Prosodic Differences in Women with the FMR1 Premutation: Subtle Expression of Autism-Related Phenotypes Through Speech
Abstract
1. Introduction
1.1. Study 1: Characterizing Prosodic Production in FMR1 PM Carriers
- Are prosodic differences evident in FMR1 PM?
- Are prosodic characteristics in FMR1 PM similar to prosodic features associated with AU and the BAP?
- Are prosodic properties of speech among FMR1 PM carriers associated with variability of the FMR1 gene?
1.2. Study 2: Studying Speech Prosodic Processing Through Neural Frequency Following Responses to Prosodic Patterns
- Is reduced accuracy in neural encoding of prosodic speech cues evident in FMR1 PM?
- Are patterns of neural encoding speech prosody in FMR1 PM similar to those observed in AU and the BAP?
2. Results
2.1. Study 1 Results
2.1.1. Group Differences: Speech Intonation
2.1.2. Group Differences: Speech Rhythm
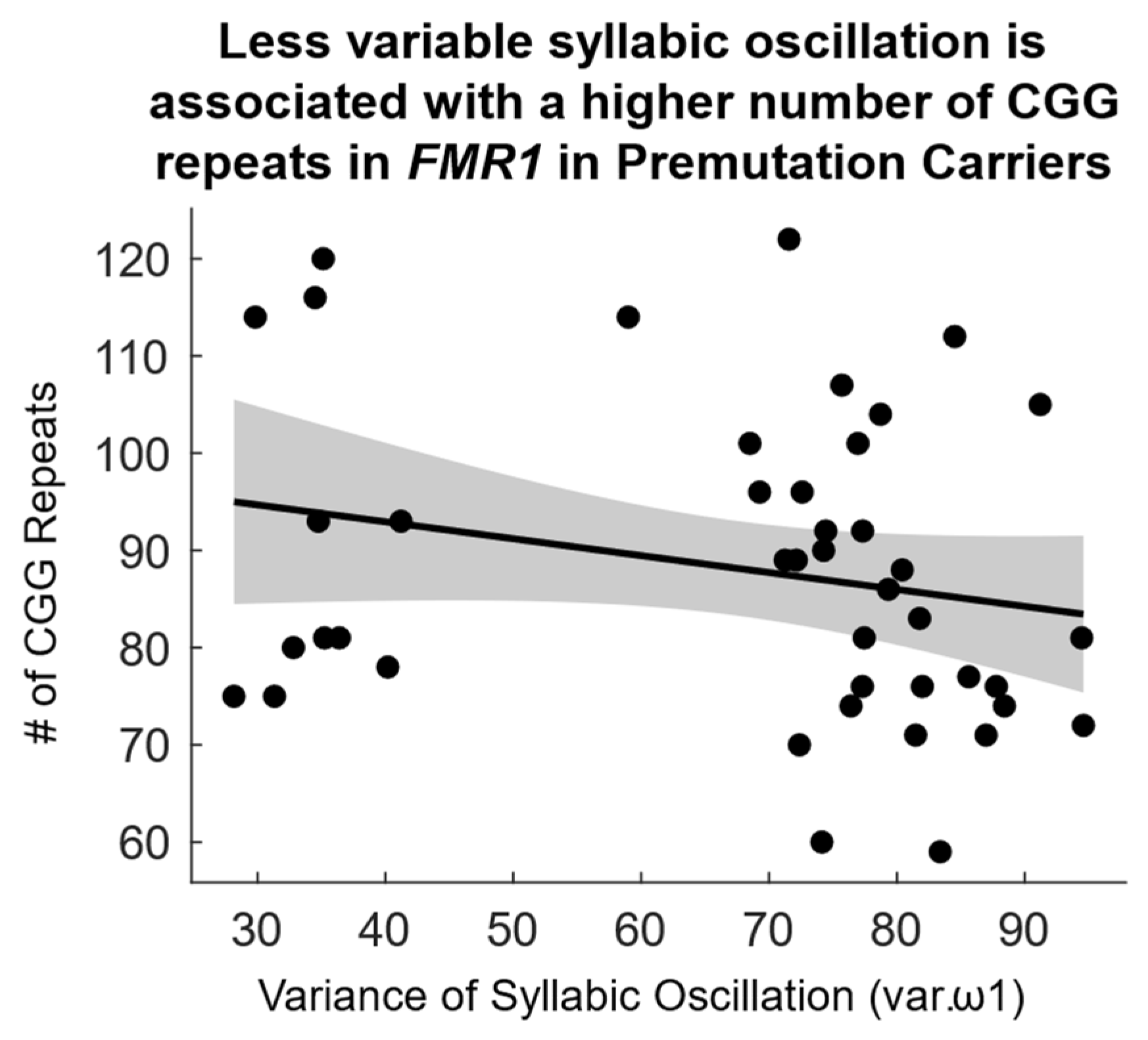
2.1.3. Correlation Between Speech Rhythm and FMR1 Molecular-Genetic Variation
2.2. Study 2 Results
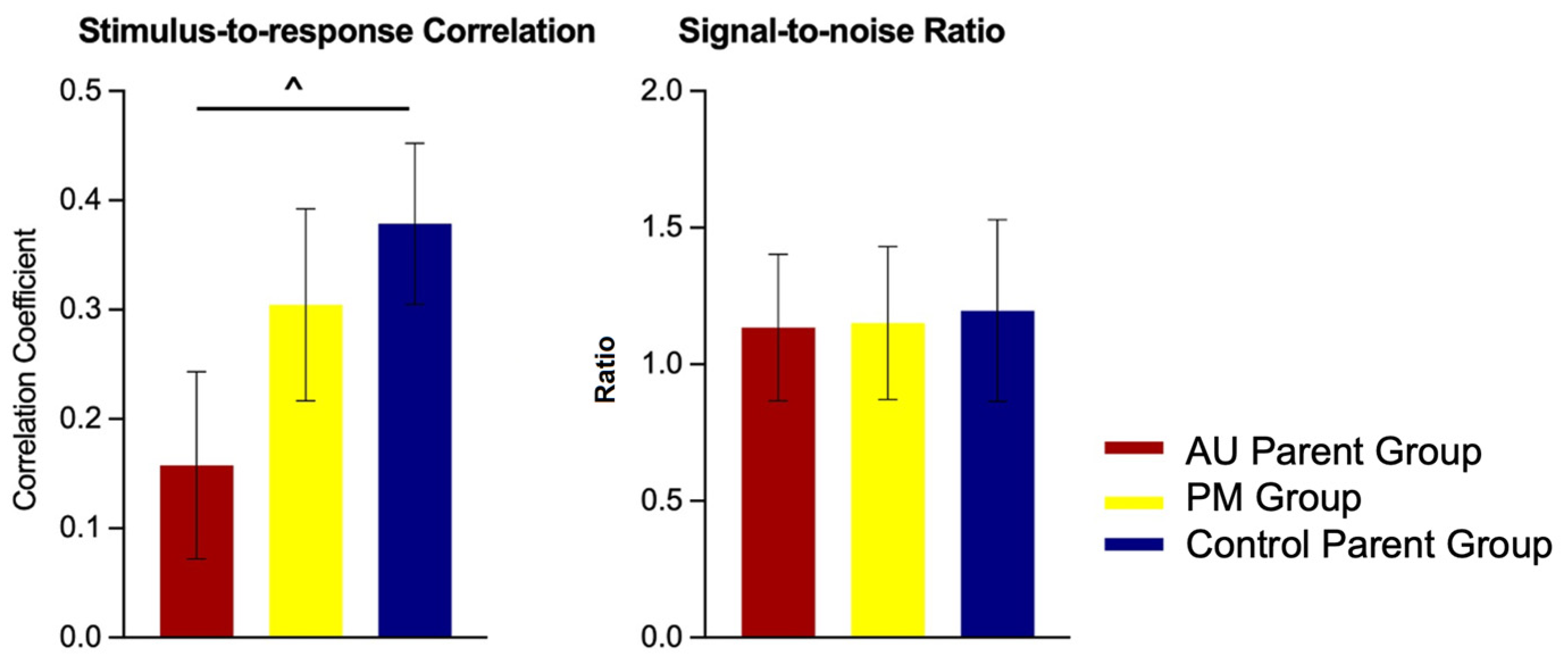
2.2.1. Group Differences: Stimulus-to-Response Correlation
2.2.2. Group Differences: Signal-to-Noise Ratio
3. Discussion
