Circ_0011446 Regulates Intramuscular Adipocyte Differentiation in Goats via the miR-27a-5p/FAM49B Axis
Abstract
1. Introduction
2. Results
2.1. Circ_0011446 Identification
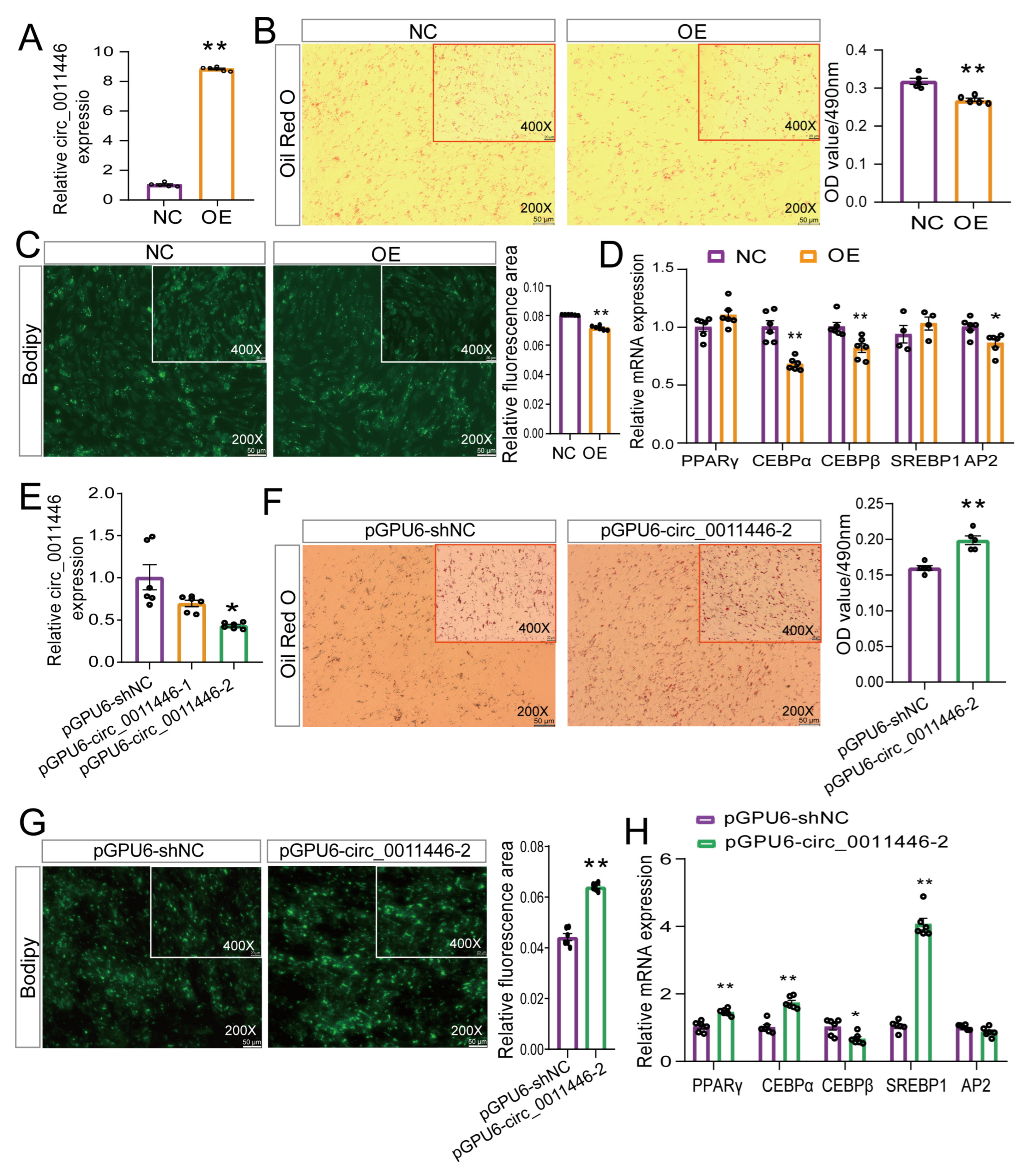
2.2. Circ_0011446 Negative Regulator Adipogenic Differentiation of Goat Preadipocytes
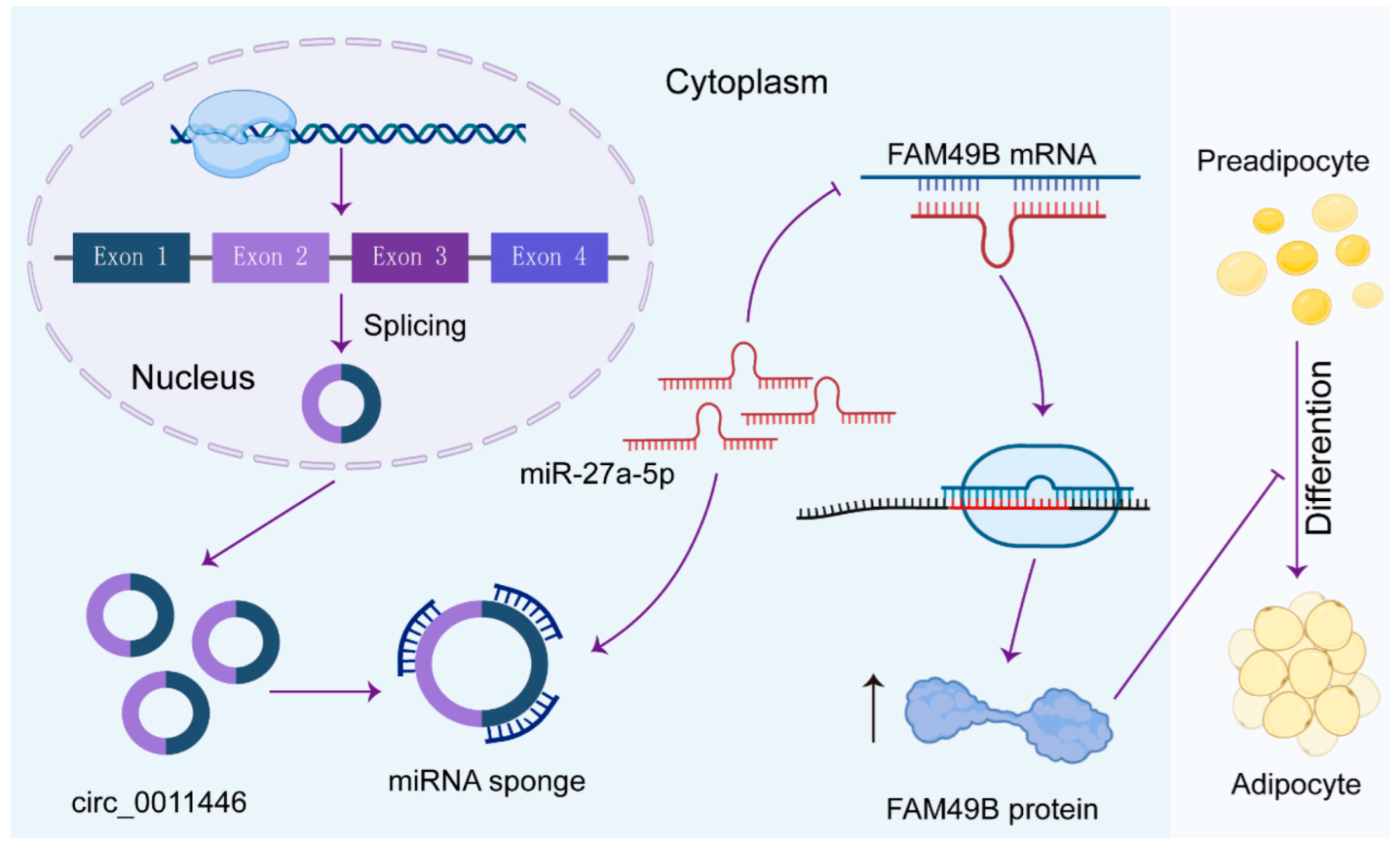
2.3. Circ_0011446 Serves as a miR-27a-5p Sponge
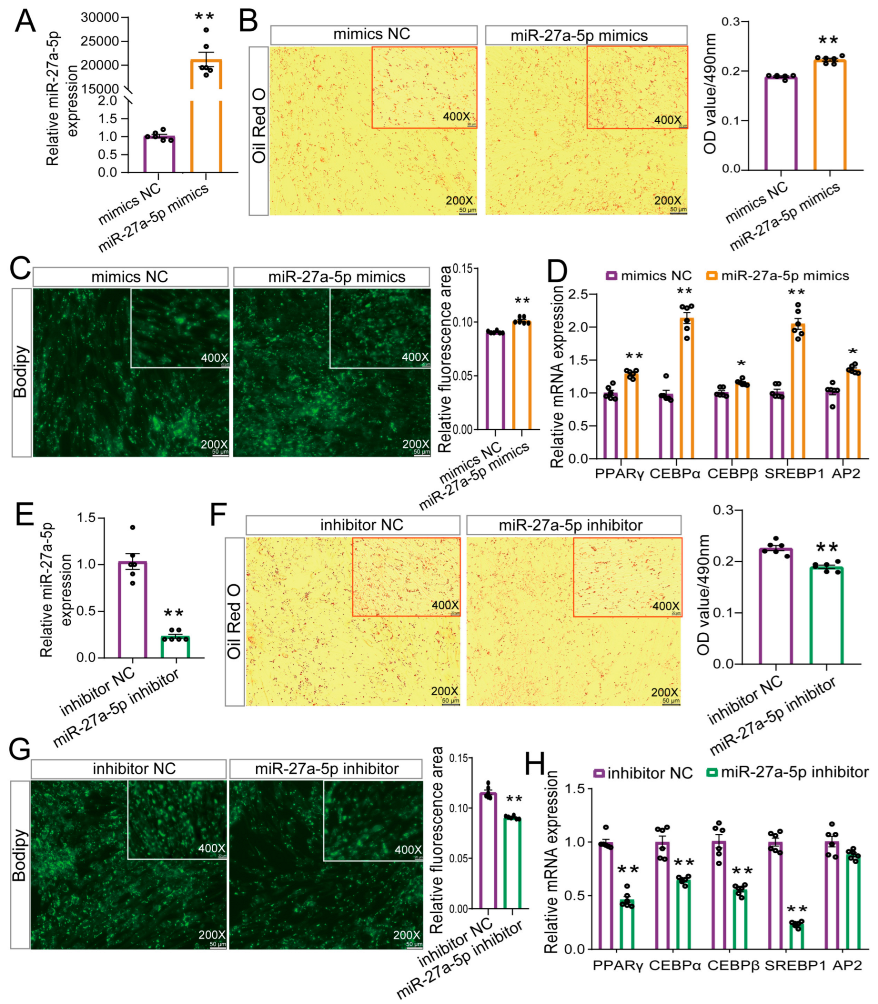
2.4. miR-27a-5p Positively Modulates Adipogenic Differentiation in Goat Preadipocytes
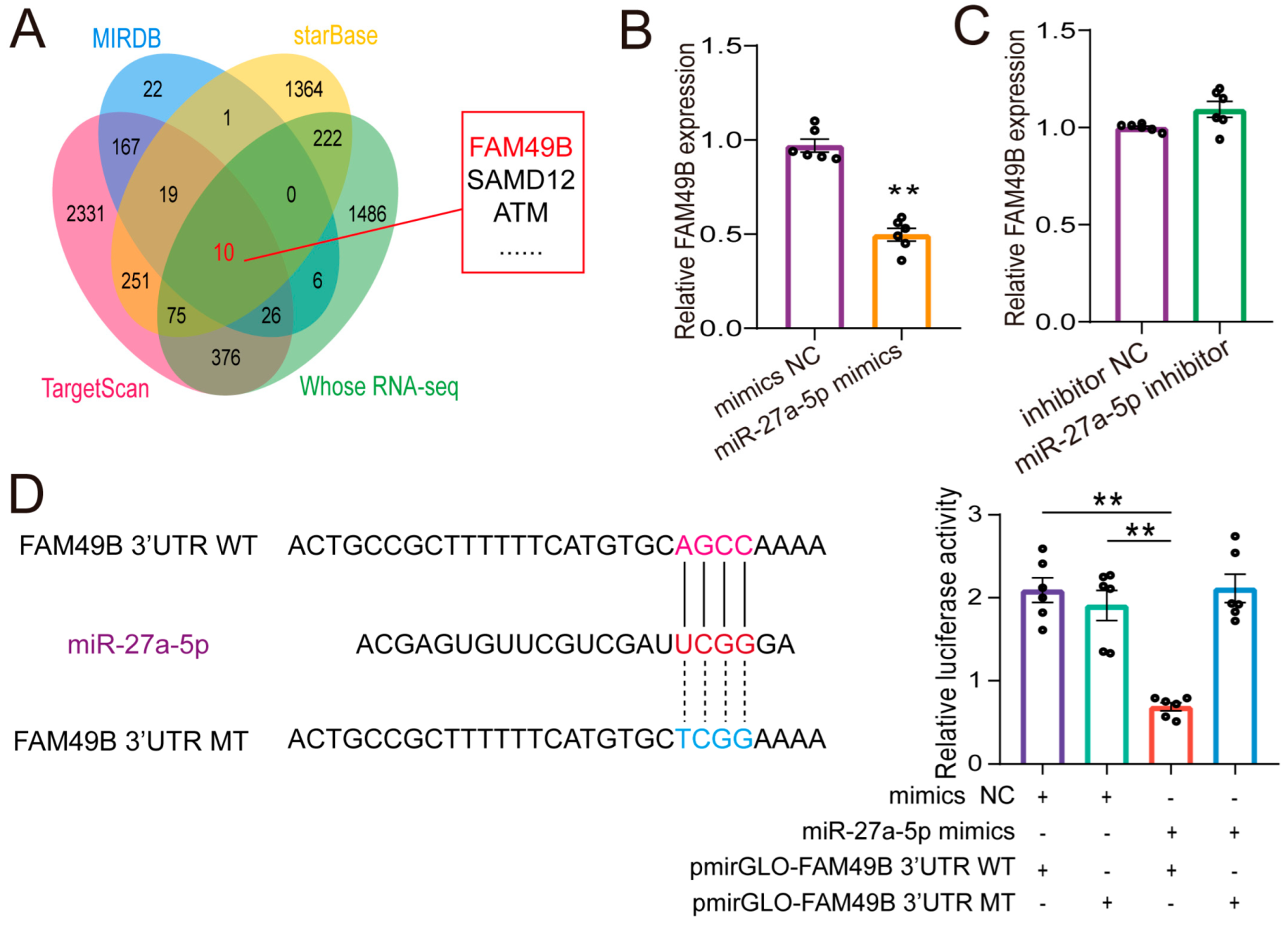
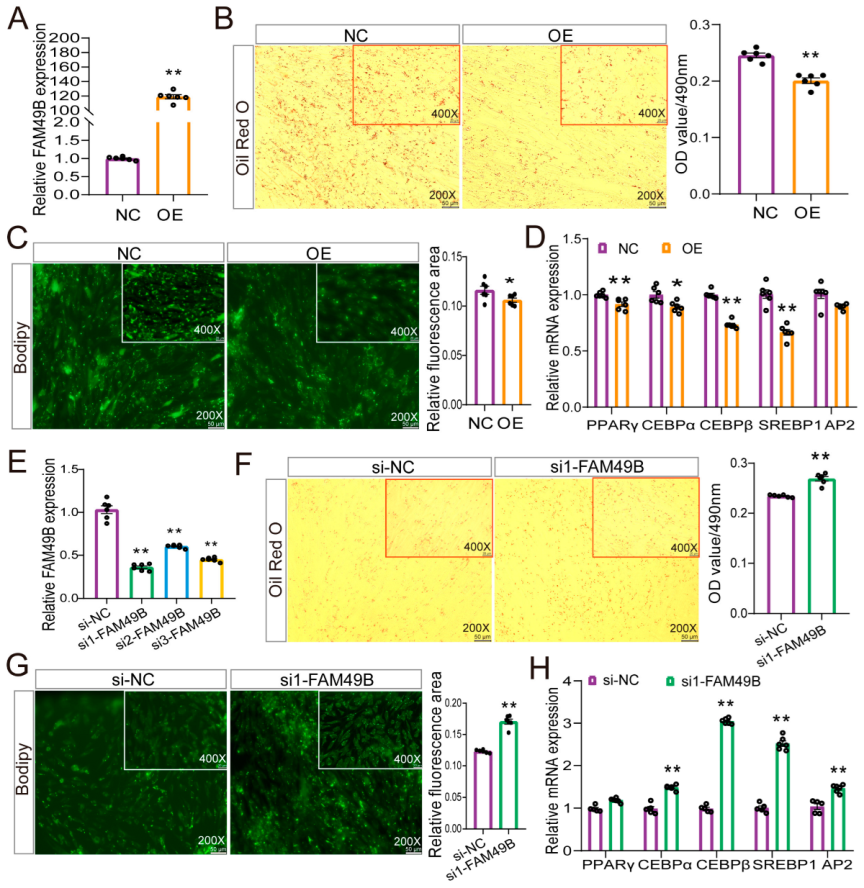
2.5. FAM49B Was the Direct Target of miR-27a-5p
2.6. FAM49B Negative Regulator Adipogenic Differentiation of Goat Preadipocytes
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture of Primary Goat Intramuscular Preadipocytes
4.2. Adenovirus Generation and Cell Infection
4.3. Chemical Synthesis of siRNA and Cell Transfection
4.4. Validation of circRNA
4.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.6. Nuclear–Cytoplasmic Isolation and Fluorescence In Situ Hybridization (FISH)
4.7. Bodipy and Oil Red O Staining
4.8. Dual-Luciferase Reporter Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, K.; Liu, Y.; He, X.; Tao, L.; Jiang, Y.; Lan, R.; Hong, Q.; Chu, M. A novel SNP in the promoter region of IGF1 associated with Yunshang black goat kidding number via promoting transcription activity by SP1. Front. Cell Dev. Biol. 2022, 10, 873095. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, J.; Zhang, K.; Cheng, G.; Mei, C.; Zan, L. Integrated multi-omics analysis reveals variation in intramuscular fat among muscle locations of Qinchuan cattle. BMC Genom. 