Modulation of Autophagy on Cinnamaldehyde Induced THP-1 Cell Activation
Abstract
1. Introduction
2. Results
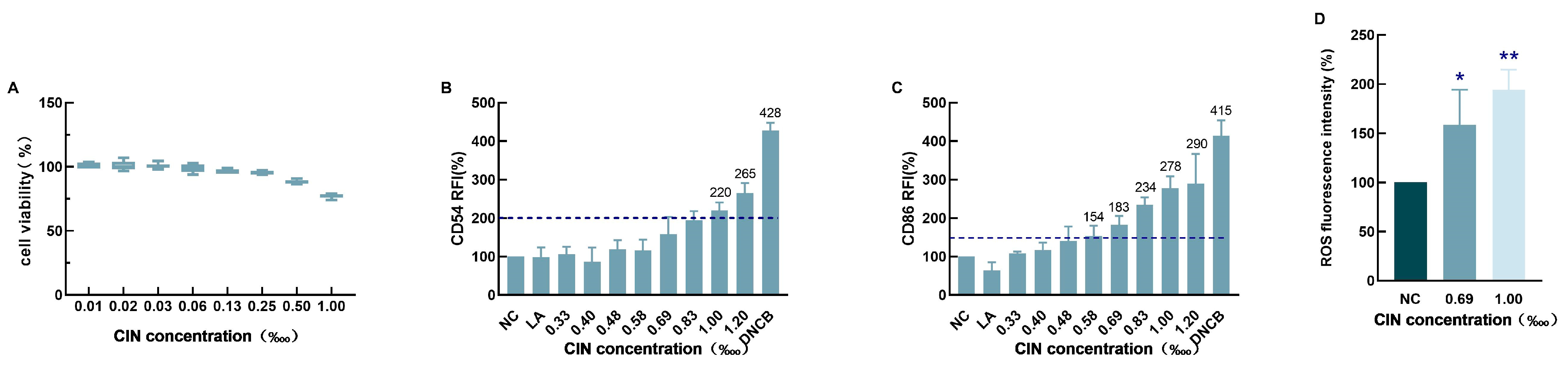
2.1. CIN-Induced THP-1 Cell Activation
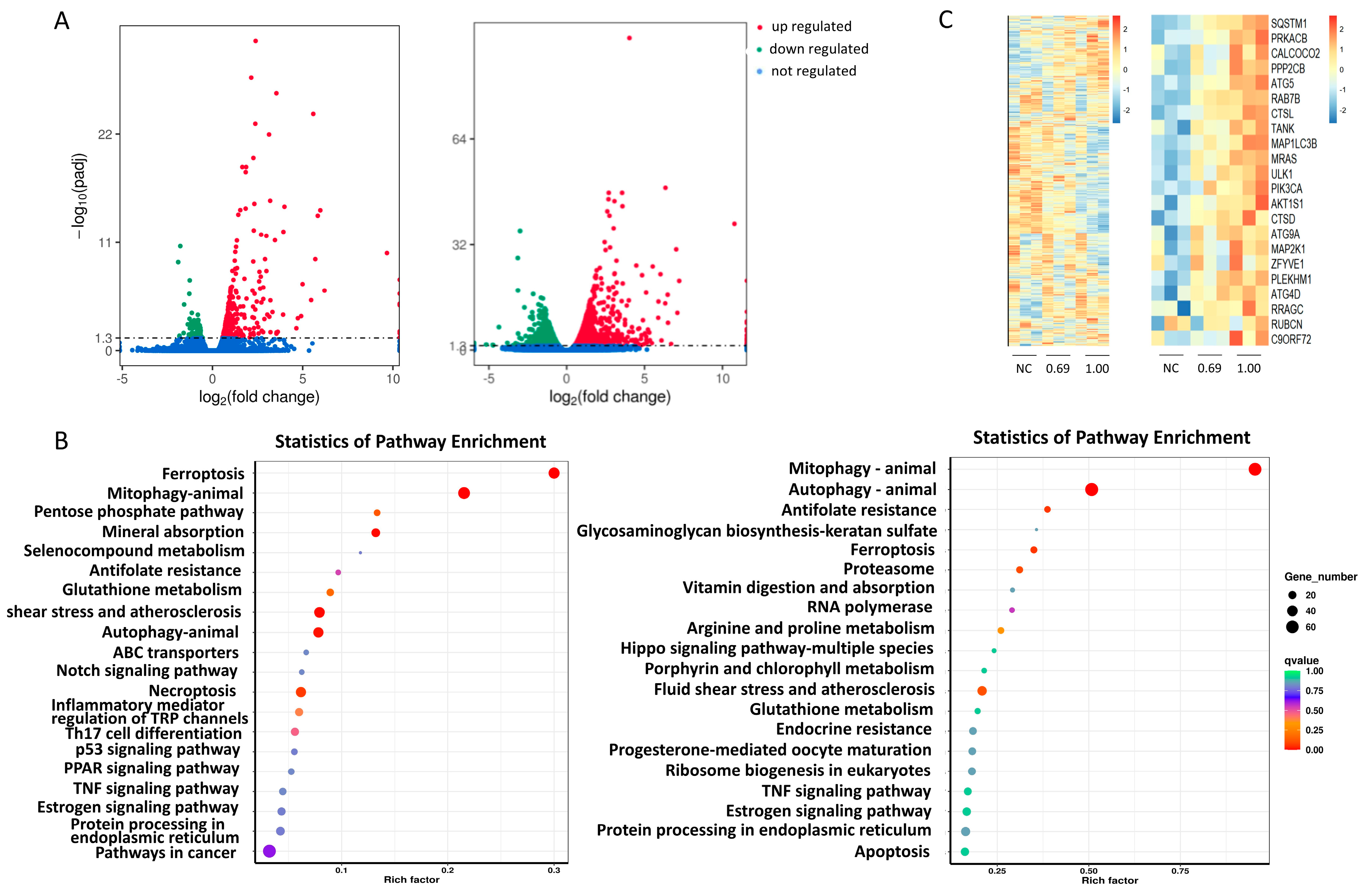
2.2. CIN Interfered with the Autophagy Pathway in THP-1 Cells
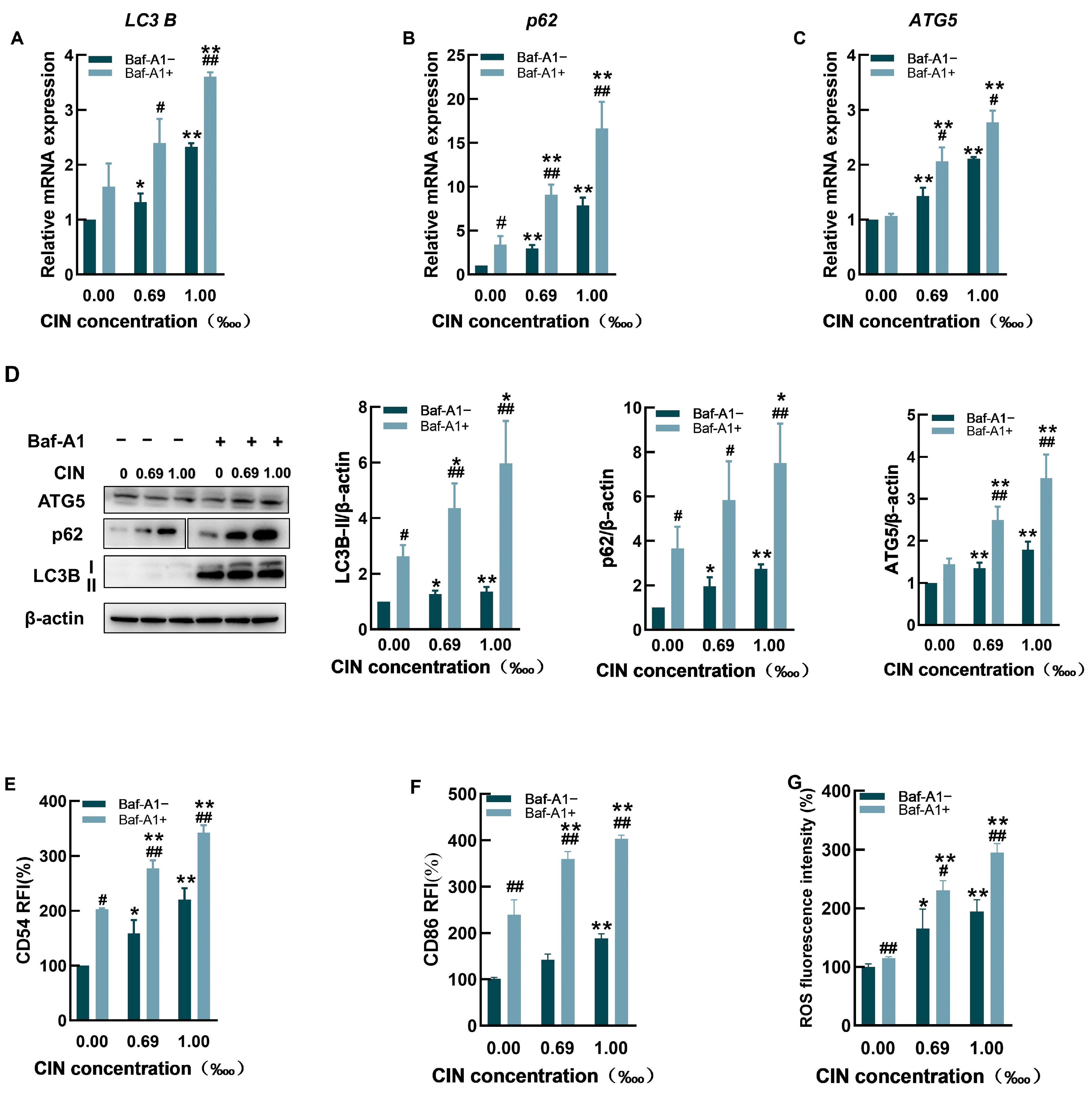
2.3. The Autophagy Inhibitor Baf-A1 Impaired Autophagic Flux and Increased CIN-Induced Activation in THP-1 Cells
2.4. The Autophagy Activator Rapamycin Promoted Autophagy and Decreased CIN-Induced Activation in THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability Assay
4.3. Flow Cytometry
4.4. Determination of Intracellular Reactive Oxygen Species (ROS)
4.5. Transcriptomics
4.6. Real-Time PCR Measurement
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olusegun, O.A.; Martincigh, B.S. Allergic contact dermatitis: A significant environmental and occupational skin disease. Int. J. Dermatol. 2021, 60, 1082–1091. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef]
- Tramontana, M.; Hansel, K.; Bianchi, L.; Sensini, C.; Malatesta, N.; Stingeni, L. Advancing the understanding of allergic contact dermatitis: From pathophysiology to novel therapeutic approaches. Front. Med. 2023, 10, 1184289. [Google Scholar] [CrossRef]
- Pastor-Nieto, M.A.; Gatica-Ortega, M.E. Ubiquity, Hazardous Effects, and Risk Assessment of Fragrances in Consumer Products. Curr. Treat. Options Allergy 2021, 8, 21–41. [Google Scholar] [CrossRef]
- De Rentiis, A.M.A.; Pink, M.; Verma, N.; Schmitz-Spanke, S. Assessment of the different skin sensitization potentials of irritants and allergens as single substances and in combination using the KeratinoSens assay. Contact Dermat. 2021, 84, 317–325. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. Corrigendum to “RIFM fragrance ingredient safety assessment, cinnamaldehyde, CAS Registry Number 104-55-2 (vol 134, 110710, 2019)”. Food Chem. Toxicol. 2020, 141, 111410. [Google Scholar] [CrossRef]
