Cartilage Oligomeric Matrix Protein Promotes Radiation Resistance in Non-Small Cell Lung Cancer In Vitro
Abstract
1. Introduction
2. Results
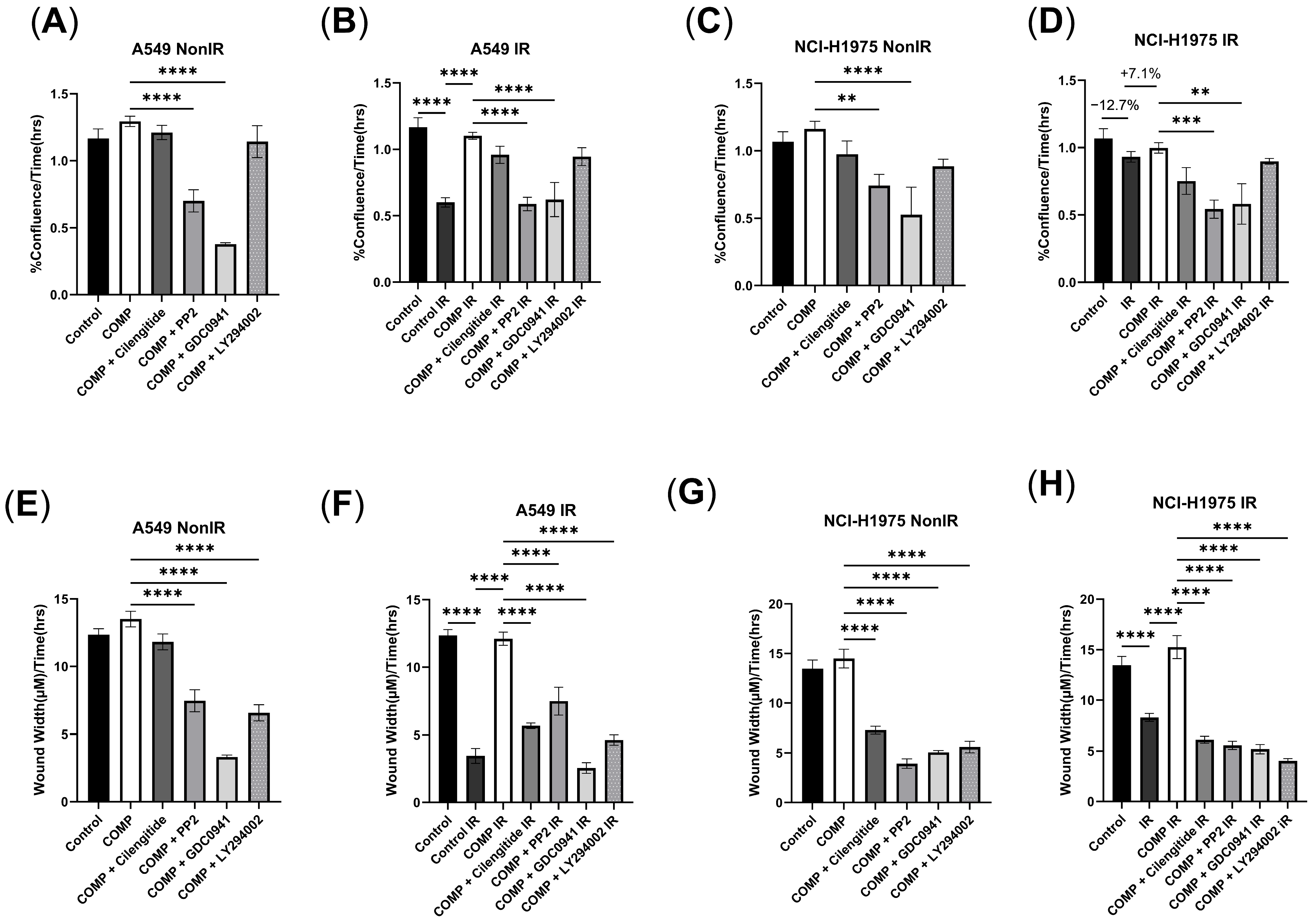
2.1. The Effect of Exogenous COMP on Cell Proliferation and Migration
2.2. Validation of COMP Overexpression
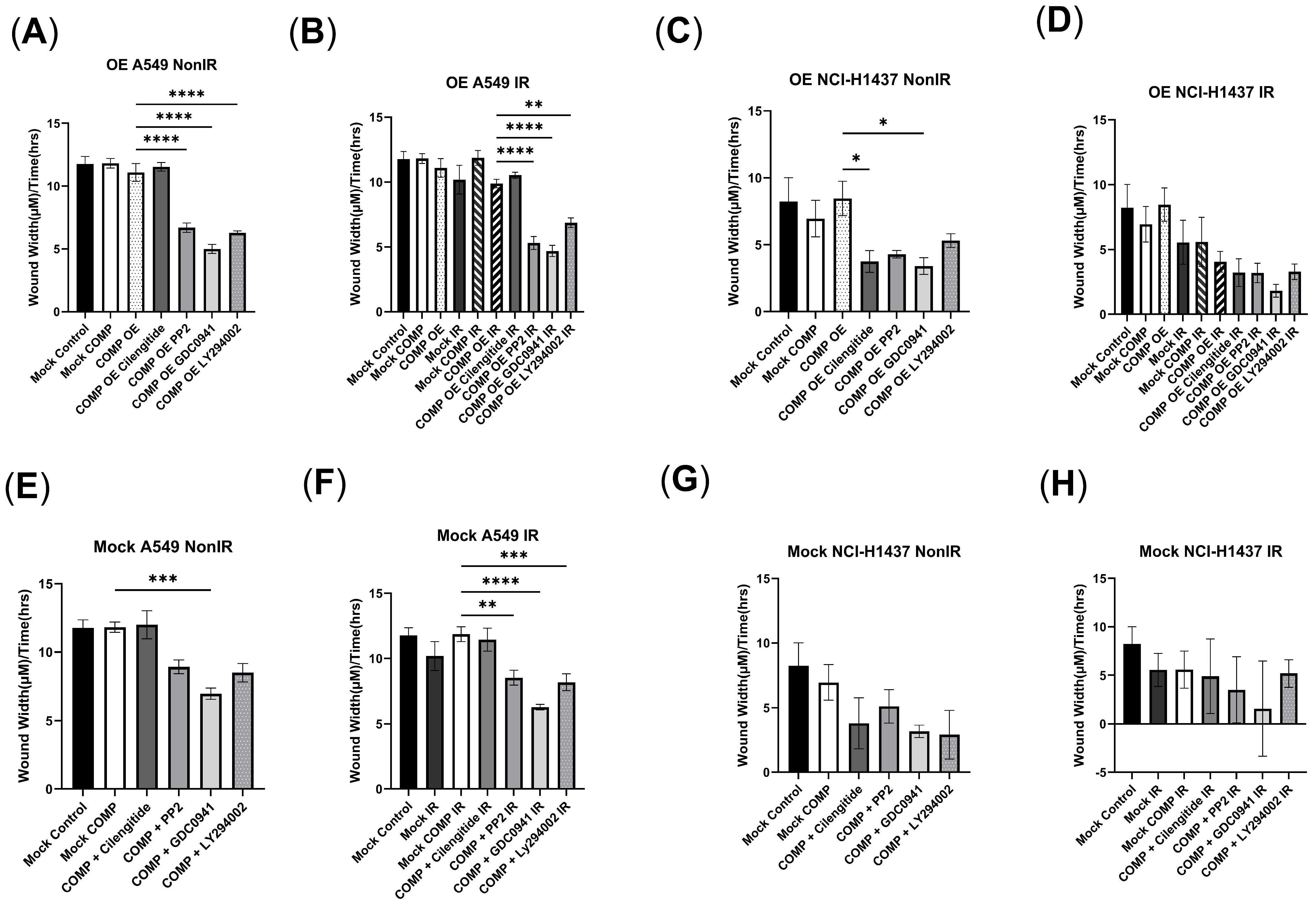
2.3. COMP Overexpression in the A549 Cell Line and the Response to Radiation with/Without COMP Signaling Inhibitors
2.4. COMP Overexpression in the NCI-H1437 Cell Line and the Response to Radiation with/Without COMP Signaling Inhibitors
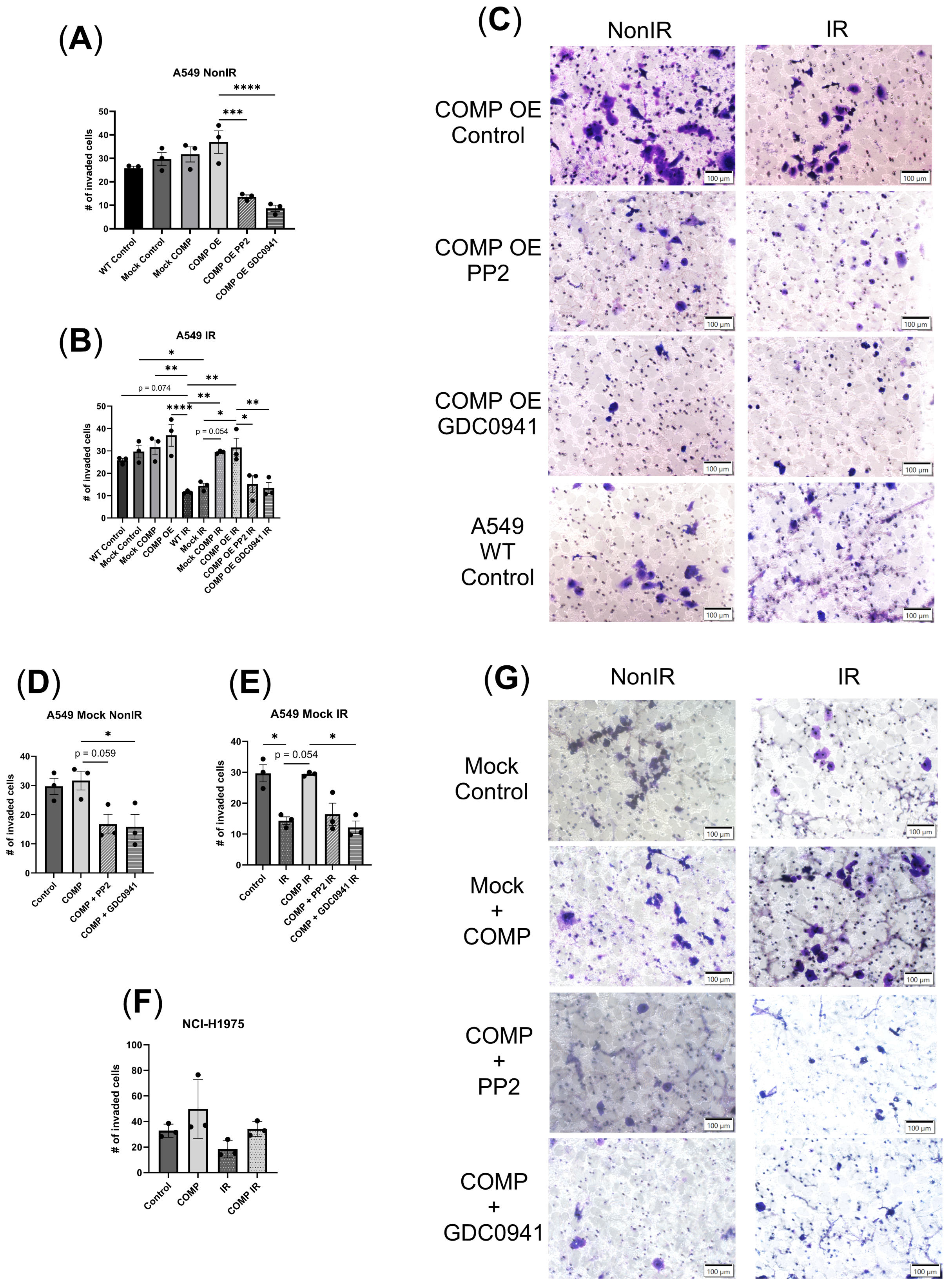
2.5. COMP Overexpression and the Invasion Response to Radiation with or Without COMP Signaling Inhibitors
2.6. Exogenous COMP Treatment or COMP Overexpression Reduce Radiation-Induced Cell Death
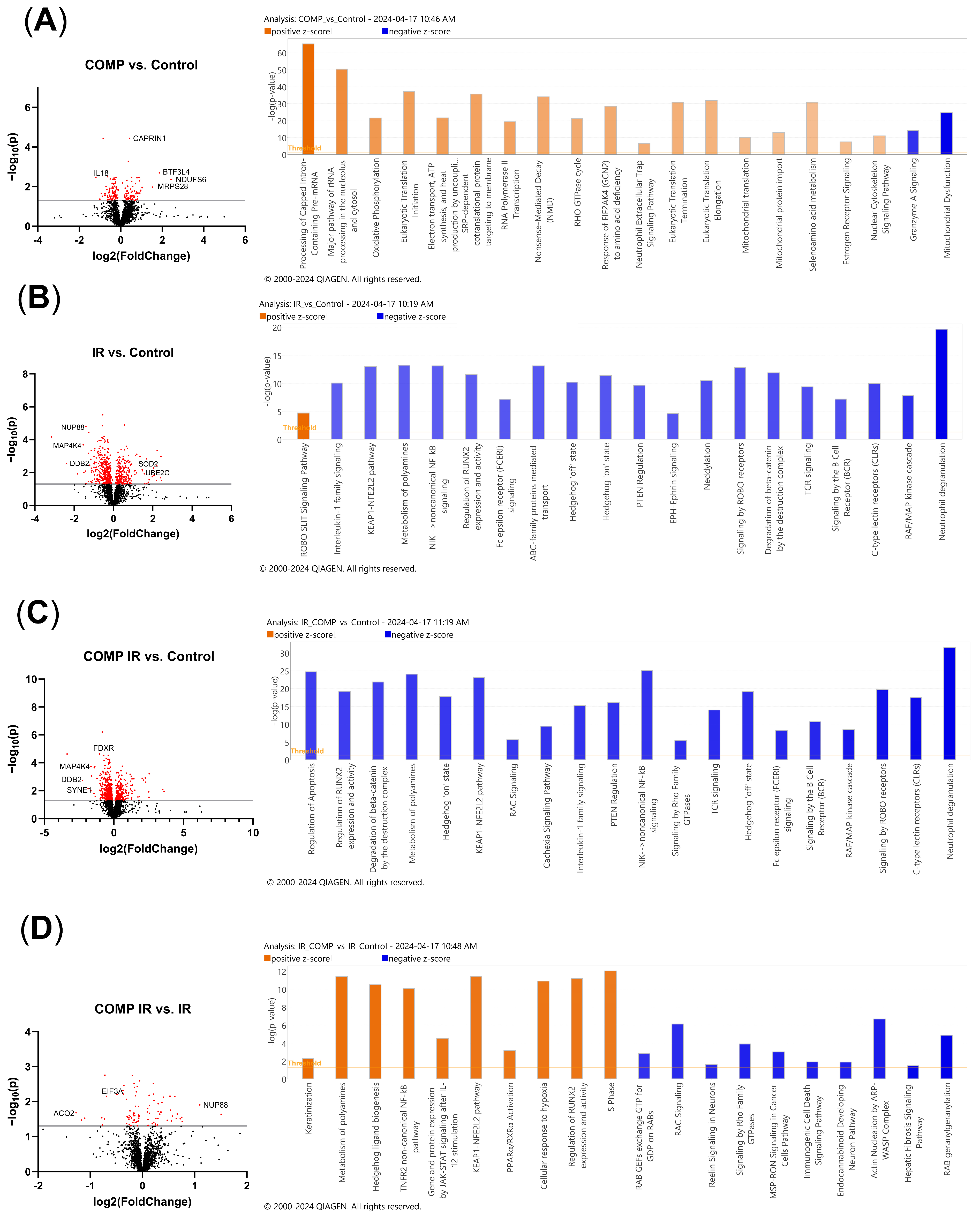
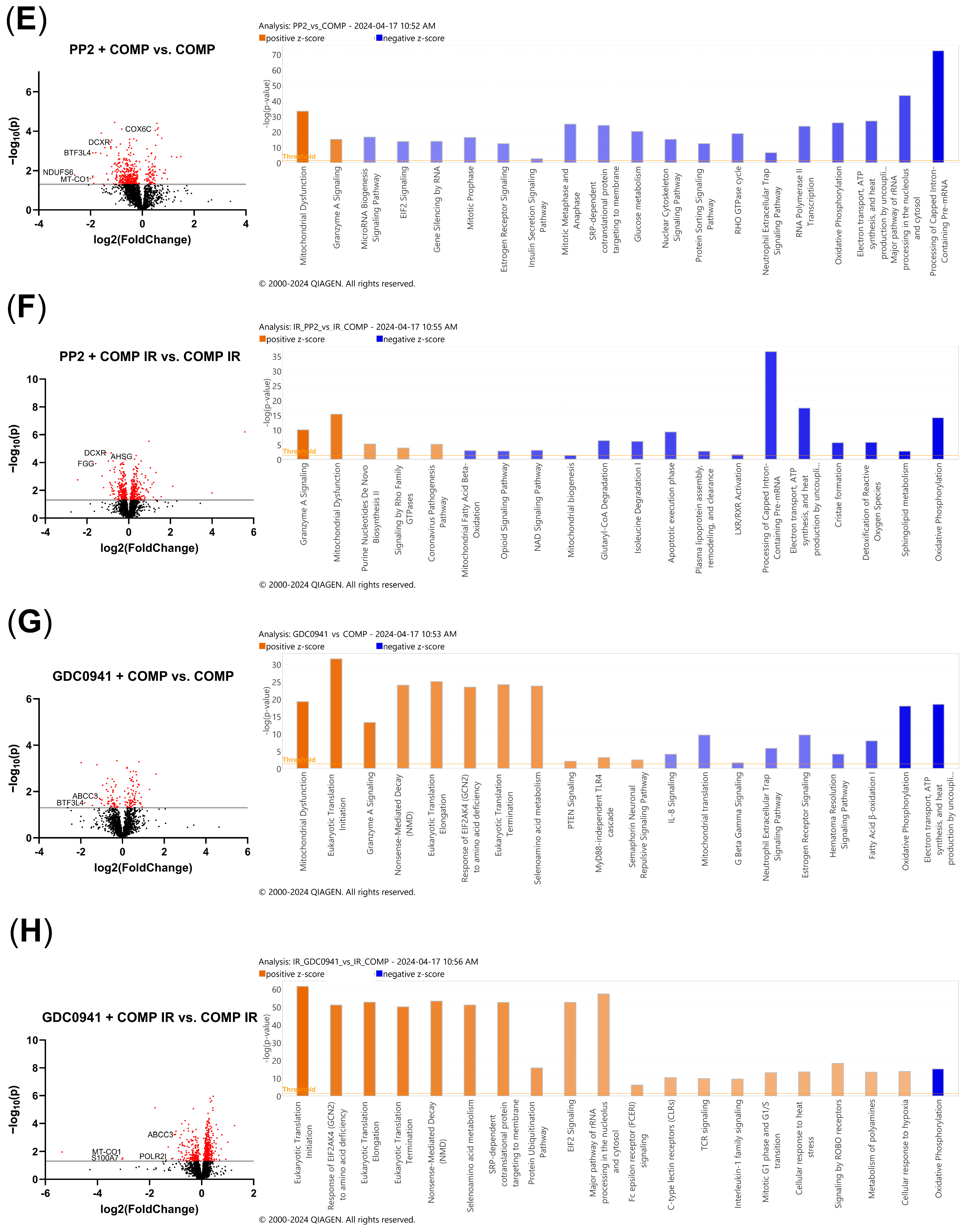
2.7. Comparative Proteomic Analysis of Non-Irradiated and Irradiated A549 Cells Treated with COMP and COMP Inhibitors
2.8. Proteomic Pathway Analysis of Non-Irradiated and Irradiated A549 Cells Treated with COMP and COMP Inhibitors to Characterize Intracellular Responses
3. Discussion
4. Materials and Methods
4.1. TCGA Data
4.2. Cell Culture, Transduction, and RT-qPCR Verification
4.3. Indirect COMP Inhibitor Treatments
4.4. Proliferation Assay
4.5. Migration Assay
4.6. Invasion Assay
4.7. Live/Dead Assay
4.8. Proteomics
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef]
- Schuurbiers, O.C.J.; Kaanders, J.H.A.M.; Van Der Heijden, H.F.M.; Dekhuijzen, R.P.N.; Oyen, W.J.G.; Bussink, J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 761–767. [Google Scholar] [CrossRef]
