Imaging of Thromboinflammation by Multispectral 19F MRI
Abstract
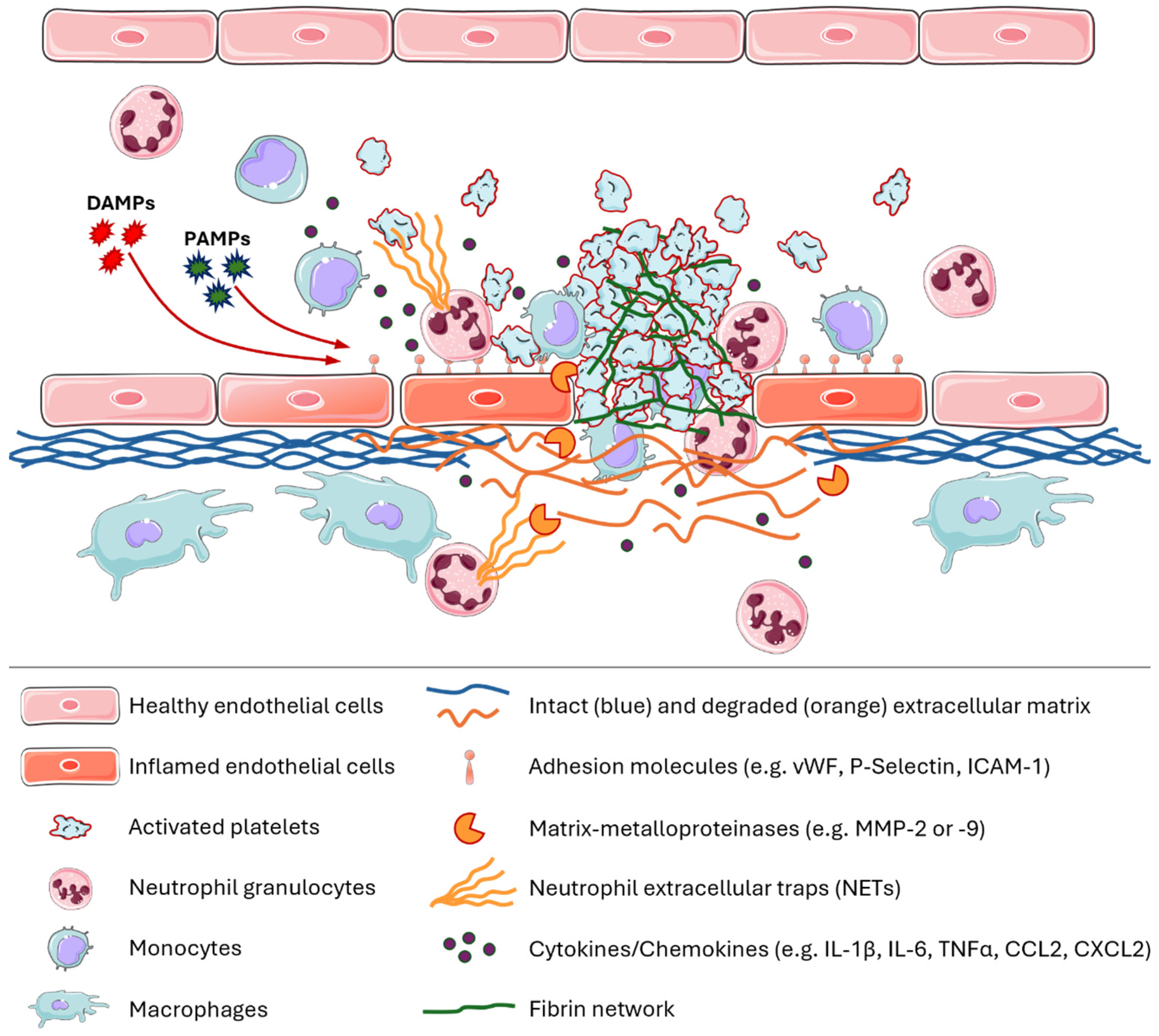
1. A Brief Introduction to Immune Thrombosis and Thromboinflammation
2. Imaging of Inflammation by Combined 1H/19F-MRI
19F MR Imaging of Monocytes and Macrophages
3. Active Targeting of Perfluorocarbon Nanoemulsions
3.1. Imaging of Thrombi by Targeting of FXIIIa, Activated Platelets, and Fibrin
3.2. Visualization of Neutrophil Trafficking
4. Multispectral 19F MRI of Thromboinflammation
4.1. Studies That Utilize Several Fluorinated Tracers for Multispectral 19F MRI
| Brief Summary of the Multispectral 19F Approach | 19F Tracer | Reference | |
|---|---|---|---|
| 1 | In vivo tracking of human hematopoietic stem/progenitor cells. The labelled cells were injected in the right and left thigh of adult C57/BL6 mice. | PFCs: PFOB and PFCE | Partlow et al., 2007 [96] |
| 2 | Multi chemical shift selective (MCSS) RARE sequence for artefact-free 19F MR imaging of two different PFCs simultaneously in vitro and in vivo. Assessment of the washout kinetics from liver and spleen after intravenous injection of a mixture of PFOB- and PFCE-PFCs into male C57BL/6 mice. | PFCs: PFOB and PFCE | Jacoby et al., 2014 [97] |
| 3 | Labelling and simultaneous visualization of two populations of blood derived human plasmacytoid and myeloid dendritic cells. The labelled cells were injected intramuscularly into the leg of a mouse. | PLGA-nanoparticles: PFCE and PFO | Srinivas et al., 2015 [102] |
| 4 | Preparation of silica nanoparticles that encapsulate several perfluorocarbons. The in vivo suitability of this approach was shown by subcutaneous injection of the nanoparticles into the back of mice followed by multicolor 19F imaging. | Silica-nanoparticles: PFCE, TPFBME and PFTBA | Akazawa et al., 2018 [99] |
| 5 | PFCE and PERFECTA were emulsified with a nonionic surfactant (Pluronic F68). These nanodroplets were used to label and track the movement and activity of mononuclear cells in C57BL/6 mice after inhibition of the colony-stimulation factor-1 receptor by multicolor 19F MRI. Nanoemulsions were taken up by mononuclear cells, including neutrophils and monocytes. | Pluronic nanoemulsions: PFCE and PERFECTA | Chirizzi et al., 2019 [100] |
| 6 | Poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with two fluorocarbons (PFCE and PERFECTA) for multicolor 19F MRI. These nanoparticles are composed of fractal blocks that contain PFCE and PERFECTA. Degradation of the PLGA results in changes in T1- and T2-relaxation of the perfluorocarbons which could be used for imaging of nanoparticle degradation in vivo. | PLGA-nanoparticles: PFCE and PERFECTA | Koshkina et al., 2019 [104] |
| 7 | Development of a method that enables the simultaneous visualization of different PFCs with a significant reduction in acquisition time and chemical shift artefacts in connection with complex 19F MR spectra. The 19F signal was also measured in mice (male C57BL/6) after intramuscular and intravascular injection of fluorine probes. | PFCs: PFCE and PFOB | Schoormans et al., 2020 [98] |
| 8 | Utilization of inhalable fluorinated anesthetics (halothane, isoflurane, sevoflurane, and fluroxene) in combination with water-soluble molecular cages (hosts) for 19F-GEST (guest exchange saturation transfer) for the simultaneous detection of micromolar concentrations of two targets. | Fluorinated anesthetics as guest: Halothane, Isoflurane, Sevoflurane, and Fluroxene | Shusterman-Krush et al., 2021 [105] |
| 9 | PFC-based multicolor 19F MRI probes for visualizing FXIIIa, fibrin, and monocytes/macrophages. PFCs with PFCH and PFTBH were PEGylated and functionalized with peptides against FXIIIa (α2APPFCH) and fibrin (fbnPFTBH). Conventional PFCE-PFCs were utilizted to image phagocytic monocytes and macrophages. HypoE mice were subjected to a high-fat diet and a combination of α2APPFCH, fbnPFTBH, and PFCE-PFCs were applied to visualize thromboinflammation in the lungs and the heart. | PFCs: PFCE, PFOB and PFCH | Flögel et al., 2021 [103] |
| 10 | Activatable 19F MRI probes for simultaneous in vivo detection and deep-tissue imaging of reactive oxygen species (ROS; O2•–) and reactive nitrogen species (RNS; ONOO–) in mice with drug-induced acute kidney injury. ROSP-1 and RNSP-2 contain paramagnetic gadolinium which reduces T1- and T2- relaxation of the fluorinated groups. ROS and RNP lead to the release of fluorinated groups from the ROSP-1 or RNSP-2 molecules and therefore the separation from the gadolinium which leads to a strong 19F signal increase. | Activatable 19F MRI molecular probes: ROSP-1 and RNSP-2 | Li et al., 2021 [106] |
