Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review
Abstract
1. Introduction
2. Background
2.1. Pathophysiology of PNH
2.2. Pathophysiology of TE in PNH
2.3. Role of Complement Inhibition in Preventing and Managing TE
3. TE in Asian PNH Patients
3.1. Incidence of TE in Asian PNH Patients
3.2. Treatment of TE in Asian PNH Patients
4. Biomarkers/Risk Factors for TE in Asian PNH Patients
5. Monitoring of TE in Asian PNH Patients
6. Anticoagulant Therapy in Asian PNH Patients
Special Situations
7. Challenges and Opportunities
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, A.; Kelly, R.J.; Hillmen, P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013, 121, 4985−4996; quiz 5105. [Google Scholar] [CrossRef] [PubMed]
- Ziakas, P.D.; Poulou, L.S.; Rokas, G.I.; Bartzoudis, D.; Voulgarelis, M. Thrombosis in paroxysmal nocturnal hemoglobinuria: Sites, risks, outcome. An overview. J. Thromb. Haemost. 2007, 5, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, R.A. Paroxysmal nocturnal hemoglobinuria. Blood 2014, 124, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Muus, P.; Dührsen, U.; Risitano, A.M.; Schubert, J.; Luzzatto, L.; Schrezenmeier, H.; Szer, J.; Brodsky, R.A.; Hill, A.; et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007, 110, 4123–4128. [Google Scholar] [CrossRef]
- Griffin, M.; Munir, T. Management of thrombosis in paroxysmal nocturnal hemoglobinuria: A clinician’s guide. Ther. Adv. Hematol. 2017, 8, 119–126. [Google Scholar] [CrossRef]
- Chatzileontiadou, S.; Hatjiharissi, E.; Angelopoulou, M.; Asimakopoulos, J.V.; Loutsidi, N.E.; Chatzikonstantinou, T.; Zikos, P.; Bouchla, A.; Bezirgiannidou, Z.; Kouvata, E.; et al. Thromboembolic events in patients with paroxysmal nocturnal hemoglobinuria (PNH): Real world data of a Greek nationwide multicenter retrospective study. Front. Oncol. 2023, 13, 1128994. [Google Scholar] [CrossRef]
- Nishimura, J.I.; Kanakura, Y.; Ware, R.E.; Shichishima, T.; Nakakuma, H.; Ninomiya, H.; Decastro, C.M.; Hall, S.; Kanamaru, A.; Sullivan, K.M.; et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine 2004, 83, 193–207. [Google Scholar] [CrossRef]
- Yu, F.; Du, Y.; Han, B. A comparative analysis of clinical characteristics of patients with paroxysmal nocturnal hemoglobinuria between Asia and Europe/America. Int. J. Hematol. 2016, 103, 649–654. [Google Scholar] [CrossRef]
- Sakurai, M.; Jang, J.H.; Chou, W.C.; Kim, J.S.; Wilson, A.; Nishimura, J.I.; Chiou, T.J.; Kanakura, Y.; Lee, J.W.; Okamoto, S. Comparative study on baseline clinical characteristics of Asian versus non-Asian patients with paroxysmal nocturnal hemoglobinuria. Int. J. Hematol. 2019, 110, 411–418. [Google Scholar] [CrossRef]
- Hill, A.; DeZern, A.E.; Kinoshita, T.; Brodsky, R.A. Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers 2017, 3, 17028. [Google Scholar] [CrossRef]
- Peacock-Young, B.; Macrae, F.L.; Newton, D.J.; Hill, A.; Ariëns, R.A.S. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: A multifaceted source. Haematologica 2018, 103, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Devalet, B.; Mullier, F.; Chatelain, B.; Dogné, J.M.; Chatelain, C. The central role of extracellular vesicles in the mechanisms of thrombosis in paroxysmal nocturnal haemoglobinuria: A review. J. Extracell. Vesicles 2014, 3, 23304. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, T.; Hall, S.E.; Ortel, T.L.; Kane, W.H.; Rosse, W.F.; Sims, P.J. Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood 1993, 82, 1192–1196. [Google Scholar] [CrossRef]
- Mannes, M.; Pechtl, V.; Hafner, S.; Dopler, A.; Eriksson, O.; Manivel, V.A.; Wohlgemuth, L.; Messerer, D.A.C.; Schrezenmeier, H.; Ekdahl, K.N.; et al. Complement and platelets: Prothrombotic cell activation requires membrane attack complex-induced release of danger signals. Blood Adv. 2023, 7, 6367–6380. [Google Scholar] [CrossRef]
- Grünewald, M.; Siegemund, A.; Grünewald, A.; Schmid, A.; Koksch, M.; Schöpflin, C.; Schauer, S.; Griesshammer, M. Plasmatic coagulation and fibrinolytic system alterations in PNH: Relation to clone size. Blood Coagul. Fibrinolysis 2003, 14, 685–695. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Ploug, M.; Plesner, T.; Rønne, E.; Ellis, V.; Høyer-Hansen, G.; Hansen, N.E.; Danø, K. The receptor for urokinase-type plasminogen activator is deficient on peripheral blood leukocytes in patients with paroxysmal nocturnal hemoglobinuria. Blood 1992, 79, 1447–1455. [Google Scholar] [CrossRef]
- Weitz, J.I. Heparan sulfate: Antithrombotic or not? J. Clin. Investig. 2003, 111, 952–954. [Google Scholar] [CrossRef]
