Supplementation with Rare Earth–Chitosan Chelate Improves Tibia Quality, Disease Resistance Capacity, and Performance in Nursery Pigs
Abstract
1. Introduction
2. Results
2.1. Effects of Dietary RECC Addition on Performance of Nursery Pigs
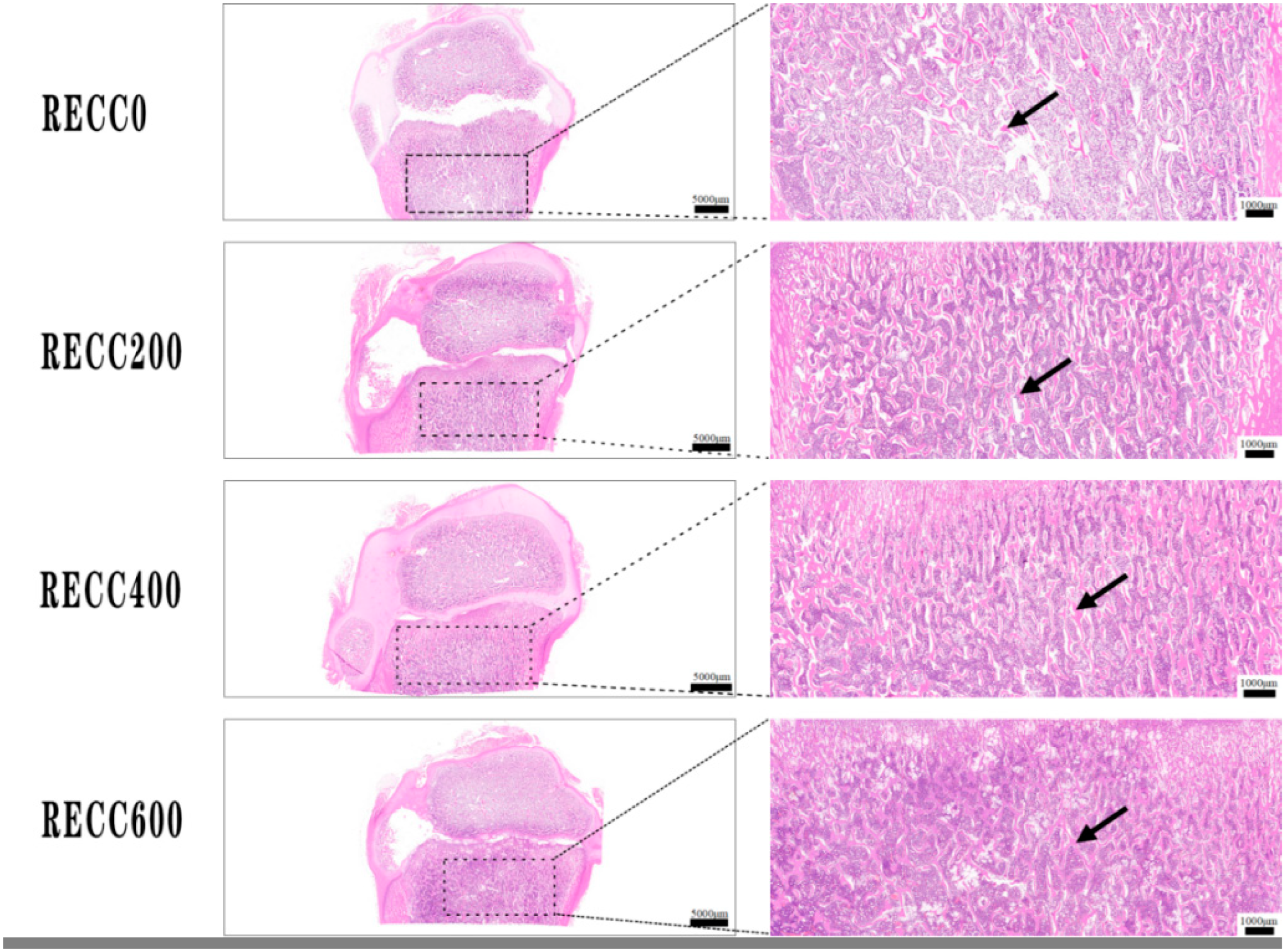
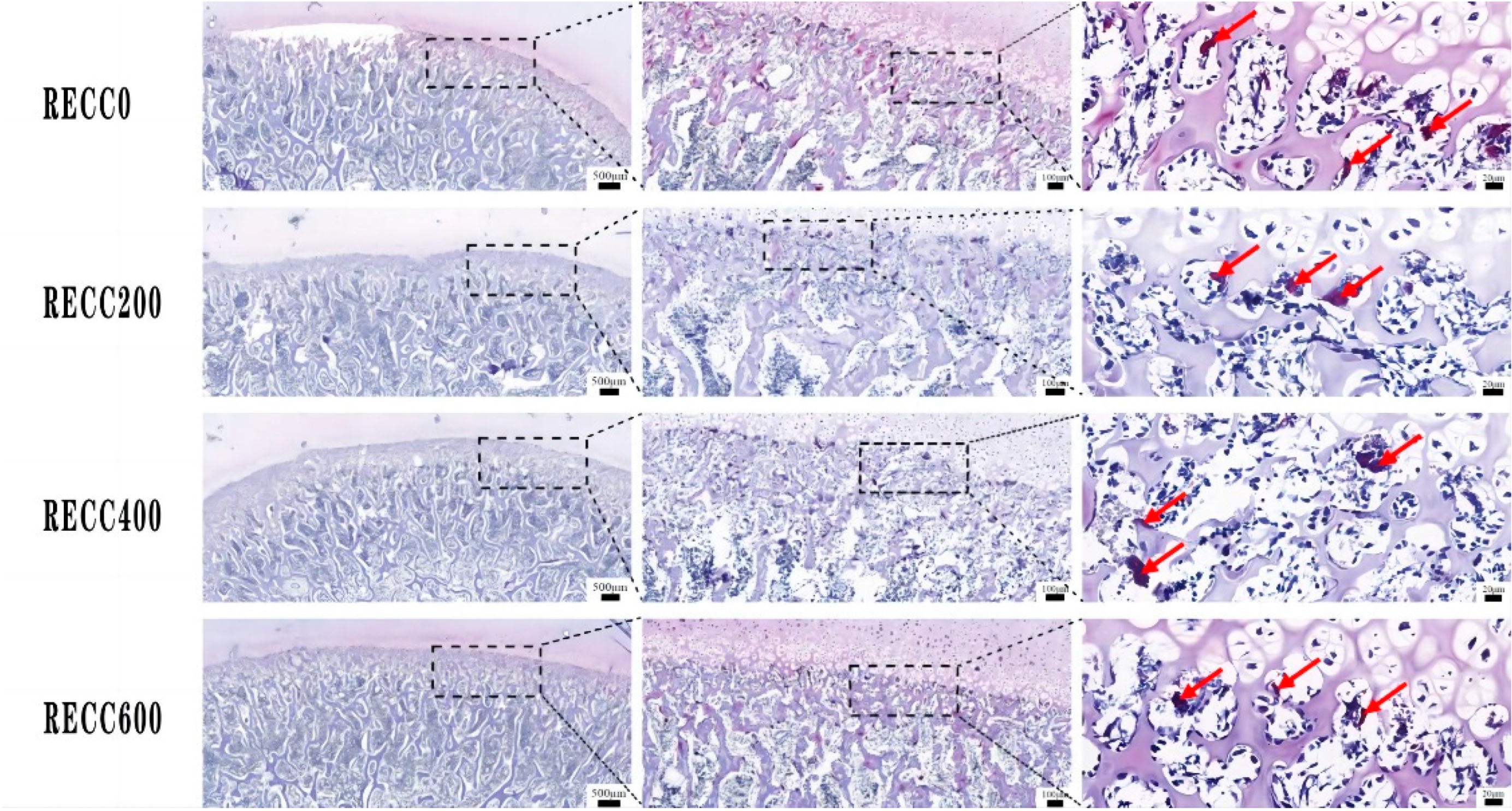
2.2. Effects of Dietary RECC Addition on Tibia Microarchitecture of Nursery Pigs
2.3. Effects of Dietary RECC Addition on Fecal Bacterial Composition of Nursery Pigs
2.4. Effects of Dietary RECC Addition on Hepatic Histomorphology of Nursery Pigs
2.5. Effects of Dietary RECC Addition on Jejunal Histomorphology of Nursery Pigs
2.6. Effects of Dietary RECC Addition on Immunoglobins and Cytokines in Serum of Nursery Pigs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experimental Design
4.3. Feed Preparation and Animal Feeding
4.4. Data Collection and Calculation
4.5. Sample Collection and Preparation
4.6. Tissue Staining
4.7. Fecal Bacteria Sequencing
4.8. Serum Sample Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RECC | Rare earth–chitosan chelate |
| IgM | Immunoglobulin M |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IL-10 | Interleukin-10 |
| IL-2 | Interleukin-2 |
| TNF-α | Tumor necrosis factor-α |
| Tb.Pm | Trabecular perimeter |
| Tb.Ar | Trabecular bone area |
| Tb.Th | Trabecular thickness |
| Tb.N | Trabecular number |
| Tb.Sp | Trabecular separation |
| N.Oc | Osteoclast number |
| ADG | Average daily gain |
| ADFI | Average daily feed intake |
References
- Boudon, A.; Karhapää, M.; Siljander-Rasi, H.; Cantaloube, E.; Brossard, L.; Le Floc’h, N.; Meunier-Salaün, M.C. Effect of moderate forced physical activity on behaviour, lameness and osteochondrosis in growing pigs from two divergent lines selected for feed efficiency. Animal 2022, 1, 100010. [Google Scholar] [CrossRef]
- Cianferotti, L. Osteomalacia is not a single disease. Int. J. Mol. Sci. 2022, 23, 14896. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, E.; Ballanti, P. Osteoporosis-bone remodeling and animal models. Toxicol. Pathol. 2014, 42, 957–969. [Google Scholar] [CrossRef]
- Regmi, P.; Nelson, N.; Haut, R.C.; Orth, M.W.; Karcher, D.M. Influence of age and housing systems on properties of tibia and humerus of Lohmann White hens(1): Bone properties of laying hens in commercial housing systems. Poult. Sci. 2017, 96, 3755–3762. [Google Scholar] [CrossRef]
- Osiak-Wicha, C.; Tomaszewska, E.; Muszyński, S.; Flis, M.; Świetlicki, M.; Arciszewski, M.B. Comparative analysis of morphometric, densitometric, and mechanical properties of skeletal locomotor elements in three duck species (Anatidae: Anatinae). Animals 2024, 14, 2191. [Google Scholar] [CrossRef]