| 11 | Inorganic small fluoride nanoparticles (~10 nm; CaF2 and SrF2) were doped with the lanthanide samarium (sm) which significantly decreased the T1 relaxation and therefore increased the 19F signal. Sm:CaF2 nanoparticles were further coated with lipids and phospholipids to enhance the water solubility. Sm:CaF2 that were further modified with lactosyl-PE (LPL-Sm:CaF2) revealed an increased uptake by macrophages and showed an enhanced accumulation in lymph nodes and within an inflammatory hot spot of mice. Multiplex 19F MRI displayed an increased accumulation of LPL-Sm:CaF2 in lymph nodes of inflamed mice after injection of a mixture of LPL-Sm:CaF2 and PL-Sm:SrF2. | CaF2 or SrF2 nanofluorides doped with paramagnetic lanthanoides (Sm3+) and coated with phospholipids | Cohen et al., 2021 [107] |
| 12 | Generation of nanoparticles that encapsulate fluorinated ionic liquids in a nanoparticle shell. The shells were designed to be opened by acidic pH (H+), glutathione (GSH) and matrix metalloproteinases (MMPs) which resulted in the release of the 19F cargo (fluoroborate, difluoroacetate, and trifluoromethanesulfonate) which is associated with turning on the 19F signal. Due to the fact that all 19F tracer have a distinct chemical shift, it was possible to image GSH- and MMP-2- activity as well as acidic pH in subcutaneously implanted tumours in mice by in vivo multiplex 19F MRI. | Encapsulated fluorinated ionic liquids. | Zhu et al., 2022 [108] |
| 13 | Imaging of tumour-associated macrophages (TAM) in mouse models of gliomagenesis. I.v. injection of PFCs revealed that the 19F signal in the tumour was mainly due to 19F labelled TAMs. Application of PFCs with individual 19F spectra bevor and after radiotherapy revealed spatially and temporally distinct TAM niches (microglia and monocytes derived tumour macrophages). | PFCs: PFCE and PFTBH | Croci et al., 2022 [101] |
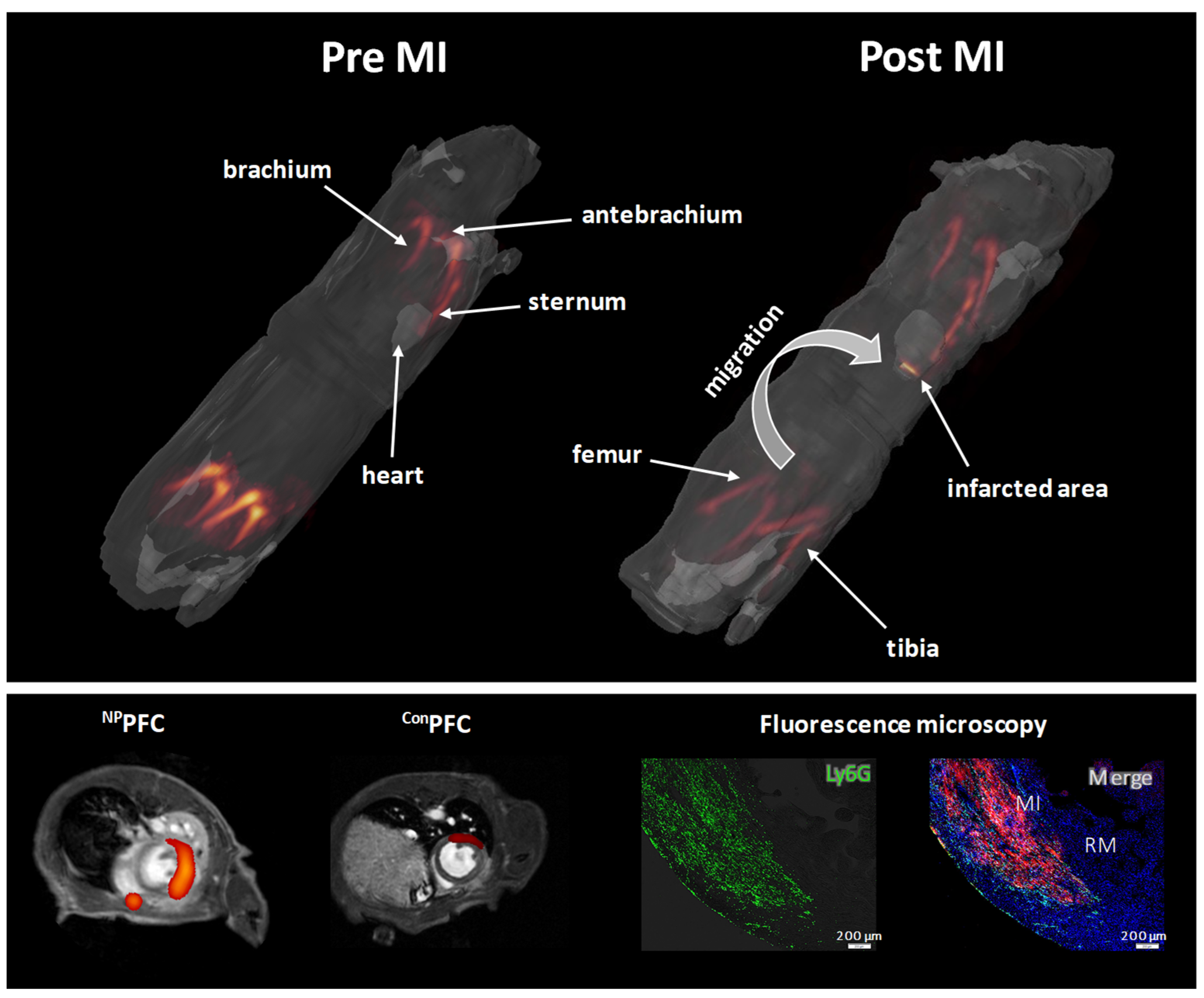
4.2. Simultaneous Visualization of Monocytes/Macrophages and Thrombi
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engelmann, B.; Massberg, S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between Inflammation and Thrombosis in Cardiovascular Pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A Link between Inflammation and Thrombosis in Atherosclerotic Cardiovascular Diseases: Clinical and Therapeutic Implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Gkaliagkousi, E.; Lazaridis, A.; Arelaki, S.; Pateinakis, P.; Ntinopoulou, M.; Mitsios, A.; Antoniadou, C.; Argyriou, C.; Georgiadis, G.S.; et al. Angiotensin II Triggers Release of Neutrophil Extracellular Traps, Linking Thromboinflammation with Essential Hypertension. JCI Insight 2021, 6, e148668. [Google Scholar] [CrossRef] [PubMed]
- Higashikuni, Y.; Liu, W.; Obana, T.; Sata, M. Pathogenic Basis of Thromboinflammation and Endothelial Injury in COVID-19: Current Findings and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 12081. [Google Scholar] [CrossRef]
- Manke, M.-C.; Ahrends, R.; Borst, O. Platelet Lipid Metabolism in Vascular Thrombo-Inflammation. Pharmacol. Ther. 2022, 237, 108258. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Assinger, A. The Concept of Thromboinflammation. Hamostaseologie 2024, 44, 21–30. [Google Scholar] [CrossRef]
- Hottz, E.D.; Martins-Gonçalves, R.; Palhinha, L.; Azevedo-Quintanilha, I.G.; de Campos, M.M.; Sacramento, C.Q.; Temerozo, J.R.; Soares, V.C.; Dias, S.S.G.; Teixeira, L.; et al. Platelet-Monocyte Interaction Amplifies Thromboinflammation through Tissue Factor Signaling in COVID-19. Blood Adv. 2022, 6, 5085–5099. [Google Scholar] [CrossRef]
- Rolling, C.C.; Barrett, T.J.; Berger, J.S. Platelet-Monocyte Aggregates: Molecular Mediators of Thromboinflammation. Front. Cardiovasc. Med. 2023, 10, 960398. [Google Scholar] [CrossRef]
- Stefanski, A.-L.; Nitschke, E.; Dörner, T. Thromboinflammation: Dynamik physiologischer und pathologischer Wechselwirkungen von Entzündung und Koagulation. Aktuelle Rheumatol. 2022, 47, 478–482. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Aggrey, A.A.; Srivastava, K.; Field, D.J.; Morrell, C.N. Platelet Induction of the Acute Phase Response Is Protective in Murine Experimental Cerebral Malaria. J. Immunol. 2013, 190, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Brand, K.; Dickfeld, T.; Pogatsa-Murray, G.; Page, S.; Bogner, C.; Koch, W.; Schömig, A.; Neumann, F. Platelets Induce Alterations of Chemotactic and Adhesive Properties of Endothelial Cells Mediated through an Interleukin-1-Dependent Mechanism. Implications for Atherogenesis. Atherosclerosis 2000, 148, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J. Tissue Factor, Blood Coagulation, and Beyond: An Overview. Int. J. Inflamm. 2011, 2011, 367284. [Google Scholar] [CrossRef]
- Segal, A.W. How Neutrophils Kill Microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ng, S.J.; Lau, H.C.; Naseer, R.; Sandhu, S.; Raynor, W.Y.; Werner, T.J.; Alavi, A. Atherosclerosis Imaging: Positron Emission Tomography. PET Clin. 2023, 18, 71–80. [Google Scholar] [CrossRef]