2023, 24, 367. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Xu, J.; Yang, Q.; Sha, Y.; Jiao, T.; Zhao, S. Effects of yeast cultures on meat quality, flavor composition and rumen microbiota in lambs. Curr. Res. Food Sci. 2024, 9, 100845. [Google Scholar] [CrossRef]
- Hocquette, J.-F.; Bernard-Capel, C.; Vidal, V.; Jesson, B.; Levéziel, H.; Renand, G.; Cassar-Malek, I. The GENOTEND chip: A new tool to analyse gene expression in muscles of beef cattle for beef quality prediction. BMC Vet. Res. 2012, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular regulation of lipogenesis, adipogenesis and fat deposition in chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, H.; Hua, Z.; Zhu, Z.; Tao, J.; Xiao, H.; Zhang, L.; Bi, Y.; Wang, H. ACSL4 directs intramuscular adipogenesis and fatty acid composition in pigs. Animals 2022, 12, 119. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Luo, Y.; Huang, Z.; Jia, G.; Liu, G.; Zhao, H. Tissue distribution of porcine FTO and its effect on porcine intramuscular preadipocytes proliferation and differentiation. PLoS ONE 2016, 11, e0151056. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Liu, H.; Zhang, W.; Li, X.; Liu, L.; Zhou, M.; Wang, J.; Su, S.; Ding, X. miR-381-3p inhibits intramuscular fat deposition through targeting FABP3 by ceRNA regulatory network. Biology 2022, 11, 1497. [Google Scholar] [CrossRef]