- Hinić-Frlog, S.; Motani, R. Relationship between osteology and aquatic locomotion in birds: Determining modes of locomotion in extinct ornithurae. J. Evol. Biol. 2010, 23, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, M.M.J.; Millet, S.; Aluwé, M.; Janssens, G.P.J. Impact of nutrition on lameness and claw health in sows. Livest. Sci. 2013, 156, 24–35. [Google Scholar] [CrossRef]
- Hasan, M.; Oster, M.; Reyer, H.; Wimmers, K.; Fischer, D.C. Efficacy of dietary vitamin D3 and 25(OH)D3 on reproductive capacities, growth performance, immunity and bone development in pigs. Br. J. Nutr. 2023, 130, 1298–1307. [Google Scholar] [CrossRef]
- Floradin, P.; Pomar, C.; Létourneau-Montminy, M.P.; Schlegel, P. Development of the mineralisation of individual bones and bone regions in replacement gilts according to dietary calcium and phosphorus. Animal 2024, 18, 101241. [Google Scholar] [CrossRef]
- Starczak, Y.; Reinke, D.C.; Barratt, K.R.; Russell, P.K.; Clarke, M.V.; Davey, R.A.; Atkins, G.J.; Anderson, P.H. Vitamin D receptor expression in mature osteoclasts reduces bone loss due to low dietary calcium intake in male mice. J. Steroid Biochem. Mol. Biol. 2021, 210, 105857. [Google Scholar] [CrossRef]
- Mahajan, A.; Alexander, L.S.; Seabolt, B.S.; Catrambone, D.E.; McClung, J.P.; Odle, J.; Pfeiler, T.W.; Loboa, E.G.; Stahl, C.H. Dietary calcium restriction affects mesenchymal stem cell activity and bone development in neonatal pigs. J. Nutr. 2011, 141, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Vötterl, J.C.; Klinsoda, J.; Koger, S.; Hennig-Pauka, I.; Verhovsek, D.; Metzler-Zebeli, B.U. Available phosphorus levels modulate gene expression related to intestinal calcium and phosphorus absorption and bone parameters differently in gilts and barrows. Anim. Biosci. 2023, 36, 740–752. [Google Scholar] [CrossRef]
- Fabà, L.; Gasa, J.; Tokach, M.D.; Font-i-Furnols, M.; Vilarrasa, E.; Solà-Oriol, D. Effects of additional organic micro-minerals and methionine on carcass composition, gait score, bone characteristics, and osteochondrosis in replacement gilts of different growth rate. Anim. Feed Sci. Technol. 2019, 256, 114262. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Mohamed, E.; Abd El-Hack, M.E.; Khafaga, A.F.; Noreldin, A.E.; Arif, M.; Chaudhry, M.T.; Losacco, C.; Abdeen, A.; Abdel-Daim, M.M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lantha. Sci. Total Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef]
- Wang, Z.R.; Zhang, Y.L.; Chen, S.S.; Qu, Y.; Tang, M.C.; Wang, W.Y.; Li, W.C.; Gu, L.S. Multifunctional CeO2 nanozymes for mitigating high-glucose induced senescence and enhancing bone regeneration in type 2 diabetes mellitus. Chem. Eng. J. 2024, 485, 149842. [Google Scholar] [CrossRef]
