 | 90.24 | 87.97 | 0.7595 |
| miR-19a-3p | 2.700 | 80.95 | 92.68 | 86.81 | 0.7363 | |
| miR-148b-3p | 1.982 | 95.24 | 90.24 | 92.74 | 0.8548 | |
| miR-34a-5p | 1.400 | 80.95 | 75.61 | 78.28 | 0.5656 | |
| miR-215-5p | 3.213 | 80.95 | 95.12 | 88.03 | 0.7607 | |
| Control vs. Stage III | miR-183-5p | 3.457 | 88.46 | 97.56 | 93.01 | 0.8602 |
| miR-19a-3p | 1.635 | 88.46 | 80.49 | 84.47 | 0.6895 | |
| miR-148b-3p | 1.982 | 96.15 | 90.24 | 93.19 | 0.8639 | |
| miR-34a-5p | 2.582 | 88.46 | 92.68 | 90.57 | 0.8114 | |
| miR-215-5p | 3.163 | 92.31 | 95.12 | 94.21 | 0.8743 | |
| Control vs. Stage IV | miR-183-5p | 3.457 | 86.36 | 97.56 | 91.96 | 0.8392 |
| miR-19a-3p | 1.873 | 90.91 | 82.93 | 86.92 | 0.7384 | |
| miR-148b-3p | 3.010 | 86.36 | 97.56 | 91.96 | 0.8392 | |
| miR-34a-5p | 2.582 | 86.36 | 92.68 | 89.52 | 0.7904 | |
| miR-215-5p | 2.669 | 95.45 | 87.80 | 91.62 | 0.8325 | |
| Group | AUC | LR+ | LR− | SE | 95% CI | p-Value |
| Control vs. HCC | 0.9233 | 11.535 | 0.167 | 0.02232 | 0.879–0.967 | <0.0001 |
| 0.9224 | 5.207 | 0.133 | 0.02281 | 0.877–0.967 | <0.0001 | |
| 0.9576 | 6.455 | 0.065 | 0.01604 | 0.926–0.989 | <0.0001 | |
| 0.9115 | 4.946 | 0.187 | 0.02537 | 0.861–0.961 | <0.0001 | |
| 0.9642 | 17.303 | 0.163 | 0.01328 | 0.938–0.990 | <0.0001 | |
| Control vs. Stage I | 0.8827 | 8.294 | 0.211 | 0.05029 | 0.784–0.981 | <0.0001 |
| 0.9199 | 5.300 | 0.114 | 0.03475 | 0.851–0.988 | <0.0001 | |
| 0.9181 | 6.184 | 0.111 | 0.03396 | 0.851–0.984 | <0.0001 | |
| 0.8705 | 4.122 | 0.121 | 0.04410 | 0.784–0.957 | <0.0001 | |
| 0.9506 | 8.781 | 0.158 | 0.02526 | 0.901–1.000 | <0.0001 | |
| Control vs. Stage II | 0.8995 | 8.781 | 0.158 | 0.04988 | 0.801–0.997 | <0.0001 |
| 0.9274 | 11.058 | 0.205 | 0.03496 | 0.858–0.995 | <0.0001 | |
| 0.9559 | 9.758 | 0.052 | 0.02877 | 0.899–1.000 | <0.0001 | |
| 0.8339 | 3.318 | 0.251 | 0.05851 | 0.719–0.948 | <0.0001 | |
| 0.9547 | 16.588 | 0.200 | 0.02591 | 0.903–1.000 | <0.0001 | |
| Control vs. Stage III | 0.9587 | 36.254 | 0.118 | 0.02505 | 0.909–1.000 | <0.0001 |
| 0.9203 | 4.534 | 0.143 | 0.03738 | 0.847–0.993 | <0.0001 | |
| 0.9822 | 9.851 | 0.042 | 0.01154 | 0.959–1.000 | <0.0001 | |
| 0.9601 | 12.084 | 0.124 | 0.02177 | 0.917–1.000 | <0.0001 | |
| 0.9737 | 18.915 | 0.080 | 0.01801 | 0.938–1.000 | <0.0001 | |
| Control vs. Stage IV | 0.9429 | 35.393 | 0.139 | 0.03764 | 0.869–1.000 | <0.0001 |
| 0.9224 | 5.325 | 0.109 | 0.03873 | 0.846–0.998 | <0.0001 | |
| 0.9678 | 35.393 | 0.139 | 0.02057 | 0.927–1.000 | <0.0001 | |
| 0.9673 | 11.797 | 0.147 | 0.01856 | 0.930–1.000 | <0.0001 | |
| 0.9751 | 7.823 | 0.051 | 0.01619 | 0.943–1.000 | <0.0001 |
| Group | miRNA | Cut-Off Value | Sensitivity (%) | Specificity (%) | Accuracy (%) | Youden’s Index (J) | |
|---|---|---|---|---|---|---|---|
| Control vs. I–II | miR-183-5p | 2.48 | 82.35 | 90.24 | 86.67 | 0.7259 | |
| miR-19a-3p | 2.515 | 79.41 | 92.68 | 86.67 | 0.7209 | ||
