Search for Disease-Specific Genetic Markers Originated from the Vitamin D Binding Protein Gene Polymorphisms in the Multiple Sclerosis Cohort in the Latvian Population
Abstract
1. Introduction
2. Results
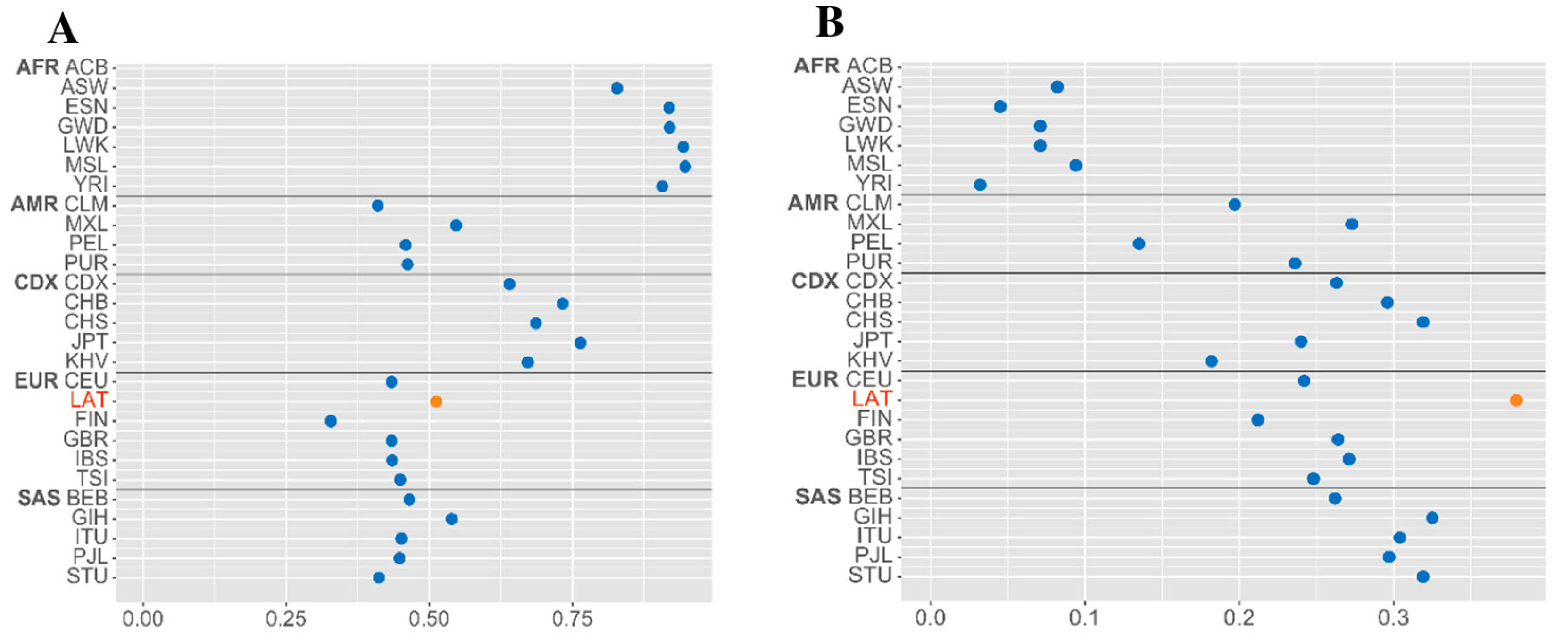
2.1. Polymorphisms Discovery and Genetic Diversity
2.2. Multi-Locus Genotypes, DBP Haplotypes, and Gc Isotype Variant Distribution
2.3. Comparison of Biochemical Data and z/Gc Isotype Distribution in MS Cohort
3. Discussion
3.1. Study Limitations
3.2. Clinical Implications
4. Materials and Methods
4.1. Study Population and Sample Recruitment
4.2. DNA Extraction and Genotyping
4.3. Measurement of Plasma 25(OH)D Concentration
4.4. Data Management and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MS International Federation. Atlas of MS, Third Edition: Global Epidemiology Data on Multiple Sclerosis. 2023. Available online: https://www.atlasofms.org (accessed on 14 October 2024).
