Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation
Abstract
1. Introduction
2. Results
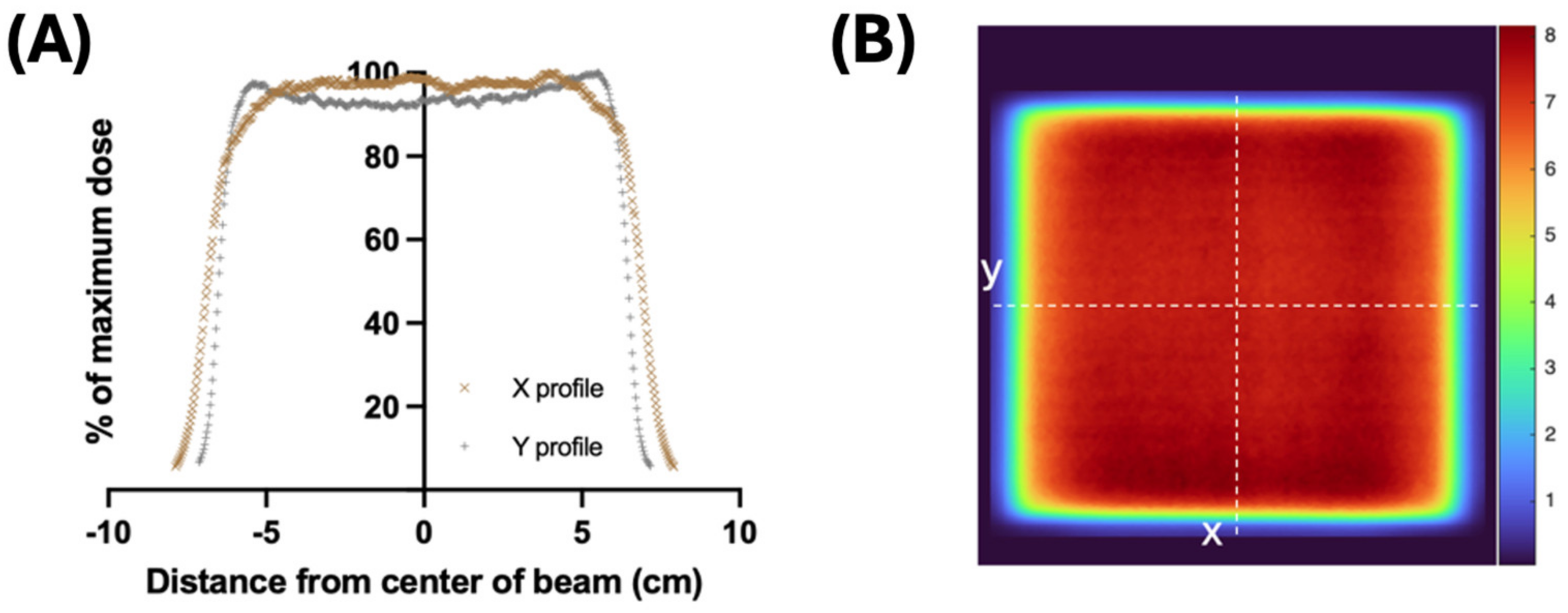
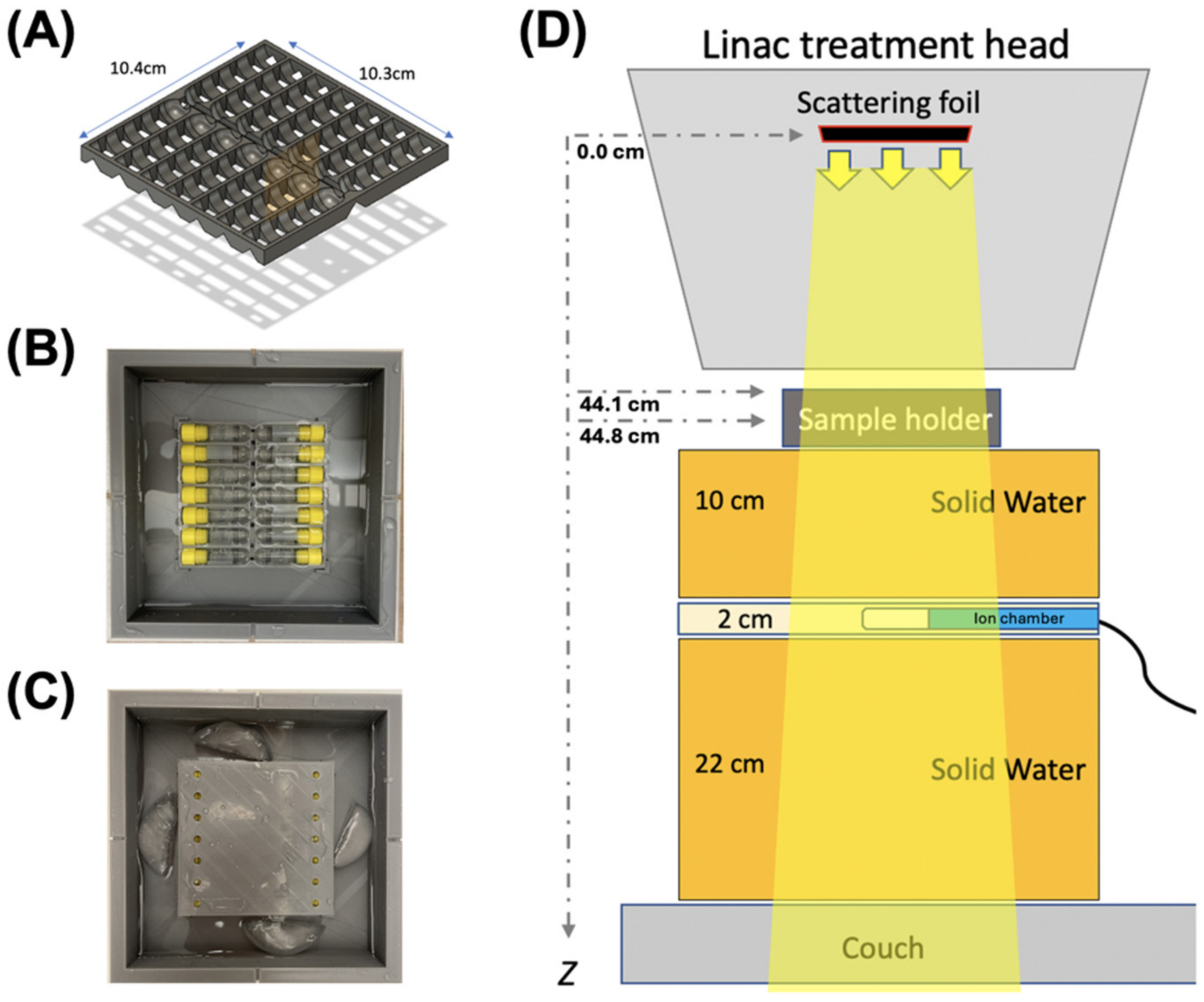
2.1. Irradiation and Dosimetry
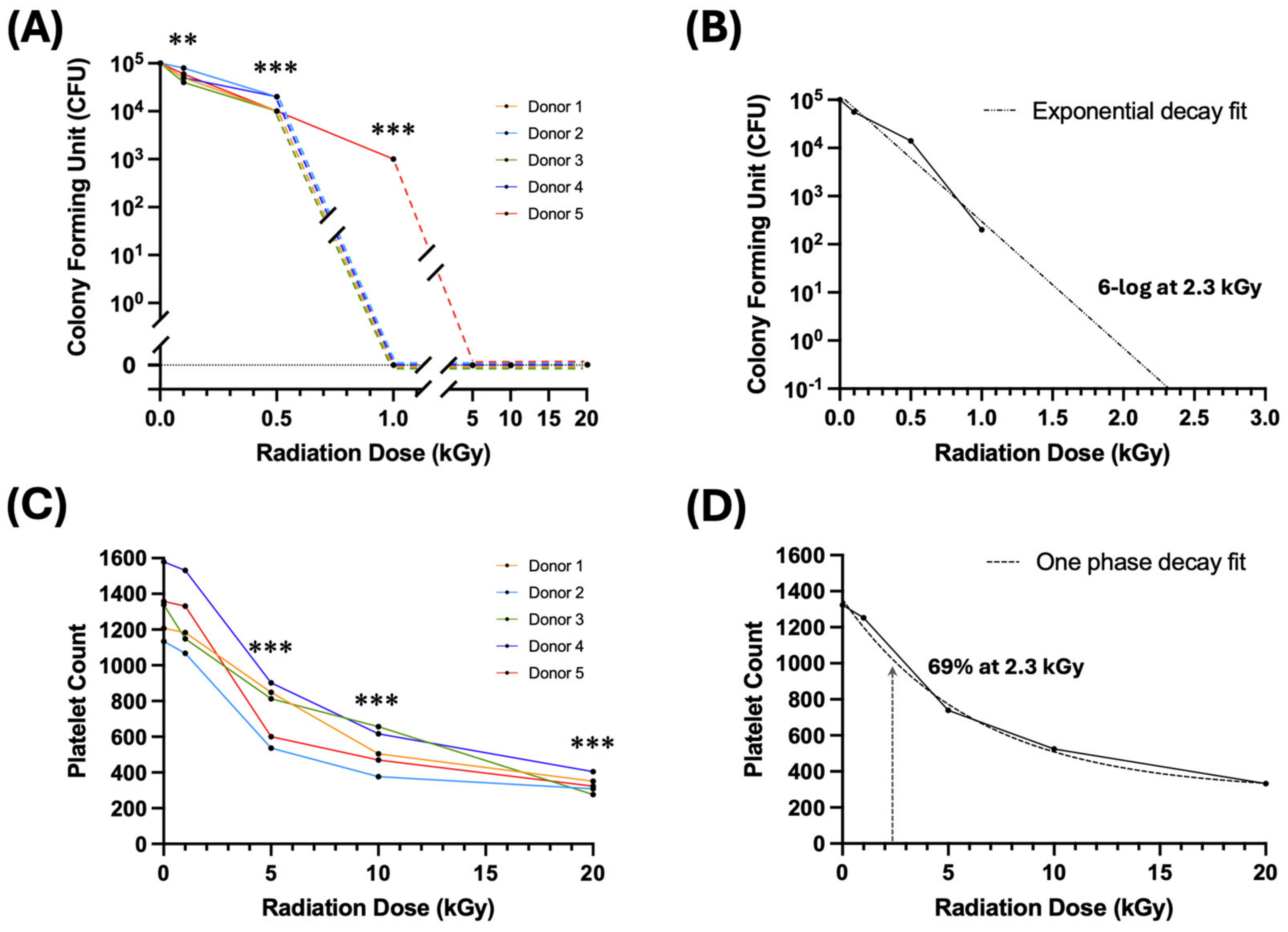
2.2. Irradiation Dose–Response on Bacteria Growth
2.3. Irradiation Dose–Response on Platelet Count
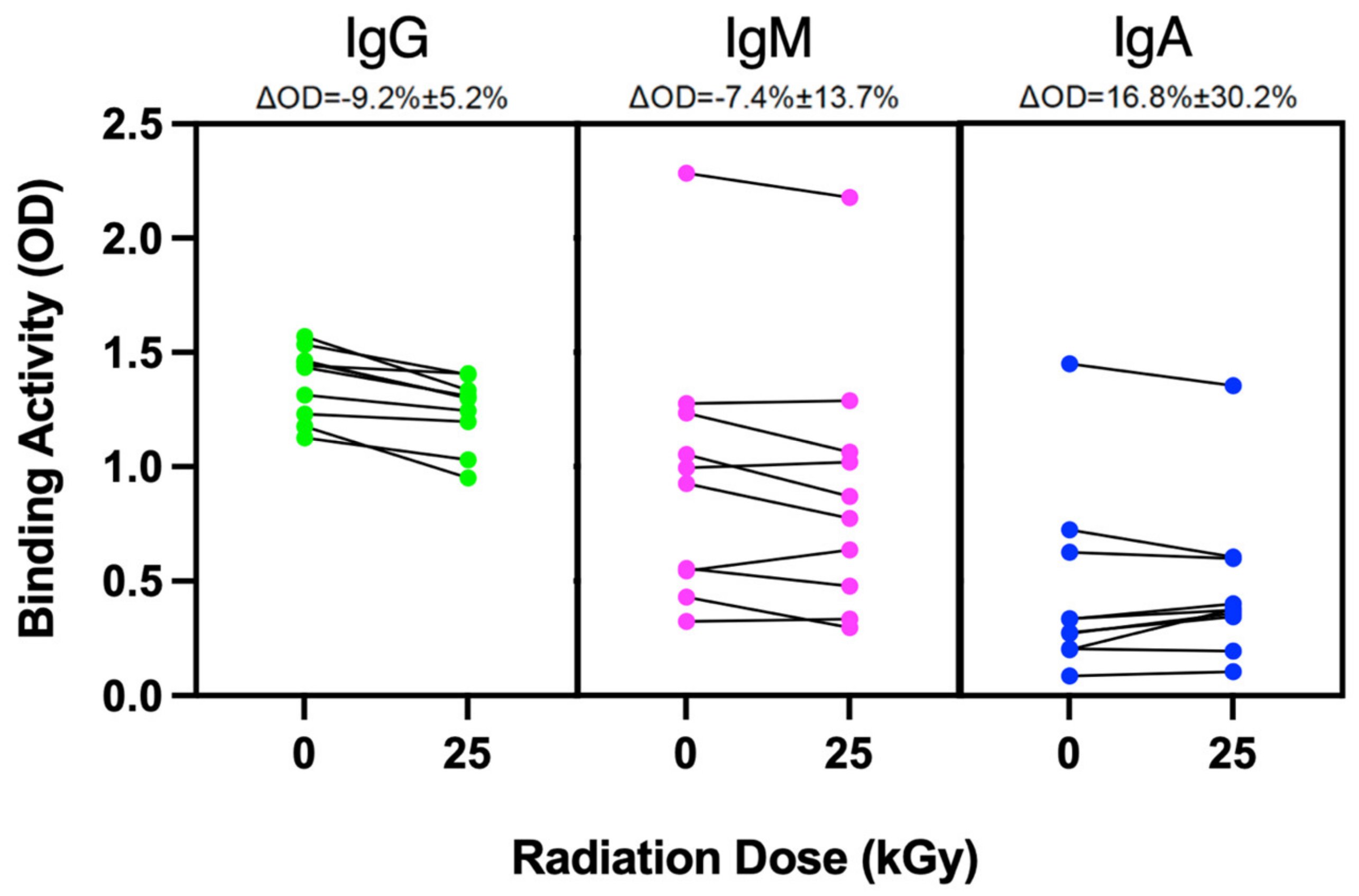
2.4. Irradiation Effects on Protein Integrity
3. Discussion
4. Materials and Methods
4.1. Irradiation
4.2. Dosimetry
4.3. Bacteria Culture and Preparation
4.4. Platelet Components
4.5. Plasma Component
4.6. SARS-CoV-2 Antibody Binding Assay
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UHDR | Ultra-High Dose Rate |
| CCP | COVID-19 Convalescent Plasma |
| PRT | Pathogen Reduction Technology |
| LVDS | Large Volume Delayed Sampling |
| FDA | Food and Drug Administration |
| TA-GVHD | Transfusion-Associated Graft Versus Host Disease |
| ISO | International Organization for Standardization |
| AATB | American Association of Tissue Banks |
| CFU | Colony Forming Units |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IgG | Immunoglobulin G |
| IgA | Immunoglobulin A |
| IgM | Immunoglobulin M |
| RBD | Receptor Binding Domain |
| HIV | Human Immunodeficiency Virus |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| WNV | West Nile Virus |
| MeV | Mega Electron Volt |
| Gy | Gray (unit of radiation dose) |
| kGy | KiloGray |
| SSD | Source-to-Surface Distance |
| OD | Optical Density |
References
