C-Terminal Extended Domain-Independent Telomere Maintenance: Modeling the Function of TIN2 Isoforms in Mus musculus
Abstract
1. Introduction
2. Results
2.1. Characterization of Alternative Splicing and Protein Sequence Homology of TIN2 Isoforms in Humans and Mice
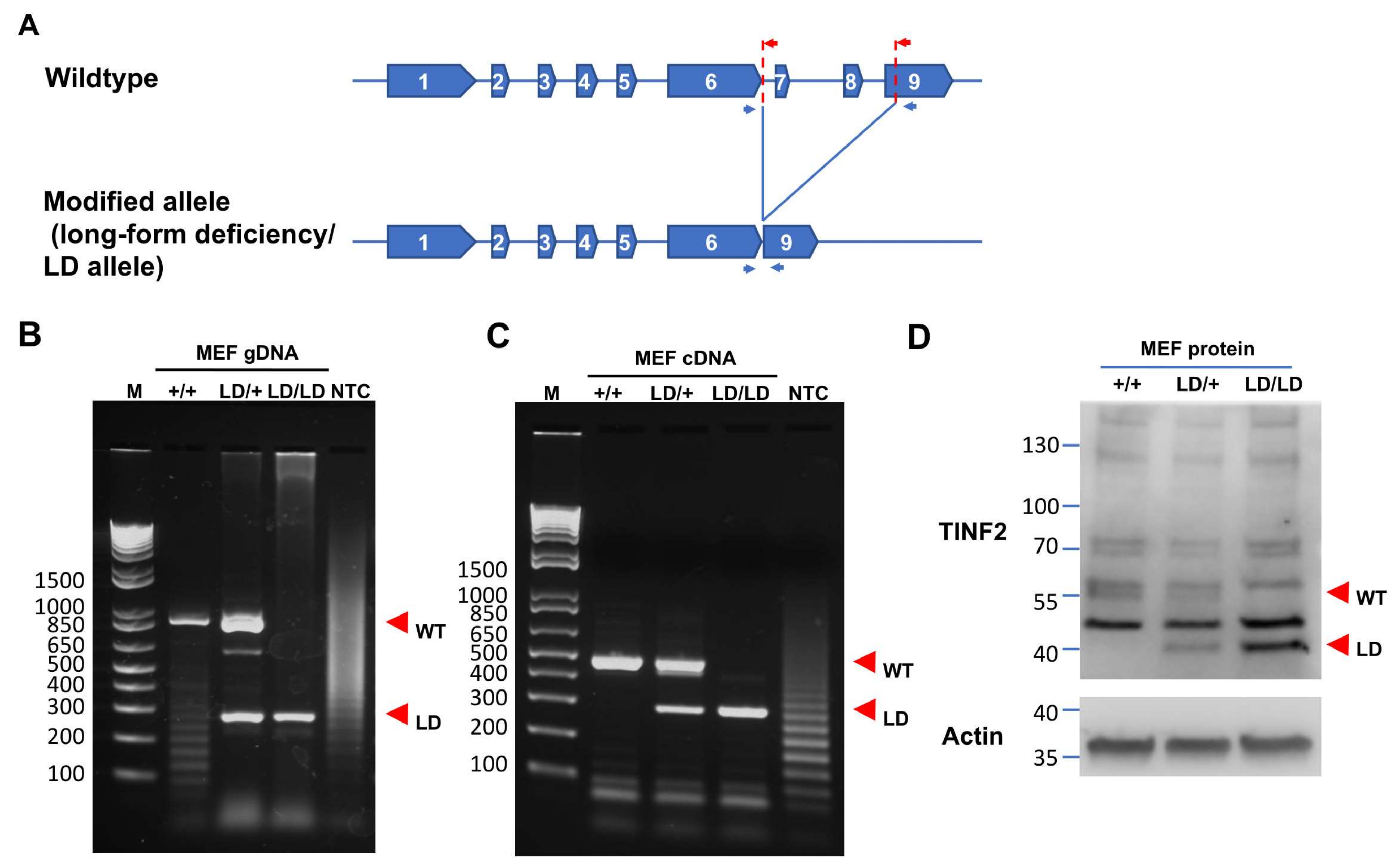
2.2. Generation of a Truncated Tinf2 Allele Expressing a TIN2S-like Protein in Mice
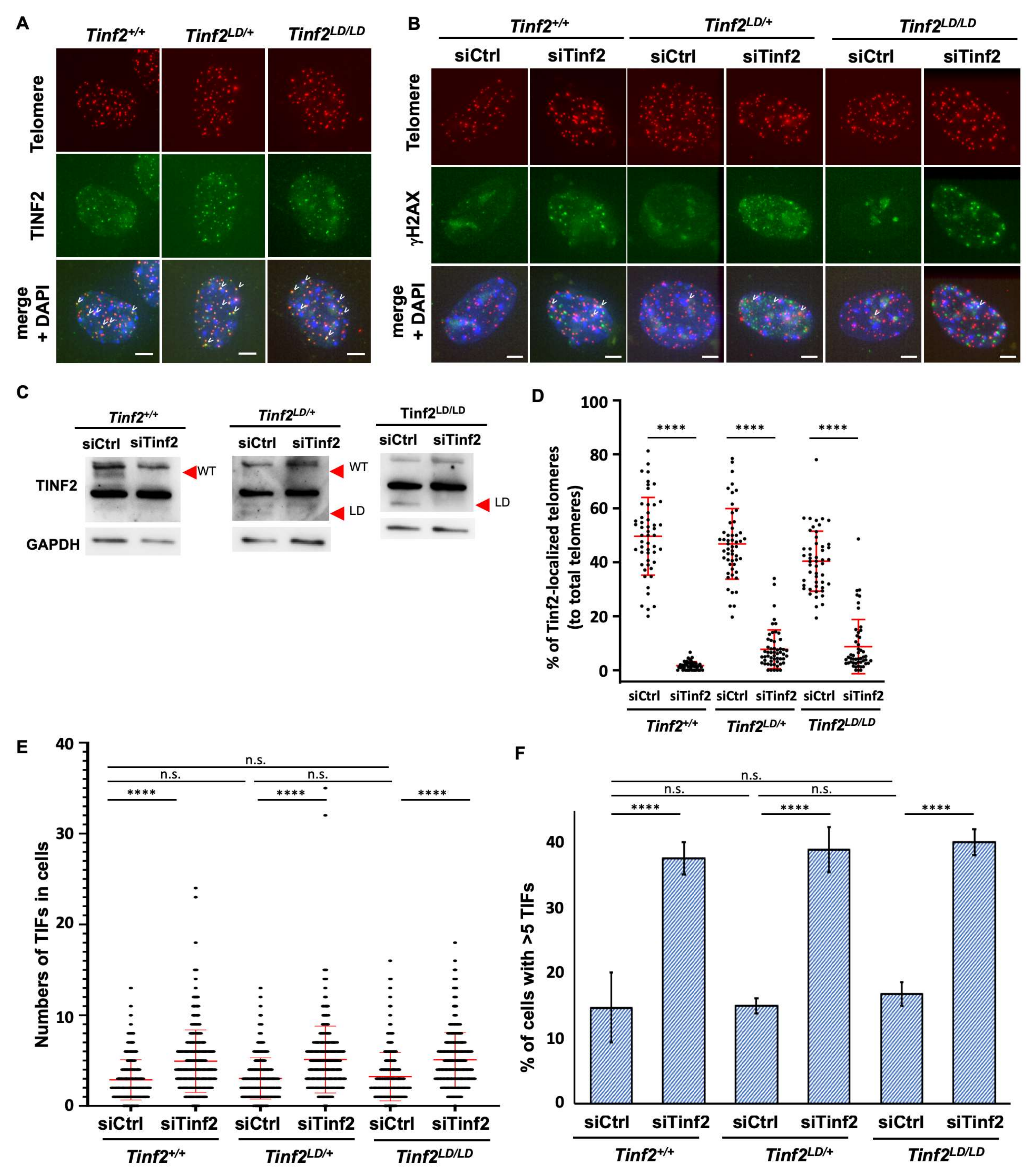
2.3. TINF2LD Protects Telomeres in MEFs
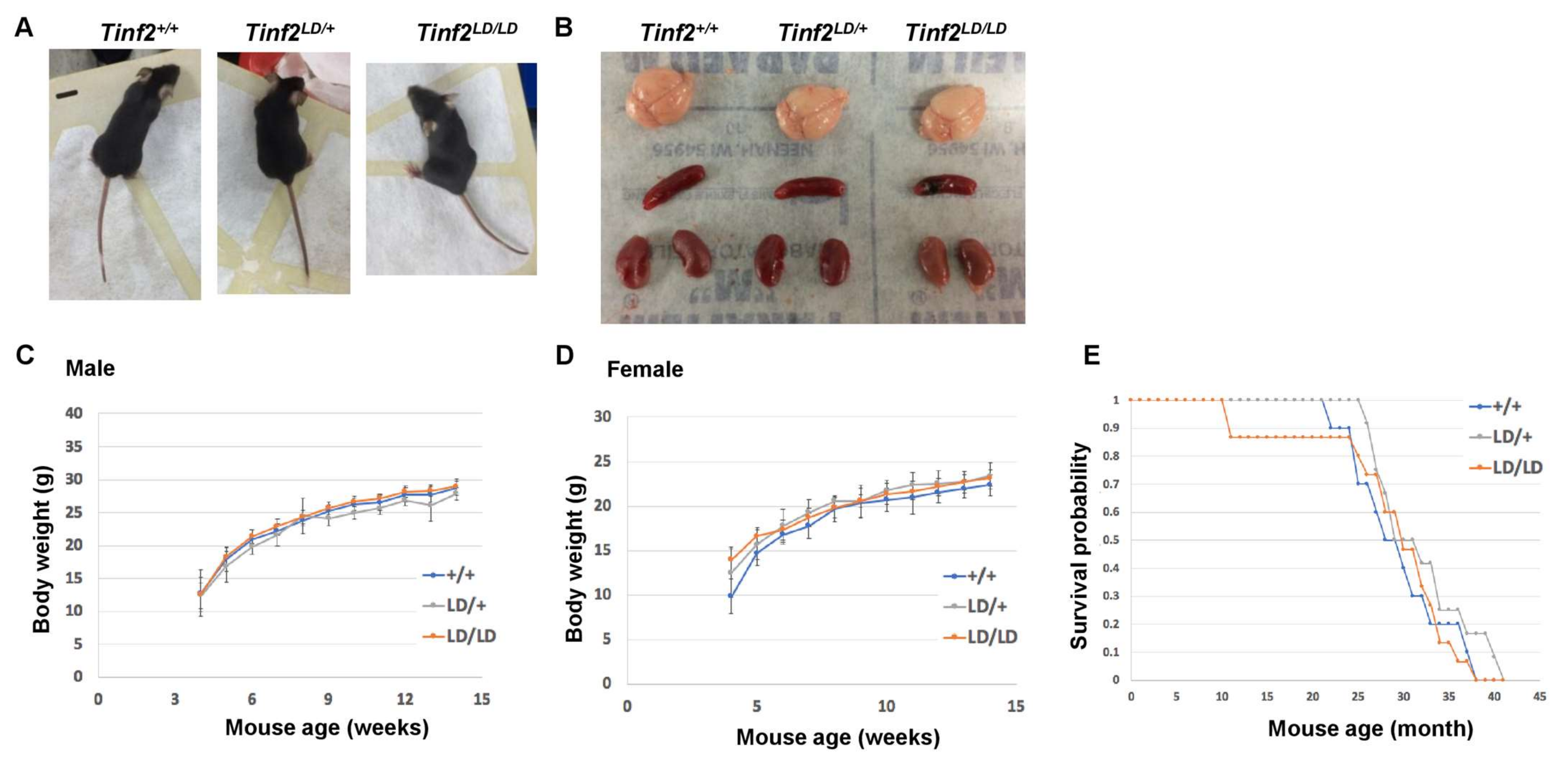
2.4. The CTED of TINF2 Is Not Essential for Embryonic Development or Growth of Mice
3. Discussion
4. Materials and Methods
4.1. Generation of the Tinf2 LD Allele in Mouse
4.2. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.3. Generation of Mouse Embryonic Fibroblasts (MEFs)
4.4. Protein Extraction and Western Blotting
4.5. Immunofluorescence and Fluorescence In Situ Hybridization (IF-FISH)
4.6. Analyses of Animal Survival, Body Weight, and Organ Structures
4.7. Stable Expression in Cells with Viral Infection
4.8. Telomere Restriction Fragment Analysis
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Giardini, M.A.; Segatto, M.; da Silva, M.S.; Nunes, V.S.; Cano, M.I. Telomere and telomerase biology. Prog. Mol. Biol. Transl. Sci. 2014, 125, 1–40. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef]
- Liu, D.; Safari, A.; O’Connor, M.S.; Chan, D.W.; Laegeler, A.; Qin, J.; Songyang, Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 2004, 6, 673–680. [Google Scholar] [CrossRef]
- Rai, R.; Gu, P.; Broton, C.; Kumar-Sinha, C.; Chen, Y.; Chang, S. The Replisome Mediates A-NHEJ Repair of Telomeres Lacking POT1-TPP1 Independently of MRN Function. Cell Rep. 2019, 29, 3708–3725.e5. [Google Scholar] [CrossRef]
- Van Steensel, B.; Smogorzewska, A.; de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 1998, 92, 401–413. [Google Scholar] [CrossRef]