- Health Policy Partnership. MS Barometer Factsheet: Latvia; Health Policy Partnership: London, UK, 2020; Available online: https://www.healthpolicypartnership.com/app/uploads/MS-Barometer-factsheet-Latvia.pdf (accessed on 10 December 2022).
- Mackey, J.D.; Young, P.; Zimmerer, R.; Miles, B. Vitamin D Deficiency as a Risk Factor for Breast Cancer Development. J. Clin. Oncol. 2023, 41 (Suppl. 16), 10559. [Google Scholar] [CrossRef]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Moreira, T.S.; Hamadeh, M.J. The role of vitamin D deficiency in the pathogenesis of type 2 diabetes mellitus. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2010, 5, e155–e165. [Google Scholar] [CrossRef]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef]
- Jaimni, V.; Shasty, B.A.; Madhyastha, S.P.; Shetty, G.V.; Acharya, R.V.; Bekur, R.; Doddamani, A. Association of Vitamin D Deficiency and Newly Diagnosed Pulmonary Tuberculosis. Pulm. Med. 2021, 2021, 5285841. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Niino, M.; Fukazawa, T.; Kikuchi, S.; Sasaki, H. Therapeutic potential of vitamin D for multiple sclerosis. Curr. Med. Chem. 2008, 15, 499–505. [Google Scholar] [CrossRef]
- Hedström, A.K.; Olsson, T.; Kockum, I.; Hillert, J.; Alfredsson, L. Low sun exposure increases multiple sclerosis risk both directly and indirectly. J. Neurol. 2020, 267, 1045–1052. [Google Scholar] [CrossRef]
- Jones, K.S.; Assar, S.; Harnpanich, D.; Bouillon, R.; Lambrechts, D.; Prentice, A.; Schoenmakers, I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J. Clin. Endocrinol. Metab. 2014, 99, 3373–3381. [Google Scholar] [CrossRef]
- Jones, K.S.; Schoenmakers, I.; Bluck, L.J.; Ding, S.; Prentice, A. Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br. J. Nutr. 2012, 107, 1128–1137. [Google Scholar] [CrossRef]
- Adams, C.; Manouchehrinia, A.; Quach, H.L.; Quach, D.L.; Olsson, T.; Kockum, I.; Schaefer, C.; Ponting, C.P.; Alfredsson, L.; Barcellos, L.F. Evidence supports a causal association between allele-specific vitamin D receptor binding and multiple sclerosis among Europeans. Proc. Natl. Acad. Sci. USA 2024, 121, e2302259121. [Google Scholar] [CrossRef] [PubMed]
- Jassil, N.K.; Sharma, A.; Bikle, D.; Wang, X. Vitamin d binding protein and 25-hydroxyvitamin D levels: Emerging clinical applications. Endocr. Pract. 2017, 23, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Cooke, N.E.; David, E.V. Serum vitamin D-binding protein is a third member of the albumin and alpha fetoprotein gene family. J. Clin. Investig. 1985, 76, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. IJMS 2023, 24, 4642. [Google Scholar] [CrossRef]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Vuolo, L.; Di Somma, C.; Faggiano, A.; Colao, A. Vitamin D and cancer. Front. Endocrinol. 2012, 3, 58. [Google Scholar] [CrossRef]
- Braithwaite, V.S.; Jones, K.S.; Schoenmakers, I.; Silver, M.; Prentice, A.; Hennig, B.J. Vitamin D binding protein genotype is associated with plasma 25OHD concentration in West African children. Bone 2015, 74, 166–170. [Google Scholar] [CrossRef]
