Negative Alterations in the Respiratory Activity of Isolated Crude Heart Mitochondria Following In Vivo Isoproterenol Injection in Rats Are Not Observed in Heart Homogenate Suggesting That the Isolation Procedure Generates Experimental Artefacts
Abstract
1. Introduction
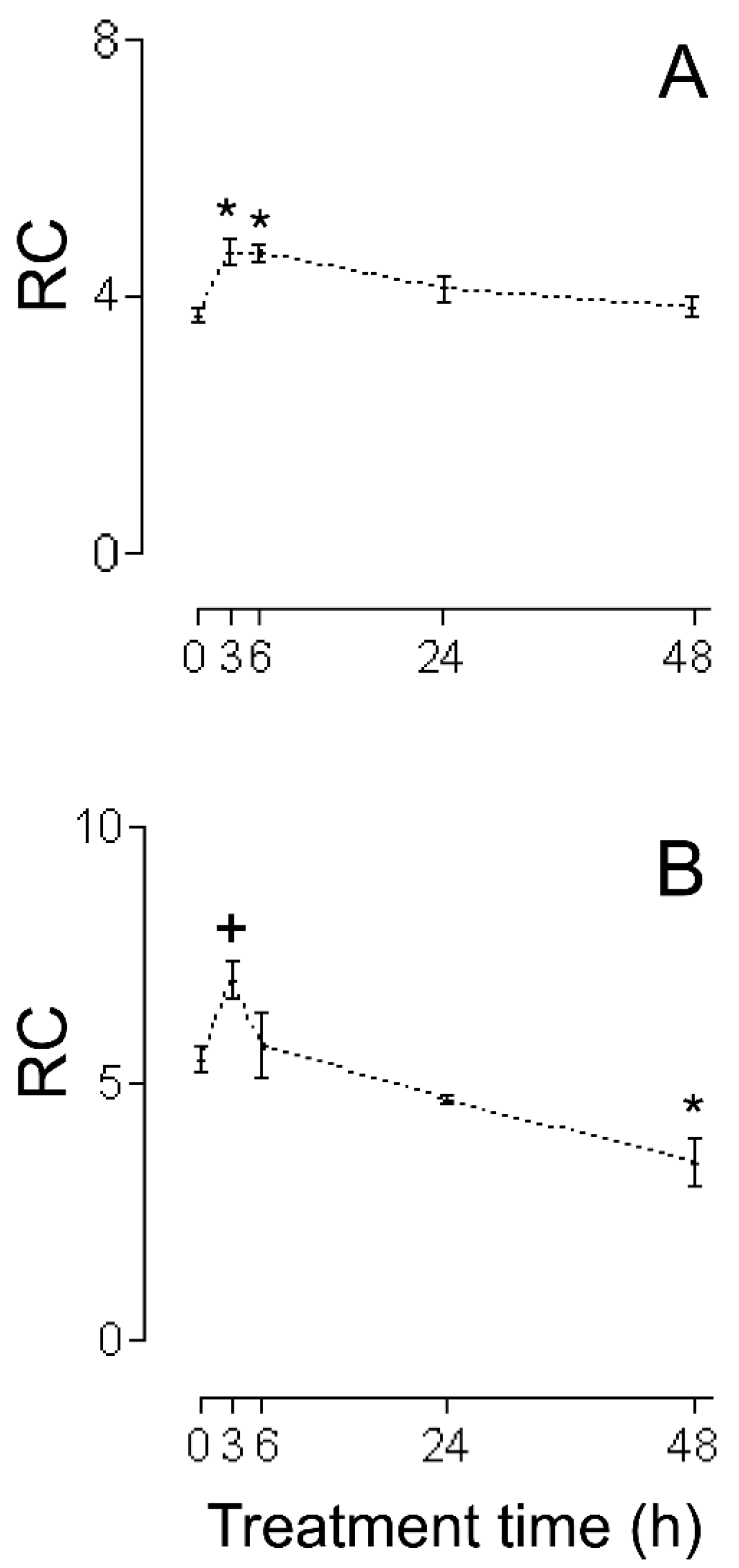
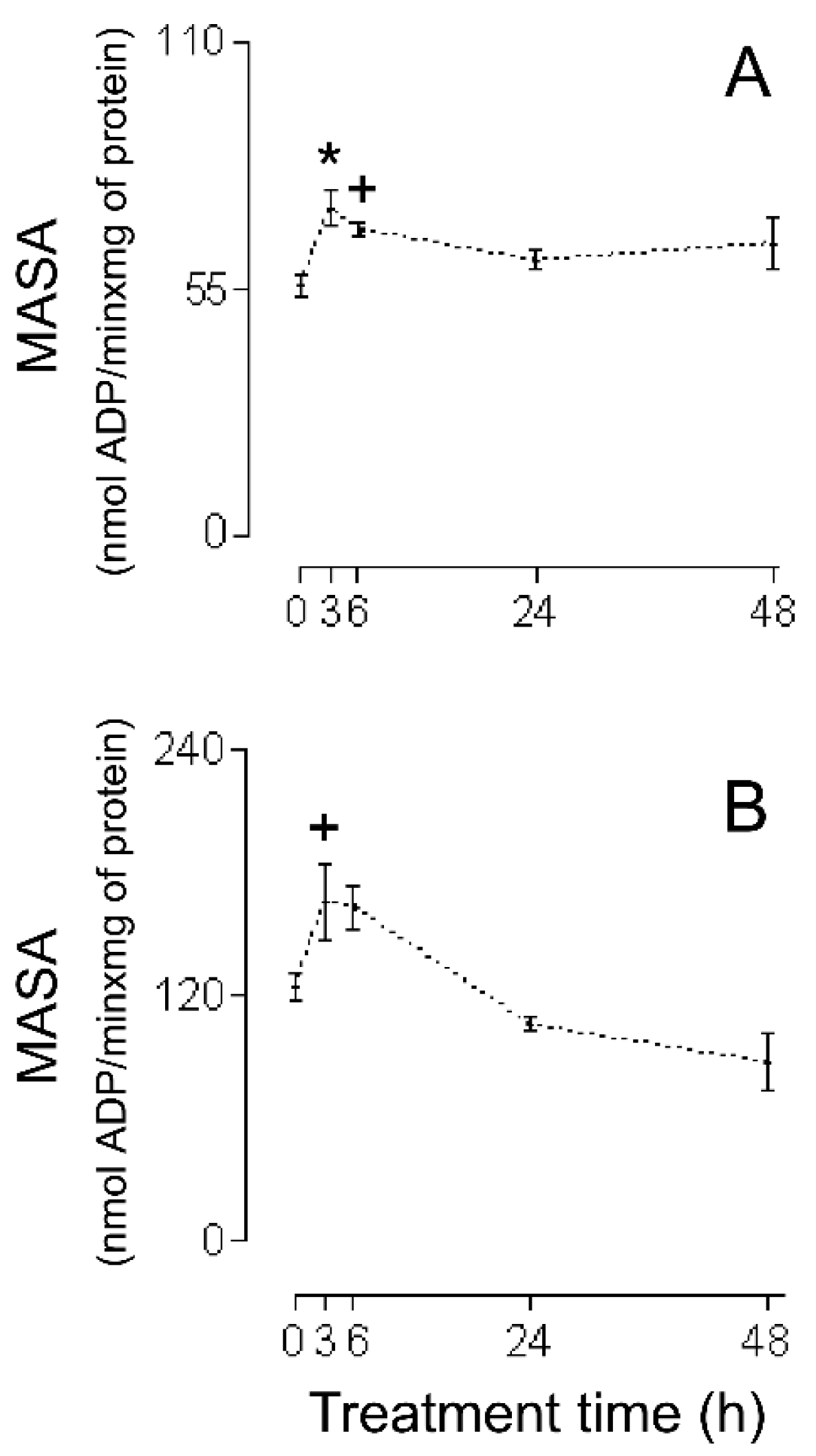
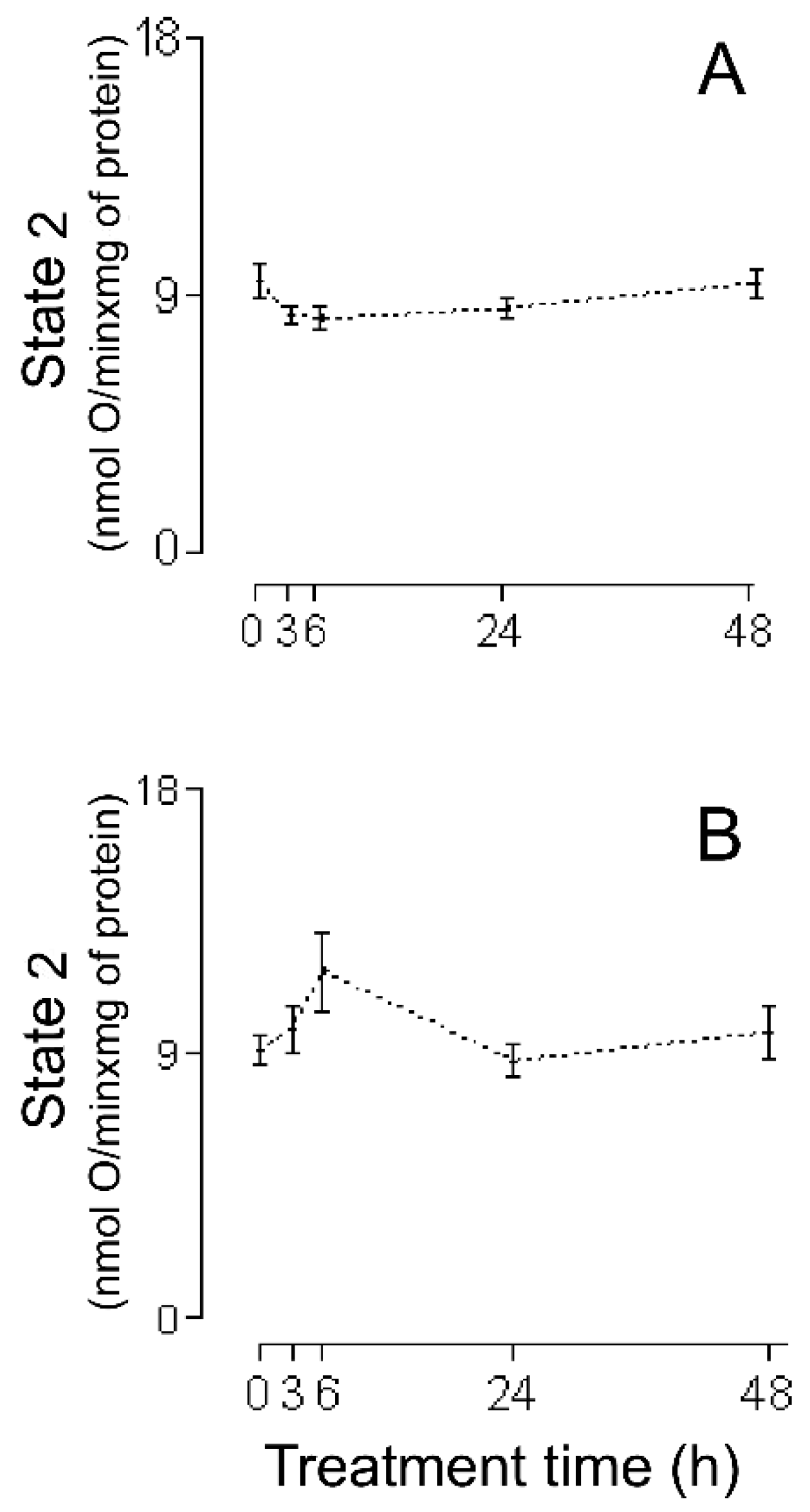
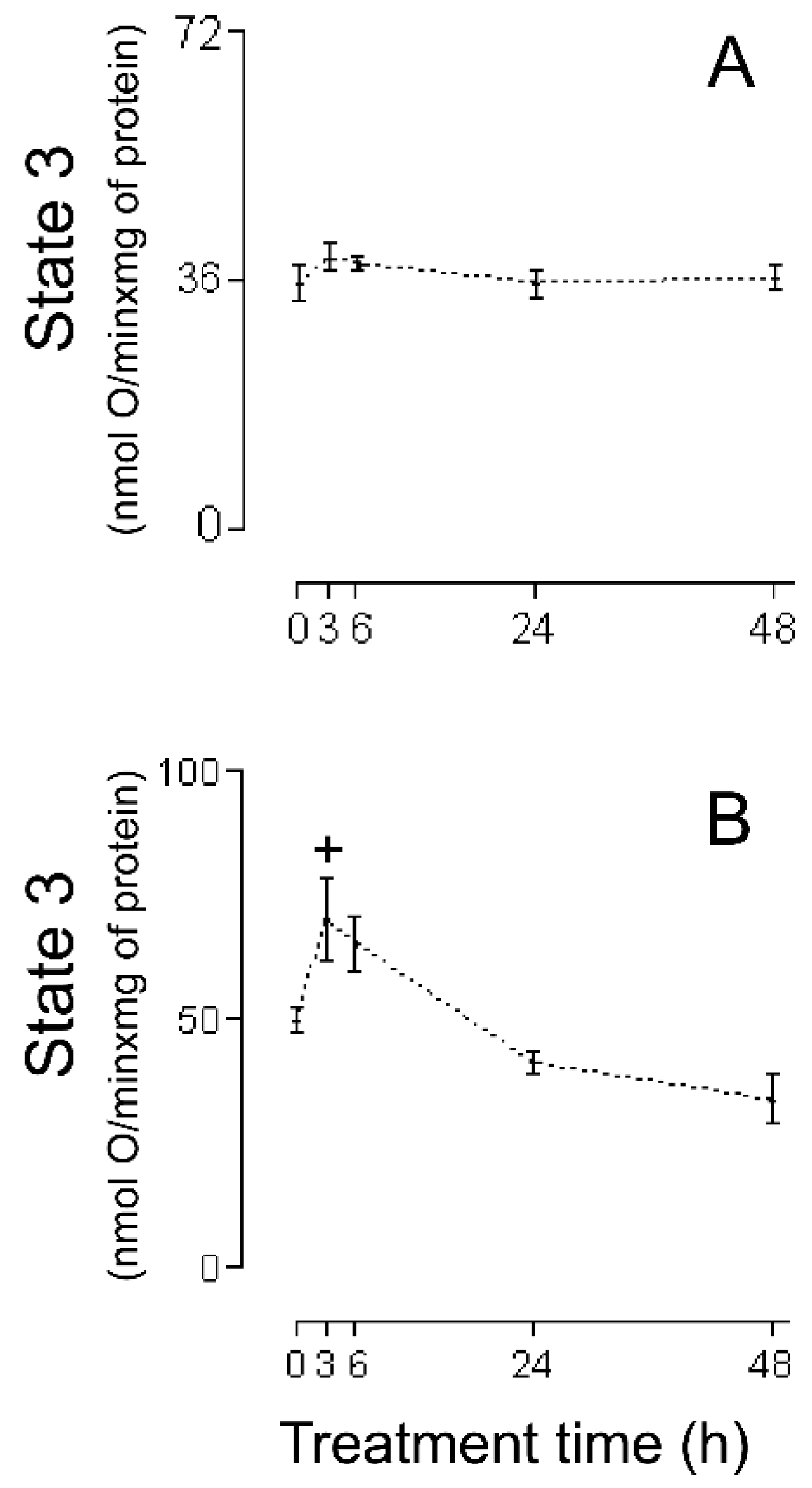
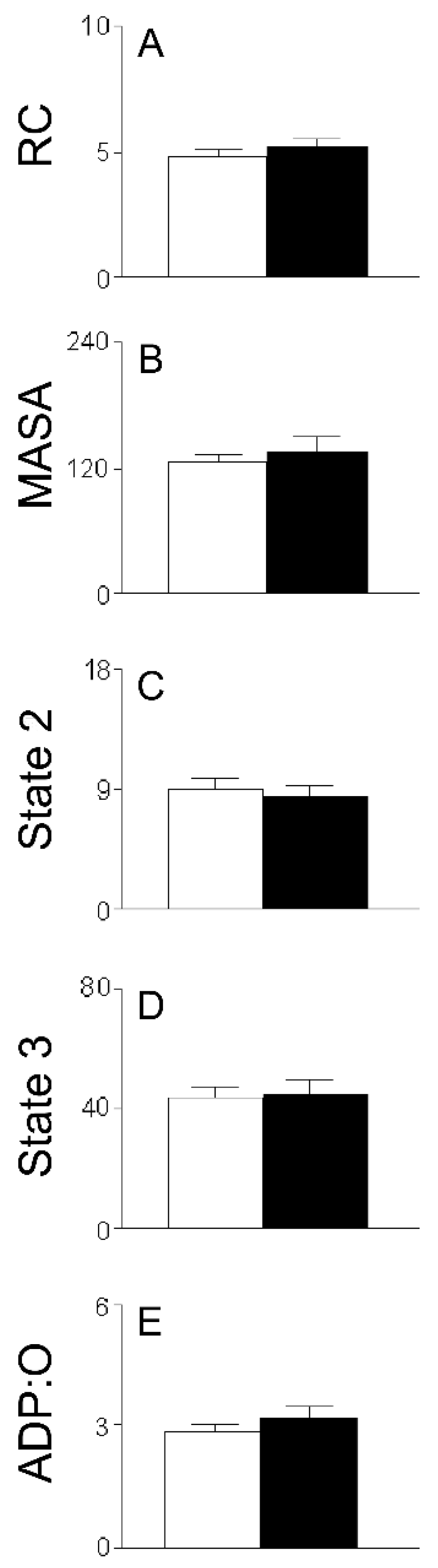
2. Results
3. Discussion
3.1. HMRA Using HHs
3.2. HMRA Using CHMs
3.3. HMRA Using IHMs
3.4. Disadvantage in the Present Study
3.5. Other Considerations
4. Materials and Methods
4.1. Experimental Design
4.2. Preparation of the HHs
4.3. Preparation of the CHMs and IHMs
4.4. Determination of the HMRA
4.5. Statistics
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frishman, W.H.; Sonnenblick, E.H. Β-adrenergic blocking drugs. In The Heart, Arteries and Veins, 7th ed.; Hurst, J.W., Schlant, R.C., Rackley, C.E., Sonnenblick, E.H., Wenger, N.K., Eds.; McGraw-Hill: New York, NY, USA, 1990; pp. 1712–1731. [Google Scholar]
- Szakacs, J.E.; Cannon, C.A. L-norepinephrine myocarditis. Am. J. Clin. Pathol. 1958, 30, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Rona, G.; Chappel, C.I.; Balazs, T.; Gaudry, R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch. Pathol. 1959, 67, 443–455. [Google Scholar] [PubMed]
- Rona, G.; Boutet, M.; Huttner, Y. Reperfusion injury: A possible link between catecholamine-induced and ischemic myocardial alterations. Adv. Cardiol. 1983, 4, 427–439. [Google Scholar]
- Cebelin, M.S.; Hirsch, C.S. Human stress cardiomyopathy. Hum. Pathol. 1980, 11, 123–132. [Google Scholar] [CrossRef]
- Mello de Oliveira, J.A. Enzyme histochemistry of the heart in chronic phase of the experimental trypanosomiasis CT, (T. Cruzi) in rats. Rev. Goiana Med. 1976, 22, 1–65. [Google Scholar]