- Tahara, N.; Mukherjee, J.; de Haas, H.J.; Petrov, A.D.; Tawakol, A.; Haider, N.; Tahara, A.; Constantinescu, C.C.; Zhou, J.; Boersma, H.H.; et al. 2-Deoxy-2-[18F]Fluoro-D-Mannose Positron Emission Tomography Imaging in Atherosclerosis. Nat. Med. 2014, 20, 215–219. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Moon, C.-H.; Muthukrishnan, A.; Furlan, A. 68Ga-DOTATATE PET/MRI for Neuroendocrine Tumors: A Pictorial Review. Clin. Nucl. Med. 2020, 45, e406–e410. [Google Scholar] [CrossRef]
- Li, X.; Bauer, W.; Israel, I.; Kreissl, M.C.; Weirather, J.; Richter, D.; Bauer, E.; Herold, V.; Jakob, P.; Buck, A.; et al. Targeting P-Selectin by Gallium-68-Labeled Fucoidan Positron Emission Tomography for Noninvasive Characterization of Vulnerable Plaques: Correlation with in Vivo 17.6T MRI. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, L.; Ahlman, M.; Lin, F.; Mena, E.; Choyke, P. Advances in PET Imaging of the CXCR4 Receptor: [68Ga]Ga-PentixaFor. Semin. Nucl. Med. 2024, 54, 163–170. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Blasi, F.; Rietz, T.A.; Rotile, N.J.; Day, H.; Caravan, P. Multimodal Molecular Imaging Reveals High Target Uptake and Specificity of 111In- and 68Ga-Labeled Fibrin-Binding Probes for Thrombus Detection in Rats. J. Nucl. Med. 2015, 56, 1587–1592. [Google Scholar] [CrossRef]
- Zhuang, Z.W.; Huang, Y.; Ju, R.; Maxfield, M.W.; Ren, Y.; Wang, X.; Wang, X.; Stacy, M.R.; Hwa, J. Molecular Imaging of Factor XIII Activity for the Early Detection of Mouse Coronary Microvascular Disease. Theranostics 2019, 9, 1474–1489. [Google Scholar] [CrossRef] [PubMed]
- Heidt, T.; Deininger, F.; Peter, K.; Goldschmidt, J.; Pethe, A.; Hagemeyer, C.E.; Neudorfer, I.; Zirlik, A.; Weber, W.A.; Bode, C.; et al. Activated Platelets in Carotid Artery Thrombosis in Mice Can Be Selectively Targeted with a Radiolabeled Single-Chain Antibody. PLoS ONE 2011, 6, e18446. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.R. Radionuclide Imaging of Atherothrombotic Diseases. Curr. Cardiovasc. Imaging Rep. 2019, 12, 17. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kuge, Y. Recent Advances in the Development of PET/SPECT Probes for Atherosclerosis Imaging. Nucl. Med. Mol. Imaging 2016, 50, 284–291. [Google Scholar] [CrossRef]
- Hammad, B.; Evans, N.R.; Rudd, J.H.F.; Tawakol, A. Molecular Imaging of Atherosclerosis with Integrated PET Imaging. J. Nucl. Cardiol. 2017, 24, 938–943. [Google Scholar] [CrossRef]
- Raynor, W.Y.; Park, P.S.U.; Borja, A.J.; Sun, Y.; Werner, T.J.; Ng, S.J.; Lau, H.C.; Høilund-Carlsen, P.F.; Alavi, A.; Revheim, M.-E. PET-Based Imaging with 18F-FDG and 18F-NaF to Assess Inflammation and Microcalcification in Atherosclerosis and Other Vascular and Thrombotic Disorders. Diagnostics 2021, 11, 2234. [Google Scholar] [CrossRef]
- Stoll, G.; Bendszus, M. New Approaches to Neuroimaging of Central Nervous System Inflammation. Curr. Opin. Neurol. 2010, 23, 282–286. [Google Scholar] [CrossRef]
- Ugga, L.; Romeo, V.; Tedeschi, E.; Brunetti, A.; Quarantelli, M. Superparamagnetic Iron Oxide Nanocolloids in MRI Studies of Neuroinflammation. J. Neurosci. Methods 2018, 310, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Swift, A.J.; Assadi, H.; Chowdhary, A.; Swoboda, P.; Sammut, E.; Dastidar, A.; Cabrero, J.B.; Del Val, J.R.; Nair, S.; et al. Myocardial Inflammation and Energetics by Cardiac MRI: A Review of Emerging Techniques. BMC Med. Imaging 2021, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Cannet, C.; Babin, A.L.; Blé, F.-X.; Zurbruegg, S.; Kneuer, R.; Dousset, V. In Vivo Visualization of Macrophage Infiltration and Activity in Inflammation Using Magnetic Resonance Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 272–298. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Idée, J.-M. A Comprehensive Literatures Update of Clinical Researches of Superparamagnetic Resonance Iron Oxide Nanoparticles for Magnetic Resonance Imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; Von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Masthoff, M.; Freppon, F.N.; Zondler, L.; Wilken, E.; Wachsmuth, L.; Niemann, S.; Schwarz, C.; Fredrich, I.; Havlas, A.; Block, H.; et al. Resolving Immune Cells with Patrolling Behaviour by Magnetic Resonance Time-Lapse Single Cell Tracking. eBioMedicine 2021, 73, 103670. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N.; Bottomley, P.A.; Hinshaw, W.S. 19F Magnetic Resonance Imaging. J. Magn. Reson. 1977, 28, 133–136. [Google Scholar] [CrossRef]
- Mattrey, R.F.; Scheible, F.W.; Gosink, B.B.; Leopold, G.R.; Long, D.M.; Higgins, C.B. Perfluoroctylbromide: A Liver/Spleen-Specific and Tumor-Imaging Ultrasound Contrast Material. Radiology 1982, 145, 759–762. [Google Scholar] [CrossRef]