- van de Stolpe, A. Quantitative Measurement of Functional Activity of the PI3K Signaling Pathway in Cancer. Cancers 2019, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Wang, Y.; Sun, L.; Liu, Z.; Wang, L.; Song, T.; Yao, Y.; Liu, Q.; Tu, K. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis 06 Biological Sciences 0601 Biochemistry and Cell Biology 11 Medical and Health Sciences 1103 Clinical Sciences. J. Exp. Clin. Cancer Res. 2018, 37, 231. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The Thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71, 161. [Google Scholar] [CrossRef]
- Chen, H.; Herndon, M.E.; Lawler, J. The cell biology of thrombospondin-1. Matrix Biol. 2000, 19, 597–614. [Google Scholar] [CrossRef]
- Acharya, C.; Yik, J.H.N.; Kishore, A.; Van Dinh, V.; Di Cesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol. 2014, 37, 102–111. [Google Scholar] [CrossRef]
- Englund, E.; Canesin, G.; Papadakos, K.S.; Vishnu, N.; Persson, E.; Reitsma, B.; Anand, A.; Jacobsson, L.; Helczynski, L.; Mulder, H.; et al. Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget 2017, 8, 98298–98311. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.J.; Holden, P.; Horton, W.A.; Cohn, D.H. Cartilage oligomeric matrix protein promotes cell attachment via two independent mechanisms involving CD47 and αVβ3 integrin. Mol. Cell. Biochem. 2010, 338, 215. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, K.S.; Darlix, A.; Jacot, W.; Blom, A.M. High Levels of Cartilage Oligomeric Matrix Protein in the Serum of Breast Cancer Patients Can Serve as an Independent Prognostic Marker. Front. Oncol. 2019, 9, 1141. [Google Scholar] [CrossRef]
- Papadakos, K.S.; Hagerling, C.; Rydén, L.; Larsson, A.M.; Blom, A.M. High Levels of Expression of Cartilage Oligomeric Matrix Protein in Lymph Node Metastases in Breast Cancer Are Associated with Reduced Survival. Cancers 2021, 13, 5876. [Google Scholar] [CrossRef]