- Benarroch-Popivker, D.; Pisano, S.; Mendez-Bermudez, A.; Lototska, L.; Kaur, P.; Bauwens, S.; Djerbi, N.; Latrick, C.M.; Fraisier, V.; Pei, B.; et al. TRF2-Mediated Control of Telomere DNA Topology as a Mechanism for Chromosome-End Protection. Mol. Cell 2016, 61, 274–286. [Google Scholar] [CrossRef]
- Karlseder, J.; Broccoli, D.; Dai, Y.; Hardy, S.; de Lange, T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 1999, 283, 1321–1325. [Google Scholar] [CrossRef]
- Kaur, P.; Barnes, R.; Pan, H.; Detwiler, A.C.; Liu, M.; Mahn, C.; Hall, J.; Messenger, Z.; You, C.; Piehler, J.; et al. TIN2 is an architectural protein that facilitates TRF2-mediated trans- and cis-interactions on telomeric DNA. Nucleic Acids Res. 2021, 49, 13000–13018. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.K.; Hooper, S.; Blackwood, S.; Gandhi, R.; de Lange, T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 2010, 285, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Rai, R.; Huang, C.; Broton, C.; Long, J.; Xu, Y.; Xue, J.; Lei, M.; Chang, S.; Chen, Y. Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res. 2017, 27, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.K.; Kibe, T.; Donigian, J.R.; Frescas, D.; de Lange, T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol. Cell 2011, 44, 647–659. [Google Scholar] [CrossRef]
- Frescas, D.; de Lange, T. TRF2-tethered TIN2 can mediate telomere protection by TPP1/POT1. Mol. Cell. Biol. 2014, 34, 1349–1362. [Google Scholar] [CrossRef]
- Abreu, E.; Aritonovska, E.; Reichenbach, P.; Cristofari, G.; Culp, B.; Terns, R.M.; Lingner, J.; Terns, M.P. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol. Cell. Biol. 2010, 30, 2971–2982. [Google Scholar] [CrossRef]