- Fumoto, T.; Ito, M.; Ikeda, K. Lanthanum carbonate stimulates bone formation in a rat model of renal insufficiency with low bone turnover. J. Bone Miner. Metab. 2014, 32, 484–493. [Google Scholar] [CrossRef]
- Jiang, C.; Shang, J.Y.; Li, Z.; Qin, A.; Ouyang, Z.X.; Qu, X.H.; Li, H.W.; Tian, B.; Wang, W.G.; Wu, C.L.; et al. Lanthanum chloride attenuates osteoclast formation and function via the downregulation of Rankl-induced Nf-kappab and Nfatc1 activities. J. Cell Physiol. 2016, 231, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef]
- Kamenaga, T.; Kuroda, Y.; Nagai, K.; Tsubosaka, M.; Takashima, Y.; Kikuchi, K.; Fujita, M.; Ikuta, K.; Anjiki, K.; Maeda, T.; et al. Cryopreserved human adipose-derived stromal vascular fraction maintains fracture healing potential via angiogenesis and osteogenesis in an immuno-deficient rat model. Stem Cell Res. Ther. 2021, 12, 110. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhou, Z.Z.; Hou, M.Z.; Xia, X.W.; Liu, Y.; Zhao, Z.J.; Wu, Y.B.; Deng, Y.G.; Zhang, Y.J.; He, F.; et al. Capturing cerium ions via hydrogel microspheres promotes vascularization for bone regeneration. Mater. Today Bio 2024, 25, 100956. [Google Scholar] [CrossRef]
- Sabir, P.S.; Abbas, K.A. Effect of strontium ranelate and cerium oxide addition in the diet on bone quality and expression level of osteocalcin and alkaline phosphatase genes in broiler chicken. Vet. Med. Sci. 2023, 9, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef]
- David Yatsonsky, I.; Pan, K.; Shendge, V.B.; Liu, J.; Ebraheim, N.A. Linkage of microbiota and osteoporosis: A mini literature review. World J. Orthop. 2019, 10, 123–127. [Google Scholar]
- Lu, L.Y.; Chen, X.X.; Liu, Y.; Yu, X.J. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Zhang, C.L.; Liu, Z.Y.; Li, C.; Ren, Z.G. Gut microbiota and bone diseases: A growing partnership. Front. Microbiol. 2022, 13, 877776. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, C.L.; Cao, X.J.; Feng, B.; Li, X.L. Intestinal population in host with metabolic syndrome during administration of chitosan and its derivatives. Molecules 2020, 25, 5857. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, S.G.; Lim, S.C.; Ong, J.L. Osteogenic activity of the mixture of chitosan and particulate dentin. J. Biomed. Mater. Res. 2008, 87, 618–623. [Google Scholar] [CrossRef]
- Lu, X.X.; Chang, X.Y.; Zhang, H.J.; Wang, J.; Qiu, K.; Wu, S.G. Effects of dietary rare earth chitosan chelate on performance, egg quality, immune and antioxidant capacity, and intestinal digestive enzyme activity of laying hens. Polymers 2023, 15, 1600. [Google Scholar] [CrossRef]