3.1. Prosodic Differences in Speech Associated with FMR1 (Pre-)Mutation
3.2. Overlap in Prosodic Characteristics Among FMR1 Premutation Carriers and Parents of Autistic Individuals
3.3. Association Between AU-Related Rhythmic Properties of Prosody and FMR1 Variability
3.4. No Differences in Neural Processing of Speech Prosodic Cues in FMR1 PM
3.5. Limitations and Future Directions
4. Materials and Methods
4.1. Study 1 Materials and Methods
4.1.1. Study 1 Participants
4.1.2. Narrative Elicitation
4.1.3. Acoustic Analysis
4.1.4. Molecular-Genetic Analysis
4.1.5. Study 1 Statistical Analysis
4.2. Study 2 Materials and Methods
4.2.1. Study 2 Participants
4.2.2. Frequency Following Response Elicitation and Data Processing
4.2.3. Frequency Following Response Data Analysis
4.2.4. Study 2 Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cutler, A.; Isard, S.D. The Production of Prosody. In Language Production; Butterworth, B., Ed.; Academic Press: London, UK, 1980; pp. 245–269. [Google Scholar]
- Fox, A. Prosodic Features and Prosodic Structure: The Phonology of Suprasegmentals; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Pub: Washington, DC, USA, 2013; ISBN 0890425574.