- Rozmus, D.; Płomiński, J.; Augustyn, K.; Cieślińska, A. rs7041 and rs4588 Polymorphisms in Vitamin D Binding Protein Gene (VDBP) and the Risk of Diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef]
- Malik, S.; Fu, L.; Juras, D.J.; Karmali, M.; Wong, B.Y.; Gozdzik, A.; Cole, D.E. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit. Rev. Clin. Lab. Sci. 2013, 50, 1–22. [Google Scholar] [CrossRef]
- Trefilio, L.M.; Bottino, L.; de Carvalho Cardoso, R.; Montes, G.C.; Fontes-Dantas, F.L. The impact of genetic variants related to vitamin D and autoimmunity: A systematic review. Heliyon 2024, 10, e27700. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, Z.; van Duijnhoven, F.J.B.; Dong, M.; Qian, Y.; Yu, H.; Yang, J.; Cui, L.; Han, R.; Su, J.; et al. Genetic Variants in Group-Specific Component (GC) Gene Are Associated with Breast Cancer Risk among Chinese Women. Biomed. Res. Int. 2019, 2019, 3295781. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.; Jørgensen, C.S.; Laursen, I.; Hirschberg, D.; Højrup, P.; Houen, G. Protein chemical characterization of Gc globulin (vitamin D-binding protein) isoforms; Gc-1f, Gc-1s and Gc-2. Biochim. Biophys. Acta 2007, 1774, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.C.; Zhu, Y.; Gong, C.; Liang, X.; Zhou, X.; Xu, Y.; Lyu, D.; Mo, J.; Xu, J.; Song, J.; et al. The GC2 haplotype of the vitamin D binding protein is a risk factor for a low plasma 25-hydroxyvitamin D concentration in a Han Chinese population. Nutr. Metab. 2019, 16, 5. [Google Scholar] [CrossRef]
- Engelen, L.; Schalkwijk, C.G.; Eussen, S.J.; Scheijen, J.L.; Soedamah-Muthu, S.S.; Chaturvedi, N.; Fuller, J.H.; Stehouwer, C.D. Low 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 levels are independently associated with macroalbuminuria, but not with retinopathy and macrovascular disease in type 1 diabetes: The EURODIAB prospective complications study. Cardiovasc. Diabetol. 2015, 14, 67. [Google Scholar] [CrossRef]
- Chen, P.; Li, M.; Gu, X.; Liu, Y.; Li, X.; Li, C.; Wang, Y.; Xie, D.; Wang, F.; Yu, C.; et al. Higher blood 25(OH)D level may reduce the breast cancer risk: Evidence from a Chinese population based case-control study and meta-analysis of the observational studies. PLoS ONE 2013, 8, e49312. [Google Scholar] [CrossRef]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Quezada-Sánchez, A.D.; Denova-Gutiérrez, E.; Torres-Ibarra, L.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Diet Modulates the Effects of Genetic Variants on the Vitamin D Metabolic Pathway and Bone Mineral Density in Mexican Postmenopausal Women. J. Nutr. 2021, 151, 1726–1735. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.; Tian, Y.; Zhang, X.; Lu, R.; Meng, H. GC Gene Polymorphisms and Vitamin D-Binding Protein Levels Are Related to the Risk of Generalized Aggressive Periodontitis. Int. J. Endocrinol. 2016, 2016, 5141089. [Google Scholar] [CrossRef]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Lucas, R.; Xiang, A.H.; Chen, L.H.; Wu, J.; Gonzalez, E.; Haraszti, S.; Smith, J.B.; Quach, H.; Barcellos, L.F. MS Sunshine Study: Sun Exposure But Not Vitamin D Is Associated with Multiple Sclerosis Risk in Blacks and Hispanics. Nutrients 2018, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Xu, B. No association between the vitamin D-binding protein (DBP) gene polymorphisms (rs7041 and rs4588) and multiple sclerosis and type 1 diabetes mellitus: A meta-analysis. PLoS ONE 2020, 15, e0242256. [Google Scholar] [CrossRef] [PubMed]