- Maroney, S.A.; Cunningham, A.C.; Ferrel, J.; Hu, R.; Haberichter, S.; Mansbach, C.M.; Brodsky, R.A.; Dietzen, D.J.; Mast, A.E. A GPI-anchored co-receptor for tissue factor pathway inhibitor controls its intracellular trafficking and cell surface expression. J. Thromb. Haemost. 2006, 4, 1114–1124. [Google Scholar] [CrossRef]
- Ritis, K.; Doumas, M.; Mastellos, D.; Micheli, A.; Giaglis, S.; Magotti, P.; Rafail, S.; Kartalis, G.; Sideras, P.; Lambris, J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006, 177, 4794–4802. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Liu, H.; Liu, Z.; Li, L.; Ding, K.; Wang, G.; Song, J.; Fu, R. Gene mutations associated with thrombosis detected by whole-exome sequencing in paroxysmal nocturnal hemoglobinuria. Int. J. Lab. Hematol. 2019, 41, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, H.; Li, L.Y.; Li, L.J.; Wang, H.Q.; Song, J.; Wu, Y.H.; Guan, J.; Xing, L.M.; Wang, G.J.; et al. Role and clinical significance of MUC4 gene mutations in thrombotic events in patients with classic paroxysmal nocturnal hemoglobinuria. Zhonghua Xue Ye Xue Za Zhi 2023, 44, 561–566. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.; Meng, X.; Wang, Y.; Liu, C.; Fu, R. Several implications for the pathogenesis and treatment of thrombosis in PNH patients according to multiomics analysis. J. Transl. Med. 2024, 22, 129. [Google Scholar] [CrossRef]
- Helley, D.; de Latour, R.P.; Porcher, R.; Rodrigues, C.A.; Galy-Fauroux, I.; Matheron, J.; Duval, A.; Schved, J.F.; Fischer, A.M.; Socié, G.; et al. Evaluation of hemostasis and endothelial function in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Haematologica 2010, 95, 574–581. [Google Scholar] [CrossRef]
- Weitz, I.C.; Razavi, P.; Rochanda, L.; Zwicker, J.; Furie, B.; Manly, D.; Mackman, N.; Green, R.; Liebman, H.A. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb. Res. 2012, 130, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Macrae, F.L.; Peacock-Young, B.; Bowman, P.; Baker, S.R.; Quested, S.; Linton, E.; Hillmen, P.; Griffin, M.; Munir, T.; Payne, D.; et al. Patients with paroxysmal nocturnal hemoglobinuria demonstrate a prothrombotic clotting phenotype which is improved by complement inhibition with eculizumab. Am. J. Hematol. 2020, 95, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Muus, P.; Röth, A.; Elebute, M.O.; Risitano, A.M.; Schrezenmeier, H.; Szer, J.; Browne, P.; Maciejewski, J.P.; Schubert, J.; et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013, 162, 62–73. [Google Scholar] [CrossRef]
- Kelly, R.J.; Hill, A.; Arnold, L.M.; Brooksbank, G.L.; Richards, S.J.; Cullen, M.; Mitchell, L.D.; Cohen, D.R.; Gregory, W.M.; Hillmen, P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: Sustained efficacy and improved survival. Blood 2011, 117, 6786–6792. [Google Scholar] [CrossRef]
- Lee, J.W.; Peffault de Latour, R.; Brodsky, R.A.; Jang, J.H.; Hill, A.; Röth, A.; Schrezenmeier, H.; Wilson, A.; Marantz, J.L.; Maciejewski, J.P. Effectiveness of eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH) with or without aplastic anemia in the International PNH Registry. Am. J. Hematol. 2019, 94, E37–E41. [Google Scholar] [CrossRef]
- Terriou, L.; Lee, J.W.; Forsyth, C.; Griffin, M.; Szer, J.; Röth, A.; Gustovic, P.; Metzger, J.; Patel, A.S.; Patriquin, C.J. Long-term effectiveness of eculizumab: Data from the International PNH Registry. Eur. J. Haematol. 2023, 111, 796–804. [Google Scholar] [CrossRef]
- Lee, J.W.; Sicre de Fontbrune, F.; Wong Lee Lee, L.; Pessoa, V.; Gualandro, S.; Füreder, W.; Ptushkin, V.; Rottinghaus, S.T.; Volles, L.; Shafner, L.; et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: The 301 study. Blood 2019, 133, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kulasekararaj, A.G.; Hill, A.; Rottinghaus, S.T.; Langemeijer, S.; Wells, R.; Gonzalez- Fernandez, F.A.; Gaya, A.; Lee, J.W.; Gutierrez, E.O.; Piatek, C.I.; et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: The 302 study. Blood 2019, 133, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kulasekararaj, A.G.; Griffin, M.; Langemeijer, S.; Usuki, K.; Kulagin, A.; Ogawa, M.; Yu, J.; Mujeebuddin, A.; Nishimura, J.I.; Lee, J.W.; et al. Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies. Eur. J. Haematol. 2022, 109, 205–214. [Google Scholar] [CrossRef]
- Kulagin, A.; Chonat, S.; Maschan, A.; Bartels, M.; Buechner, J.; Punzalan, R.; Richards, M.; Ogawa, M.; Hicks, E.; Yu, J.; et al. Pharmacokinetics, pharmacodynamics, efficacy and safety of ravulizumab in children and adolescents with paroxysmal nocturnal hemoglobinuria: Interim analysis of a phase 3, open-label study. In Proceedings of the European Hematology Association (EHA) 2021, Virtual Congress, 9–17 June 2021. Poster number: EP590. [Google Scholar]