- Volkmar, F.R.; Lord, C.; Bailey, A.; Schultz, R.T.; Klin, A. Autism and Pervasive Developmental Disorders. J. Child Psychol. Psychiatry 2004, 45, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Roselló, B.; Berenguer, C.; Navío, P.; Baixauli, I.; Miranda, A. Executive Functioning, Social Cognition, Pragmatics, and Social Interaction in Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder. Curr. Dev. Disord. Rep. 2017, 4, 72–77. [Google Scholar] [CrossRef]
- Sturrock, A.; Marsden, A.; Adams, C.; Freed, J. Observational and Reported Measures of Language and Pragmatics in Young People with Autism: A Comparison of Respondent Data and Gender Profiles. J. Autism Dev. Disord. 2020, 50, 812–830. [Google Scholar] [CrossRef]
- Peppé, S.; McCann, J.; Gibbon, F.; O’Hare, A.; Rutherford, M. Assessing Prosodic and Pragmatic Ability in Children with High-Functioning Autism. J. Pragmat. 2006, 38, 1776–1791. [Google Scholar] [CrossRef]
- McCann, J.; Peppé, S. Prosody in Autism Spectrum Disorders: A Critical Review. Int. J. Lang. Commun. Disord. 2003, 38, 325–350. [Google Scholar] [CrossRef]
- Asghari, S.Z.; Farashi, S.; Bashirian, S.; Jenabi, E. Distinctive Prosodic Features of People with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis Study. Sci. Rep. 2021, 11, 23093. [Google Scholar] [CrossRef]
- Fusaroli, R.; Lambrechts, A.; Bang, D.; Bowler, D.M.; Gaigg, S.B. Is Voice a Marker for Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. Autism Res. 2017, 10, 384–407. [Google Scholar] [CrossRef]
- Loveall, S.J.; Hawthorne, K.; Gaines, M. A Meta-Analysis of Prosody in Autism, Williams Syndrome, and Down Syndrome. J. Commun. Disord. 2021, 89, 106055. [Google Scholar] [CrossRef]
- Haigh, S.M.; Brosseau, P.; Eack, S.M.; Leitman, D.I.; Salisbury, D.F.; Behrmann, M. Hyper-Sensitivity to Pitch and Poorer Prosody Processing in Adults with Autism: An ERP Study. Front. Psychiatry 2022, 13, 844830. [Google Scholar] [CrossRef]
- McCann, J.; Peppé, S.; Gibbon, F.E.; O’Hare, A.; Rutherford, M. Prosody and Its Relationship to Language in School-Aged Children with High-Functioning Autism. Int. J. Lang. Commun. Disord. 2006, 42, 682–702. [Google Scholar] [CrossRef]
- Nadig, A.; Shaw, H. Acoustic and Perceptual Measurement of Expressive Prosody in High-Functioning Autism: Increased Pitch Range and What It Means to Listeners. J. Autism Dev. Disord. 2012, 42, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Peppé, S.; Cleland, J.; Gibbon, F.; O’Hare, A.; Castilla, P.M. Expressive Prosody in Children with Autism Spectrum Conditions. J. Neurolinguist. 2011, 24, 41–53. [Google Scholar] [CrossRef]
- Diehl, J.J.; Watson, D.; Bennetto, L.; Mcdonough, J.; Gunlogson, C. An Acoustic Analysis of Prosody in High-Functioning Autism. Appl. Psycholinguist. 2009, 30, 385–404. [Google Scholar] [CrossRef]
- Paul, R.; Augustyn, A.; Klin, A.; Volkmar, F.R. Perception and Production of Prosody by Speakers with Autism Spectrum Disorders. J. Autism Dev. Disord. 2005, 35, 205–220. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Paul, R.; McSweeny, J.L.; Klin, A.; Cohen, D.J.; Volkmar, F.R. Speech and Prosody Characteristics of Adolescents and Adults with High-Functioning Autism and Asperger Syndrome. J. Speech Lang. Hear. Res. 2001, 44, 1097–1115. [Google Scholar] [CrossRef]
- Patel, S.P.; Nayar, K.; Martin, G.E.; Franich, K.; Crawford, S.; Diehl, J.J.; Losh, M. An Acoustic Characterization of Prosodic Differences in Autism Spectrum Disorder and First-Degree Relatives. J. Autism Dev. Disord. 2020, 50, 3032–3045. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.C.Y.; Losh, M.; Speights, M. Differences in Speech Articulatory Timing and Associations with Pragmatic Language Ability in Autism. Res. Autism Spectr. Disord. 2023, 102, 102118. [Google Scholar] [CrossRef]
- Peppé, S.; McCann, J. Assessing Intonation and Prosody in Children with Atypical Language Development: The PEPS-C Test and the Revised Version. Clin. Linguist. Phon. 2003, 17, 345–354. [Google Scholar] [CrossRef]
- Scheerer, N.E.; Shafai, F.; Stevenson, R.A.; Iarocci, G. Affective Prosody Perception and the Relation to Social Competence in Autistic and Typically Developing Children. J. Abnorm. Child. Psychol. 2020, 48, 965–975. [Google Scholar] [CrossRef]
- Lau, J.C.Y.; To, C.K.S.; Kwan, J.S.K.; Kang, X.; Losh, M.; Wong, P.C.M. Lifelong Tone Language Experience Does Not Eliminate Deficits in Neural Encoding of Pitch in Autism Spectrum Disorder. J. Autism Dev. Disord. 2021, 51, 3291–3310. [Google Scholar] [CrossRef]