- Rosas, S.; Hughes, R.T.; Farris, M.; Lee, H.; McTyre, E.R.; Plate, J.F.; Shi, L.; Emory, C.L.; Blackstock, A.W.; Kerr, B.A.; et al. Cartilage oligomeric matrix protein in patients with osteoarthritis is independently associated with metastatic disease in prostate cancer. Oncotarget 2019, 10, 4776–4785. [Google Scholar] [CrossRef]
- Englund, E.; Bartoschek, M.; Reitsma, B.; Jacobsson, L.; Escudero-Esparza, A.; Orimo, A.; Leandersson, K.; Hagerling, C.; Aspberg, A.; Storm, P.; et al. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene 2016, 35, 5585–5596. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.S.; Kerr, B.A. Prostate Cancer Stem Cell Markers Drive Progression, Therapeutic Resistance, and Bone Metastasis. Stem Cells Int. 2017, 2017, 8629234. [Google Scholar] [CrossRef]
- Nfonsam, V.N.; Jecius, H.C.; Janda, J.; Omesiete, P.N.; Elquza, E.; Scott, A.J.; Nfonsam, L.E.; Jandova, J. Cartilage oligomeric matrix protein (COMP) promotes cell proliferation in early-onset colon cancer tumorigenesis. Surg. Endosc. 2020, 34, 3992–3998. [Google Scholar] [CrossRef]
- Oliveira-Ferrer, L.; Hauschild, J.; Fiedler, W.; Bokemeyer, C.; Nippgen, J.; Celik, I.; Schuch, G. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J. Exp. Clin. Cancer Res. 2008, 27, 86. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Wang, B.; Zhang, R.; Wu, Z.; Huang, Y.; Zhang, X.; Zhou, J.; Yi, J.; Shen, J.; Li, M.-Y.; et al. The activated CD36-Src axis promotes lung adenocarcinoma cell proliferation and actin remodeling-involved metastasis in high-fat environment. Cell Death Dis. 2023, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, X.; Ren, Y.; Yin, Z.; Zhou, B. Coexisting EGFR and TP53 Mutations in Lung Adenocarcinoma Patients Are Associated With COMP and ITGB8 Upregulation and Poor Prognosis. Front. Mol. Biosci. 2020, 7, 30. [Google Scholar] [CrossRef]