| miR-148b-3p | 1.515 | 91.18 | 85.37 | 88.00 | 0.7655 | ||
| miR-34a-5p | 1.24 | 88.24 | 73.17 | 80.00 | 0.6141 | ||
| miR-215-5p | 2.833 | 82.35 | 90.24 | 86.67 | 0.7259 | ||
| Control vs. III- IV | miR-183-5p | 3.582 | 87.5 | 100 | 93.26 | 0.875 | |
| miR-19a-3p | 1.801 | 89.29 | 82.93 | 86.60 | 0.7222 | ||
| miR-148b-3p | 1.982 | 92.86 | 90.24 | 91.75 | 0.831 | ||
| miR-34a-5p | 2.582 | 85.96 | 92.68 | 88.78 | 0.7864 | ||
| miR-215-5p | 3.163 | 89.58 | 95.12 | 92.13 | 0.847 | ||
| Group | miRNA | LR+ | LR− | AUC | SE | 95% CI | p-Value |
| Control vs. I–II | miR-183-5p | 8.44 | 0.12 | 0.892 | 0.03877 | 0.8161–0.9680 | <0.0001 |
| miR-19a-3p | 10.85 | 0.09 | 0.9186 | 0.03082 | 0.8582–0.9790 | <0.0001 | |
| miR-148b-3p | 6.23 | 0.16 | 0.9258 | 0.02952 | 0.8679–0.9836 | <0.0001 | |
| miR-34a-5p | 3.29 | 0.30 | 0.8375 | 0.04707 | 0.7453–0.9298 | <0.0001 | |
| miR-215-5p | 8.44 | 0.12 | 0.9426 | 0.02386 | 0.8958–0.9894 | <0.0001 | |
| Control vs. III- IV | miR-183-5p | 87.50 | 0.00 | 0.9515 | 0.02246 | 0.9074–0.9955 | <0.0001 |
| miR-19a-3p | 5.23 | 0.19 | 0.9247 | 0.02624 | 0.8732–0.9761 | <0.0001 | |
| miR-148b-3p | 9.51 | 0.11 | 0.9769 | 0.01139 | 0.9546–0.9992 | <0.0001 | |
| miR-34a-5p | 11.74 | 0.09 | 0.9572 | 0.01746 | 0.9230–0.9914 | <0.0001 | |
| miR-215-5p | 18.36 | 0.05 | 0.9743 | 0.01323 | 0.9484–1.000 | <0.0001 | |
References
- Farazi, P.A.; DePinho, R.A. Hepatocellular Carcinoma Pathogenesis: From Genes to Environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, C. Advances in the Early Diagnosis of Hepatocellular Carcinoma. Genes Dis. 2020, 7, 308–319. [Google Scholar] [CrossRef]
- Rao, A.; Rich, N.E.; Marrero, J.A.; Yopp, A.C.; Singal, A.G. Diagnostic and Therapeutic Delays in Patients with Hepatocellular Carcinoma. JNCCN J. Natl. Compr. Cancer Netw. 2021, 19, 1063–1071. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of Hepatocellular Carcinoma: An Update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Di Tommaso, L.; Spadaccini, M.; Donadon, M.; Personeni, N.; Elamin, A.; Aghemo, A.; Lleo, A. Role of Liver Biopsy in Hepatocellular Carcinoma. World J. Gastroenterol. 2019, 25, 6041–6052. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Ayuso, C.; Bianchi, L.; Solé, M.; Ayuso, J.R.; Boix, L.; Sala, M.; Varela, M.; Llovet, J.M.; et al. Diagnosis of Hepatic Nodules 20 Mm or Smaller in Cirrhosis: Prospective Validation of the Noninvasive Diagnostic Criteria for Hepatocellular Carcinoma. Hepatology 2008, 47, 97–104. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From Diagnosis to Treatment of Hepatocellular Carcinoma: An Epidemic Problem for Both Developed and Developing World. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Debes, J.D.; Romagnoli, P.A.; Prieto, J.; Arrese, M.; Mattos, A.Z.; Boonstra, A. Serum Biomarkers for the Prediction of Hepatocellular Carcinoma. Cancers 2021, 13, 1681. [Google Scholar] [CrossRef] [PubMed]