- Patel, S.P.; Winston, M.; Guilfoyle, J.; Nicol, T.; Martin, G.E.; Nayar, K.; Kraus, N.; Losh, M. Neural Processing of Speech Sounds in ASD and First-Degree Relatives. J. Autism Dev. Disord. 2022, 53, 3257–3271. [Google Scholar] [CrossRef]
- Bidelman, G.M. Multichannel Recordings of the Human Brainstem Frequency-Following Response: Scalp Topography, Source Generators, and Distinctions from the Transient ABR. Hear. Res. 2015, 323, 68–80. [Google Scholar] [CrossRef]
- Bidelman, G.M.; Gandour, J.T.; Krishnan, A. Cross-Domain Effects of Music and Language Experience on the Representation of Pitch in the Human Auditory Brainstem. J. Cogn. Neurosci. 2011, 23, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.M.; Skoe, E.; Trommer, B.; Nicol, T.; Zecker, S.; Bradlow, A.; Kraus, N. Deficient Brainstem Encoding of Pitch in Children with Autism Spectrum Disorders. Clin. Neurophysiol. 2008, 119, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.; Nicol, T.; Trommer, B.; Zecker, S.; Kraus, N. Brainstem Transcription of Speech Is Disrupted in Children with Autism Spectrum Disorders. Dev. Sci. 2009, 12, 557–567. [Google Scholar] [CrossRef]
- Otto-Meyer, S.; Krizman, J.; White-Schwoch, T.; Kraus, N. Children with Autism Spectrum Disorder Have Unstable Neural Responses to Sound. Exp. Brain Res. 2018, 236, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.; Macdonald, H.; Pickles, A.; Rios, P.; Goode, S.; Crowson, M.; Bailey, A.; Rutter, M. A Case-Control Family History Study of Autism. J. Child. Psychol. Psychiatry 1994, 35, 877–900. [Google Scholar] [CrossRef]
- Losh, M.; Childress, D.; Lam, K.; Piven, J. Defining Key Features of the Broad Autism Phenotype: A Comparison across Parents of Multiple- and Single-Incidence Autism Families. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147B, 424–433. [Google Scholar] [CrossRef]
- Piven, J.; Palmer, P.; Landa, R.; Santangelo, S.; Jacobi, D.; Childress, D. Personality and Language Characteristics in Parents from Multiple-incidence Autism Families. Am. J. Med. Genet. 1997, 74, 398–411. [Google Scholar] [CrossRef]
- Landa, R.; Piven, J.; Wzorek, M.M.; Gayle, J.O.; Chase, G.A.; Folstein, S.E. Social Language Use in Parents of Autistic Individuals. Psychol. Med. 1992, 22, 245–254. [Google Scholar] [CrossRef]
- Losh, M.; Martin, G.E.; Klusek, J.; Hogan-Brown, A.L. Pragmatic Language in Autism and Fragile X Syndrome: Genetic and Clinical Applications. Perspect. Lang. Learn. Educ. 2012, 19, 48–55. [Google Scholar] [CrossRef]
- Patel, S.P.; Cole, J.; Lau, J.C.Y.; Fragnito, G.; Losh, M. Verbal Entrainment in Autism Spectrum Disorder and First-Degree Relatives. Sci. Rep. 2022, 12, 11496. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Kaufmann, W.E. What Can We Learn about Autism from Studying Fragile X Syndrome? Dev. Neurosci. 2011, 33, 379–394. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Rivera, S.M.; Hagerman, P.J. The Fragile X Family of Disorders: A Model for Autism and Targeted Treatments. Curr. Pediatr. Rev. 2008, 4, 40–52. [Google Scholar] [CrossRef]
- Kazdoba, T.M.; Leach, P.T.; Crawley, J.N. Behavioral Phenotypes of Genetic Mouse Models of Autism. Genes. Brain Behav. 2016, 15, 7–26. [Google Scholar] [CrossRef]
- Yu, T.W.; Chahrour, M.H.; Coulter, M.E.; Jiralerspong, S.; Okamura-Ikeda, K.; Ataman, B.; Schmitz-Abe, K.; Harmin, D.A.; Adli, M.; Malik, A.N.; et al. Using Whole-Exome Sequencing to Identify Inherited Causes of Autism. Neuron 2013, 77, 259–273. [Google Scholar] [CrossRef]
- Ascano, M.; Mukherjee, N.; Bandaru, P.; Miller, J.B.; Nusbaum, J.D.; Corcoran, D.L.; Langlois, C.; Munschauer, M.; Dewell, S.; Hafner, M.; et al. FMRP Targets Distinct MRNA Sequence Elements to Regulate Protein Expression. Nature 2012, 492, 382–386. [Google Scholar] [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.S.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP Stalls Ribosomal Translocation on MRNAs Linked to Synaptic Function and Autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef]
- Steinberg, J.; Webber, C. The Roles of FMRP-Regulated Genes in Autism Spectrum Disorder: Single- and Multiple-Hit Genetic Etiologies. Am. J. Hum. Genet. 2013, 93, 825–839. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, Transcriptional and Chromatin Genes Disrupted in Autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Timothy, W.Y.; Berry-Kravis, E. Autism and Fragile X Syndrome. In Proceedings of the Seminars in Neurology; Thieme Medical Publishers: New York, NY, USA, 2014; Volume 34, pp. 258–265. [Google Scholar]