- Griffin, M.; Gandhi, S.; Hicks, E.; Jain, D.; Kelly, R.J.; Munir, T.; Muus, P.; Ogawa, M.; Trikha, R.; Yu, J.; et al. Terminal complement inhibition and control of hemolysis in paroxysmal nocturnal hemoglobinuria following switching from high-dose eculizumab to ravulizumab: An interim analysis. In Proceedings of the American Society of Hematology Annual Meeting & Exposition, New Orleans, LA, USA, 10–13 December 2022. Poster number: 1251. [Google Scholar]
- Van Bijnen, S.T.; Østerud, B.; Barteling, W.; Verbeek-Knobbe, K.; Willemsen, M.; van Heerde, W.L.; Muus, P. Alterations in markers of coagulation and fibrinolysis in patients with paroxysmal nocturnal hemoglobinuria before and during treatment with eculizumab. Thromb. Res. 2015, 136, 274–281. [Google Scholar] [CrossRef]
- Kanakura, Y.; Ohyashiki, K.; Shichishima, T.; Okamoto, S.; Ando, K.; Ninomiya, H.; Kawaguchi, T.; Nakao, S.; Nakakuma, H.; Nishimura, J.; et al. Safety and efficacy of the terminal complement inhibitor eculizumab in Japanese patients with paroxysmal nocturnal hemoglobinuria: The AEGIS clinical trial. Int. J. Hematol. 2011, 93, 36–46. [Google Scholar] [CrossRef]
- Lee, J.W.; Jang, J.H.; Kim, J.S.; Yoon, S.S.; Lee, J.H.; Kim, Y.K.; Jo, D.Y.; Chung, J.; Sohn, S.K. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int. J. Hematol. 2013, 97, 749–757. [Google Scholar] [CrossRef]
- Wang, H.C.; Kuo, C.Y.; Liu, I.T.; Chen, T.Y.; Chang, Y.H.; Lin, S.J.; Cho, S.F.; Liu, Y.C.; Liu, T.C.; Lin, S.F.; et al. Distinct clinical characteristics of paroxysmal nocturnal hemoglobinuria in patients in Southern Taiwan: A multicenter investigation. Kaohsiung J. Med. Sci. 2017, 33, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Li, L.; Li, L.; Liu, H.; Zhang, T.; Ding, S.; Wang, G.; Song, J.; Wang, H.; Xing, L.; et al. Analysis of clinical characteristics of 92 patients with paroxysmal nocturnal hemoglobinuria: A single institution experience in China. J. Clin. Lab. Anal. 2020, 34, e23008. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Y.; Yang, C.; Chen, M.; Han, B. Clinical characteristics of 512 eculizumab-naive paroxysmal nocturnal hemoglobinuria patients in China: A single-center observational study. Hematology 2022, 27, 113–121. [Google Scholar] [CrossRef]
- Schrezenmeier, H.; Röth, A.; Araten, D.J.; Kanakura, Y.; Larratt, L.; Shammo, J.M.; Wilson, A.; Shayan, G.; Maciejewski, J.P. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): Updated analysis from the International PNH Registry. Ann. Hematol. 2020, 99, 1505–1514. [Google Scholar] [CrossRef]
- Hillmen, P.; Lewis, S.M.; Bessler, M.; Luzzatto, L.; Dacie, J.V. Natural history of paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 1995, 333, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Mary, J.Y.; de Gramont, A.; Rio, B.; Leporrier, M.; Rose, C.; Heudier, P.; Rochant, H.; Cahn, J.Y.; Gluckman, E. Paroxysmal nocturnal haemoglobinuria: Long-term follow-up and prognostic factors. French Society of Haematology. Lancet 1996, 348, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Linares, C.; Ojeda, E.; Forés, R.; Pastrana, M.; Cabero, M.; Morillo, D.; Bautista, G.; Baños, I.; Monteserín, C.; Bravo, P.; et al. Paroxysmal nocturnal hemoglobinuria: A single Spanish center’s experience over the last 40 yr. Eur. J. Haematol. 2014, 93, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, H.; Muus, P.; Socié, G.; Szer, J.; Urbano-Ispizua, A.; Maciejewski, J.P.; Brodsky, R.A.; Bessler, M.; Kanakura, Y.; Rosse, W.; et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 2014, 99, 922–929. [Google Scholar] [CrossRef]
- Chou, W.C.; Huang, W.H.; Wang, M.C.; Chang, C.S.; Yeh, S.P.; Chiou, T.J.; Chen, Y.C.; Lin, T.H.; Shen, M.C.; Taiwan PNH Study Group. Characteristics of Taiwanese patients of PNH in the international PNH registry. Thromb. J. 2016, 14 (Suppl. 1), 39. [Google Scholar] [CrossRef]
- Kruatrachue, M.; Wasi, P.; Na-Nakorn, S. Paroxysmal nocturnal haemoglobinuria in Thailand with special reference to as association with aplastic anaemia. Br. J. Haematol. 1978, 39, 267–276. [Google Scholar] [CrossRef]
- Naithani, R.; Mahapatra, M.; Dutta, P.; Kumar, R.; Pati, H.P.; Choudhry, V.P. Paroxysmal nocturnal hemoglobinuria in childhood and adolescence—A retrospective analysis of 18 cases. Indian J. Pediatr. 2008, 75, 575–578. [Google Scholar] [CrossRef]
- Ge, M.; Shi, J.; Li, X.; Shao, Y.; Huang, J.; Huang, Z.; Zhang, J.; Nie, N.; Zheng, Y. Clinical features and survival of asian pediatric patients with paroxysmal nocturnal hemoglobinuria: Results from a single center in China. Acta Haematol. 2015, 134, 1–6. [Google Scholar] [CrossRef]