- Beudeker, B.J.B.; Boonstra, A. Circulating Biomarkers for Early Detection of Hepatocellular Carcinoma. Ther. Adv. Gastroenterol. 2020, 13, 1756284820931734. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Cerione, R.A.; Antonyak, M.A. Probing the Mechanisms of Extracellular Vesicle Biogenesis and Function in Cancer. Biochem. Soc. Trans. 2018, 46, 1137–1146. [Google Scholar] [CrossRef]
- Lin, S.; Gregory, R.I. MicroRNA Biogenesis Pathways in Cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Bronte, F.; Bronte, G.; Fanale, D.; Caruso, S.; Bronte, E.; Bavetta, M.G.; Fiorentino, E.; Rolfo, C.; Bazan, V.; Marco, V.D.; et al. HepatomiRNoma: The Proposal of a New Network of Targets for Diagnosis, Prognosis and Therapy in Hepatocellular Carcinoma. Crit. Rev. Oncol. Hematol. 2016, 97, 312–321. [Google Scholar] [CrossRef]
- Corsini, L.R.; Bronte, G.; Terrasi, M.; Amodeo, V.; Fanale, D.; Fiorentino, E.; Cicero, G.; Bazan, V.; Russo, A. The Role of MicroRNAs in Cancer: Diagnostic and Prognostic Biomarkers and Targets of Therapies. Expert Opin. Ther. Targets 2012, 16, S103–S109. [Google Scholar] [CrossRef]
- Yang, N.; Ekanem, N.R.; Sakyi, C.A.; Ray, S.D. Hepatocellular Carcinoma and MicroRNA: New Perspectives on Therapeutics and Diagnostics. Adv. Drug Deliv. Rev. 2015, 81, 62–74. [Google Scholar] [CrossRef]
- Jansson, M.D.; Lund, A.H. MicroRNA and Cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef]
- Thurnherr, T.; Mah, W.C.; Lei, Z.; Jin, Y.; Rozen, S.G.; Lee, C.G. Differentially Expressed MiRNAs in Hepatocellular Carcinoma Target Genes in the Genetic Information Processing and Metabolism Pathways. Sci. Rep. 2016, 6, 20065. [Google Scholar] [CrossRef]
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; Van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Lin, W.L.; Yan, I.K.; Braconi, C.; Patel, T. Intercellular Nanovesicle-Mediated MicroRNA Transfer: A Mechanism of Environmental Modulation of Hepatocellular Cancer Cell Growth. Hepatology 2011, 54, 1237–1248. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G. Proteomic Profiling of NCI-60 Extracellular Vesicles Uncovers Common Protein Cargo and Cancer Type-Specific Biomarkers. Oncotarget 2016, 7, 86999. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, Q.; Cao, K.; Sun, Y. Exosomes: Small Vesicles with Big Roles in Hepatocellular Carcinoma. Oncotarget 2016, 7, 60687–60697. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Berardocco, M.; Radeghieri, A.; Busatto, S.; Gallorini, M.; Raggi, C.; Gissi, C.; D’Agnano, I.; Bergese, P.; Felsani, A.; Berardi, A.C. RNA-Seq Reveals Distinctive RNA Profiles of Small Extracellular Vesicles from Different Human Liver Cancer Cell Lines. Oncotarget 2017, 8, 82920–82939. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Zhou, J.; Flores-Bellver, M.; Pan, J.; Benito-Martin, A.; Shi, C.; Onwumere, O.; Mighty, J.; Qian, J.; Zhong, X.; Hogue, T.; et al. Human Retinal Organoids Release Extracellular Vesicles That Regulate Gene Expression in Target Human Retinal Progenitor Cells. Sci. Rep. 2021, 11, 21128. [Google Scholar] [CrossRef]