- Mattrey, R.F.; Long, D.M.; Multer, F.; Mitten, R.; Higgins, C.B. Perfluoroctylbromide: A Reticuloendothelial-Specific and Tumor-Imaging Agent for Computed Tomography. Radiology 1982, 145, 755–758. [Google Scholar] [CrossRef]
- Mattrey, R.F.; Leopold, G.R.; vanSonnenberg, E.; Gosink, B.B.; Scheible, F.W.; Long, D.M. Perfluorochemicals as Liver- and Spleen-Seeking Ultrasound Contrast Agents. J. Ultrasound Med. 1983, 2, 173–176. [Google Scholar] [CrossRef]
- Longmaid, H.E.; Adams, D.F.; Neirinckx, R.D.; Harrison, C.G.; Brunner, P.; Seltzer, S.E.; Davis, M.A.; Neuringer, L.; Geyer, R.P. In Vivo 19F NMR Imaging of Liver, Tumor, and Abscess in Rats. Preliminary Results. Investig. Radiol. 1985, 20, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, E.T.; Flores, R.; Xu, H.; Morel, P.A. In Vivo Imaging Platform for Tracking Immunotherapeutic Cells. Nat. Biotechnol. 2005, 23, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Flögel, U.; Ding, Z.; Hardung, H.; Jander, S.; Reichmann, G.; Jacoby, C.; Schubert, R.; Schrader, J. In Vivo Monitoring of Inflammation After Cardiac and Cerebral Ischemia by Fluorine Magnetic Resonance Imaging. Circulation 2008, 118, 140–148. [Google Scholar] [CrossRef]
- Ebner, B.; Behm, P.; Jacoby, C.; Burghoff, S.; French, B.A.; Schrader, J.; Flögel, U. Early Assessment of Pulmonary Inflammation by 19F MRI In Vivo. Circ. Cardiovasc. Imaging 2010, 3, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.E.; Smailagic, A.; Önnervik, P.-O.; Hockings, P.D. 1H and Hyperpolarized 3He MR Imaging of Mouse with LPS-Induced Inflammation. J. Magn. Reson. Imaging 2009, 29, 977–981. [Google Scholar] [CrossRef]
- Quintana, H.K.; Cannet, C.; Schaeublin, E.; Zurbruegg, S.; Sugar, R.; Mazzoni, L.; Page, C.P.; Fozard, J.R.; Beckmann, N. Identification with MRI of the Pleura as a Major Site of the Acute Inflammatory Effects Induced by Ovalbumin and Endotoxin Challenge in the Airways of the Rat. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 291, L651–L657. [Google Scholar] [CrossRef][Green Version]
- van Heeswijk, R.B.; Pellegrin, M.; Flögel, U.; Gonzales, C.; Aubert, J.-F.; Mazzolai, L.; Schwitter, J.; Stuber, M. Fluorine MR Imaging of Inflammation in Atherosclerotic Plaque in Vivo. Radiology 2015, 275, 421–429. [Google Scholar] [CrossRef]
- Kadayakkara, D.K.; Ranganathan, S.; Young, W.-B.; Ahrens, E.T. Assaying Macrophage Activity in a Murine Model of Inflammatory Bowel Disease Using Fluorine-19 MRI. Lab. Investig. 2012, 92, 636–645. [Google Scholar] [CrossRef]
- Rothe, M.; Jahn, A.; Weiss, K.; Hwang, J.-H.; Szendroedi, J.; Kelm, M.; Schrader, J.; Roden, M.; Flögel, U.; Bönner, F. In Vivo 19F MR Inflammation Imaging after Myocardial Infarction in a Large Animal Model at 3 T. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 5–13. [Google Scholar] [CrossRef]
- Jacoby, C.; Borg, N.; Heusch, P.; Sauter, M.; Bönner, F.; Kandolf, R.; Klingel, K.; Schrader, J.; Flögel, U. Visualization of Immune Cell Infiltration in Experimental Viral Myocarditis by 19F MRI in Vivo. Magn. Reson. Mater. Phys. Biol. Med. 2014, 27, 101–106. [Google Scholar] [CrossRef]
- Temme, S.; Jacoby, C.; Ding, Z.; Bönner, F.; Borg, N.; Schrader, J.; Flögel, U. Technical Advance: Monitoring the Trafficking of Neutrophil Granulocytes and Monocytes during the Course of Tissue Inflammation by Noninvasive 19F MRI. J. Leukoc. Biol. 2014, 95, 689–697. [Google Scholar] [CrossRef]
- Ding, Z.; Temme, S.; Quast, C.; Friebe, D.; Jacoby, C.; Zanger, K.; Bidmon, H.-J.; Grapentin, C.; Schubert, R.; Flögel, U.; et al. Epicardium-Derived Cells Formed After Myocardial Injury Display Phagocytic Activity Permitting In Vivo Labeling and Tracking. Stem Cells Transl. Med. 2016, 5, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. (Ed.) Chapter 3—The Reactions of Bioconjugation. In Bioconjugate Techniques, 3rd ed.; Academic Press: Boston, MA, USA, 2013; pp. 229–258. ISBN 978-0-12-382239-0. [Google Scholar]
- Lesch, H.P.; Kaikkonen, M.U.; Pikkarainen, J.T.; Ylä-Herttuala, S. Avidin-Biotin Technology in Targeted Therapy. Expert Opin. Drug Deliv. 2010, 7, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Al-Seragi, M.; Chen, Y.; Duong van Hoa, F. Advances in Nanobody Multimerization and Multispecificity: From in Vivo Assembly to in Vitro Production. Biochem. Soc. Trans. 2025, 53, BST20241419. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.-U.; Langer, K. Effect of Nanoparticle Size and PEGylation on the Protein Corona of PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to PEGylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Koshkina, O.; Rheinberger, T.; Flocke, V.; Windfelder, A.; Bouvain, P.; Hamelmann, N.M.; Paulusse, J.M.J.; Gojzewski, H.; Flögel, U.; Wurm, F.R. Biodegradable Polyphosphoester Micelles Act as Both Background-Free 31P Magnetic Resonance Imaging Agents and Drug Nanocarriers. Nat. Commun. 2023, 14, 4351. [Google Scholar] [CrossRef]