- Pentinga, S.E.; Kuik, D.J.; Bruynzeel, D.P.; Rustemeyer, T. Do ’cinnamon-sensitive’ patients react to cinnamate UV filters? Contact Dermat. 2009, 60, 210–213. [Google Scholar] [CrossRef]
- Cheung, C.; Hotchkiss, S.A.M.; Pease, C.K.S. Cinnamic compound metabolism in human skin and the role metabolism may play in determining relative sensitisation potency. J. Dermatol. Sci. 2003, 31, 9–19. [Google Scholar] [CrossRef]
- de Groot, A.C. Fragrances: Contact Allergy and Other Adverse Effects. Dermatitis 2020, 31, 13–35. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Ofenloch, R.; Bruze, M.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of fragrance contact allergy in the general population of five European countries: A cross-sectional study. Br. J. Dermatol. 2015, 173, 1411–1419. [Google Scholar] [CrossRef]
- Guarneri, F. Occupational allergy to cinnamal in a baker. Contact Dermat. 2010, 63, 294. [Google Scholar] [CrossRef]
- Ackermann, L.; Aalto-Korte, K.; Jolanki, R.; Alanko, K. Occupational allergic contact dermatitis from cinnamon including one case from airborne exposure. Contact Dermat. 2009, 60, 96–99. [Google Scholar] [CrossRef]
- Filaire, E.; Nachat-Kappes, R.; Laporte, C.; Harmand, M.-F.; Simon, M.; Poinsot, C. Alternative in vitro models used in the main safety tests of cosmetic products and new challenges. Int. J. Cosmet. Sci. 2022, 44, 604–613. [Google Scholar] [CrossRef]
- OECD. Test Guideline No. 442E In Vitro Skin Sensitisation, In Vitro Skin Sensitisation Assays Addressingthe Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation, OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2024. [Google Scholar]
- OECD. Test Guideline No. 442C: In Chemico Skin Sensitisation, Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins, OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2024. [Google Scholar]