- Mills, J.; Capece, M.; Cocucci, E.; Tessari, A.; Palmieri, D. Cancer-Derived Extracellular Vesicle-Associated MicroRNAs in Intercellular Communication: One Cell’s Trash Is Another Cell’s Treasure. Int. J. Mol. Sci. 2019, 20, 6109. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Woo, H.K.; Lee, C.; Min, Y.; Kumar, S.; Sunkara, V.; Jo, H.G.; Lee, Y.J.; Kim, J.; Ha, H.K.; et al. EV-Ident: Identifying Tumor-Specific Extracellular Vesicles by Size Fractionation and Single-Vesicle Analysis. Anal. Chem. 2020, 92, 6010–6018. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Endothelial Cell-Derived Extracellular Vesicles in Atherosclerosis: The Emerging Value for Diagnosis, Risk Stratification and Prognostication. Vessel Plus 2020, 4, 15. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Rai, A.; Ji, H.; Simpson, R.J. Highly-Purified Exosomes and Shed Microvesicles Isolated from the Human Colon Cancer Cell Line LIM1863 by Sequential Centrifugal Ultrafiltration Are Biochemically and Functionally Distinct. Methods 2015, 87, 11–25. [Google Scholar] [CrossRef]
- Soltész, B.; Buglyó, G.; Németh, N.; Szilágyi, M.; Pös, O.; Szemes, T.; Balogh, I.; Nagy, B. The Role of Exosomes in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 8. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, S.N. Insulin-like Growth Factor-1 Prevents MiR-122 Production in Neighbouring Cells to Curtail Its Intercellular Transfer to Ensure Proliferation of Human Hepatoma Cells. Nucleic Acids Res. 2014, 42, 7170–7185. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, L.L.; Wen, D.; Liu, D.L.; Dong, L.L.; Gao, D.M.; Bian, X.Y.; Zhou, J.; Fan, J.; Wu, W.Z. MiR-612 Regulates Invadopodia of Hepatocellular Carcinoma by HADHA-Mediated Lipid Reprogramming. J. Hematol. Oncol. 2020, 13, 12. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef]
- Sorop, A.; Constantinescu, D.; Cojocaru, F.; Dinischiotu, A.; Cucu, D.; Dima, S.O. Exosomal MicroRNAs as Biomarkers and Therapeutic Targets for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4997. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, I.H.; Wang, C.C.; Chen, P.J.; Tseng, H.P.; Huang, K.T.; Hu, T.H.; Li, L.C.; Goto, S.; Cheng, Y.F.; et al. Circulating Exosomal MiR-92b: Its Role for Cancer Immunoediting and Clinical Value for Prediction of Posttransplant Hepatocellular Carcinoma Recurrence. Am. J. Transplant. 2019, 19, 3250–3262. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, H.F.; Liu, M.Y.; Xu, Y.J.; He, J.C.; Zhou, Y.; Cang, S.D. Mechanism of Exosomal MicroRNA-224 in Development of Hepatocellular Carcinoma and Its Diagnostic and Prognostic Value. World J. Gastroenterol. 