- Xiong, Y.; Pang, J.M.; Lv, L.K.; Wu, Y.J.; Li, N.; Huang, S.M.; Feng, Z.; Ren, Y.; Wang, J.J. Effects of maternal supplementation with rare earth elements during late gestation and lactation on performances, health, and fecal microbiota of the sows and their offspring. Animals 2019, 9, 738. [Google Scholar] [CrossRef]
- Feng, S.F.; Du, X.Y.; Wang, C.; Ye, D.D.; Ma, G.J.; Zhao, S.H.; Wang, H.Y.; Liu, X.D. Identification of mRNAs related to tibial cartilage development of Yorkshire piglets. Biomed Res. Int. 2019, 2019, 2365416. [Google Scholar] [CrossRef] [PubMed]
- Bioletto, F.; Sibilla, M.; Berton, A.M.; Prencipe, N.; Varaldo, E.; Maiorino, F.; Cuboni, D.; Pusterla, A.; Gasco, V.; Grottoli, S.; et al. Mild hyponatremia is not associated with degradation of trabecular bone microarchitecture despite bone mass loss. J. Clin. Endocrinol. Metab. 2024, 12, dgae234. [Google Scholar] [CrossRef]
- Bisazza, K.T.; Nelson, B.B.; Sikes, K.J.; Nakamura, L.; Easley, J.T. Computed tomography provides improved quantification of trabecular lumbar spine bone loss compared to dual-energy X-ray absorptiometry in ovariectomized sheep. JBMR Plus 2023, 7, e10807. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.L.; Xu, P.; Zhong, Y.H.; Zhou, Z.G.; Zhang, W.C. Interleukin-21 knockout reduces bone loss in ovariectomized mice by inhibiting osteoclastogenesis. Biosci. Biotechnol. Biochem. 2023, 87, 1265–1273. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yao, H.; Tjahjono, A.W.; Xiang, L.; Li, K.; Tang, J.H.; Gao, Y.X. Si-Zhi Wan regulates osteoclast autophagy in osteoporosis through the AMPK signaling pathway to attenuate osteoclastogenesis. J. Pharm. Pharmacol. 2024, 76, 236–244. [Google Scholar] [CrossRef]
- Zou, D.D.; Lin, R.L.; Han, Y.; Jia, J.; Zhou, G.Q.; Zhang, H.S.; Ge, K. Lanthanum promoting bone formation by regulating osteogenesis, osteoclastogenesis and angiogenesis. J. Rare Earths 2024, 42, 621–628. [Google Scholar] [CrossRef]
- Xu, W.; Wei, K.; Lin, Z.; Wu, T.; Li, G.; Wang, L. Storage and release of rare earth elements in microsphere-based scaffolds for enhancing osteogenesis. Sci. Rep. 2022, 12, 6383. [Google Scholar] [CrossRef]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological reaction in TNF-alpha-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013, 2013, 181849. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Frey, B.; Hess, A.; Zwerina, J.; Luther, J.; Nimmerjahn, F.; Engelke, K.; Kollias, G.; Hünig, T.; Schett, G.; et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J. Immunol. 2010, 184, 7238–7246. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Bone remodeling and the microbiome. Cold Spring Harb. Perspect. Med. 2018, 8, a031203. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R.; Probiotic, L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Bubnov, R.; Babenko, L.; Lazarenko, L.; Kryvtsova, M.; Shcherbakov, O.; Zholobak, N.; Golubnitschaja, O.; Spivak, M. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J. 2019, 10, 317–335. [Google Scholar] [CrossRef]