- Alhomsi, B.; Aboualchamat, G.; Alkadi, I. Assessment of vitamin D-binding protein (DBP) gene polymorphisms and their correlation with multiple sclerosis: A case-control study in a sample of the Syrian population. Egypt. J. Med. Hum. Genet. 2020, 21, 32. [Google Scholar] [CrossRef]
- Paramonova, N.; Trapina, I.; Gradauskiene (Sitkauskiene), B.; Plavina, S.; Tamasauskiene, L.; Bastyte, D.; Rumba-Rozenfelde, I.; Tapina, S.; Stakaitiene, I.; Ugenskiene, R.; et al. Genetic Diversity in Bronchial Asthma Susceptibility: Exploring the Role of Vitamin D Receptor Gene Polymorphisms in Varied Geographic Contexts. IJMS 2024, 25, 1943. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Fawzy, M.S.; Elgazzaz, M.G.; Ibrahim, A.; Hussein, M.H.; Khashana, M.S.; Toraih, E.A. Association of group-specific component exon 11 polymorphisms with bronchial asthma in children and adolescents. Scand. J. Immunol. 2019, 89, e12740. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Ma, T. Associations of Genetic Polymorphisms Relevant to Metabolic Pathway of Vitamin D3 with Development and Prognosis of Childhood Bronchial Asthma. DNA Cell Biol. 2017, 36, 682–692. [Google Scholar] [CrossRef]
- Moy, K.A.; Mondul, A.M.; Zhang, H.; Weinstein, S.J.; Wheeler, W.; Chung, C.C.; Männistö, S.; Yu, K.; Chanock, S.J.; Albanes, D. Genome-wide association study of circulating vitamin D-binding protein. Am. J. Clin. Nutr. 2014, 99, 1424–1431. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Costa, A.S.; Clerici, M. Vitamin D-binding protein gene polymorphisms are not associated with MS risk in an Italian cohort. J. Neuroimmunol. 2017, 305, 92–95. [Google Scholar] [CrossRef]
- Lafi, Z.M.; Irshaid, Y.M.; El-Khateeb, M.; Ajlouni, K.M.; Hyassat, D. Association of rs7041 and rs4588 Polymorphisms of the Vitamin D Binding Protein and the rs10741657 Polymorphism of CYP2R1 with Vitamin D Status Among Jordanian Patients. Genet. Test. Mol. Biomark. 2015, 19, 629–636. [Google Scholar] [CrossRef]
- Jassil, N.K.; Sharma, A.; Bikle, D.; Wang, X. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D Supplementation: Upper Limit for Safety Revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, K.; Shen, J.; Xu, S.; Wang, Q.; Gong, Y.; Xia, Y.; Wang, X.; Chen, L.; Yan, S.; et al. Correlation between meteorological factors and vitamin D status under different season. Sci. Rep. 2023, 13, 4762. [Google Scholar] [CrossRef] [PubMed]