- Gheibi Hayat, S.M.; Bianconi, V.; Pirro, M.; Sahebkar, A. Stealth Functionalization of Biomaterials and Nanoparticles by CD47 Mimicry. Int. J. Pharm. 2019, 569, 118628. [Google Scholar] [CrossRef]
- Vandchali, N.R.; Moadab, F.; Taghizadeh, E.; Tajbakhsh, A.; Gheibihayat, S.M. CD47 Functionalization of Nanoparticles as a Poly(Ethylene Glycol) Alternative: A Novel Approach to Improve Drug Delivery. Curr. Drug Targets 2021, 22, 1750–1759. [Google Scholar] [CrossRef]
- Hu, G.; Lijowski, M.; Zhang, H.; Partlow, K.C.; Caruthers, S.D.; Kiefer, G.; Gulyas, G.; Athey, P.; Scott, M.J.; Wickline, S.A.; et al. Imaging of Vx-2 Rabbit Tumors with Alpha(Nu)Beta3-Integrin-Targeted 111In Nanoparticles. Int. J. Cancer 2007, 120, 1951–1957. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kang, S.-T.; Lee, Y.-H.; Luo, Y.-L.; Huang, Y.-F.; Yeh, C.-K. Aptamer-Conjugated and Drug-Loaded Acoustic Droplets for Ultrasound Theranosis. Biomaterials 2012, 33, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Akers, W.J.; Zhang, Z.; Berezin, M.; Ye, Y.; Agee, A.; Guo, K.; Fuhrhop, R.W.; Wickline, S.A.; Lanza, G.M.; Achilefu, S. Targeting of Alpha(Nu)Beta(3)-Integrins Expressed on Tumor Tissue and Neovasculature Using Fluorescent Small Molecules and Nanoparticles. Nanomedicine 2010, 5, 715–726. [Google Scholar] [CrossRef]
- Winter, P.M.; Caruthers, S.D.; Yu, X.; Song, S.-K.; Chen, J.; Miller, B.; Bulte, J.W.M.; Robertson, J.D.; Gaffney, P.J.; Wickline, S.A.; et al. Improved Molecular Imaging Contrast Agent for Detection of Human Thrombus. Magn. Reson. Med. 2003, 50, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Flacke, S.; Fischer, S.; Scott, M.J.; Fuhrhop, R.J.; Allen, J.S.; McLean, M.; Winter, P.; Sicard, G.A.; Gaffney, P.J.; Wickline, S.A.; et al. Novel MRI Contrast Agent for Molecular Imaging of Fibrin: Implications for Detecting Vulnerable Plaques. Circulation 2001, 104, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.M.; Yu, X.; Winter, P.M.; Abendschein, D.R.; Karukstis, K.K.; Scott, M.J.; Chinen, L.K.; Fuhrhop, R.W.; Scherrer, D.E.; Wickline, S.A. Targeted Antiproliferative Drug Delivery to Vascular Smooth Muscle Cells with a Magnetic Resonance Imaging Nanoparticle Contrast Agent: Implications for Rational Therapy of Restenosis. Circulation 2002, 106, 2842–2847. [Google Scholar] [CrossRef]
- Temme, S.; Grapentin, C.; Quast, C.; Jacoby, C.; Grandoch, M.; Ding, Z.; Owenier, C.; Mayenfels, F.; Fischer, J.W.; Schubert, R.; et al. Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging with Targeted Perfluorocarbon Nanoemulsions. Circulation 2015, 131, 1405–1414. [Google Scholar] [CrossRef]
- Krämer, W.; Grapentin, C.; Bouvain, P.; Temme, S.; Flögel, U.; Schubert, R. Rational Manufacturing of Functionalized, Long-Term Stable Perfluorocarbon-Nanoemulsions for Site-Specific 19F Magnetic Resonance Imaging. Eur. J. Pharm. Biopharm. 2019, 142, 114–122. [Google Scholar] [CrossRef]
- Wang, X.; Temme, S.; Grapentin, C.; Palasubramaniam, J.; Walsh, A.; Krämer, W.; Kleimann, P.; Havlas, A.; Schubert, R.; Schrader, J.; et al. Fluorine-19 Magnetic Resonance Imaging of Activated Platelets. J. Am. Heart Assoc. 2020, 9, e016971. [Google Scholar] [CrossRef]
- Flögel, U.; Burghoff, S.; van Lent, P.L.E.M.; Temme, S.; Galbarz, L.; Ding, Z.; El-Tayeb, A.; Huels, S.; Bönner, F.; Borg, N.; et al. Selective Activation of Adenosine A2A Receptors on Immune Cells by a CD73-Dependent Prodrug Suppresses Joint Inflammation in Experimental Rheumatoid Arthritis. Sci. Transl. Med. 2012, 4, 146ra108. [Google Scholar] [CrossRef]
- Temme, S.; Baran, P.; Bouvain, P.; Grapentin, C.; Krämer, W.; Knebel, B.; Al-Hasani, H.; Moll, J.M.; Floss, D.; Schrader, J.; et al. Synthetic Cargo Internalization Receptor System for Nanoparticle Tracking of Individual Cell Populations by Fluorine Magnetic Resonance Imaging. ACS Nano 2018, 12, 11178–11192. [Google Scholar] [CrossRef] [PubMed]
- Straub, T.; Nave, J.; Bouvain, P.; Akbarzadeh, M.; Dasa, S.S.K.; Kistner, J.; Ding, Z.; Marzoq, A.; Stepanow, S.; Becker, K.; et al. MRI-Based Molecular Imaging of Epicardium-Derived Stromal Cells (EpiSC) by Peptide-Mediated Active Targeting. Sci. Rep. 2020, 10, 21669. [Google Scholar] [CrossRef]
- Martínez, A.A.E.; Bergmann, A.K.; Tellkamp, F.; Schott-Verdugo, S.; Bouvain, P.; Steinhausen, J.; Bahr, J.; Kmietczyk, V.; Flögel, U.; Distler, J.H.W.; et al. CD63 as Novel Target for Nanoemulsion-Based 19F MRI Imaging and Drug Delivery to Activated Cardiac Fibroblasts. Theranostics 2024, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bouvain, P.; Ding, Z.; Kadir, S.; Kleimann, P.; Kluge, N.; Tiren, Z.-B.; Steckel, B.; Flocke, V.; Zalfen, R.; Petzsch, P.; et al. Non-Invasive Mapping of Systemic Neutrophil Dynamics upon Cardiovascular Injury. Nat. Cardiovasc. Res. 2023, 2, 126–143. [Google Scholar] [CrossRef]