- Niu, M.; Han, Y.; Dy, A.B.C.; Du, J.; Jin, H.; Qin, J.; Zhang, J.; Li, Q.; Hagerman, R.J. Autism Symptoms in Fragile X Syndrome. J. Child. Neurol. 2017, 32, 903–909. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Hagerman, P.J. Fragile X Syndrome: Diagnosis, Treatment, and Research; Taylor & Francis US: New York, NY, USA, 2002; ISBN 0801868440. [Google Scholar]
- Losh, M.; Martin, G.E.; Klusek, J.; Hogan-Brown, A.L.; Sideris, J. Social Communication and Theory of Mind in Boys with Autism and Fragile x Syndrome. Front. Psychol. 2012, 3, 266. [Google Scholar] [CrossRef]
- Zajac, D.J.; Harris, A.A.; Roberts, J.E.; Martin, G.E. Direct Magnitude Estimation of Articulation Rate in Boys with Fragile X Syndrome. J. Speech Lang. Hear. Res. 2009, 52, 1370–1379. [Google Scholar] [CrossRef]
- Zajac, D.J.; Roberts, J.E.; Hennon, E.A.; Harris, A.A.; Barnes, E.F.; Misenheimer, J. Articulation Rate and Vowel Space Characteristics of Young Males with Fragile X Syndrome: Preliminary Acoustic Findings. J. Speech Lang. Hear. Res. 2006, 49, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.L.; Freund, L. Behavioral Phenotype of Fragile X Syndrome: DSM-III-R Autistic Behavior in Male Children. Am. J. Med. Genet. 1992, 43, 35–46. [Google Scholar] [CrossRef]
- Bangert, K.; Scott, K.S.; Adams, C.; Kisenwether, J.S.; Giuffre, L.; Reed, J.; Thurman, A.J.; Abbeduto, L.; Klusek, J. Cluttering in the Speech of Young Men with Fragile X Syndrome. J. Speech Lang. Hear. Res. 2022, 65, 954–969. [Google Scholar] [CrossRef]
- Wall, C.A.; Shic, F.; Varanasi, S.; Roberts, J.E. Distinct Social Attention Profiles in Preschoolers with Autism Contrasted to Fragile X Syndrome. Autism Res. 2023, 16, 340–354. [Google Scholar] [CrossRef]
- Farzin, F.; Rivera, S.M.; Hessl, D. Brief Report: Visual Processing of Faces in Individuals with Fragile X Syndrome: An Eye Tracking Study. J. Autism Dev. Disord. 2009, 39, 946–952. [Google Scholar] [CrossRef]
- Tassone, F.; Hall, D.A. FXTAS, FXPOI, and Other Premutation Disorders; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3319338986. [Google Scholar]
- Hagerman, R.J.; Hagerman, P.J. The Fragile X Premutation: Into the Phenotypic Fold. Curr. Opin. Genet. Dev. 2002, 12, 278–283. [Google Scholar] [CrossRef]
- Clifford, S.; Dissanayake, C.; Bui, Q.M.; Huggins, R.; Taylor, A.K.; Loesch, D.Z. Autism Spectrum Phenotype in Males and Females with Fragile X Full Mutation and Premutation. J. Autism Dev. Disord. 2007, 37, 738–747. [Google Scholar] [CrossRef]
- Hagerman, R.; Au, J.; Hagerman, P. FMR1 Premutation and Full Mutation Molecular Mechanisms Related to Autism. J. Neurodev. Disord. 2011, 3, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Maltman, N.; Guilfoyle, J.; Nayar, K.; Martin, G.E.; Winston, M.; Lau, J.C.Y.; Bush, L.; Patel, S.; Lee, M.; Sideris, J.; et al. The Phenotypic Profile Associated with the FMR1 Premutation in Women: An Investigation of Clinical-Behavioral, Social-Cognitive, and Executive Abilities. Front. Psychiatry 2021, 12, 718485. [Google Scholar] [CrossRef]
- Sterling, A.M.; Mailick, M.; Greenberg, J.; Warren, S.F.; Brady, N. Language Dysfluencies in Females with the FMR1 Premutation. Brain Cogn. 2013, 82, 84–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klusek, J.; Thurman, A.J.; Abbeduto, L. Maternal Pragmatic Language Difficulties in the FMR1 Premutation and the Broad Autism Phenotype: Associations with Individual and Family Outcomes. J. Autism Dev. Disord. 2022, 52, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Nayar, K.; McKinney, W.; Hogan, A.L.; Martin, G.E.; La Valle, C.; Sharp, K.; Berry-Kravis, E.; Norton, E.S.; Gordon, P.C.; Losh, M. Language Processing Skills Linked to FMR1 Variation: A Study of Gaze-Language Coordination during Rapid Automatized Naming among Women with the FMR1 Premutation. PLoS ONE 2019, 14, e0219924. [Google Scholar] [CrossRef]
- Klusek, J.; Schmidt, J.; Fairchild, A.J.; Porter, A.; Roberts, J.E. Altered Sensitivity to Social Gaze in the FMR1 Premutation and Pragmatic Language Competence. J. Neurodev. Disord. 2017, 9, 31. [Google Scholar] [CrossRef]
- Winston, M.; Nayar, K.; Hogan, A.L.; Barstein, J.; La Valle, C.; Sharp, K.; Berry-Kravis, E.; Losh, M. Physiological Regulation and Social-Emotional Processing in Female Carriers of the FMR1 Premutation. Physiol. Behav. 2020, 214, 112746. [Google Scholar] [CrossRef]
- Norris, J.E.; Schmitt, L.M.; De Stefano, L.A.; Pedapati, E.V.; Erickson, C.A.; Sweeney, J.A.; Ethridge, L.E. Neuropsychiatric Feature-Based Subgrouping Reveals Neural Sensory Processing Spectrum in Female FMR1 Premutation Carriers: A Pilot Study. Front. Integr. Neurosci. 2023, 17, 898215. [Google Scholar] [CrossRef]