- OECD. Test Guideline No. 442D: In Vitro Skin Sensitisation, Assays Addressing the Adverse Outcome Pathway Key Event on Keratinocyte Activation, OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2024. [Google Scholar]
- Galbiati, V.; Marinovich, M.; Corsini, E. Mechanistic understanding of dendritic cell activation in skin sensitization: Additional evidences to support potency classification. Toxicol. Lett. 2020, 322, 50–57. [Google Scholar] [CrossRef]
- Gądarowska, D.; Kalka, J.; Daniel-Wójcik, A.; Mrzyk, I. Alternative Methods for Skin-Sensitization Assessment. Toxics 2022, 10, 740. [Google Scholar] [CrossRef]
- Lichter, J.; Silva, E.S.M.; Peter, N.; Sahli, F.; Vileno, B.; Kuresepi, S.; Gourlaouen, C.; Giménez-Arnau, E.; Blömeke, B. Skin sensitization to fragrance hydroperoxides: Interplay between dendritic cells, keratinocytes and free radicals. Br. J. Dermatol. 2021, 184, 1143–1152. [Google Scholar] [CrossRef]
- Lindberg, T.; de Ávila, R.I.; Zeller, K.S.; Levander, F.; Eriksson, D.; Chawade, A.; Lindstedt, M. An integrated transcriptomic- and proteomic-based approach to evaluate the human skin sensitization potential of glyphosate and its commercial agrochemical formulations. J. Proteom. 2020, 217, 103647. [Google Scholar] [CrossRef]
- Luís, A.; Martins, J.D.; Silva, A.; Ferreira, I.; Cruz, M.T.; Neves, B.M. Oxidative stress-dependent activation of the eIF2α–ATF4 unfolded protein response branch by skin sensitizer 1-fluoro-2,4-dinitrobenzene modulates dendritic-like cell maturation and inflammatory status in a biphasic manner. Free Radic. Biol. Med. 2014, 77, 217–229. [Google Scholar] [CrossRef]
- Changotra, H.; Kaur, S.; Yadav, S.S.; Gupta, G.L.; Parkash, J.; Duseja, A. ATG5: A central autophagy regulator implicated in various human diseases. Cell Biochem. Funct. 2022, 40, 650–667. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, H.; Liao, S.; He, J.; Zhou, Y.; Lei, Y. LC3B: A microtubule-associated protein influences disease progression and prognosis. Cytokine Growth Factor Rev. 2024, 81, 16–26. [Google Scholar] [CrossRef]