- Forouhari, A.; Heidari-Beni, M.; Veisi, S.; Poursafa, P.; Kelishadi, R. Effect of epigenetics on vitamin D levels: A systematic review until December 2020. Arch. Public Health 2023, 81, 106. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; Li, X.; Mao, Z.; Yu, F.; Wang, L.; Ba, Y.; Wang, C.; Li, W. Methylation in 3′ Near Region of GC Gene and Its Association with the Level of Vitamin D Binding Protein and Type 2 Diabetes Mellitus. Nutr. Res. 2018, 54, 52–59. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Kilikevicius, A.; Meister, G.; Corey, D.R. Reexamining Assumptions about miRNA-Guided Gene Silencing. Nucleic Acids Res. 2022, 50, 617–634. [Google Scholar] [CrossRef]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Mazur, A.; Frączek, P.; Tabarkiewicz, J. Vitamin D as a Nutri-Epigenetic Factor in Autoimmunity—A Review of Current Research and Reports on Vitamin D Deficiency in Autoimmune Diseases. Nutrients 2022, 14, 4286. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Lewis, C.M. Genetic association studies: Design, analysis and interpretation. Brief. Bioinform. 2002, 3, 146–153. [Google Scholar] [CrossRef]
| Gene Location in the Genome | SNP 1 ID | SNP 2 ID | Distance in the Genome (bp) | LD Analyse | |||||
|---|---|---|---|---|---|---|---|---|---|
| MS | Control | ||||||||
| D′ | r2 | p-Value | D′ | r2 | p-Value | ||||
| GC chr4:71741693-71805520 | rs4588 | rs7041 | 72,618,323–72,618,334 | 0.99 | 0.60 | <0.00001 | 0.80 | 0.39 | <0.0001 |
| GC SNPs | MAF/Genotype | Distribution of Genotypes | Data on Association | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MS (296) | Control (253) | Additive Model | Multiplicative Model | ||||||
| N * | % | N * | % | ORA [95% CI] | p-Value | ORM [95% CI] | p-Value | ||
| rs4588 | A | 178 | 30.07 | 192 | 37.94 | 0.65 [0.51–0.83] | 5.00 × 10−4 | - | |
| CC | 136 | 45.95 | 91 | 35.97 | reference | 1.50 [1.02–2.19] | 3.63 × 10−2 | ||
| CA | 142 | 47.97 | 132 | 52.17 | 0.82 [0.55–1.23] | 0.33 | 1.22 [0.87–1.70] | 0.26 | |
| AA | 18 | 6.08 | 30 | 11.86 | 0.39 [0.24–0.64] | 2.08 × 10−4 | 0.62 [0.40–0.96] | 2.95 × 10−2 | |
| rs7041 | T | 244 | 41.22 | 259 | 51.19 | 0.70 [0.54–0.90] | 5.25 × 10−3 | - | |
| GG | 94 | 31.76 | 60 | 23.72 | reference | 1.52 [1.08–2.15] | 1.59 × 10−2 | ||
| GT | 160 | 54.05 | 123 | 48.62 | 0.71 [0.50–1.02] | 6.36 × 10−2 | 0.84 [0.60–1.17] | 0.31 | |
| TT | 42 | 14.19 | 68 | 26.88 | 0.40 [0.21–0.76] | 4.15 × 10−3 | 0.60 [0.32–1.11] | 0.10 | |
| Multi- Locus Group * | Alleles/ Genotypes | Distribution of Haplotypes and Genotypes | Statistical Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Control | Additive Model | Multiplicative Model | ||||||||
| rs7041 | rs4588 | N | % | N | % | ORA [95% CI] | p-Value | ORM [95% CI] | p-Value | ||
| Haplotypes | |||||||||||
| H1 | 1S | G | C | 347 | 58.61 | 229 | 45.26 | reference | 1.72 [1.36–2.19] | 7.90 × 10−6 | |
| H2 | 1F | T | C | 67 | 11.32 | 85 | 16.80 | 0.52 [0.36–0.74] | 3.04 × 10−4 | 0.63 [0.45–0.89] | 8.20 × 10−3 |
| H3 | 2 | T | A | 177 | 29.90 | 176 | 34.78 | 0.66 [0.51–0.86] | 2.23 × 10−3 | 0.80 [0.62–1.03] | 0.078 |
| H4 # | 2 | G | A | 1 | 0.17 | 16 | 3.16 | 0.04 [0.01–0.31] | 6.80 × 10−6 | 0.05 [0.01–0.39] | 6.03 × 10−5 |
| DBP(Gc) isotype variations | |||||||||||
| G1 | 1S/2 | TG | CA | 120 | 40.54 | 87 | 34.66 | reference | 1.28 [0.90–1.81] | 0.17 | |
| G2 | 1S/1S | GG | CC | 93 | 31.42 | 50 | 19.92 | 1.36 [0.88–2.12] | 0.17 | 1.86 [1.25–2.76] | 1.88 × 10−3 |