- Frank, A.K.; Tran, D.C.; Qu, R.W.; Stohr, B.A.; Segal, D.J.; Xu, L. The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres. PLoS Genet. 2015, 11, e1005410. [Google Scholar] [CrossRef]
- Canudas, S.; Houghtaling, B.R.; Bhanot, M.; Sasa, G.; Savage, S.A.; Bertuch, A.A.; Smith, S. A role for heterochromatin protein 1gamma at human telomeres. Genes Dev. 2011, 25, 1807–1819. [Google Scholar] [CrossRef]
- Kaminker, P.; Plachot, C.; Kim, S.H.; Chung, P.; Crippen, D.; Petersen, O.W.; Bissell, M.J.; Campisi, J.; Lelievre, S.A. Higher-order nuclear organization in growth arrest of human mammary epithelial cells: A novel role for telomere-associated protein TIN2. J. Cell Sci. 2005, 118 Pt 6, 1321–1330. [Google Scholar] [CrossRef][Green Version]
- Sasa, G.S.; Ribes-Zamora, A.; Nelson, N.D.; Bertuch, A.A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin. Genet. 2012, 81, 470–478. [Google Scholar] [CrossRef]
- Yang, D.; He, Q.; Kim, H.; Ma, W.; Songyang, Z. TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase. J. Biol. Chem. 2011, 286, 23022–23030. [Google Scholar] [CrossRef] [PubMed]
- Walne, A.J.; Vulliamy, T.; Beswick, R.; Kirwan, M.; Dokal, I. TINF2 mutations result in very short telomeres: Analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood 2008, 112, 3594–3600. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.P.; Talcott, K.E.; Kim, D.Y.; Agarwal, S.; Mukai, S. Retinal findings and a novel TINF2 mutation in Revesz syndrome: Clinical and molecular correlations with pediatric retinal vasculopathies. Ophthalmic Genet. 2017, 38, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Glousker, G.; Touzot, F.; Revy, P.; Tzfati, Y.; Savage, S.A. Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder. Br. J. Haematol. 2015, 170, 457–471. [Google Scholar] [CrossRef]