- Robinson, B.R.; Houng, A.K.; Reed, G.L. Catalytic Life of Activated Factor XIII in Thrombi. Implications for Fibrinolytic Resistance and Thrombus Aging. Circulation 2000, 102, 1151–1157. [Google Scholar] [CrossRef]
- Tung, C.-H.; Ho, N.-H.; Zeng, Q.; Tang, Y.; Jaffer, F.A.; Reed, G.L.; Weissleder, R. Novel Factor XIII Probes for Blood Coagulation Imaging. Chembiochem 2003, 4, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Miserus, R.-J.J.H.M.; Herías, M.V.; Prinzen, L.; Lobbes, M.B.I.; Van Suylen, R.-J.; Dirksen, A.; Hackeng, T.M.; Heemskerk, J.W.M.; van Engelshoven, J.M.A.; Daemen, M.J.A.P.; et al. Molecular MRI of Early Thrombus Formation Using a Bimodal Alpha2-Antiplasmin-Based Contrast Agent. JACC Cardiovasc. Imaging 2009, 2, 987–996. [Google Scholar] [CrossRef]
- Gantert, M.; Lewrick, F.; Adrian, J.E.; Rössler, J.; Steenpass, T.; Schubert, R.; Peschka-Süss, R. Receptor-Specific Targeting with Liposomes in Vitro Based on Sterol-PEG(1300) Anchors. Pharm. Res. 2009, 26, 529–538. [Google Scholar] [CrossRef]
- Schwarz, M.; Röttgen, P.; Takada, Y.; Le Gall, F.; Knackmuss, S.; Bassler, N.; Büttner, C.; Little, M.; Bode, C.; Peter, K. Single-Chain Antibodies for the Conformation-Specific Blockade of Activated Platelet Integrin alphaIIbbeta3 Designed by Subtractive Selection from Naive Human Phage Libraries. FASEB J. 2004, 18, 1704–1706. [Google Scholar] [CrossRef]
- Schwarz, M.; Meade, G.; Stoll, P.; Ylanne, J.; Bassler, N.; Chen, Y.C.; Hagemeyer, C.E.; Ahrens, I.; Moran, N.; Kenny, D.; et al. Conformation-Specific Blockade of the Integrin GPIIb/IIIa: A Novel Antiplatelet Strategy That Selectively Targets Activated Platelets. Circ. Res. 2006, 99, 25–33. [Google Scholar] [CrossRef]
- Wang, X.; Peter, K. Molecular Imaging of Atherothrombotic Diseases. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hagemeyer, C.E.; Hohmann, J.D.; Leitner, E.; Armstrong, P.C.; Jia, F.; Olschewski, M.; Needles, A.; Peter, K.; Ahrens, I. Novel Single-Chain Antibody-Targeted Microbubbles for Molecular Ultrasound Imaging of Thrombosis. Circulation 2012, 125, 3117–3126. [Google Scholar] [CrossRef]
- Spuentrup, E.; Fausten, B.; Kinzel, S.; Wiethoff, A.J.; Botnar, R.M.; Graham, P.B.; Haller, S.; Katoh, M.; Parsons, E.C.; Manning, W.J.; et al. Molecular Magnetic Resonance Imaging of Atrial Clots in a Swine Model. Circulation 2005, 112, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Spuentrup, E.; Katoh, M.; Wiethoff, A.J.; Parsons, E.C.; Botnar, R.M.; Mahnken, A.H.; Günther, R.W.; Buecker, A. Molecular Magnetic Resonance Imaging of Pulmonary Emboli with a Fibrin-Specific Contrast Agent. Am. J. Respir. Crit. Care Med. 2005, 172, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Sirol, M.; Aguinaldo, J.G.S.; Graham, P.B.; Weisskoff, R.; Lauffer, R.; Mizsei, G.; Chereshnev, I.; Fallon, J.T.; Reis, E.; Fuster, V.; et al. Fibrin-Targeted Contrast Agent for Improvement of in Vivo Acute Thrombus Detection with Magnetic Resonance Imaging. Atherosclerosis 2005, 182, 79–85. [Google Scholar] [CrossRef]
- Vymazal, J.; Spuentrup, E.; Cardenas-Molina, G.; Wiethoff, A.J.; Hartmann, M.G.; Caravan, P.; Parsons, E.C. Thrombus Imaging with Fibrin-Specific Gadolinium-Based MR Contrast Agent EP-2104R: Results of a Phase II Clinical Study of Feasibility. Investig. Radiol. 2009, 44, 697–704. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Basu, S.; Hodgson, G.; Katz, M.; Dunn, A.R. Evaluation of Role of G-CSF in the Production, Survival, and Release of Neutrophils from Bone Marrow into Circulation. Blood 2002, 100, 854–861. [Google Scholar] [CrossRef]
- Ulich, T.R.; del Castillo, J.; Souza, L. Kinetics and Mechanisms of Recombinant Human Granulocyte-Colony Stimulating Factor-Induced Neutrophilia. Am. J. Pathol. 1988, 133, 630–638. [Google Scholar]
- Mazzucchelli, L.; Burritt, J.B.; Jesaitis, A.J.; Nusrat, A.; Liang, T.W.; Gewirtz, A.T.; Schnell, F.J.; Parkos, C.A. Cell-Specific Peptide Binding by Human Neutrophils. Blood 1999, 93, 1738–1748. [Google Scholar] [CrossRef]
- Goldschmeding, R.; van Dalen, C.M.; Faber, N.; Calafat, J.; Huizinga, T.W.; van der Schoot, C.E.; Clement, L.T.; von dem Borne, A.E. Further Characterization of the NB 1 Antigen as a Variably Expressed 56–62 kD GPI-Linked Glycoprotein of Plasma Membranes and Specific Granules of Neutrophils. Br. J. Haematol. 1992, 81, 336–345. [Google Scholar] [CrossRef]
- Miettinen, H.M.; Gripentrog, J.M.; Lord, C.I.; Nagy, J.O. CD177-Mediated Nanoparticle Targeting of Human and Mouse Neutrophils. PLoS ONE 2018, 13, e0200444. [Google Scholar] [CrossRef] [PubMed]
- Grapentin, C.; Mayenfels, F.; Barnert, S.; Süss, R.; Schubert, R.; Temme, S.; Jacoby, C.; Schrader, J.; Flögel, U. Optimization of Perfluorocarbon Nanoemulsions for Molecular Imaging by 19F MRI. In Nanomedicine; Seifalin, A., de Mel, A., Kalaskar, D.M., Eds.; One Central Press: Manchester, UK, 2014; pp. 268–286. [Google Scholar]