- Chagoya de Sànchez, V.; Hernández-Muñoz, R.; López-Barrera, F.; Yañez, L.; Vidrio, S.; Suárez, J.; Cota-Garza, M.D.; Aranda-Fraustro, A.; Cruz, D. Sequential changes of energy metabolism and mitochondrial function in myocardial infarction induced by isoproterenol in rats: A long-term and integrative study. Can. J. Physiol. Pharmacol. 1997, 75, 1300–1311. [Google Scholar] [CrossRef]
- Heather, L.C.; Catchpole, A.F.; Stuckey, D.J.; Cole, M.A.; Carr, C.A.; Clarke, K. Isoproterenol induces in vivo functional and metabolic abnormalities: Similar to those found in the infarcted rat heart. J. Physiol. Pharmacol. 2009, 60, 31–39. [Google Scholar]
- Sachdeva, J.; Dai, W.; Kloner, R.A.; Lovenberg, W. Functional and histological assessment of an experimental model of Takotsubo’s cardiomyopathy. J. Am. Heart Assoc. 2014, 3, e000921. [Google Scholar] [CrossRef]
- Sobel, B.; Jeguier, F.; Sjoesdsma, A.; Lovenberg, W. Effect of catecholamines and adrenergic blocking agents on oxidative phosphorylation in rat heart mitochondria. Circ. Res. 1966, 19, 1050–1061. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Yates, J.C.; Lee, S.L.; Singh, A. Functional and subcellular changes in the isolated rat heart perfused with oxidized isoproterenol. J. Mol. Cell. Cardiol. 1978, 10, 31–41. [Google Scholar] [CrossRef]
- Ishiyama, T.; Morita, Y.; Tsukamoto, N.; Yamamura, Y. A correlative study of the experimental cardiac dynamics and myocardial energy metabolism. Jpn. Circ. J. 1975, 39, 1313–1320. [Google Scholar] [CrossRef][Green Version]
- Grieve, S.J.; Williams, A.J. The isolation and characterization of cardiac mitochondrial fractions from isoprenaline treated rats. J. Mol. Cell. Cardiol. 1981, 13, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Miyazaki, Y.; Ogawa, K.; Satake, T.; Sugiyama, S.; Ozawa, T. The effects of β-blocking agents on mitochondrial function in ischemic myocardium. Jpn. Circ. J. 1984, 48, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Curti, C.; Uyemura, S.A.; Grecchi, M.J.; Leone, F.A. Kinetic properties of mitochondrial ATPase during isoproterenol-induced cardiomyopathy. Int. J. Biochem. 