- Lo Russo, G.; Prelaj, A.; Dolezal, J.; Beninato, T.; Agnelli, L.; Triulzi, T.; Fabbri, A.; Lorenzini, D.; Ferrara, R.; Brambilla, M.; et al. PEOPLE (NTC03447678), a phase II trial to test pembrolizumab as first-line treatment in patients with advanced NSCLC with PD-L1 <50%: A multiomics analysis. J. ImmunoTherapy Cancer 2023, 11, e006833. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Bang, W.; Cho, J.H.; Lee, R.H.; Kim, S.-H.; Kim, M.S.; Park, S.-M.; Shin, J.-C.; Chung, H.-J.; Oh, K.B.; et al. Kahweol induces apoptosis by suppressing BTF3 expression through the ERK signaling pathway in non-small cell lung cancer cells. Int. J. Oncol. 2016, 49, 2294–2302. [Google Scholar] [CrossRef]
- Lu, B.; Lu, T.; Shi, J.; Sun, P.; Zhang, X.; Yang, L.; Wang, Z.; Huang, J. Basic Transcription Factor 3 Like 4 Enhances Malignant Phenotypes through Modulating Tumor Cell Function and Immune Microenvironment in Glioma. Am. J. Pathol. 2024, 194, 772–784. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, X.; Liu, R.; Shi, L.; Jin, H.; Yang, D.; Zhang, X.; Shen, Y.; Feng, Y.; Zhang, P.; et al. PRMT1 methylation of WTAP promotes multiple myeloma tumorigenesis by activating oxidative phosphorylation via m6A modification of NDUFS6. Cell Death Dis. 2023, 14, 512. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, W.; Tang, W.; Long, T.; Zhang, J.; Xie, X.; Kuang, Y.; Chen, M.; Su, J.; Chen, X. CD147 Interacts with NDUFS6 in Regulating Mitochondrial Complex I Activity and the Mitochondrial Apoptotic Pathway in Human Malignant Melanoma Cells. Curr. Mol. Med. 2014, 14, 1252–1264. [Google Scholar] [CrossRef]