- Bonnet, L.V.; Palandri, A.; Flores-Martin, J.B.; Hallak, M.E. Arginyltransferase 1 modulates p62-driven autophagy via mTORC1/AMPk signaling. Cell Commun. Signal. 2024, 22, 87. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Ashikaga, T.; Sakaguchi, H.; Sono, S.; Kosaka, N.; Ishikawa, M.; Nukada, Y.; Miyazawa, M.; Ito, Y.; Nishiyama, N.; Itagaki, H. A comparative evaluation of in vitro skin sensitisation tests: The human cell-line activation test (h-CLAT) versus the local lymph node assay (LLNA). Altern. Lab. Anim. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Hölken, J.M.; Teusch, N. The Monocytic Cell Line THP-1 as a Validated and Robust Surrogate Model for Human Dendritic Cells. Int. J. Mol. Sci. 2023, 24, 1452. [Google Scholar] [CrossRef]
- Foti, C.; Bonamonte, D.; Conserva, A.; Stingeni, L.; Lisi, P.; Lionetti, N.; Rigano, L.; Angelini, G. Allergic and photoallergic contact dermatitis from ketoprofen: Evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr. Pharm. Des. 2008, 14, 2833–2839. [Google Scholar] [CrossRef]
- Karkhanis, A.V.; Chan, E.C.Y.; Ren, E.C. Preliminary discovery of novel markers for human cell line activation test (h-CLAT). Toxicol. In Vitro 2021, 74, 105154. [Google Scholar] [CrossRef]
- Otsubo, Y.; Nishijo, T.; Miyazawa, M.; Saito, K.; Mizumachi, H.; Sakaguchi, H. Binary test battery with KeratinoSensTM and h-CLAT as part of a bottom-up approach for skin sensitization hazard prediction. Regul. Toxicol. Pharmacol. 2017, 88, 118–124. [Google Scholar] [CrossRef]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM fragrance ingredient safety assessment, cinnamaldehyde, CAS Registry Number 104-55-2. Food Chem. Toxicol. 2019, 134 (Suppl. S1), 110710. [Google Scholar] [CrossRef]