- Guo, L.; Chang, Y.; Sun, Z.; Deng, J.; Jin, Y.; Shi, M.; Zhang, J.; Miao, Z. Effects of Chinese Yam Polysaccharide on Intramuscular Fat and Fatty Acid Composition in Breast and Thigh Muscles of Broilers. Foods 2023, 12, 1479. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circ RNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.-O.; Chen, T.; Xiang, J.-F.; Yin, Q.-F.; Xing, Y.-H.; Zhu, S.; Yang, L.; Chen, L.-L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Zganiacz, D.; Milanowski, R. Characteristics of circular ribonucleic acid molecules (circRNA). Postep. Biochem. 2017, 63, 221–232. [Google Scholar]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Tu, C.-Y.; Jin, K.-D.; Shao, C.-C.; Liu, B.-N.; Zhang, Y.-Q.; Xie, J.-H.; Shen, Y.-W. Research Progress of CircRNA and its Application Prospect in Forensic Medicine. J. Forensic Med. 2018, 34, 73–78. [Google Scholar]
- Jiang, R.; Li, H.; Yang, J.; Shen, X.; Song, C.; Yang, Z.; Wang, X.; Huang, Y.; Lan, X.; Lei, C. circRNA profiling reveals an abundant circFUT10 that promotes adipocyte proliferation and inhibits adipocyte differentiation via sponging let-7. Mol. Ther.-Nucleic Acids 2020, 20, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Li, Y.; Mao, R.; Yang, H.; Zhang, Y.; Zhang, Y.; Guo, P.; Zhan, D.; Zhang, T. Circular RNA SAMD4A controls adipogenesis in obesity through the miR-138-5p/EZH2 axis. Theranostics 2020, 10, 4705. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Xu, Q.; Zhang, H.; Wang, Y.; Zhu, J.; Lin, Y. Key circRNAs from goat: Discovery, integrated regulatory network and their putative roles in the differentiation of intramuscular adipocytes. BMC Genom. 