- Kim, S.H.; Kaminker, P.; Campisi, J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 1999, 23, 405–412. [Google Scholar] [CrossRef]
- Schmutz, I.; Mensenkamp, A.R.; Takai, K.K.; Haadsma, M.; Spruijt, L.; de Voer, R.M.; Choo, S.S.; Lorbeer, F.K.; van Grinsven, E.J.; Hockemeyer, D.; et al. TINF2 is a haploinsufficient tumor suppressor that limits telomere length. Elife 2020, 9, e61235. [Google Scholar] [CrossRef]
- Choo, S.; Lorbeer, F.K.; Regalado, S.G.; Short, S.B.; Wu, S.; Rieser, G.; Bertuch, A.A.; Hockemeyer, D. Editing TINF2 as a potential therapeutic approach to restore telomere length in dyskeratosis congenita. Blood 2022, 140, 608–618. [Google Scholar] [CrossRef]
- Chiang, Y.J.; Kim, S.H.; Tessarollo, L.; Campisi, J.; Hodes, R.J. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Mol. Cell. Biol. 2004, 24, 6631–6634. [Google Scholar] [CrossRef]
- Frescas, D.; de Lange, T. A TIN2 dyskeratosis congenita mutation causes telomerase-independent telomere shortening in mice. Genes Dev. 2014, 28, 153–166. [Google Scholar] [CrossRef]
- Kaminker, P.G.; Kim, S.H.; Desprez, P.Y.; Campisi, J. A novel form of the telomere-associated protein TIN2 localizes to the nuclear matrix. Cell Cycle 2009, 8, 931–939. [Google Scholar] [CrossRef]
- Kim, S.H.; Parrinello, S.; Kim, J.; Campisi, J. Mus musculus and Mus spretus homologues of the human telomere-associated protein TIN2. Genomics 2003, 81, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.D.; Dodson, L.M.; Escudero, L.; Sukumar, A.T.; Williams, C.L.; Mihalek, I.; Baldan, A.; Baird, D.M.; Bertuch, A.A. The C-Terminal Extension Unique to the Long Isoform of the Shelterin Component TIN2 Enhances Its Interaction with TRF2 in a Phosphorylation- and Dyskeratosis Congenita Cluster-Dependent Fashion. Mol. Cell Biol. 2018, 38, e00025-18. [Google Scholar] [CrossRef] [PubMed]