- Ye, Q.R.; Jia, D.T.; Ji, J.; Liu, Y.; Wu, G. Effects of nano-cerium dioxide on intestinal microflora in rats by oral subchronic exposure. PLoS ONE 2024, 19, e0298917. [Google Scholar] [CrossRef]
- Cai, L.; Nyachoti, C.M.; Kim, I.H. Impact of rare earth element enriched yeast on growth performance, nutrient digestibility, blood profile, and fecal microflora in finishing pigs. Can. J. Anim. Sci. 2018, 98, 347–353. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.J.; Tang, G.J.; Deng, P.; Qin, Y.Y.; Han, J.L.; Wang, S.L.; Sun, X.J.; Li, D.X.; Chen, Z.J. The causal relationship between gut microbiota and bone mineral density: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1268935. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Wang, T.F.; Guan, K.F.; Su, Q.J.; Wang, X.T.; Yan, Z.Q.; Kuang, K.L.; Wang, Y.; Zhang, Q.D.; Zhou, X.; Liu, B. Change of gut microbiota in PRRSV-resistant pigs and PRRSV-susceptible pigs from Tongcheng pigs and Large white pigs crossed population upon PRRSV infection. Animals 2022, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Wu, B.; He, T.S.; Xie, L.; Liu, Z.P. Dock2 affects the host susceptibility to Citrobacter rodentium infection through regulating gut microbiota. Gut Pathog. 2021, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Aliakbari, A.; Zemb, O.; Billon, Y.; Barilly, C.; Ahn, I.; Riquet, J.; Gilbert, H. Genetic relationships between feed efficiency and gut microbiome in pig lines selected for residual feed intake. J. Anim. Breed. Genet. 2021, 138, 491–507. [Google Scholar] [CrossRef]
- Hua, C.F.; Tian, J.; Tian, P.; Cong, R.; Luo, Y.W.; Geng, Y.; Tao, S.Y.; Ni, Y.D.; Zhao, R.Q. Feeding a high concentration diet induces unhealthy alterations in the composition and metabolism of ruminal microbiota and host response in a goat model. Front. Microbiol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Gualdrón-Duarte, L.B.; Allen, M.A. Effects of acetic acid or sodium acetate infused into the rumen or abomasum on feeding behavior and metabolic response of cows in the postpartum period. J. Dairy Sci. 2018, 101, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, G.; Liu, Z.; Wu, P.; Yu, Z.; Wang, J. Repeated inoculation with fresh rumen fluid before or during weaning modulates the microbiota composition and co-occurrence of the rumen and colon of lambs. BMC Microbiol. 2020, 20, 29. [Google Scholar] [CrossRef]