1990, 22, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Uyemura, S.; Curti, C. Respiration and mitochondrial ATPase in energized mitochondria during isoproterenol-induced cell injury of myocardium. Int. J. Biochem. 1991, 23, 1143–1149. [Google Scholar] [CrossRef]
- Capozza, G.; Muolo, L.; Jirillo, E.; Guerrieri, F. Isoproterenol causes changes in the mitochondrial energy metabolism in the rat heart. Cardiologia 1992, 37, 663–665. [Google Scholar]
- Poderoso, J.J.; Fernandez, S.; Carreras, M.C.; Del Bosco, C.G.; Boveris, A. Isoproterenol-dependent decrease in oxygen uptake and respiratory enzyme activities in rat myocardial tissue and mitochondria. Crit. Care Med. 1995, 23, 1726–1733. [Google Scholar] [CrossRef]
- David, C.E.B.; Lucas, A.M.B.; Cunha, P.L.O.; Viana, Y.I.P.; Yoshinaga, M.Y.; Miyamoto, S.; Filho, A.B.C.; Varela, A.L.N.; Kowaltowski, A.J.; Facundo, H.T. Calorie restriction changes lipidomic profiles and maintains mitochondrial function and redox balance during isoproterenol-induced cardiac hypertrophy. J. Physiol. Biochem. 2022, 78, 283–294. [Google Scholar] [CrossRef]
- Rendon, D.A.; Lopez, L.F. Mitochondrial oligomycin-sensitive ATPase during isoproterenol-induced cell injury of myocardium. Arch. Inst. Cardiol. Mex. 2000, 70, 130–135. [Google Scholar]
- Rendon, D.A.; Lopez, L.F. Activation of mitochondrial oxidative phosphorylation during (±)-isoproterenol-induced cell injury of myocardium. Arch. Cardiol. Mex. 2001, 71, 13–19. [Google Scholar]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Rendon, D.A. Important methodological aspects that should be taken into account during the research of isolated mitochondria. Anal. Biochem. 2020, 15, 113492. [Google Scholar] [CrossRef] [PubMed]
- Somani, P.; Laddu, A.R.; Hardman, H. Nutritional circulation in the heart. III. Effects of isoproterenol and beta adrenergic blockade on myocardial hemodynamics and rubidium-86 extraction in the isolated supported heart preparation. J. Pharmacol. Exp. Ther. 1970, 175, 577–592. [Google Scholar] [CrossRef]