2023, 24, 51. [Google Scholar]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; Wang, Y.; Zhu, J.; Lin, Y. Screening of key miRNAs related with the differentiation of subcutaneous adipocytes and the validation of miR-133a-3p functional significance in goats. Anim. Biosci. 2022, 36, 144–155. [Google Scholar] [CrossRef]
- Kos, A.; Dijkema, R.; Arnberg, A.; Van der Meide, P.; Schellekens, H. The hepatitis delta (δ) virus possesses a circular RNA. Nature 1986, 323, 558–560. [Google Scholar] [CrossRef]
- Cocquerelle, C.; Mascrez, B.; Hétuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Danan, M.; Schwartz, S.; Edelheit, S.; Sorek, R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012, 40, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.-M.; Meitinger, T.; Engelhardt, S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell. Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Che, S.; Cui, J.; Ma, X.; An, X.; Cao, B.; Song, Y. Endometrial epithelial cell apoptosis is inhibited by a ciR8073-miR181a-neurotensis pathway during embryo implantation. Mol. Ther. Nucleic Acids 2019, 14, 262–273. [Google Scholar] [CrossRef]
- Shen, J.; Jin, X.; Hao, Z.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Zhao, F.; Li, M.; Zhao, Z. Identification and screening of circular RNAs during adipogenic differentiation of ovine preadipocyte by RNA-seq. J. Anim. Sci. 2024, 102, skae042. [Google Scholar] [CrossRef]
- Jin, W.; Zhao, Y.; Zhai, B.; Li, Y.; Fan, S.; Yuan, P.; Sun, G.; Jiang, R.; Wang, Y.; Liu, X. Characteristics and expression profiles of circRNAs during abdominal adipose tissue development in Chinese Gushi chickens. PLoS ONE 2021, 16, e0249288. [Google Scholar] [CrossRef]