- Pike, A.M.; Strong, M.A.; Ouyang, J.P.T.; Greider, C.W. TIN2 Functions with TPP1/POT1 To Stimulate Telomerase Processivity. Mol. Cell. Biol. 2019, 39, e00593-18. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Deng, Y.; Lin, Y.; Cosme-Blanco, W.; Chan, S.; He, H.; Yuan, G.; Brown, E.J.; Chang, S. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 2007, 26, 4709–4719. [Google Scholar] [CrossRef]
- Chen, L.Y.; Liu, D.; Songyang, Z. Telomere maintenance through spatial control of telomeric proteins. Mol. Cell. Biol. 2007, 27, 5898–5909. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, F.; Lin, S.; Chen, W.; Weng, K.; Liu, D.; Wang, C.; He, Z.; Chen, Y.; Ma, W.; et al. TIN2 deficiency leads to ALT-associated phenotypes and differentiation defects in embryonic stem cells. Stem Cell Rep. 2022, 17, 1183–1197. [Google Scholar] [CrossRef]
- Dejager, L.; Libert, C.; Montagutelli, X. Thirty years of Mus spretus: A promising future. Trends Genet. 2009, 25, 234–241. [Google Scholar] [CrossRef]
- Inoue, H.; Horiguchi, M.; Ono, K.; Kanoh, J. Casein kinase 2 regulates telomere protein complex formation through Rap1 phosphorylation. Nucleic Acids Res. 2019, 47, 6871–6884. [Google Scholar] [CrossRef]
- Inoue, H.; Sugimoto, S.; Takeshita, Y.; Takeuchi, M.; Hatanaka, M.; Nagao, K.; Hayashi, T.; Kokubu, A.; Yanagida, M.; Kanoh, J. CK2 phospho-independent assembly of the Tel2-associated stress-signaling complexes in Schizosaccharomyces pombe. Genes Cells 2017, 22, 59–70. [Google Scholar] [CrossRef]
- Horejsi, Z.; Takai, H.; Adelman, C.A.; Collis, S.J.; Flynn, H.; Maslen, S.; Skehel, J.M.; de Lange, T.; Boulton, S.J. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol. Cell 2010, 39, 839–850. [Google Scholar] [CrossRef]