- Bloch, E.M.; Busch, M.P.; Corash, L.M.; Dodd, R.; Hailu, B.; Kleinman, S.; O’Brien, S.; Petersen, L.; Stramer, S.L.; Katz, L. Leveraging Donor Populations to Study the Epidemiology and Pathogenesis of Transfusion-Transmitted and Emerging Infectious Diseases. Transfus. Med. Rev. 2023, 37, 150769. [Google Scholar] [CrossRef] [PubMed]
- Godbey, E.A. Whole Blood Transfusion: Past, Present, and Future. Clin. Lab. Med. 2021, 41, 659–667. [Google Scholar] [CrossRef]
- Steele, W.R.; Dodd, R.Y.; Notari, E.P.; Xu, M.; Nelson, D.; Kessler, D.A.; Reik, R.; Williams, A.E.; Custer, B.; Stramer, S.L.; et al. Prevalence of Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus in United States Blood Donations, 2015 to 2019: The Transfusion-Transmissible Infections Monitoring System (TTIMS). Transfusion 2020, 60, 2327–2339. [Google Scholar] [CrossRef]
- Dodd, R.Y.; Crowder, L.A.; Haynes, J.M.; Notari, E.P.; Stramer, S.L.; Steele, W.R. Screening Blood Donors for HIV, HCV, and HBV at the American Red Cross: 10-Year Trends in Prevalence, Incidence, and Residual Risk, 2007 to 2016. Transfus. Med. Rev. 2020, 34, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Stramer, S.L.; Dodd, R.Y. Donor Testing and Risk: Current Prevalence, Incidence, and Residual Risk of Transfusion-Transmissible Agents in US Allogeneic Donations. Transfus. Med. Rev. 2012, 26, 119–128. [Google Scholar] [CrossRef]
- McDonald, C.P. Bacterial Risk Reduction by Improved Donor Arm Disinfection, Diversion and Bacterial Screening. Transfus. Med. 2006, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cook, D.N.; Wiesehahn, G.P.; Alfonso, R.; Behrman, B.; Cimino, G.D.; Corten, L.; Damonte, P.B.; Dikeman, R.; Dupuis, K.; et al. Photochemical Inactivation of Viruses and Bacteria in Platelet Concentrates by Use of a Novel Psoralen and Long-Wavelength Ultraviolet Light. Transfusion 1997, 37, 423–435. [Google Scholar] [CrossRef]