- Frafix, T.A.; Heineman, F.W.; Balaban, R.S. Effect of work on intracellular calcium of the intact heart. Am. J. Physiol. 1991, 261, 54–59. [Google Scholar] [CrossRef]
- Das, A.M.; Harris, D.A. Control of mitochondrial ATPsynthase in heart cells: Inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc. Res. 1990, 24, 411–417. [Google Scholar] [CrossRef]
- De Rasmo, D.; Gattoni, G.; Papa, F.; Santeramo, A.; Pacelli, C.; Cocco, T.; Micelli, L.; Sardaro, N.; Larizza, M.; Scivetti, M.; et al. The β-adrenoceptor agonist isoproterenol promotes the activity of respiratory chain complex I and lowers cellular reactive oxygen species in fibroblast and heart myoblast. Eur. J. Pharmacol. 2011, 652, 15–22. [Google Scholar] [CrossRef]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: Role of the Fo/F1 ATPase. Am. J. Physiol. Cell Physiol. 2000, 278, C423–C435. [Google Scholar] [CrossRef]
- Bernardi, P.; Lisa, F.D.; Fogolari, F.; Lippe, G. From ATP to PTP and back: A dual function for the mitochondrial ATP synthase. Circ. Res. 2015, 116, 1850–1862. [Google Scholar] [CrossRef]
- Díaz-Muñoz, M.; Alvarez-Pérez, M.A.; Yáñez, L.; Vidrio, S.; Martínez, L.; Rosas, G.; Yáñez, M.; Ramírez, S.; de Sánchez, V.C. Correlation between oxidative stress of alteration of intracellular calcium handling in isoproterenol-induced myocardial infarction. Mol. Cell Biochem. 2006, 289, 125–136. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Ferguson, S.J. The chemiosmotic proton circuit in isolated organelles. In Bioenergetics 4, 4th ed.; Nicholls, D.G., Ferguson, S.J., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 53–83. [Google Scholar]