- Lin, A.L.; Yan, X.X.; Wang, H.Y.; Su, Y.; Zhu, W.Y. Effects of lactic acid bacteria-fermented formula milk supplementation on ileal microbiota, transcriptomic profile, and mucosal immunity in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 113. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Xiao, K.P.; Jiang, F.; Wang, H.C.; Tang, D.Z.; Liu, D.; Liu, B.; Liu, Y.S.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef]
- Rao, Z.Y.; Li, Y.; Yang, X.P.; Guo, Y.P.; Zhang, W.; Wang, Z.X. Diet xylo-oligosaccharide supplementation improves growth performance, immune function, and intestinal health of broilers. Anim. Nutr. 2024, 17, 165–176. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Smith, P.; Howitt, M.; Panikov, N.; Michaud, M.; Gallini, C.; Bohlooly-Y., M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Wang, H.Y.; Xia, P.K.; Lu, Z.Y.; Su, Y.; Zhu, W.Y. Metabolome-microbiome responses of growing pigs induced by time-restricted feeding. Front. Vet. Sci. 2021, 8, 681202. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.; Cotter, P. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.N.; Ma, Y.; Xiong, K.; Wang, Y.R.; Liu, Y.J.; Sun, Y.; Yang, Y.X.; Ma, A.G. Ameliorating effects of vitamin K2 on dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Mol. Sci. 2023, 24, 2986. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functionalinteractions between the gut microbiota and host metabolism. Nature 2012, 489, 242249. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Siegerstetter, S.C.; Magowan, E.; Lawlor, P.G.; O’Connell, N.E.; Zebeli, Q. Fecal microbiota transplant from highly feed efficient donors affects cecal physiology and microbiota in low-and high-feed efficient chickens. Front. Microbiol. 2019, 10, 1576. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Roongsitthichai, A.; Tummaruk, P. Importance of backfat thickness to reproductive performance in female pigs. Thai J. Vet. Med. 2014, 44, 171–178. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, Y.; Yang, T.; Ao, H.; Chen, S.K.; Xing, K.; Zhang, F.X.; Zhao, X.T.; Liu, J.F.; Wang, C.D. Differences in gut microbiota composition in finishing Landrace pigs with low and high feed conversion ratios. Antonie Leeuwenhoek 2018, 111, 1673–1685. [Google Scholar] [CrossRef]
- Zhou, Z.; Niu, H.J.; Bian, M.; Zhu, C.S. Kidney tea [Orthosiphon aristatus (Blume) Miq.] improves diabetic nephropathy via regulating gut microbiota and ferroptosis. Front. Pharmacol. 2024, 15, 1392123. [Google Scholar] [CrossRef]
- Kusumawati, A.; Mustopa, A.Z.; Wibawan, I.W.T.; Setiyono, A.; Sudarwanto, M.B. Metagenomic analysis of pathogen mastitis in cow’s milk from Cicurug, Sukabumi, West Java, Indonesia. Earth Environ. Sci. 2021, 762, 012064. [Google Scholar] [CrossRef]