- Makise, M.; Uchimura, R.; Higashi, K.; Mashiki, Y.; Shiraishi, R.; Shutoku, Y.; Kuniyasu, A. Overexpression of the nucleoporin Nup88 stimulates migration and invasion of HeLa cells. Histochem. Cell Biol. 2021, 156, 409–421. [Google Scholar] [CrossRef]
- Naylor, R.M.; Jeganathan, K.B.; Cao, X.; Deursen, J.M.v. Nuclear pore protein NUP88 activates anaphase-promoting complex to promote aneuploidy. J. Clin. Investig. 2016, 126, 543–559. [Google Scholar] [CrossRef]
- Giroux-Leprieur, E.; Costantini, A.; Ding, V.W.; He, B. Hedgehog Signaling in Lung Cancer: From Oncogenesis to Cancer Treatment Resistance. Int. J. Mol. Sci. 2018, 19, 2835. [Google Scholar] [CrossRef] [PubMed]
- Binkley, M.S.; Jeon, Y.-J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S.; et al. KEAP1/NFE2L2 mutations predict lung cancer radiation resistance that can be targeted by glutaminase inhibition. Cancer Discov. 2020, 10, 1826–1841. [Google Scholar] [CrossRef]
- Cho-Vega, J.H.; Vega, F.; Schwartz, M.R.; Prieto, V.G. Expression of dicarbonyl/l-xylulose reductase (DCXR) in human skin and melanocytic lesions: Morphological studies supporting cell adhesion function of DCXR. J. Cutan. Pathol. 2007, 34, 535–542. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, M.; Tong, Y.; Qiu, L.; Ye, Y.; Zhao, B. DCXR promotes cell proliferation by promoting the activity of aerobic glycolysis in breast cancer. Mol. Med. Rep. 2023, 27, 31. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cosmes, A.; Reyes-Jiménez, E.; Zertuche-Martínez, C.; Hernández-Hernández, C.A.; García-Román, R.; Romero-Díaz, R.I.; Manuel-Martínez, A.E.; Elizarrarás-Rivas, J.; Vásquez-Garzón, V.R. The implications of ABCC3 in cancer drug resistance: Can we use it as a therapeutic target? Am. J. Cancer Res. 2021, 11, 4127–4140. [Google Scholar]