- Ware, R.E.; Hall, S.E.; Rosse, W.F. Paroxysmal nocturnal hemoglobinuria with onset in childhood and adolescence. N. Engl. J. Med. 1991, 325, 991–996. [Google Scholar] [CrossRef]
- van den Heuvel-Eibrink, M.M.; Bredius, R.G.; te Winkel, M.L.; Tamminga, R.; de Kraker, J.; Schouten-van Meeteren, A.Y.; Bruin, M.; Korthof, E.T. Childhood paroxysmal nocturnal haemoglobinuria (PNH), a report of 11 cases in the Netherlands. Br. J. Haematol. 2005, 128, 571–577. [Google Scholar] [CrossRef]
- Curran, K.J.; Kernan, N.A.; Prockop, S.E.; Scaradavou, A.; Small, T.N.; Kobos, R.; Castro-Malaspina, H.; Araten, D.; DiMichele, D.; O’Reilly, R.J.; et al. Paroxysmal nocturnal hemoglobinuria in pediatric patients. Pediatr. Blood Cancer 2012, 59, 525–529. [Google Scholar] [CrossRef]
- Kim, J.S.; Jang, J.H.; Yoon, S.S.; Lee, J.H.; Kim, Y.K.; Jo, D.Y.; Chung, J.S.; Sohn, S.K.; Lee, J.W. Distinct subgroups of paroxysmal nocturnal hemoglobinuria (PNH) with cytopenia: Results from South Korean National PNH Registry. Ann. Hematol. 2016, 95, 125–133. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, J.S.; Yoon, S.S.; Lee, J.H.; Kim, Y.K.; Jo, D.Y.; Chung, J.; Sohn, S.K.; Lee, J.W. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): Results from a Korean PNH Registry. J. Korean Med. Sci. 2016, 31, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, J.S.; Lim, C.T.K.; Kleinman, N.J.; Myren, K.J.; Wang, A.; Patel, Y.; Lee, J.W. Impact of lactate dehydrogenase and hemoglobin levels on clinical outcomes in patients with paroxysmal nocturnal hemoglobinuria: Results from the National Korean PNH Registry. J. Korean Med. Sci. 2024, 39, e81. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Kim, S.; Suh, H.S. Efficacy of complement inhibitors for patients with paroxysmal nocturnal hemoglobinuria: A systematic review and meta-analysis. Ther. Adv. Hematol. 2023, 14, 20406207231216080. [Google Scholar] [CrossRef] [PubMed]
- Kanakura, Y.; Ohyashiki, K.; Shichishima, T.; Okamoto, S.; Ando, K.; Ninomiya, H.; Kawaguchi, T.; Nakao, S.; Nakakuma, H.; Nishimura, J.; et al. Long-term efficacy and safety of eculizumab in Japanese patients with PNH: AEGIS trial. Int. J. Hematol. 2013, 98, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, H.; Obara, N.; Chiba, S.; Usuki, K.; Nishiwaki, K.; Matsumura, I.; Shichishima, T.; Okamoto, S.; Nishimura, J.I.; Ohyashiki, K.; et al. Interim analysis of post-marketing surveillance of eculizumab for paroxysmal nocturnal hemoglobinuria in Japan. Int. J. Hematol. 2016, 104, 548–558. [Google Scholar] [CrossRef]
- Choi, C.W.; Jang, J.H.; Kim, J.S.; Jo, D.Y.; Lee, J.H.; Kim, S.H.; Kim, Y.K.; Won, J.H.; Chung, J.S.; Kim, H.; et al. Efficacy of eculizumab in paroxysmal nocturnal hemoglobinuria patients with or without aplastic anemia: Prospective study of a Korean PNH cohort. Blood Res. 2017, 52, 207–211. [Google Scholar] [CrossRef]
- Kang, K.W.; Moon, H.; Lee, B.H.; Jeon, M.J.; Yu, E.S.; Kim, D.S.; Lee, S.R.; Sung, H.J.; Choi, C.W.; Kim, B.S.; et al. Nationwide study of paroxysmal nocturnal hemoglobinuria in South Korea: Paradox of eculizumab. Ann. Hematol. 2021, 99, 1493–1503, Erratum in Ann. Hematol. 2021, 100, 3083. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Noji, H.; Ueda, Y.; Kanda, Y.; Okamoto, S.; Usuki, K.; Matsuda, T.; Akiyama, H.; Shimono, A.; Yonemura, Y.; et al. Long-term follow-up of patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab: Post-marketing surveillance in Japan. Int. J. Hematol. 2022, 115, 470–480. [Google Scholar] [CrossRef]
- Usuki, K.; Ikezoe, T.; Ishiyama, K.; Kanda, Y.; Gotoh, A.; Hayashi, H.; Shimono, A.; Kitajima, A.; Obara, N.; Nishimura, J.I. Interim analysis of post-marketing surveillance of ravulizumab for paroxysmal nocturnal hemoglobinuria in Japan. Int. J. Hematol. 2023, 118, 311–322. [Google Scholar] [CrossRef]
- Kim, J.S.; Jang, J.H.; Jo, D.Y.; Ahn, S.Y.; Yoon, S.S.; Lee, J.H.; Kim, S.H.; Choi, C.W.; Shin, H.J.; Kim, M.K.; et al. Long-term efficacy and safety of eculizumab in patients with paroxysmal nocturnal hemoglobinuria and high disease burden: Real-world data from Korea. J. Korean Med. Sci. 2023, 38, e328. [Google Scholar] [CrossRef] [PubMed]
- Hosen, N.; Takamori, H.; Nishimura, J.; Bessler, M.; Ware, R.; Hillmen, P.; Luzzatto, L.; Young, N.; Kinoshita, T.; for the Research Group on Idiopathic Hematopoietic Disorder. Reference Guide on Clinical Practice for Paroxysmal Nocturnal Hemoglobinuria (2022 Revision). March 2023. Available online: http://zoketsushogaihan.umin.jp/file/2022/Paroxysmal_nocturnal_hemoglobinuria.pdf (accessed on 16 December 2024).