- Tian, W.; Liu, Y.; Zhang, W.; Nie, R.; Ling, Y.; Zhang, B.; Zhang, H.; Wu, C. CircDOCK7 facilitates the proliferation and adipogenic differentiation of chicken abdominal preadipocytes through the gga-miR-301b-3p/ACSL1 axis. J. Anim. Sci. Biotechnol. 2023, 14, 91. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Zuo, Y.; Qiang, L.; Farmer, S.R. Activation of CCAAT/enhancer-binding protein (C/EBP) α expression by C/EBPβ during adipogenesis requires a peroxisome proliferator-activated receptor-γ-associated repression of HDAC1 at the C/ebpα gene promoter. J. Biol. Chem. 2006, 281, 7960–7967. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, S.; Jiang, E.; Wang, X.; Wang, Z.; Chen, H.; Lan, X. circFLT1 and lncCCPG1 Sponges miR-93 to Regulate the Proliferation and Differentiation of Adipocytes by Promoting lncSLC30A9 Expression. Mol. Ther.-Nucleic Acids 2020, 22, 484–499. [Google Scholar] [CrossRef]
- Ding, J.; Wen, Q.; Huo, Z.; Nie, H.; Qin, Y.; Yan, X. Identification of shell-color-related microRNAs in the Manila clam Ruditapes philippinarum using high-throughput sequencing of small RNA transcriptomes. Sci. Rep. 2021, 11, 8044. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Swaminathan, S.K.; Kirtane, A.; Srivastava, S.K.; Bhardwaj, A.; Singh, S.; Panyam, J.; Singh, A.P. Synthesis, characterization, and evaluation of poly (D,L-lactide-co-glycolide)-based nanoformulation of miRNA-150: Potential implications for pancreatic cancer therapy. Int. J. Nanomed. 2014, 9, 2933–2942. [Google Scholar]