- Schmitt, L.M.; Dominick, K.C.; Liu, R.; Pedapati, E.V.; Ethridge, L.E.; Smith, E.; Sweeney, J.A.; Erickson, C.A. Evidence for Three Subgroups of Female FMR1 Premutation Carriers Defined by Distinct Neuropsychiatric Features: A Pilot Study. Front. Integr. Neurosci. 2021, 15, 797546. [Google Scholar] [CrossRef]
- Garcia-Pino, E.; Gessele, N.; Koch, U. Enhanced Excitatory Connectivity and Disturbed Sound Processing in the Auditory Brainstem of Fragile X Mice. J. Neurosci. 2017, 37, 7403–7419. [Google Scholar] [CrossRef]
- Ruby, K.; Falvey, K.; Kulesza, R.J. Abnormal Neuronal Morphology and Neurochemistry in the Auditory Brainstem of Fmr1 Knockout Rats. Neuroscience 2015, 303, 285–298. [Google Scholar] [CrossRef]
- Lau, J.C.Y.; Patel, S.; Kang, X.; Nayar, K.; Martin, G.E.; Choy, J.; Wong, P.C.; Losh, M. Cross-Linguistic Patterns of Impaired Speech Prosody in Autism: A Machine Learning Study. PLoS ONE 2022, 17, e0269637. [Google Scholar] [CrossRef]
- Tilsen, S.; Arvaniti, A. Speech Rhythm Analysis with Decomposition of the Amplitude Envelope: Characterizing Rhythmic Patterns within and across Languages. J. Acoust. Soc. Am. 2013, 134, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Borghgraef, M.; Fryns, J.P.; Dielkens, A.; Pyck, K.; Van den Berghe, H. Fragile X Syndrome: A Study of the Psychological Profile in 23 Prepubertal Patients. Clin. Genet. 1987, 32, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Brun-Gasca, C.; Artigas-Pallarés, J. Psycholinguistic Aspects of Fragile X Chromosome Syndrome. Rev. Neurol. 2001, 33 (Suppl. S1), S29–S32. [Google Scholar] [CrossRef]
- Hanson, D.M.; Jackson, A.W.; Hagerman, R.J. Speech Disturbances (Cluttering) in Mildly Impaired Males with the Martin-Bell/Fragile X Syndrome. Am. J. Med. Genet. 1986, 23, 195–206. [Google Scholar] [CrossRef]
- Barnes, E.F.; Roberts, J.; Mirrett, P.; Sideris, J.; Misenheimer, J. A Comparison of Oral Structure and Oral-Motor Function in Young Males with Fragile X Syndrome and Down Syndrome. J. Speech Lang. Hear. Res. 2006, 49, 903–917. [Google Scholar] [CrossRef]
- McKinney, W.S.; Bartolotti, J.; Khemani, P.; Wang, J.Y.; Hagerman, R.J.; Mosconi, M.W. Cerebellar-Cortical Function and Connectivity during Sensorimotor Behavior in Aging FMR1 Gene Premutation Carriers. Neuroimage Clin. 2020, 27, 102332. [Google Scholar] [CrossRef]
- Kraan, C.M.; Hocking, D.R.; Georgiou-Karistianis, N.; Metcalfe, S.A.; Archibald, A.D.; Fielding, J.; Trollor, J.; Bradshaw, J.L.; Cohen, J.; Cornish, K.M. Cognitive-Motor Interference during Postural Control Indicates at-Risk Cerebellar Profiles in Females with the FMR1 Premutation. Behav. Brain Res. 2013, 253, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Wang, Z.; McKinney, W.; Khemani, P.; Lui, S.; Christou, E.A.; Mosconi, M.W. Functional Motor Control Deficits in Older FMR1 Premutation Carriers. Exp. Brain Res. 2019, 237, 2269–2278. [Google Scholar] [CrossRef]
- Grigsby, J.; Leehey, M.A.; Jacquemont, S.; Brunberg, J.A.; Hagerman, R.J.; Wilson, R.; Epstein, J.H.; Greco, C.M.; Tassone, F.; Hagerman, P.J. Cognitive Impairment in a 65-Year-Old Male with the Fragile X-Associated Tremor-Ataxia Syndrome (FXTAS). Cogn. Behav. Neurol. 2006, 19, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Hagerman, R. The Fragile X-Associated Tremor Ataxia Syndrome. Results Probl. Cell Differ. 2012, 54, 337–357. [Google Scholar] [CrossRef]
- Grigsby, J.; Brega, A.G.; Seritan, A.L.; Bourgeois, J.A. FXTAS: Neuropsychological/Neuropsychiatric Phenotypes. In The Fragile X-Associated Tremor Ataxia Syndrome (FXTAS); Springer: New York, NY, USA, 2010; pp. 31–53. [Google Scholar]
- Friedman, L.; Lauber, M.; Behroozmand, R.; Fogerty, D.; Kunecki, D.; Berry-Kravis, E.; Klusek, J. Atypical Vocal Quality in Women with the FMR1 Premutation: An Indicator of Impaired Sensorimotor Control. Exp. Brain Res. 2023, 241, 1975–1987. [Google Scholar] [CrossRef]
- Nooteboom, S. The Prosody of Speech: Melody and Rhythm. Handb. Phon. Sci. 1997, 5, 640–673. [Google Scholar]
- Wilson, D.; Wharton, T. Relevance and Prosody. J. Pragmat. 2006, 38, 1559–1579. [Google Scholar] [CrossRef]
- Cole, J.; Hilger, A.; Patel, S. Prosody: Linguistic and Clinical Perspectives. In Clinical Applications of Linguistics to Speech-Language Pathology: A Guide for Clinicians; Gurevich, N., Grindrod, C., Eds.; Routledge: London, UK, 2022. [Google Scholar]