- Goodrich, R.P. The Use of Riboflavin for the Inactivation of Pathogens in Blood Products. Vox Sang. 2000, 78 (Suppl. 2), 211–215. [Google Scholar] [CrossRef]
- Ciaravi, V.; McCullough, T.; Dayan, A.D. Pharmacokinetic and Toxicology Assessment of INTERCEPT (S-59 and UVA Treated) Platelets. Hum. Exp. Toxicol. 2001, 20, 533–550. [Google Scholar] [CrossRef]
- Picker, S.M. Current Methods for the Reduction of Blood-Borne Pathogens: A Comprehensive Literature Review. Blood Transfus. 2013, 11, 343–348. [Google Scholar] [CrossRef]
- Investigational COVID-19 Convalescent Plasma; Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-covid-19-convalescent-plasma (accessed on 16 September 2024).
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Wirz, O.F.; Röltgen, K.; Pandey, S.; Tolentino, L.; Boyd, S.D.; Pham, T.D. Efficient Identification of High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Plasma Samples by Pooling Method. Arch. Pathol. Lab. Med. 2021, 145, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Recommendations for Investigational and Licensed COVID-19 Convalescent Plasma; Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-investigational-and-licensed-covid-19-convalescent-plasma (accessed on 16 September 2024).
- House, C.; House, J.A.; Yedloutschnig, R.J. Inactivation of Viral Agents in Bovine Serum by Gamma Irradiation. Can. J. Microbiol. 1990, 36, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Hume, A.J.; Ames, J.; Rennick, L.J.; Duprex, W.P.; Marzi, A.; Tonkiss, J.; Mühlberger, E. Inactivation of RNA Viruses by Gamma Irradiation: A Study on Mitigating Factors. Viruses 2016, 8, 204. [Google Scholar] [CrossRef]
- Sullivan, R.; Fassolitis, A.C.; Larkin, E.P.; Read, R.B.; Peeler, J.T. Inactivation of Thirty Viruses by Gamma Radiation. Appl. Microbiol. 1971, 22, 61–65. [Google Scholar] [CrossRef]
- Kitchen, A.D.; Mann, G.F.; Harrison, J.F.; Zuckerman, A.J. Effect of Gamma Irradiation on the Human Immunodeficiency Virus and Human Coagulation Proteins. Vox Sang. 1989, 56, 223–229. [Google Scholar] [CrossRef]
- Hiemstra, H.; Tersmette, M.; Vos, A.H.; Over, J.; van Berkel, M.P.; de Bree, H. Inactivation of Human Immunodeficiency Virus by Gamma Radiation and Its Effect on Plasma and Coagulation Factors. Transfusion 1991, 31, 32–39. [Google Scholar] [CrossRef]
- Grieb, T.; Forng, R.-Y.; Brown, R.; Owolabi, T.; Maddox, E.; McBain, A.; Drohan, W.N.; Mann, D.M.; Burgess, W.H. Effective Use of Gamma Irradiation for Pathogen Inactivation of Monoclonal Antibody Preparations. Biologicals 2002, 30, 207–216. [Google Scholar] [CrossRef]
- Feldmann, F.; Shupert, W.L.; Haddock, E.; Twardoski, B.; Feldmann, H. Gamma Irradiation as an Effective Method for Inactivation of Emerging Viral Pathogens. Am. J. Trop. Med. Hyg. 2019, 100, 1275–1277. [Google Scholar] [CrossRef]
- Schuler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W.; Maxim, P.G. Experimental Platform for Ultra-High Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 195–203. [Google Scholar] [CrossRef]