- Lee, Y.H.; Su, S.B.; Huang, C.C.; Sheu, H.M.; Tsai, J.C.; Lin, C.H.; Wang, Y.J.; Wang, B.J. N-acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through inhibition of cell death, ROS-related signaling and cytokine expression. PLoS ONE 2014, 9, e108317. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Cao, W.Y.; Li, J.H.; Yang, K.P.; Cao, D.L. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Li, Y.; Liu, T.; Yang, B.; Liu, L.; Wu, R. Autophagy and Inflammation: Regulatory Roles in Viral Infections. Biomolecules 2023, 13, 1454. [Google Scholar] [CrossRef]
- Gan, T.; Qu, S.; Zhang, H.; Zhou, X.J. Modulation of the immunity and inflammation by autophagy. MedComm 2023, 4, e311. [Google Scholar] [CrossRef]
- Gerada, C.; Ryan, K.M. Autophagy, the innate immune response and cancer. Mol. Oncol. 2020, 14, 1913–1929. [Google Scholar] [CrossRef]
- Liu, C.; Gu, L.; Ding, J.; Meng, Q.; Li, N.; Dai, G.; Li, Q.; Wu, X. Autophagy in skin barrier and immune-related skin diseases. J. Dermatol. 2021, 48, 1827–1837. [Google Scholar] [CrossRef]
- Wu, M.; Cong, Y.; Wang, K.; Yu, H.; Zhang, X.; Ma, M.; Duan, Z.; Pei, X. Bisphenol A impairs macrophages through inhibiting autophagy via AMPK/mTOR signaling pathway and inducing apoptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113395. [Google Scholar] [CrossRef]
- Hegdekar, N.; Sarkar, C.; Bustos, S.; Ritzel, R.M.; Hanscom, M.; Ravishankar, P.; Philkana, D.; Wu, J.; Loane, D.J.; Lipinski, M.M. Inhibition of autophagy in microglia and macrophages exacerbates innate immune responses and worsens brain injury outcomes. Autophagy 2023, 19, 2026–2044. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, J.; Wang, Q.; Fu, Y.; Gu, L.; Tian, X.; Yu, B.; Fu, X.; Zheng, H.; Li, C.; et al. Rosmarinic acid alleviates fungal keratitis caused by Aspergillus fumigatus by inducing macrophage autophagy. Exp. Eye Res. 2024, 244, 109944. [Google Scholar] [CrossRef]