- Fusaroli, R.; Grossman, R.; Bilenberg, N.; Cantio, C.; Jepsen, J.R.M.; Weed, E. Toward a Cumulative Science of Vocal Markers of Autism: A Cross-Linguistic Meta-Analysis-Based Investigation of Acoustic Markers in American and Danish Autistic Children. Autism Res. 2022, 15, 653–664. [Google Scholar] [CrossRef]
- Losh, M.; Klusek, J.; Martin, G.E.; Sideris, J.; Parlier, M.; Piven, J. Defining Genetically Meaningful Language and Personality Traits in Relatives of Individuals with Fragile X Syndrome and Relatives of Individuals with Autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Berman, R.F.; Martin, R.M.; Tassone, F.; Schwartz, P.H.; Chang, A.; Trapp, B.D.; Iwahashi, C.; Brunberg, J.; Grigsby, J. Neuropathology of Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). Brain 2006, 129, 243–255. [Google Scholar] [CrossRef]
- McKinney, W.S.; Wang, Z.; Kelly, S.; Khemani, P.; Lui, S.; White, S.P.; Mosconi, M.W. Precision Sensorimotor Control in Aging FMR1 Gene Premutation Carriers. Front. Integr. Neurosci. 2019, 13, 56. [Google Scholar] [CrossRef]
- Ahissar, E.; Nagarajan, S.; Ahissar, M.; Protopapas, A.; Mahncke, H.; Merzenich, M.M. Speech Comprehension Is Correlated with Temporal Response Patterns Recorded from Auditory Cortex. Proc. Natl. Acad. Sci. USA 2001, 98, 13367–13372. [Google Scholar] [CrossRef]
- Giraud, A.-L.; Poeppel, D. Cortical Oscillations and Speech Processing: Emerging Computational Principles and Operations. Nat. Neurosci. 2012, 15, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, D.; Assaneo, M.F. Speech Rhythms and Their Neural Foundations. Nat. Rev. Neurosci. 2020, 21, 322–334. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The MGluR Theory of Fragile X Mental Retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Li, K.E.; Dimitrijevic, A.; Gordon, K.A.; Pang, E.W.; Greiner, H.M.; Kadis, D.S. Age-Related Increases in Right Hemisphere Support for Prosodic Processing in Children. Sci. Rep. 2023, 13, 15849. [Google Scholar] [CrossRef]
- Leipold, S.; Abrams, D.A.; Karraker, S.; Menon, V. Neural Decoding of Emotional Prosody in Voice-Sensitive Auditory Cortex Predicts Social Communication Abilities in Children. Cereb. Cortex 2023, 33, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Beebe, K.; Wang, Y.; Kulesza, R. Distribution of Fragile X Mental Retardation Protein in the Human Auditory Brainstem. Neuroscience 2014, 273, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; Anderson, S.; White-Schwoch, T. The Frequency-Following Response: A Window into Human Communication. In The Frequency-Following Response; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–15. [Google Scholar]
- Coffey, E.B.J.; Nicol, T.; White-Schwoch, T.; Chandrasekaran, B.; Krizman, J.; Skoe, E.; Zatorre, R.J.; Kraus, N. Evolving Perspectives on the Sources of the Frequency-Following Response. Nat. Commun. 2019, 10, 5036. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L.; Luyster, R.; Gutrie, W. ADOS-2. Manual (Part. I): Modules; Western Psychological Services (WPS): Torrance, CA, USA, 2015; pp. 1–4. [Google Scholar]
- Rutter, M.; Le Couteur, A.; Lord, C. Autism Diagnostic Interview-Revised; Western Psychological Services: Los Angeles, CA, USA, 2003; pp. 29–30. [Google Scholar]
- Wechsler, D. Manual for the Wechsler Abbreviated Intelligence Scale (WASI); The Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Mayer, M. Frog, Where Are You? Dial Books: New York, NY, USA, 1969. [Google Scholar]
- Capps, L.; Losh, M.; Thurber, C. “The Frog Ate the Bug and Made His Mouth Sad”: Narrative Competence in Children with Autism. J. Abnorm. Child. Psychol. 2000, 28, 193–204. [Google Scholar] [CrossRef]
- Diehl, J.J.; Bennetto, L.; Young, E.C. Story Recall and Narrative Coherence of High-Functioning Children with Autism Spectrum Disorders. J. Abnorm. Child. Psychol. 2006, 34, 87–102. [Google Scholar] [CrossRef]
- Losh, M.; Capps, L. Narrative Ability in High-Functioning Children with Autism or Asperger’s Syndrome. J. Autism Dev. Disord. 2003, 33, 239–251. [Google Scholar] [CrossRef]
- Reilly, J.; Losh, M.; Bellugi, U.; Wulfeck, B. “Frog, Where Are You?” Narratives in Children with Specific Language Impairment, Early Focal Brain Injury, and Williams Syndrome. Brain Lang. 2004, 88, 229–247. [Google Scholar] [CrossRef]
- Tager-Flusberg, H.; Sullivan, K. Attributing Mental States to Story Characters: A Comparison of Narratives Produced by Autistic and Mentally Retarded Individuals. Appl. Psycholinguist. 1995, 16, 241–256. [Google Scholar] [CrossRef]
- Boersma, P. Praat, a System for Doing Phonetics by Computer. Glot. Int. 2001, 5, 341–345. [Google Scholar]