- Shao, J.; Jiang, G.; Li, Y.; Wang, M.; Tang, T.; Wang, J.; Jia, X.; Lai, S. Let-7a-5p Regulates Animal Lipid Accumulation by Targeting Srebf2 and Thbs1 Signaling. Int. J. Mol. Sci. 2024, 25, 894. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Fernández-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 2104–2110. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, T.; Zhang, L.; Fu, L.; Hu, Y.; Li, H.; Li, C.; Zhang, J.; Liang, B.; Liu, J. miR-669a-5p promotes adipogenic differentiation and induces browning in preadipocytes. Adipocyte 2022, 11, 120–132. [Google Scholar] [CrossRef]
- Lima, V.M.; Liu, J.; Brandão, B.B.; Lino, C.A.; Silva, C.S.B.; Ribeiro, M.A.; Oliveira, T.E.; Real, C.C.; de Paula Faria, D.; Cederquist, C. miRNA-22 deletion limits white adipose expansion and activates brown fat to attenuate high-fat diet-induced fat mass accumulation. Metabolism 2021, 117, 154723. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, L.; Jiao, H.; Zhu, H.; Xu, K.; Guo, S.; Shi, Q.; Zhao, T.; Pang, F.; Jia, X. Mmu-miR-27a-5p-dependent upregulation of MCPIP1 inhibits the inflammatory response in LPS-induced RAW264.7 macrophage cells. BioMed Res. Int. 2015, 2015, 607692. [Google Scholar] [CrossRef]
- Xing, Y.; Li, J.; Li, S.p.; Xi, J.; Ma, N.; Liu, L.; Wang, J.S.; Cai, J.Z. MiR-27a-5p regulates apoptosis of liver ischemia-reperfusion injury in mice by targeting Bach1. J. Cell. Biochem. 2018, 119, 10376–10383. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Wang, F. MicroRNA MiR-27a-5p alleviates the cerulein-induced cell apoptosis and inflammatory injury of AR42J cells by targeting Traf3 in acute pancreatitis. Inflammation 2020, 43, 1988–1998. [Google Scholar] [CrossRef]