- National Health Insurance Administration, Ministry of Health and Welfare. NHI Payment Guidelines, Update on March 25, 2024. Available online: https://www.nhi.gov.tw/ch/sp-search-form-1.html?q=eculizumab (accessed on 16 December 2024).
- Hall, C.; Richards, S.; Hillmen, P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2003, 102, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Moyo, V.M.; Mukhina, G.L.; Garrett, E.S.; Brodsky, R.A. Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br. J. Haematol. 2004, 126, 133–138. [Google Scholar] [CrossRef]
- Höchsmann, B.; Peffault de Latour, R.; Hill, A.; Röth, A.; Devos, T.; Patriquin, C.J.; Chou, W.C.; Jain, D.; Zu, K.; Wu, C.; et al. Risk factors for thromboembolic events in patients with paroxysmal nocturnal hemoglobinuria (PNH): A nested case-control study in the International PNH Registry. Ann. Hematol. 2023, 102, 2979–2988. [Google Scholar] [CrossRef]
- Dingli, D.; Maciejewski, J.P.; Larratt, L.; Go, R.S.; Höchsmann, B.; Zu, K.; Gustovic, P.; Kulagin, A.D. Relationship of paroxysmal nocturnal hemoglobinuria (PNH) granulocyte clone size to disease burden and risk of major vascular events in untreated patients: Results from the International PNH Registry. Ann. Hematol. 2023, 102, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- de Latour, R.P.; Mary, J.Y.; Salanoubat, C.; Terriou, L.; Etienne, G.; Mohty, M.; Roth, S.; de Guibert, S.; Maury, S.; Cahn, J.Y.; et al. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood 2008, 112, 3099–3106. [Google Scholar] [CrossRef]
- Bais, J.; Pel, M.; von dem Borne, A.; van der Lelie, H. Pregnancy and paroxysmal nocturnal hemoglobinuria. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 53, 211–214. [Google Scholar] [CrossRef]
- Fieni, S.; Bonfanti, L.; Gramellini, D.; Benassi, L.; Delsignore, R. Clinical management of paroxysmal nocturnal hemoglobinuria in pregnancy: A case report and updated review. Obstet. Gynecol. Surv. 2006, 61, 593–601. [Google Scholar] [CrossRef]
- de Guibert, S.; Peffault de Latour, R.; Varoqueaux, N.; Labussière, H.; Rio, B.; Jaulmes, D.; Eveillard, J.R.; Dulucq, S.; Stoppa, A.M.; Bouscary, D.; et al. Paroxysmal nocturnal hemoglobinuria and pregnancy before the eculizumab era: The French experience. Haematologica 2011, 96, 1276–1283. [Google Scholar] [CrossRef]
- Kim, H.K.; Tantry, U.S.; Park, H.W.; Shin, E.S.; Geisler, T.; Gorog, D.A.; Gurbel, P.A.; Jeong, Y.H. Ethnic difference of thrombogenicity in patients with cardiovascular disease: A Pandora Box to explain prognostic differences. Korean Circ. J. 2021, 51, 202–221. [Google Scholar] [CrossRef] [PubMed]
- JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ. J. 2011, 75, 1258–1281. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Akay, O.M.; Ayer, M.; Dal, M.S.; Ertop, S.; Ilhan, O.; Karakus, V.; Ozcan, M.A.; Ozkocaman, V.; Ozsan, H.; et al. Pesg PNH diagnosis, follow-up and treatment guidelines. Am. J. Blood Res. 2016, 6, 19–27. [Google Scholar]
- Risitano, A.M.; Peffault de Latour, R. How we(‘ll) treat paroxysmal nocturnal haemoglobinuria: Diving into the future. Br. J. Haematol. 2022, 196, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913, Erratum in Circulation 2024, 149, e1164. [Google Scholar] [CrossRef]
- Devos, T.; Meers, S.; Boeckx, N.; Gothot, A.; Deeren, D.; Chatelain, B.; Chatelain, C.; Devalet, B. Diagnosis and management of PNH: Review and recommendations from a Belgian expert panel. Eur. J. Haematol. 2018, 101, 737–749. [Google Scholar] [CrossRef]
- Dhawan, R.; Ahluwalia, J.; Malhotra, P.; Mahapatra, M.; Varma, N.; Varma, S. Markers of thrombin generation and inflammation in patients with paroxysmal nocturnal hemoglobinuria. Indian J. Hematol. Blood Transfus. 2021, 37, 204–209. [Google Scholar] [CrossRef]
- Weitz, J.I.; Fredenburgh, J.C.; Eikelboom, J.W. A test in context: D-dimer. J. Am. Coll. Cardiol. 2017, 70, 2411–2420. [Google Scholar] [CrossRef]
- Patriquin, C.J.; Kiss, T.; Caplan, S.; Chin-Yee, I.; Grewal, K.; Grossman, J.; Larratt, L.; Marceau, D.; Nevill, T.; Sutherland, D.R.; et al. How we treat paroxysmal nocturnal hemoglobinuria: A consensus statement of the Canadian PNH Network and review of the national registry. Eur. J. Haematol. 2019, 102, 36–52. [Google Scholar] [CrossRef]
- Malato, A.; Saccullo, G.; Coco, L.L.; Mancuso, S.; Santoro, M.; Martino, S.; Zammit, V.; Sprini, D.; Siragusa, S. Thrombotic complications in paroxysmal nocturnal haemoglobinuria: A literature review. Blood Transfus. 2012, 10, 428–435. [Google Scholar] [CrossRef]
- Lee, L.H.; Gallus, A.; Jindal, R.; Wang, C.; Wu, C.C. Incidence of venous thromboembolism in Asian populations: A systematic review. Thromb. Haemost. 2017, 117, 2243–2260. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Wang, Y.Q.; Wang, C.; Oh, D.; Yin, W.H.; Kimura, T.; Miyazaki, K.; Abe, K.; Mercuri, M.; Lee, L.H.; et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: A subanalysis of East Asian patients in the Hokusai-VTE trial. J. Thromb. Haemost. 2015, 13, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Wang, K.L.; Chiang, C.E. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: Time for a reappraisal. Int. J. Cardiol. 2015, 180, 246–254. [Google Scholar] [CrossRef]
- Schubert, J.; Bettleheim, P.; Brümmendorf, T.H.; Höchsmann, B.; Panse, J.; Röth, A.; Schrezenmeier, H.; Stüssi, G. Paroxysmal Nocturnal Haemoglobinuria (PNH). Guideline: Recommendations of the Professional Society for Diagnostics and Therapy for Haematological and Oncological Diseases. November 2019. Available online: https://www.onkopedia.com/de/onkopedia/guidelines/paroxysmale-naechtliche-haemoglobinurie-pnh/@@guideline/html/index.html (accessed on 16 December 2024).