- Rao, F.; Cha, J.; Xu, J.; Xu, R.; Vandiver, M.S.; Tyagi, R.; Tokhunts, R.; Koldobskiy, M.A.; Fu, C.; Barrow, R.; et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol. Cell 2014, 54, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Zhang, Y.; Zhang, Q.; Li, H.; Luo, Z.; Fang, H.; Kim, S.H.; Qin, L.; Yotnda, P.; Xu, J.; et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol. Cell 2012, 47, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Hristova, K.; Wimley, W.C. Determining the statistical significance of the difference between arbitrary curves: A spreadsheet method. PLoS ONE 2023, 18, e0289619. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-M.; Shen, Y.-L.; Ho, C.-L.; Chen, T.-E.; Hsia, H.-Y.; Songyang, Z.; Chen, L.-Y. C-Terminal Extended Domain-Independent Telomere Maintenance: Modeling the Function of TIN2 Isoforms in Mus musculus. Int. J. Mol. Sci. 2025, 26, 2414. https://doi.org/10.3390/ijms26062414
Huang C-M, Shen Y-L, Ho C-L, Chen T-E, Hsia H-Y, Songyang Z, Chen L-Y. C-Terminal Extended Domain-Independent Telomere Maintenance: Modeling the Function of TIN2 Isoforms in Mus musculus. International Journal of Molecular Sciences. 2025; 26(6):2414. https://doi.org/10.3390/ijms26062414
Chicago/Turabian StyleHuang, Chiao-Ming, Yi-Ling Shen, Chia-Lo Ho, Tzeng-Erh Chen, Hsuan-Yu Hsia, Zhou Songyang, and Liuh-Yow Chen. 2025. "C-Terminal Extended Domain-Independent Telomere Maintenance: Modeling the Function of TIN2 Isoforms in Mus musculus" International Journal of Molecular Sciences 26, no. 6: 2414. https://doi.org/10.3390/ijms26062414
APA StyleHuang, C.-M., Shen, Y.-L., Ho, C.-L., Chen, T.-E., Hsia, H.-Y., Songyang, Z., & Chen, L.-Y. (2025). C-Terminal Extended Domain-Independent Telomere Maintenance: Modeling the Function of TIN2 Isoforms in Mus musculus. International Journal of Molecular Sciences, 26(6), 2414. https://doi.org/10.3390/ijms26062414