- Wang, J.; Melemenidis, S.; Manjappa, R.; Viswanathan, V.; Ashraf, R.M.; Levy, K.; Skinner, L.B.; Soto, L.A.; Chow, S.; Lau, B.; et al. Dosimetric Calibration of Anatomy-Specific Ultra-High Dose Rate Electron Irradiation Platform for Preclinical FLASH Radiobiology Experiments. Med. Phys. 2024, 51, 9166–9178. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.R.; Melemenidis, S.; Liu, K.; Grilj, V.; Jansen, J.; Velasquez, B.; Connell, L.; Schulz, J.B.; Bailat, C.; Libed, A.; et al. Multi-Institutional Audit of FLASH and Conventional Dosimetry with a 3D-Printed Anatomically Realistic Mouse Phantom. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Standards for Blood Banks and Transfusion Services. QRB Qual. Rev. Bull. 1977, 3, 17,22. [PubMed]
- Pathogen-Reduced Platelets. Available online: https://professionaleducation.blood.ca/en/transfusion/clinical-guide/pathogen-reduced-platelets (accessed on 16 June 2024).
| Parameter | Value |
|---|---|
| Beam energy [MeV] | 16.6 |
| Source-to-surface distance (cm) | 44.8 |
| Field size (cm2) | 10 × 10 |
| Target dose [kGy] | 0.1, 0.5, 1, 5, 10, 15, 20, 25 |
| Pulse rate [Hz] | 180 |
| Mean dose per pulse [Gy] | 0.53 |
| Mean dose rate [Gy/s] | 95.5 |
| Pulse length [s] | 3.75 × 10−6 |
| Mean delivery time per kGy [s] | 10.44 |
| Intra-pulse dose rate [Gy/s] | 1.41 × 105 |
| Irradiation (kGy) | PLT (A) | PLT (B) | PLT (C) | PLT (D) | PLT (E) | p-Value (Compared to 0 kGy) |
|---|---|---|---|---|---|---|
| 0 | 100% | 100% | 100% | 100% | 100% | |