- Brodsky, R.A. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2021, 137, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Gerber, G.F.; DeZern, A.E.; Chaturvedi, S.; Brodsky, R.A. A 15-year, single institution experience of anticoagulation management in paroxysmal nocturnal hemoglobinuria patients on terminal complement inhibition with history of thromboembolism. Am. J. Hematol. 2022, 97, E59–E62. [Google Scholar] [CrossRef]
- Brodsky, R.A. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2009, 113, 6522–6527. [Google Scholar] [CrossRef]
- Weitz, J.I. Factor Xa and thrombin as targets for new oral anticoagulants. Thromb. Res. 2011, 127 (Suppl. 2), S5–S12. [Google Scholar] [CrossRef]
- Orphananesthesia. Anaesthesia Recommendations for Patients Suffering from Paroxysmal Nocturnal Haemoglobinuria (PNH). Available online: https://www.orphananesthesia.eu/en/rare-diseases/published-guidelines/paroxysmal-nocturnal-haemoglobinuria/222-paroxysmal-nocturnal-haemoglobinuria/file.html (accessed on 3 March 2025).
- Gurnari, C.; Awada, H.; Pagliuca, S.; Dima, D.; Ullah, F.; Kawashima, N.; Kubota, Y.; Colak, C.; Visconte, V.; Patel, B.J.; et al. Paroxysmal nocturnal hemoglobinu-ria-related thrombosis in the era of novel therapies: A 2043-patient-year analysis. Blood 2024, 144, 145–155. [Google Scholar] [CrossRef]
- Manning, J.E.; Anderson, R.M.; Hill, A.; Zeidan, D.; Ciantar, E. Pregnancy outcomes in women receiving eculizumab for the management of paroxysmal nocturnal haemoglobinuria. Obstet. Med. 2022, 15, 45–49. [Google Scholar] [CrossRef]
- Kelly, R.J.; Höchsmann, B.; Szer, J.; Kulasekararaj, A.; de Guibert, S.; Röth, A.; Weitz, I.C.; Armstrong, E.; Risitano, A.M.; Patriquin, C.J.; et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2015, 373, 1032–1039. [Google Scholar] [CrossRef]
- Miyasaka, N.; Miura, O.; Kawaguchi, T.; Arima, N.; Morishita, E.; Usuki, K.; Morita, Y.; Nishiwaki, K.; Ninomiya, H.; Gotoh, A.; et al. Pregnancy outcomes of patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab: A Japanese experience and updated review. Int. J. Hematol. 2016, 103, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chu, R.; Huang, C.; Song, X.; Wang, J.; Li, L.; Xu, Y.; Ma, Y. Progress in the management of pregnancy with paroxysmal nocturnal hemoglobinuria: A review. J. Womens Health 2024, 33, 98–104. [Google Scholar] [CrossRef]
- Kuo, G.P.; Brodsky, R.A.; Kim, H.S. Catheter-directed thrombolysis and thrombectomy for the Budd-Chiari syndrome in paroxysmal nocturnal hemoglobinuria in three patients. J. Vasc. Interv. Radiol. 2006, 17 Pt 1, 383–387. [Google Scholar] [CrossRef]
- Singer, A.L.; Locke, J.E.; Stewart, Z.A.; Lonze, B.E.; Hamilton, J.P.; Scudiere, J.R.; Anders, R.A.; Rother, R.P.; Brodsky, R.A.; Cameron, A.M. Successful liver transplantation for Budd-Chiari syndrome in a patient with paroxysmal nocturnal hemoglobinuria treated with the anti-complement antibody eculizumab. Liver Transpl. 2009, 15, 540–543. [Google Scholar] [CrossRef]