- Ravikumar, B.; Vacher, C.; Berger, Z.; Davies, J.E.; Luo, S.; Oroz, L.G.; Scaravilli, F.; Easton, D.F.; Duden, R.; O’Kane, C.J.; et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 2004, 36, 585–595. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X.; Li, L.; Chen, H.; Chai, Y. Autophagy Inhibition Contributes to ROS-Producing NLRP3-Dependent Inflammasome Activation and Cytokine Secretion in High Glucose-Induced Macrophages. Cell. Physiol. Biochem. 2017, 43, 247–256. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, C.; Chen, H.; Chai, Y. Rapamycin Attenuates High Glucose-Induced Inflammation Through Modulation of mTOR/NF-κB Pathways in Macrophages. Front. Pharmacol. 2019, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qu, S.; Ma, J.; Wang, X.; Yang, Y. Metformin Suppresses LPS-Induced Inflammatory Responses in Macrophage and Ameliorates Allergic Contact Dermatitis in Mice via Autophagy. Biol. Pharm. Bull. 2020, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Wu, F.; Wang, R.; Wang, J.; Zhang, J.; Pan, Y. Modulation of Autophagy on Cinnamaldehyde Induced THP-1 Cell Activation. Int. J. Mol. Sci. 2025, 26, 2377. https://doi.org/10.3390/ijms26062377
Qin Y, Wu F, Wang R, Wang J, Zhang J, Pan Y. Modulation of Autophagy on Cinnamaldehyde Induced THP-1 Cell Activation. International Journal of Molecular Sciences. 2025; 26(6):2377. https://doi.org/10.3390/ijms26062377
Chicago/Turabian StyleQin, Yi, Fan Wu, Rui Wang, Jun Wang, Jiaqi Zhang, and Yao Pan. 2025. "Modulation of Autophagy on Cinnamaldehyde Induced THP-1 Cell Activation" International Journal of Molecular Sciences 26, no. 6: 2377. https://doi.org/10.3390/ijms26062377
APA StyleQin, Y., Wu, F., Wang, R., Wang, J., Zhang, J., & Pan, Y. (2025). Modulation of Autophagy on Cinnamaldehyde Induced THP-1 Cell Activation. International Journal of Molecular Sciences, 26(6), 2377. https://doi.org/10.3390/ijms26062377