- Bouvain, P.; Flocke, V.; Krämer, W.; Schubert, R.; Schrader, J.; Flögel, U.; Temme, S. Dissociation of 19F and Fluorescence Signal upon Cellular Uptake of Dual-Contrast Perfluorocarbon Nanoemulsions. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 133–145. [Google Scholar] [CrossRef]
- Partlow, K.C.; Chen, J.; Brant, J.A.; Neubauer, A.M.; Meyerrose, T.E.; Creer, M.H.; Nolta, J.A.; Caruthers, S.D.; Lanza, G.M.; Wickline, S.A. 19F Magnetic Resonance Imaging for Stem/Progenitor Cell Tracking with Multiple Unique Perfluorocarbon Nanobeacons. FASEB J. 2007, 21, 1647–1654. [Google Scholar] [CrossRef]
- Jacoby, C.; Oerther, T.; Temme, S.; Schrader, J.; Flögel, U. Simultaneous 19F MR Imaging at Different Resonance Frequencies Using Multi Chemical Shift Selective RARE. In Proceedings of the ISMRM, Milan, Italy, 10–16 May 2014. Abstract #2927. [Google Scholar]
- Schoormans, J.; Calcagno, C.; Daal, M.R.R.; Wüst, R.C.I.; Faries, C.; Maier, A.; Teunissen, A.J.P.; Naidu, S.; Sanchez-Gaytan, B.L.; Nederveen, A.J.; et al. An Iterative Sparse Deconvolution Method for Simultaneous Multicolor 19F-MRI of Multiple Contrast Agents. Magn. Reson. Med. 2020, 83, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, K.; Sugihara, F.; Nakamura, T.; Matsushita, H.; Mukai, H.; Akimoto, R.; Minoshima, M.; Mizukami, S.; Kikuchi, K. Perfluorocarbon-Based 19F MRI Nanoprobes for In Vivo Multicolor Imaging. Angew. Chem. Int. Ed. 2018, 57, 16742–16747. [Google Scholar] [CrossRef] [PubMed]
- Chirizzi, C.; De Battista, D.; Tirotta, I.; Metrangolo, P.; Comi, G.; Bombelli, F.B.; Chaabane, L. Multispectral MRI with Dual Fluorinated Probes to Track Mononuclear Cell Activity in Mice. Radiology 2019, 291, 351–357. [Google Scholar] [CrossRef]
- Croci, D.; Santalla Méndez, R.; Temme, S.; Soukup, K.; Fournier, N.; Zomer, A.; Colotti, R.; Wischnewski, V.; Flögel, U.; van Heeswijk, R.B.; et al. Multispectral Fluorine-19 MRI Enables Longitudinal and Noninvasive Monitoring of Tumor-Associated Macrophages. Sci. Transl. Med. 2022, 14, eabo2952. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Tel, J.; Schreibelt, G.; Bonetto, F.; Cruz, L.-J.; Amiri, H.; Heerschap, A.; Figdor, C.G.; de Vries, I.J.M. PLGA-Encapsulated Perfluorocarbon Nanoparticles for Simultaneous Visualization of Distinct Cell Populations by 19F MRI. Nanomedicine 2015, 10, 2339–2348. [Google Scholar] [CrossRef]
- Flögel, U.; Temme, S.; Jacoby, C.; Oerther, T.; Keul, P.; Flocke, V.; Wang, X.; Bönner, F.; Nienhaus, F.; Peter, K.; et al. Multi-Targeted 1H/19F MRI Unmasks Specific Danger Patterns for Emerging Cardiovascular Disorders. Nat. Commun. 2021, 12, 5847. [Google Scholar] [CrossRef]
- Koshkina, O.; White, P.B.; Staal, A.H.J.; Schweins, R.; Swider, E.; Tirotta, I.; Tinnemans, P.; Fokkink, R.; Veltien, A.; van Riessen, N.K.; et al. Nanoparticles for “Two Color” 19F Magnetic Resonance Imaging: Towards Combined Imaging of Biodistribution and Degradation. J. Colloid Interface Sci. 2019, 565, 278–287. [Google Scholar] [CrossRef]
- Shusterman-Krush, R.; Tirukoti, N.D.; Bandela, A.K.; Avram, L.; Allouche-Arnon, H.; Cai, X.; Gibb, B.C.; Bar-Shir, A. Single Fluorinated Agent for Multiplexed 19F-MRI with Micromolar Detectability Based on Dynamic Exchange. Angew. Chem. Int. Ed. 2021, 60, 15405–15411. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Luo, X.; Li, L.; Chen, D.; Liu, X.; Yang, Z.; Yang, L.; Gao, J.; Lin, H. Activatable Multiplexed 19F Magnetic Resonance Imaging Visualizes Reactive Oxygen and Nitrogen Species in Drug-Induced Acute Kidney Injury. Anal. Chem. 2021, 93, 16552–16561. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Mashiach, R.; Houben, L.; Galisova, A.; Addadi, Y.; Kain, D.; Lubart, A.; Blinder, P.; Allouche-Arnon, H.; Bar-Shir, A. Glyconanofluorides as Immunotracers with a Tunable Core Composition for Sensitive Hotspot Magnetic Resonance Imaging of Inflammatory Activity. ACS Nano 2021, 15, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiong, H.; Wang, S.; Li, Y.; Chi, J.; Wang, X.; Li, T.; Zhou, Q.; Gao, J.; Shi, S. Fluorinated Ionic Liquid Based Multicolor 19F MRI Nanoprobes for In Vivo Sensing of Multiple Biological Targets. Adv. Healthc. Mater. 2022, 11, 2102079. [Google Scholar] [CrossRef]
- Zhang, S.; Picard, M.H.; Vasile, E.; Zhu, Y.; Raffai, R.L.; Weisgraber, K.H.; Krieger, M. Diet-Induced Occlusive Coronary Atherosclerosis, Myocardial Infarction, Cardiac Dysfunction, and Premature Death in Scavenger Receptor Class B Type I-Deficient, Hypomorphic Apolipoprotein ER61 Mice. Circulation 2005, 111, 3457–3464. [Google Scholar] [CrossRef]
- Hermann, S.; Kuhlmann, M.T.; Starsichova, A.; Eligehausen, S.; Schäfers, K.; Stypmann, J.; Tiemann, K.; Levkau, B.; Schäfers, M. Imaging Reveals the Connection Between Spontaneous Coronary Plaque Ruptures, Atherothrombosis, and Myocardial Infarctions in HypoE/SRBI-/- Mice. J. Nucl. Med. 2016, 57, 1420–1427. [Google Scholar] [CrossRef]