- Brodsky, A.; Mazzocchi, O.; Sánchez, F.; Khursigara, G.; Malhotra, S.; Volpacchio, M. Eculizumab in paroxysmal nocturnal hemoglobinuria with Budd-Chiari syndrome progressing despite anticoagulation. Exp. Hematol. Oncol. 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Mandala, E.; Lafaras, C.; Goulis, I.; Tsioni, K.; Georgopoulou, V.; Ilonidis, G. Treatment of a patient with classical paroxysmal nocturnal hemoglobinuria and Budd-Chiari syndrome, with complement inhibitor eculizumab: Case report. Hippokratia 2013, 17, 81–84. [Google Scholar]
- Kim, H.; Kim, I.S.; Cho, S.H.; Lee, H.J.; Chang, C.L.; Yoon, K.T. The first case of paroxysmal nocturnal hemoglobinuria and Budd-Chiari syndrome treated with complement inhibitor eculizumab in Korea. Blood Res. 2017, 52, 145–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hillmen, P.; Szer, J.; Weitz, I.; Röth, A.; Höchsmann, B.; Panse, J.; Usuki, K.; Griffin, M.; Kiladjian, J.J.; de Castro, C.; et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2021, 384, 1028–1037, Erratum in N. Engl. J. Med. 2024, 390, 1060. [Google Scholar] [CrossRef]
- Risitano, A.M.; Röth, A.; Soret, J.; Frieri, C.; de Fontbrune, F.S.; Marano, L.; Alashkar, F.; Benajiba, L.; Marotta, S.; Rozenberg, I.; et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: An open-label, single-arm, phase 2, proof-of-concept trial. Lancet Haematol. 2021, 8, e344–e354. [Google Scholar] [CrossRef]
- Notaro, R.; Luzzatto, L. Breakthrough hemolysis in PNH with proximal or terminal complement inhibition. N. Engl. J. Med. 2022, 387, 160–166. [Google Scholar] [CrossRef]
- Griffin, M.; Kelly, R.J.; Panse, J.; de Castro, C.; Szer, J.; Horneff, R.; Tan, L.; Yeh, M.; Peffault de Latour, R. Management of acute breakthrough hemolysis with intensive pegcetacoplan dosing in patients with PNH. Blood Adv. 2024, 8, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- De Latour, R.P.; Kulasekararaj, A.; Scheinberg, P.; Ferber, P.; Lawniczek, T.; Thorburn, C.; Dahlke, M.; Risitano, A.M. Clinical breakthrough hemolysis (BTH) during monotherapy with the oral factor B inhibitor iptacopan is generally not severe and managed without treatment discontinuation: 48-week data from the phase III apply-PNH and appoint-PNH trials in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2023, 142 (Suppl. 1), 1338. [Google Scholar] [CrossRef]
- Nishimori, H.; Nakazawa, H.; Tamura, S.; Uchida, T.; Usuki, K.; Szamosi, J.; de Latour, R.P.; Röth, A.; Panse, J. Efficacy, Safety, and Quality of Life of Pegcetacoplan in Japanese Patients with Paroxysmal Nocturnal Hemoglobinuria Treated within the Phase 3 PEGASUS Trial. Acta Haematol. 2025, 148, 22–35. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Reference | Asian | Non-Asian | p-Value |
|---|---|---|---|---|
| Incidence rate | [8] a | 11.5% | 32.5% | 0.000 |
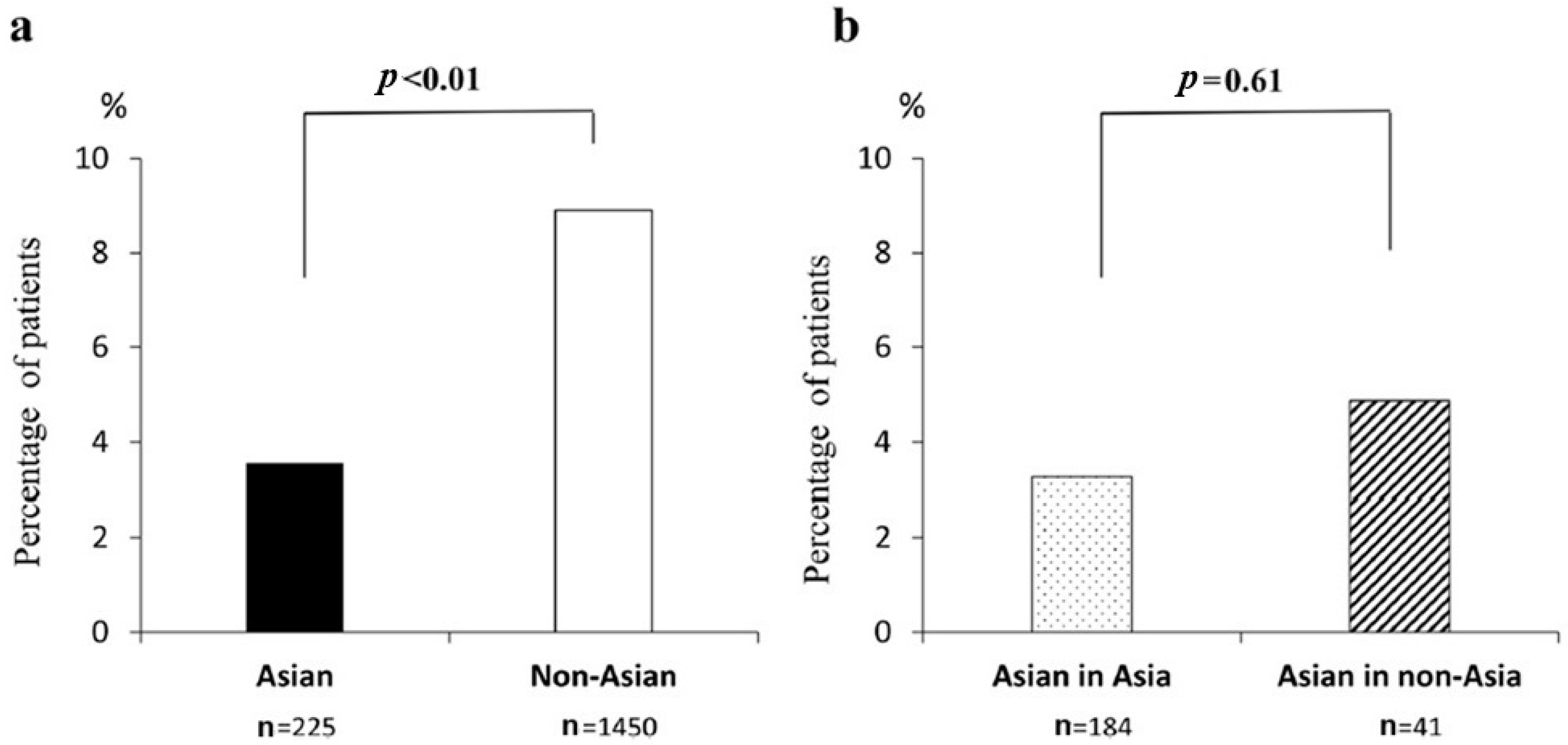
| History of TE | [9] b | 3.6% | 8.9% | <0.01 |
| Type of TE, frequency | [9] b | |||
| Venous | 3.1% | 7.3% | 0.02 | |
| Arterial | 0.4% | 2.1% | 0.09 | |
| Sites of thrombosis, proportion in all TE events | [8] a | |||
| Venous (abdominal) | 35.1% | 50.5% | 0.024 | |
| Arterial | 23.0% | 1.1% | 0.000 | |
| Death rate | [8] a | 19.4% | 19.4% | 0.968 |
| Death from TE | [8] a | 6.9% | 43.7% | 0.000 |
| Use of anticoagulant therapy c | [9] b | 8.5% | 16.2% | 0.002 |
| Parameter | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| LDH ≥ 1.5 × ULN | 7.0 (1.5–32) a 12.21 (1.61–92.86) b | 0.013 0.016 |
| Male sex | 2.19 (1.02–4.72) b | 0.045 |