2019, 25, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wu, J.; Wu, J.; Ji, A.; Qiang, G.; Jiang, Y.; Jiang, C.; Ding, Y. Exosomal MiR-665 as a Novel Minimally Invasive Biomarker for Hepatocellular Carcinoma Diagnosis and Prognosis. Oncotarget 2017, 8, 80666. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jiang, Y.; Yang, L.; Yan, S.; Wang, Y.G.; Lu, X.J. Decreased Levels of Serum Exosomal MiR-638 Predict Poor Prognosis in Hepatocellular Carcinoma. J. Cell. Biochem. 2018, 119, 4711–4716. [Google Scholar] [CrossRef]
- Li, B.; Hao, K.; Li, M.; Wang, A.; Tang, H.; Xu, L.; Ma, C.; Du, W.; Sun, L.; Hou, X.; et al. Five MiRNAs Identified in Fucosylated Extracellular Vesicles as Non-Invasive Diagnostic Signatures for Hepatocellular Carcinoma. Cell Rep. Med. 2024, 5, 101716. [Google Scholar] [CrossRef]
- Cho, H.J.; Eun, J.W.; Baek, G.O.; Seo, C.W.; Ahn, H.R.; Kim, S.S.; Cho, S.W.; Cheong, J.Y. Serum Exosomal MicroRNA, MiR-10b-5p, as a Potential Diagnostic Biomarker for Early-Stage Hepatocellular Carcinoma. J. Clin. Med. 2020, 9, 281. [Google Scholar] [CrossRef]
- Chen, S.; Mao, Y.; Chen, W.; Liu, C.; Wu, H.; Zhang, J.; Wang, S.; Wang, C.; Lin, Y.; Lv, Y. Serum Exosomal MiR-34a as a Potential Biomarker for the Diagnosis and Prognostic of Hepatocellular Carcinoma. J. Cancer 2022, 13, 1410–1417. [Google Scholar] [CrossRef]
- Qi, P.; Cheng, S.Q.; Wang, H.; Li, N.; Chen, Y.F.; Gao, C. fang Serum MicroRNAs as Biomarkers for Hepatocellular Carcinoma in Chinese Patients with Chronic Hepatitis B Virus Infection. PLoS ONE 2011, 6, e28486. [Google Scholar] [CrossRef]
- Boonkaew, B.; Satthawiwat, N.; Pinjaroen, N.; Chuaypen, N.; Tangkijvanich, P. Circulating Extracellular Vesicle-Derived MicroRNAs as Novel Diagnostic and Prognostic Biomarkers for Non-Viral-Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 16043. [Google Scholar] [CrossRef]
- Eldosoky, M.A.; Hammad, R.; Elmadbouly, A.A.; Aglan, R.B.; Abdel-Hamid, S.G.; Alboraie, M.; Hassan, D.A.; Shaheen, M.A.; Rushdi, A.; Ahmed, R.M.; et al. Diagnostic Significance of Hsa-MiR-21-5p, Hsa-MiR-192-5p, Hsa-MiR-155-5p, Hsa-MiR-199a-5p Panel and Ratios in Hepatocellular Carcinoma on Top of Liver Cirrhosis in HCV-Infected Patients. Int. J. Mol. Sci. 2023, 24, 3157. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Z. Diagnostic Value of a MicroRNA Signature Panel in Exosomes for Patients with Hepatocellular Carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 1478. [Google Scholar] [PubMed]
- Xue, X.; Zhao, Y.; Wang, X.; Qin, L.; Hu, R. Development and Validation of Serum Exosomal MicroRNAs as Diagnostic and Prognostic Biomarkers for Hepatocellular Carcinoma. J. Cell. Biochem. 2019, 120, 135–142. [Google Scholar] [CrossRef]