- Bouvain, P.; Temme, S.; Flögel, U. Hot Spot 19F Magnetic Resonance Imaging of Inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2020, 12, e1639. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Bulte, J.W.M. Tracking Immune Cells in Vivo Using Magnetic Resonance Imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef]
- Nienhaus, F.; Walz, M.; Rothe, M.; Jahn, A.; Pfeiler, S.; Busch, L.; Stern, M.; Heiss, C.; Vornholz, L.; Cames, S.; et al. Quantitative Assessment of Angioplasty-Induced Vascular Inflammation with 19F Cardiovascular Magnetic Resonance Imaging. J. Cardiovasc. Magn. Reson. 2023, 25, 54. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Helfer, B.M.; O’Hanlon, C.F.; Schirda, C. Clinical Cell Therapy Imaging Using a Perfluorocarbon Tracer and Fluorine-19 MRI. Magn. Reson. Med. 2014, 72, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- van Heeswijk, R.B.; Bauer, W.R.; Bönner, F.; Janjic, J.M.; Mulder, W.J.M.; Schreiber, L.M.; Schwitter, J.; Flögel, U. Cardiovascular Molecular Imaging With Fluorine-19 MRI: The Road to the Clinic. Circ. Cardiovasc. Imaging 2023, 16, e014742. [Google Scholar] [CrossRef]
- Zhong, J.; Mills, P.H.; Hitchens, T.K.; Ahrens, E.T. Accelerated Fluorine-19 MRI Cell Tracking Using Compressed Sensing. Magn. Reson. Med. 2013, 69, 1683–1690. [Google Scholar] [CrossRef]
- Liang, S.; Dresselaers, T.; Louchami, K.; Zhu, C.; Liu, Y.; Himmelreich, U. Comparison of Different Compressed Sensing Algorithms for Low SNR 19F MRI Applications-Imaging of Transplanted Pancreatic Islets and Cells Labeled with Perfluorocarbons. NMR Biomed. 2017, 30, e3776. [Google Scholar] [CrossRef] [PubMed]
- Waiczies, S.; Millward, J.M.; Starke, L.; Delgado, P.R.; Huelnhagen, T.; Prinz, C.; Marek, D.; Wecker, D.; Wissmann, R.; Koch, S.P.; et al. Enhanced Fluorine-19 MRI Sensitivity Using a Cryogenic Radiofrequency Probe: Technical Developments and Ex Vivo Demonstration in a Mouse Model of Neuroinflammation. Sci. Rep. 2017, 7, 9808. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, D.E.; Vercellotti, G.M. Limitation of Complement Activation by Perfluorocarbon Emulsions: Superiority of Lecithin-Emulsified Preparations. Biomater. Artif. Cells Artif. Organs 1988, 16, 431–438. [Google Scholar] [CrossRef]
- Ingram, D.A.; Forman, M.B.; Murray, J.J. Activation of Complement by Fluosol Attributable to the Pluronic Detergent Micelle Structure. J. Cardiovasc. Pharmacol. 1993, 22, 456–461. [Google Scholar] [CrossRef]
- Noveck, R.J.; Shannon, E.J.; Leese, P.T.; Shorr, J.S.; Flaim, K.E.; Keipert, P.E.; Woods, C.M. Randomized Safety Studies of Intravenous Perflubron Emulsion. II. Effects on Immune Function in Healthy Volunteers. Anesth. Analg. 2000, 91, 812–822. [Google Scholar] [CrossRef]
- Keipert, P.E.; Otto, S.; Flaim, S.F.; Weers, J.G.; Schutt, E.A.; Pelura, T.J.; Klein, D.H.; Yaksh, T.L. Influence of Perflubron Emulsion Particle Size on Blood Half-Life and Febrile Response in Rats. Artif. Cells Blood Substit. Biotechnol. 1994, 22, 1169–1174. [Google Scholar] [CrossRef]
- Staal, A.H.J.; Becker, K.; Tagit, O.; Koen van Riessen, N.; Koshkina, O.; Veltien, A.; Bouvain, P.; Cortenbach, K.R.G.; Scheenen, T.; Flögel, U.; et al. In Vivo Clearance of 19F MRI Imaging Nanocarriers Is Strongly Influenced by Nanoparticle Ultrastructure. Biomaterials 2020, 261, 120307. [Google Scholar] [CrossRef]
- Becker, K.; Ding, Z.; Bouvain, P.; Koshy, J.; Massold, T.; Kleimann, P.; Flögel, U.; Temme, S. Inflammatory Stimuli Impact on Cellular Uptake and Biodistribution of Perfluorocarbon Nanoemulsions. J. Leukoc. Biol. 2024, 117, qiae199. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjug. Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temme, S.; Kleimann, P.; Tiren, Z.-B.; Bouvain, P.; Zielinski, A.; Dollmeyer, W.; Poth, S.; Görges, J.; Flögel, U. Imaging of Thromboinflammation by Multispectral 19F MRI. Int. J. Mol. Sci. 2025, 26, 2462. https://doi.org/10.3390/ijms26062462
Temme S, Kleimann P, Tiren Z-B, Bouvain P, Zielinski A, Dollmeyer W, Poth S, Görges J, Flögel U. Imaging of Thromboinflammation by Multispectral 19F MRI. International Journal of Molecular Sciences. 2025; 26(6):2462. https://doi.org/10.3390/ijms26062462
Chicago/Turabian StyleTemme, Sebastian, Patricia Kleimann, Zeynep-Büsra Tiren, Pascal Bouvain, Arthur Zielinski, William Dollmeyer, Sarah Poth, Juliana Görges, and Ulrich Flögel. 2025. "Imaging of Thromboinflammation by Multispectral 19F MRI" International Journal of Molecular Sciences 26, no. 6: 2462. https://doi.org/10.3390/ijms26062462
APA StyleTemme, S., Kleimann, P., Tiren, Z.-B., Bouvain, P., Zielinski, A., Dollmeyer, W., Poth, S., Görges, J., & Flögel, U. (2025). Imaging of Thromboinflammation by Multispectral 19F MRI. International Journal of Molecular Sciences, 26(6), 2462. https://doi.org/10.3390/ijms26062462