- Nef, H.M.; Möllmann, H.; Troidl, C.; Kostin, S.; Böttger, T.; Voss, S.; Hilpert, P.; Krause, N.; Weber, M.; Rolf, A.; et al. Expression profiling of cardiac genes in Tako-Tsubo cardiomyopathy: Insight into a new cardiac entity. J. Mol. Cell. Cardiol. 2008, 44, 395–404. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, S.B.; Singh, U.R. Beta-adrenoreceptor agonist isoproterenol alters oxidative status, inflammatory signaling, injury markers and apoptotic cell death in myocardium of rats. Indian J. Clin. Biochem. 2015, 30, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rendon, D.A. Alterations of mitochondria in liver but not in heart homogenates after treatment of rats with benznidazole. Hum. Exp. Toxicol. 2014, 33, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Izem-Meziane, M.; Djerdjouri, B.; Rimbaud, S.; Caffin, F.; Fortin, D.; Garnier, A.; Veksler, V.; Joubert, F.; Ventura-Clapier, R. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: Protective effect of curcumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H665–H674. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.T.; Thisted, L.; Zois, N.E.; Thrane, S.T.; West, J.A.; Fosgerau, K.; Griffin, J.L.; Fink, L.N.; Murray, A.J. Beta-adrenergic agonism protects mitochondrial metabolism in the pancreatectomised rat heart. Sci. Rep. 2024, 14, 19383. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef]
- Tamura, K.; Hayatsu, H.; Watanabe, I.; Nakano, T.; Sugawara, Y.; Nishii, Y. Evaluation of mitochondrial function by measuring the heat production in state 3 and state 4 respiration. Chem. Pharm. Bull. 1989, 37, 3033–3036. [Google Scholar] [CrossRef]
- Krestinina, O.; Baburina, Y.; Krestinin, R.; Odinokova, I.; Fadeeva, I.; Sotnikova, L. Astaxanthin prevents mitochondrial impairment induced by isoproterenol in isolated rat heart mitochondria. Antioxidants 2020, 9, 262. [Google Scholar] [CrossRef]
- Krestinin, R.; Baburina, Y.; Odinokova, I.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Krestinina, O. Isoproterenol-induced permeability transition pore-related dysfunction of heart mitochondria is attenuated by Astaxanthin. Biomedicines 2020, 8, 437. [Google Scholar] [CrossRef]