| 1 | 98% | 94% | 86% | 97% | 98% | 0.0784 |
| 5 | 70% | 47% | 61% | 57% | 44% | 0.0007 |
| 10 | 42% | 33% | 49% | 39% | 35% | 0.0000 |
| 20 | 29% | 27% | 21% | 26% | 24% | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melemenidis, S.; Nguyen, K.D.; Baraceros-Pineda, R.; Barclay, C.K.; Bautista, J.; Lau, H.D.; Ashraf, M.R.; Manjappa, R.; Dutt, S.; Soto, L.A.; et al. Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation. Int. J. Mol. Sci. 2025, 26, 2424. https://doi.org/10.3390/ijms26062424
Melemenidis S, Nguyen KD, Baraceros-Pineda R, Barclay CK, Bautista J, Lau HD, Ashraf MR, Manjappa R, Dutt S, Soto LA, et al. Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation. International Journal of Molecular Sciences. 2025; 26(6):2424. https://doi.org/10.3390/ijms26062424
Chicago/Turabian StyleMelemenidis, Stavros, Khoa D. Nguyen, Rosella Baraceros-Pineda, Cherie K. Barclay, Joanne Bautista, Hubert D. Lau, M. Ramish Ashraf, Rakesh Manjappa, Suparna Dutt, Luis A. Soto, and et al. 2025. "Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation" International Journal of Molecular Sciences 26, no. 6: 2424. https://doi.org/10.3390/ijms26062424
APA StyleMelemenidis, S., Nguyen, K. D., Baraceros-Pineda, R., Barclay, C. K., Bautista, J., Lau, H. D., Ashraf, M. R., Manjappa, R., Dutt, S., Soto, L. A., Katila, N., Lau, B., Viswanathan, V., Yu, A. S., Surucu, M., Skinner, L. B., Engleman, E. G., Loo, B. W., Jr., & Pham, T. D. (2025). Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation. International Journal of Molecular Sciences, 26(6), 2424. https://doi.org/10.3390/ijms26062424