| G3 | 1F/2 | TT | CA | 21 | 13.51 | 36 | 14.34 | 0.42 [0.23–0.77] | 4.63 × 10−3 | 0.45 [0.26–0.80] | 5.46 × 10−3 |
| G4 | 1F/1S | TG | CC | 40 | 13.51 | 33 | 13.15 | 0.88 [0.51–1.50] | 0.64 | 1.03 [0.63–1.69] | 0.91 |
| G5 | 2/2 | TT | AA | 18 | 6.08 | 24 | 9.56 | 0.54 [0.28–1.06] | 0.072 | 0.61 [0.32–1.15] | 0.12 |
| G6 # | 1F/1F | TT | CC | 3 | 1.01 | 8 | 3.19 | 0.27 [0.07–1.05] | 0.061 | 0.31 [0.08–1.18] | 0.12 |
| G7 # | 1S/2 | GG | CA | 1 | 0.34 | 7 | 2.79 | 0.10 [0.01–0.86] | 2.27 × 10−2 | 0.12 [0.01–0.96] | 2.67 × 10−2 |
| G8 # | 2/2 | TG | AA | - | - | 3 | 1.20 | 0.58 [0.51–0.65] | 4.40 × 10−2 | 0.54 [0.50–0.59] | 0.10 |
| G9 # | 2/2 | GG | AA | - | - | 3 | 1.20 | 0.58 [0.51–0.65] | 4.40 × 10−2 | 0.54 [0.50–0.59] | 0.10 |
| Genotype ^ | 25(OH)D Levels, ng/mL | Statistical Analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N, 131 | % | Two-Locus Genotype * | Gc Isotype | Average | SD | Min | Max | CI95% | Median | IQR | |||
| G1 | 59 | 45.04 | TG-CA | Gc1S/2 | 21.53 | 6.55 | 12.20 | 39.49 | 19.82 | 23.24 | 20.34 | 10.95 | 0.26 |
| G2 | 35 | 26.72 | GG-CC | Gc1S/1S | 24.12 | 7.46 | 7.37 | 49.26 | 21.56 | 26.68 | 23.16 | 8.55 | |
| G3 | 10 | 7.63 | TT-CA | Gc1F/2 | 24.48 | 10.10 | 11.76 | 39.89 | 17.26 | 31.71 | 21.32 | 18.95 | |
| G4 | 18 | 13.74 | TG-CC | Gc1F/1S | 24.20 | 7.63 | 14.86 | 39.52 | 20.41 | 27.99 | 21.30 | 13.35 | |
| G5 | 7 | 5.34 | TT-AA | Gc2/2 | 23.66 | 5.72 | 15.11 | 33.41 | 18.37 | 28.96 | 23.11 | 6.57 | |
| G6 | 2 | 1.53 | TT-CC | Gc1F/1F | 29.31 | 0.24 | 29.14 | 29.48 | 27.15 | 31.47 | 29.31 | 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalnina, J.; Trapina, I.; Plavina, S.; Leonova, E.; Paramonovs, J.; Sjakste, N.; Paramonova, N. Search for Disease-Specific Genetic Markers Originated from the Vitamin D Binding Protein Gene Polymorphisms in the Multiple Sclerosis Cohort in the Latvian Population. Int. J. Mol. Sci. 2025, 26, 2555. https://doi.org/10.3390/ijms26062555
Kalnina J, Trapina I, Plavina S, Leonova E, Paramonovs J, Sjakste N, Paramonova N. Search for Disease-Specific Genetic Markers Originated from the Vitamin D Binding Protein Gene Polymorphisms in the Multiple Sclerosis Cohort in the Latvian Population. International Journal of Molecular Sciences. 2025; 26(6):2555. https://doi.org/10.3390/ijms26062555
Chicago/Turabian StyleKalnina, Jolanta, Ilva Trapina, Samanta Plavina, Elina Leonova, Jegors Paramonovs, Nikolajs Sjakste, and Natalia Paramonova. 2025. "Search for Disease-Specific Genetic Markers Originated from the Vitamin D Binding Protein Gene Polymorphisms in the Multiple Sclerosis Cohort in the Latvian Population" International Journal of Molecular Sciences 26, no. 6: 2555. https://doi.org/10.3390/ijms26062555
APA StyleKalnina, J., Trapina, I., Plavina, S., Leonova, E., Paramonovs, J., Sjakste, N., & Paramonova, N. (2025). Search for Disease-Specific Genetic Markers Originated from the Vitamin D Binding Protein Gene Polymorphisms in the Multiple Sclerosis Cohort in the Latvian Population. International Journal of Molecular Sciences, 26(6), 2555. https://doi.org/10.3390/ijms26062555