- Ruiz-López, E.; Jovčevska, I.; González-Gómez, R.; Tejero, H.; Al-Shahrour, F.; Muyldermans, S.; Schuhmacher, A.J.; Ruiz-López, E.; Jovčevska, I.; González-Gómez, R.; et al. Nanobodies targeting ABCC3 for immunotargeted applications in glioblastoma. Sci. Rep. 2022, 12, 22581. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Yan, A.; Yang, Y.; Meng, Q.; Sun, L.; Pang, H.; Li, C.; Dong, X.; Cai, L. ABCC3 as a marker for multidrug resistance in non-small cell lung cancer. Sci. Rep. 2013, 3, 3120. [Google Scholar] [CrossRef]
- Eskuri, M.; Kemi, N.; Kauppila, J.H. Monocarboxylate Transporters 1 and 4 and MTCO1 in Gastric Cancer. Cancers 2021, 13, 2142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Mei, Y.; Wang, Z.; He, W. The Effect of Oxidative Phosphorylation on Cancer Drug Resistance. Cancers 2023, 15, 62. [Google Scholar] [CrossRef]
- Uslu, C.; Kapan, E.; Lyakhovich, A. Cancer resistance and metastasis are maintained through oxidative phosphorylation. Cancer Lett. 2024, 587, 216705. [Google Scholar] [CrossRef]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J. Mol. Biol. 2019, 431, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Alemi, F.; Raei Sadigh, A.; Malakoti, F.; Elhaei, Y.; Ghaffari, S.H.; Maleki, M.; Asemi, Z.; Yousefi, B.; Targhazeh, N.; Majidinia, M. Molecular mechanisms involved in DNA repair in human cancers: An overview of PI3k/Akt signaling and PIKKs crosstalk. J. Cell. Physiol. 2022, 237, 313–328. [Google Scholar] [CrossRef]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned So Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Schott, A.F.; Barlow, W.E.; Poznak, C.H.V.; Hayes, D.F.; Moinpour, C.M.; Lew, D.L.; Dy, P.A.; Keller, E.T.; Keller, J.M.; Hortobagyi, G.N. Phase II Studies of Two Different Schedules of Dasatinib in Bone-Metastasis Predominant Metastatic Breast Cancer: SWOG S0622. Breast Cancer Res. Treat. 2016, 159, 87–95. [Google Scholar] [CrossRef]