- Filipovic-Sadic, S.; Sah, S.; Chen, L.; Krosting, J.; Sekinger, E.; Zhang, W.; Hagerman, P.J.; Stenzel, T.T.; Hadd, A.G.; Latham, G.J.; et al. A Novel FMR1 PCR Method for the Routine Detection of Low Abundance Expanded Alleles and Full Mutations in Fragile X Syndrome. Clin. Chem. 2010, 56, 399–408. [Google Scholar] [CrossRef]
- Chen, L.; Hadd, A.; Sah, S.; Houghton, J.F.; Filipovic-Sadic, S.; Zhang, W.; Hagerman, P.J.; Tassone, F.; Latham, G.J. High-Resolution Methylation Polymerase Chain Reaction for Fragile X Analysis: Evidence for Novel FMR1 Methylation Patterns Undetected in Southern Blot Analyses. Genet. Med. 2011, 13, 528–538. [Google Scholar] [CrossRef]
- LaFauci, G.; Adayev, T.; Kascsak, R.; Kascsak, R.; Nolin, S.; Mehta, P.; Brown, W.T.; Dobkin, C. Fragile X Screening by Quantification of FMRP in Dried Blood Spots by a Luminex Immunoassay. J. Mol. Diagn. 2013, 15, 508–517. [Google Scholar] [CrossRef]
- Mailick, M.R.; Hong, J.; Greenberg, J.; Smith, L.; Sherman, S. Curvilinear Association of CGG Repeats and Age at Menopause in Women with FMR1 Premutation Expansions. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 705–711. [Google Scholar] [CrossRef]
- Klusek, J.; Porter, A.; Abbeduto, L.; Adayev, T.; Tassone, F.; Mailick, M.R.; Glicksman, A.; Tonnsen, B.L.; Roberts, J.E. Curvilinear Association between Language Disfluency and FMR1 CGG Repeat Size across the Normal, Intermediate, and Premutation Range. Front. Genet. 2018, 9, 344. [Google Scholar] [CrossRef]
- Seltzer, M.M.; Barker, E.T.; Greenberg, J.S.; Hong, J.; Coe, C.; Almeida, D. Differential Sensitivity to Life Stress in FMR1 Premutation Carrier Mothers of Children with Fragile X Syndrome. Health Psychol. 2012, 31, 612. [Google Scholar] [CrossRef]
- Krizman, J.; Kraus, N. Analyzing the FFR: A Tutorial for Decoding the Richness of Auditory Function. Hear. Res. 2019, 382, 107779. [Google Scholar] [CrossRef]
- Tichko, P.; Skoe, E. Frequency-Dependent Fine Structure in the Frequency-Following Response: The Byproduct of Multiple Generators. Hear. Res. 2017, 348, 1–15. [Google Scholar] [CrossRef]

| Mean (SD) | AU Parent Group (n = 98) | PM Group (n = 52) | Control Parent Group (n = 53) |
|---|---|---|---|
| Chronological Age | 46.43 (8.72) | 46.40 (10.97) | 44.00 (8.24) |
| Verbal IQ ^ | 110.99 (12.35) | 113.71 (10.50) | 115.48 (9.98) |
| Number of Utterances Elicited | 43.78 (17.44) | 38.87 (20.27) | 39.60 (15.60) |
| Mean (SD) | AU Parent Group (n = 18) | PM Group (n = 17) | Control Parent Group (n = 13) |
|---|---|---|---|
| Chronological Age | 52.14 (10.17) | 52.67 (12.73) | 47.72 (7.07) |
| FSIQ | 115.11 (11.69) | 115.29 (11.53) | 117.31 (10.07) |
| Spearman’s ρ (p) | Quantitative FMRP | Activation Ratio | Number of CGG Repeats |
|---|---|---|---|
| Pitch (F0) range | 0.142 (0.394) | −0.070 (0.668) | 0.175 (0.261) |
| Syllabic Oscillation Variability (var.ω1) | −0.052 (0.755) | −0.102 (0.531) | −0.311 (* 0.043) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, J.C.Y.; Guilfoyle, J.; Crawford, S.; Johnson, G.; Landau, E.; Xing, J.; Kumareswaran, M.; Ethridge, S.; Butler, M.; Goldman, L.; et al. Prosodic Differences in Women with the FMR1 Premutation: Subtle Expression of Autism-Related Phenotypes Through Speech. Int. J. Mol. Sci. 2025, 26, 2481. https://doi.org/10.3390/ijms26062481
Lau JCY, Guilfoyle J, Crawford S, Johnson G, Landau E, Xing J, Kumareswaran M, Ethridge S, Butler M, Goldman L, et al. Prosodic Differences in Women with the FMR1 Premutation: Subtle Expression of Autism-Related Phenotypes Through Speech. International Journal of Molecular Sciences. 2025; 26(6):2481. https://doi.org/10.3390/ijms26062481
Chicago/Turabian StyleLau, Joseph C. Y., Janna Guilfoyle, Stephanie Crawford, Grace Johnson, Emily Landau, Jiayin Xing, Mitra Kumareswaran, Sarah Ethridge, Maureen Butler, Lindsay Goldman, and et al. 2025. "Prosodic Differences in Women with the FMR1 Premutation: Subtle Expression of Autism-Related Phenotypes Through Speech" International Journal of Molecular Sciences 26, no. 6: 2481. https://doi.org/10.3390/ijms26062481
APA StyleLau, J. C. Y., Guilfoyle, J., Crawford, S., Johnson, G., Landau, E., Xing, J., Kumareswaran, M., Ethridge, S., Butler, M., Goldman, L., Martin, G. E., Zhou, L., Krizman, J., Nicol, T., Kraus, N., Berry-Kravis, E., & Losh, M. (2025). Prosodic Differences in Women with the FMR1 Premutation: Subtle Expression of Autism-Related Phenotypes Through Speech. International Journal of Molecular Sciences, 26(6), 2481. https://doi.org/10.3390/ijms26062481