- Odinokova, I.; Baburina, Y.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Akatov, V.; Krestinina, O. Effect of melatonin on rat heart mitochondria in acute heart failure in aged rats. Int. J. Mol. Sci. 2018, 19, 1555. [Google Scholar] [CrossRef]
- Popova, T.A.; Muzyko, E.A.; Kustova, M.V.; Bychenkova, M.A.; Perfilova, V.N.; Prokofiev, I.I.; Samoylova, M.A.; Tyurenkov, I.N.; Latypova, G.M.; Kataev, V.A. Influence of the dense extract from herb of Primula veris, L. On the oxidative stress development and the functional state of the cardiomyocytes mitochondria of rats with experimental chronic heart failure. Biomed. Khim. 2018, 64, 334–343. [Google Scholar] [CrossRef]
- Panasiuk, O.S.; Shysh, A.M.; Moĭbenko, O.O. The influence of dietary omega-3 polyunsaturated fatty acids on functional parameters of myocardial mitochondria during isoproterenol-induced heart injury. Fiziolohichnyi Zhurnal 2014, 60, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Babu, P.V.A.; Shyamaladevi, C.S. Protective effect of aspartate and glutamate on cardiac mitochondrial function during myocardial infarction in experimental rats. Chem. Biol. Interact. 2008, 176, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Suchalatha, S.; Srinivasan, P.; Devi, C.S. Effect of T. Chebula on mitochondrial alterations in experimental myocardial injury. Chem. Biol. Interact. 2007, 169, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Shyamala Devi, C.S. Effect of mangiferin on mitochondrial energy production in experimentally induced myocardial infarcted rats. Vascul Pharmacol. 2006, 44, 519–525. [Google Scholar] [CrossRef]
- Padma, V.V.; Devi, C.S. Effect of fish oil on mitochondrial respiration in isoproterenol induced myocardial infarction in rats. Indian J. Exp. Biol. 2002, 40, 268–272. [Google Scholar]
- Han, L.; Chen, K.J. The protective effects of yi-xin-kang capsule on the structure and function of rat myocardial mitochondria. Zhongguo Zhong Yao Za Zhi 2001, 26, 773–777. [Google Scholar]