- Kaye, S.; Aamdal, S.; Jones, R.; Freyer, G.; Pujade-Lauraine, E.; Vries, E.G.E.d.; Barriuso, J.; Sandhu, S.; Tan, D.S.-W.; Hartog, V.; et al. Phase I study of saracatinib (AZD0530) in combination with paclitaxel and/or carboplatin in patients with solid tumours. Br. J. Cancer 2012, 106, 1728–1734. [Google Scholar] [CrossRef]
- Campone, M.; Bondarenko, I.; Brincat, S.; Hotko, Y.; Munster, P.N.; Chmielowska, E.; Fumoleau, P.; Ward, R.; Bardy-Bouxin, N.; Leip, E.; et al. Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann. Oncol. 2012, 23, 610–617. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Xu, Z.; Yang, B.; He, Q.; Luo, P.; Yan, H.; Yang, X. Development and safety of PI3K inhibitors in cancer. Arch. Toxicol. 2023, 97, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef]
- Markham, A.; Markham, A. Copanlisib: First Global Approval. Drugs 2017, 77, 2057–2062. [Google Scholar] [CrossRef]
- Schöffski, P.; Cresta, S.; Mayer, I.A.; Wildiers, H.; Damian, S.; Gendreau, S.; Rooney, I.; Morrissey, K.M.; Spoerke, J.M.; Ng, V.W.; et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018, 20, 109. [Google Scholar] [CrossRef]
- Seol, M.Y.; Choi, S.H.; Yoon, H.I. Combining radiation with PI3K isoform-selective inhibitor administration increases radiosensitivity and suppresses tumor growth in non-small cell lung cancer. J. Radiat. Res. 2022, 63, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.C.; Bradley, J.D.; Robinson, C.G. Optimizing radiation dose and fractionation for the definitive treatment of locally advanced non-small cell lung cancer. J. Thorac. Dis. 2018, 10, S2465. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Wang, Y.; Li, D.; Guo, F.; Xu, L.; Zeng, Z.; Zhong, X.; Qian, K. Rapamycin-induced autophagy sensitizes A549 cells to radiation associated with DNA damage repair inhibition. Thorac. Cancer 2016, 7, 379–386. [Google Scholar] [CrossRef]
- Justus, C.R.; Marie, M.A.; Sanderlin, E.J.; Yang, L.V. Transwell in vitro cell migration and invasion assays. In Cell Viability Assays: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 349–359. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reno, K.E.; Costa-Terryll, A.; Park, S.H.; Hughes, R.T.; Farris, M.K.; Xing, F.; Willey, J.S. Cartilage Oligomeric Matrix Protein Promotes Radiation Resistance in Non-Small Cell Lung Cancer In Vitro. Int. J. Mol. Sci. 2025, 26, 2465. https://doi.org/10.3390/ijms26062465
Reno KE, Costa-Terryll A, Park SH, Hughes RT, Farris MK, Xing F, Willey JS. Cartilage Oligomeric Matrix Protein Promotes Radiation Resistance in Non-Small Cell Lung Cancer In Vitro. International Journal of Molecular Sciences. 2025; 26(6):2465. https://doi.org/10.3390/ijms26062465
Chicago/Turabian StyleReno, Kaitlyn E., Alicia Costa-Terryll, Sun H. Park, Ryan T. Hughes, Michael K. Farris, Fei Xing, and Jeffrey S. Willey. 2025. "Cartilage Oligomeric Matrix Protein Promotes Radiation Resistance in Non-Small Cell Lung Cancer In Vitro" International Journal of Molecular Sciences 26, no. 6: 2465. https://doi.org/10.3390/ijms26062465
APA StyleReno, K. E., Costa-Terryll, A., Park, S. H., Hughes, R. T., Farris, M. K., Xing, F., & Willey, J. S. (2025). Cartilage Oligomeric Matrix Protein Promotes Radiation Resistance in Non-Small Cell Lung Cancer In Vitro. International Journal of Molecular Sciences, 26(6), 2465. https://doi.org/10.3390/ijms26062465