| Pain | 2.79 (1.09–7.17) b | 0.033 |
| LDH ≥ 1.5 × ULN plus abdominal pain | 17.79 (2.33–36.01) a | 0.006 |
| LDH ≥ 1.5 × ULN plus chest pain | 19.04 (3.74–96.99) a | <0.001 |
| LDH ≥ 1.5 × ULN plus dyspnea | 10.35 (2.31–46.45) a | 0.002 |
| LDH ≥ 1.5 × ULN plus hemoglobinuria | 10.28 (1.34–79.02) a | 0.025 |
| TE History | Complement Inhibitor | |
|---|---|---|
| Yes | No | |
| Yes | Administer anticoagulant therapy Patients already receiving a complement inhibitor who experience an acute TE should be given anticoagulation in addition [10,80,89]. Consider discontinuing anticoagulation after 3–6 months of concomitant therapy provided that the TE has fully resolved, PNH is well controlled, and there are no persistent risk factors for TE [89,90]. | Administer anticoagulant therapy Patients not receiving a complement inhibitor who experience an acute TE should be given anticoagulation and commence treatment with a complement inhibitor [10,78,80,83,89]. In settings where complement inhibitors are not available, anticoagulation should be continued for secondary prophylaxis. |
| No | No anticoagulant therapy Patients without a history of TE who are receiving a complement inhibitor do not need to receive anticoagulation as primary prophylaxis [10,78]. As anticoagulant therapy can be dangerous in patients with low platelet counts, prophylactic anticoagulation should not be initiated in patients without a history of TE who do not meet the eligibility criteria for complement inhibitor treatment [91]. | Consider anticoagulant therapy Prophylactic anticoagulation can be considered in patients with persistently elevated D-dimer levels, pregnant women, and during preoperative periods [84,88,91]. In settings where complement inhibitors are not available, consider anticoagulant primary prophylaxis for patients with a substantial PNH clone, platelet count × 109/L, and no known risk factor for hemorrhage [10,67,84]. Primary prophylactic anticoagulation should also be considered in patients who do not respond to complement inhibitors [88]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, Y.; Chou, W.-C.; Goh, Y.-T.; Rojnuckarin, P.; Kim, J.S.; Wong, R.S.M.; Lee Wong, L.L.; Jang, J.H.; Chiou, T.-J.; Kanakura, Y.; et al. Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 2504. https://doi.org/10.3390/ijms26062504
Ueda Y, Chou W-C, Goh Y-T, Rojnuckarin P, Kim JS, Wong RSM, Lee Wong LL, Jang JH, Chiou T-J, Kanakura Y, et al. Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(6):2504. https://doi.org/10.3390/ijms26062504
Chicago/Turabian StyleUeda, Yasutaka, Wen-Chien Chou, Yeow-Tee Goh, Ponlapat Rojnuckarin, Jin Seok Kim, Raymond Siu Ming Wong, Lily Lee Lee Wong, Jun Ho Jang, Tzeon-Jye Chiou, Yuzuru Kanakura, and et al. 2025. "Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review" International Journal of Molecular Sciences 26, no. 6: 2504. https://doi.org/10.3390/ijms26062504
APA StyleUeda, Y., Chou, W.-C., Goh, Y.-T., Rojnuckarin, P., Kim, J. S., Wong, R. S. M., Lee Wong, L. L., Jang, J. H., Chiou, T.-J., Kanakura, Y., & Lee, J. W. (2025). Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review. International Journal of Molecular Sciences, 26(6), 2504. https://doi.org/10.3390/ijms26062504