- Ithayarasi, A.P.; Shyamala Devi, C. Effect of alpha-tocopherol on mitochondrial electron transport in experimental myocardial infarction in rats. Indian J. Biochem. Biophys. 1998, 35, 115–119. [Google Scholar]
- Belosludtseva, N.V.; Uryupina, T.A.; Pavlik, L.L.; Mikheeva, I.B.; Talanov, E.Y.; Venediktova, N.I.; Serov, D.A.; Stepanov, M.R.; Ananyan, M.A.; Mironova, G.D. Pathological alterations in heart mitochondria in a rat model of isoprenaline-induced myocardial injury and their correction with water-soluble taxifolin. Int. J. Mol. Sci. 2024, 25, 11596. [Google Scholar] [CrossRef]
- Ash, C.E.; Merry, B.J. The molecular basis by which dietary restricted feeding reduces mitochondrial reactive oxygen species generation. Mech. Ageing Dev. 2011, 132, 43–54. [Google Scholar] [CrossRef]
- David, C.E.B.; Lucas, A.M.B.; Araújo, M.T.S.; Coelho, B.N.; Neto, J.B.S.; Portela, B.R.C.; Varela, A.L.N.; Kowaltowski, A.J.; Facundo, H.T. Calorie restriction attenuates hypertrophy-induced redox imbalance and mitochondrial ATP-sensitive K+ channel repression. J. Nutr. Biochem. 2018, 62, 87–94. [Google Scholar] [CrossRef]
- Serna, J.D.C.; Caldeira da Silva, C.C.; Kowaltowski, A.J. Functional changes induced by caloric restriction in cardiac and skeletal muscle mitochondria. J. Bioenerg. Biomembr. 2020, 52, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rendon, D.A. Letter to the Editor: The bioenergetics of hepatic mitochondria isolated from avocado oil-treated rats: Typical experimental errors in the study of the bioenergetics of isolated mitochondria. J. Bioenerg. Biomembr. 2015, 47, 451–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rendon, D.A. Mitochondria isolated from the striatum brain of acute paraquat treated rats exhibit a higher degree of oxidative phosphorylation coupling, which shows that they are not subject to energetic dysfunction upon acute paraquat administration. J. Bioenerg. Biomembr. 2016, 48, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Rendon, D.A.; Alvarez-Bustamante, J.A. Early hyperglycemia following alloxan administration in vivo is not associated with altered hepatic mitochondrial function: Acceptable model for type 1 diabetes? Can. J. Physiol. Pharmacol. 2011, 89, 477–484. [Google Scholar] [CrossRef]