- Qian, F.; Wang, J.; Wang, Y.; Gao, Q.; Yan, W.; Lin, Y.; Shen, L.; Xie, Y.; Jiang, X.; Shen, B. MiR-378a-3p as a Putative Biomarker for Hepatocellular Carcinoma Diagnosis and Prognosis: Computational Screening with Experimental Validation. Clin. Transl. Med. 2021, 11, e307. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Huang, H.; Wang, Z.; Zou, W.; Lv, Y.; Zhou, Z.; Zhang, Q.; Qiao, L.; Wu, F.; Shao, S. Extracellular Vesicle-Associated Mir-21 and Mir-144 Are Markedly Elevated in Serum of Patients with Hepatocellular Carcinoma. Front. Physiol. 2018, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Agricultura, S.D.; Ganadería, D.R. Norma Oficial Mexicana, NOM -062- ZOO- 1999, Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. Diario Oficial de la Federación, 2021. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 Guidelines for Reporting Diagnostic Accuracy Studies: Explanation and Elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Pelayo, F.A.; Zarate-Lopez, D.; García-Carrillo, R.; Rodríguez-Beas, C.; Íñiguez-Palomares, R.; Rodríguez-Mejía, J.L.; Soto-Guzmán, A.; Velasco-Loyden, G.; Sierra-Martínez, M.; Virgen-Ortiz, A.; et al. miRNAs-Set of Plasmatic Extracellular Vesicles as Novel Biomarkers for Hepatocellular Carcinoma Diagnosis Across Tumor Stage and Etiologies. Int. J. Mol. Sci. 2025, 26, 2563. https://doi.org/10.3390/ijms26062563
Molina-Pelayo FA, Zarate-Lopez D, García-Carrillo R, Rodríguez-Beas C, Íñiguez-Palomares R, Rodríguez-Mejía JL, Soto-Guzmán A, Velasco-Loyden G, Sierra-Martínez M, Virgen-Ortiz A, et al. miRNAs-Set of Plasmatic Extracellular Vesicles as Novel Biomarkers for Hepatocellular Carcinoma Diagnosis Across Tumor Stage and Etiologies. International Journal of Molecular Sciences. 2025; 26(6):2563. https://doi.org/10.3390/ijms26062563
Chicago/Turabian StyleMolina-Pelayo, Francisco A., David Zarate-Lopez, Rosendo García-Carrillo, César Rodríguez-Beas, Ramón Íñiguez-Palomares, José L. Rodríguez-Mejía, Adriana Soto-Guzmán, Gabriela Velasco-Loyden, Mónica Sierra-Martínez, Adolfo Virgen-Ortiz, and et al. 2025. "miRNAs-Set of Plasmatic Extracellular Vesicles as Novel Biomarkers for Hepatocellular Carcinoma Diagnosis Across Tumor Stage and Etiologies" International Journal of Molecular Sciences 26, no. 6: 2563. https://doi.org/10.3390/ijms26062563
APA StyleMolina-Pelayo, F. A., Zarate-Lopez, D., García-Carrillo, R., Rodríguez-Beas, C., Íñiguez-Palomares, R., Rodríguez-Mejía, J. L., Soto-Guzmán, A., Velasco-Loyden, G., Sierra-Martínez, M., Virgen-Ortiz, A., Sánchez-Pastor, E., Magaña-Vergara, N. E., Baltiérrez-Hoyos, R., Alamilla, J., Chagoya de Sánchez, V., Dagnino-Acosta, A., Chávez, E., & Castro-Sánchez, L. (2025). miRNAs-Set of Plasmatic Extracellular Vesicles as Novel Biomarkers for Hepatocellular Carcinoma Diagnosis Across Tumor Stage and Etiologies. International Journal of Molecular Sciences, 26(6), 2563. https://doi.org/10.3390/ijms26062563