- Kuehbacher, T.; Rehman, A.; Lepage, P.; Hellmig, S.; lsch, U.R.F.; Schreiber, S.; Ott, S.J. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J. Med. Microbiol. 2008, 57, 1569–1576. [Google Scholar] [CrossRef]
- Miao, Z.G.; Zhao, W.X.; Guo, L.P.; Wang, S.; Zhang, J.Z. Effects of dietary supplementation of chitosan on immune function in growing Huoyan geese. Poult. Sci. 2020, 99, 95–100. [Google Scholar] [CrossRef]
- Zeng, Y.D.; Wang, Z.R.; Zou, T.D.; Chen, J.; Li, G.H.; Zheng, L.Z.; Li, S.; You, J.M. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Yan, L.W.; Cao, X.H.; Luo, Y.M.; Peng, X.T.; Wang, Z.R.; Zou, T.D.; Chen, J.; You, J.M. Effects of rare earth as feed additive on production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota in late-phase laying hens. Front. Sustain. Food Syst. 2023, 7, 1155543. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, X.Q.; Xu, Z.; Yao, W.X.; Leng, X.J. Dietary Azomite, a natural trace mineral complex, improved the growth, immunity response, intestine health and resistance against bacterial infection in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 108, 53–62. [Google Scholar] [CrossRef]
- Sun, P.; Li, D.; Li, Z.; Dong, B.; Wang, F. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J. Nutr. Biochem. 2008, 19, 627–633. [Google Scholar] [CrossRef]
- Zhou, H.M.; Wu, H.D.; Chen, Y.X.; Zou, W.J.; Lu, W.; He, Y.Y. Administration of all-trans retinoic acid to pregnant sows alters gut bacterial community of neonatal piglets with different hoxa1 genotypes. Front. Microbiol. 2021, 12, 712212. [Google Scholar] [CrossRef]



| RECC0 | RECC200 | RECC400 | RECC600 | |
|---|---|---|---|---|
| Initial live body weight (kg) | 7.53 ± 0.05 | 7.67 ± 0.10 | 7.78 ± 0.09 | 7.71 ± 0.07 |
| Final live body weight (kg) | 18.59 ± 0.09 C | 20.17 ± 0.02 A | 19.59 ± 0.08 B | 19.47 ± 0.10 B |
| Average daily gain (g/d) | 395.14 ± 4.78 c | 446.25 ± 3.04 a | 421.93 ± 0.22 b | 420.12 ± 1.19 b |
| Average daily feed intake (g/d) | 645.45 ± 0.74 | 669.94 ± 4.61 | 678.18 ± 12.01 | 693.29 ± 13.39 |
| Feed intake/body weight gain | 1.63 ± 0.02 a | 1.50 ± 0.01 b | 1.61 ± 0.03 ab | 1.65 ± 0.05 a |
| Diarrhea incidence (%) | 18.39 ± 1.61 Aa | 5.89 ± 1.25 Bb | 9.46 ± 1.25 ABb | 15.00 ± 1.43 Aa |
| RECC0 | RECC200 | RECC400 | RECC600 | |
|---|---|---|---|---|
| Tb.Pm (mm) | 522.90 ± 60.23 b | 731.88 ± 61.39 a | 726.55 ± 44.34 a | 645.41 ± 41.14 ab |
| Tb.Ar (mm2) | 7.01 ± 0.54 Cc | 9.50 ± 0.69 ABb | 11.31 ± 0.62 Aa | 7.92 ± 0.46 BCbc |
| Tb.Th (um) | 24.79 ± 2.13 ab | 22.22 ± 1.35 ab | 26.36 ± 1.11 a | 21.18 ± 1.44 b |
| Tb.N (1/mm) | 6.42 ± 0.74 b | 8.99 ± 0.75 a | 8.92 ± 0.54 a | 7.93 ± 0.51 ab |