- Rendon, D.A. The bioenergetics of isolated mitochondria from different animal models for diabetes. Curr. Diabetes Rev. 2016, 12, 66–80. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Human Experimental Techniques; Methuen and Co., Ltd.: London, UK, 1959. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendon, D.A. Negative Alterations in the Respiratory Activity of Isolated Crude Heart Mitochondria Following In Vivo Isoproterenol Injection in Rats Are Not Observed in Heart Homogenate Suggesting That the Isolation Procedure Generates Experimental Artefacts. Int. J. Mol. Sci. 2025, 26, 2388. https://doi.org/10.3390/ijms26062388
Rendon DA. Negative Alterations in the Respiratory Activity of Isolated Crude Heart Mitochondria Following In Vivo Isoproterenol Injection in Rats Are Not Observed in Heart Homogenate Suggesting That the Isolation Procedure Generates Experimental Artefacts. International Journal of Molecular Sciences. 2025; 26(6):2388. https://doi.org/10.3390/ijms26062388
Chicago/Turabian StyleRendon, Dairo Alonso. 2025. "Negative Alterations in the Respiratory Activity of Isolated Crude Heart Mitochondria Following In Vivo Isoproterenol Injection in Rats Are Not Observed in Heart Homogenate Suggesting That the Isolation Procedure Generates Experimental Artefacts" International Journal of Molecular Sciences 26, no. 6: 2388. https://doi.org/10.3390/ijms26062388
APA StyleRendon, D. A. (2025). Negative Alterations in the Respiratory Activity of Isolated Crude Heart Mitochondria Following In Vivo Isoproterenol Injection in Rats Are Not Observed in Heart Homogenate Suggesting That the Isolation Procedure Generates Experimental Artefacts. International Journal of Molecular Sciences, 26(6), 2388. https://doi.org/10.3390/ijms26062388