| Tb.Sp (um) | 167.14 ± 28.07 Aa | 97.84 ± 8.36 Bb | 91.88 ± 8.02 Bb | 112.83 ± 9.51 ABb |
| N.Oc (N/mm2) | 81.11 ± 9.93 A | 19.31 ± 3.36 B | 27.04 ± 3.84 B | 28.14 ± 5.93 B |
| RECC0 | RECC200 | RECC400 | RECC600 | |
|---|---|---|---|---|
| Ace | 649.25 ± 36.65 a | 654.37 ± 11.04 a | 550.39 ± 26.89 b | 571.06 ± 16.78 b |
| Chao1 | 651.23 ± 33.69 a | 660.39 ± 10.06 a | 553.89 ± 28.63 b | 575.48 ± 17.54 b |
| Shannon | 6.35 ± 0.28 a | 6.17 ± 0.09 ab | 5.79 ± 0.16 b | 5.64 ± 0.09 b |
| Simpson | 0.96 ± 0.01 | 0.95 ± 0.01 | 0.95 ± 0.01 | 0.95 ± 0.01 |
| RECC0 | RECC200 | RECC400 | RECC600 | |
|---|---|---|---|---|
| Villus height (mm) | 0.38 ± 0.02 B | 0.49 ± 0.03 A | 0.39 ± 0.01 B | 0.37 ± 0.02 B |
| Crypt depth (mm) | 0.28 ± 0.01 B | 0.35 ± 0.01 A | 0.33 ± 0.01 A | 0.34 ± 0.01 A |
| Villus height/crypt depth | 1.40 ± 0.09 Aa | 1.42 ± 0.09 Aa | 1.19 ± 0.04 ABb | 1.09 ± 0.04 Bb |
| RECC0 | RECC200 | RECC400 | RECC600 | |
|---|---|---|---|---|
| IgM (μg/mL) | 104.51 ± 5.55 B | 145.98 ± 6.61 A | 146.81 ± 3.55 A | 106.24 ± 3.81 B |
| IgA (μg/mL) | 40.00 ± 1.98 C | 66.13 ± 1.68 A | 62.30 ± 2.04 A | 52.16 ± 2.46 B |
| IgG (g/L) | 14.07 ± 0.72 B | 21.59 ± 1.25 A | 22.21 ± 0.81 A | 19.76 ± 0.54 A |
| IL-10 (pg/mL) | 147.65 ± 3.29 Cb | 184.23 ± 4.07 ABa | 175.99 ± 6.43 Ba | 157.57 ± 6.98 BCb |
| IL-2 (pg/mL) | 282.07 ± 16.52 | 254.14 ± 11.43 | 273.08 ± 11.44 | 269.84 ± 11.28 |
| TNF-α (pg/mL) | 222.95 ± 5.95 Aa | 167.67 ± 10.83 Bc | 205.31 ± 5.71 Aab | 193.92 ± 7.75 Ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Sun, W.; Wei, P.; Wu, H.; Lu, W.; He, Y. Supplementation with Rare Earth–Chitosan Chelate Improves Tibia Quality, Disease Resistance Capacity, and Performance in Nursery Pigs. Int. J. Mol. Sci. 2025, 26, 2409. https://doi.org/10.3390/ijms26062409
Hao S, Sun W, Wei P, Wu H, Lu W, He Y. Supplementation with Rare Earth–Chitosan Chelate Improves Tibia Quality, Disease Resistance Capacity, and Performance in Nursery Pigs. International Journal of Molecular Sciences. 2025; 26(6):2409. https://doi.org/10.3390/ijms26062409
Chicago/Turabian StyleHao, Shaobin, Wenchen Sun, Panting Wei, Huadong Wu, Wei Lu, and Yuyong He. 2025. "Supplementation with Rare Earth–Chitosan Chelate Improves Tibia Quality, Disease Resistance Capacity, and Performance in Nursery Pigs" International Journal of Molecular Sciences 26, no. 6: 2409. https://doi.org/10.3390/ijms26062409
APA StyleHao, S., Sun, W., Wei, P., Wu, H., Lu, W., & He, Y. (2025). Supplementation with Rare Earth–Chitosan Chelate Improves Tibia Quality, Disease Resistance Capacity, and Performance in Nursery Pigs. International Journal of Molecular Sciences, 26(6), 2409. https://doi.org/10.3390/ijms26062409