- Yang, W.; Tang, K.; Wang, Y.; Zan, L. MiR-27a-5p increases steer fat deposition partly by targeting calcium-sensing receptor (CASR). Sci. Rep. 2018, 8, 3012. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yuan, Q.; Liu, C.; Zhang, C.; Yuan, D. MiR-155/GSK-3β mediates anti-inflammatory effect of Chikusetsusaponin IVa by inhibiting NF-κB signaling pathway in LPS-induced RAW264. 7 cell. Sci. Rep. 2020, 10, 18303. [Google Scholar] [CrossRef]
- Melnik, S.; Gabler, J.; Dreher, S.I.; Hecht, N.; Hofmann, N.; Großner, T.; Richter, W. MiR-218 affects hypertrophic differentiation of human mesenchymal stromal cells during chondrogenesis via targeting RUNX2, MEF2C, and COL10A1. Stem Cell Res. Ther. 2020, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, K.; Yan, H.; Feng, H.; Wang, T.; Chai, L.; Xu, G. MicroRNA expression signature and the therapeutic effect of the microRNA-21 antagomir in hypertrophic scarring. Mol. Med. Rep. 2017, 15, 1211–1221. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Wang, Z.; Han, J.; Shi, S.; He, J.; Shen, N.; Wu, W.; Wang, R.; Lv, W. FAM49B promotes breast cancer proliferation, metastasis, and chemoresistance by stabilizing ELAVL1 protein and regulating downstream Rab10/TLR4 pathway. Cancer Cell Int. 2021, 21, 534. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lin, S.; Wang, Y.; Zhu, J.; Lin, Y. Fibroblast growth factor 10 (FGF10) promotes the adipogenesis of intramuscular preadipocytes in goat. Mol. Biol. Rep. 2018, 45, 1881–1888. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, S.; Zhu, J.; Wang, Y.; Lin, Y. The expression stability analysis of reference genes in the process of goat intramuscular preadipocytes differentiation in goat. Acta Vet. Zootech. Sin. 2018, 49, 907–918. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; Wang, Y.; Zhu, J.; Lin, Y. Chi-Circ_0006511 positively regulates the differentiation of goat intramuscular adipocytes via novel-miR-87/CD36 axis. Int. J. Mol. Sci. 2022, 23, 12295. [Google Scholar] [CrossRef] [PubMed]
 and
and  represent the convergent prime and divergent prime, respectively. (C). Relative circ_0011446 expression during goat intramuscular adipocyte differentiation. Different lowercase letters indicate significant differences between groups according to one-way ANOVA (p < 0.01).
represent the convergent prime and divergent prime, respectively. (C). Relative circ_0011446 expression during goat intramuscular adipocyte differentiation. Different lowercase letters indicate significant differences between groups according to one-way ANOVA (p < 0.01).
 and
and  represent the convergent prime and divergent prime, respectively. (C). Relative circ_0011446 expression during goat intramuscular adipocyte differentiation. Different lowercase letters indicate significant differences between groups according to one-way ANOVA (p < 0.01).
represent the convergent prime and divergent prime, respectively. (C). Relative circ_0011446 expression during goat intramuscular adipocyte differentiation. Different lowercase letters indicate significant differences between groups according to one-way ANOVA (p < 0.01).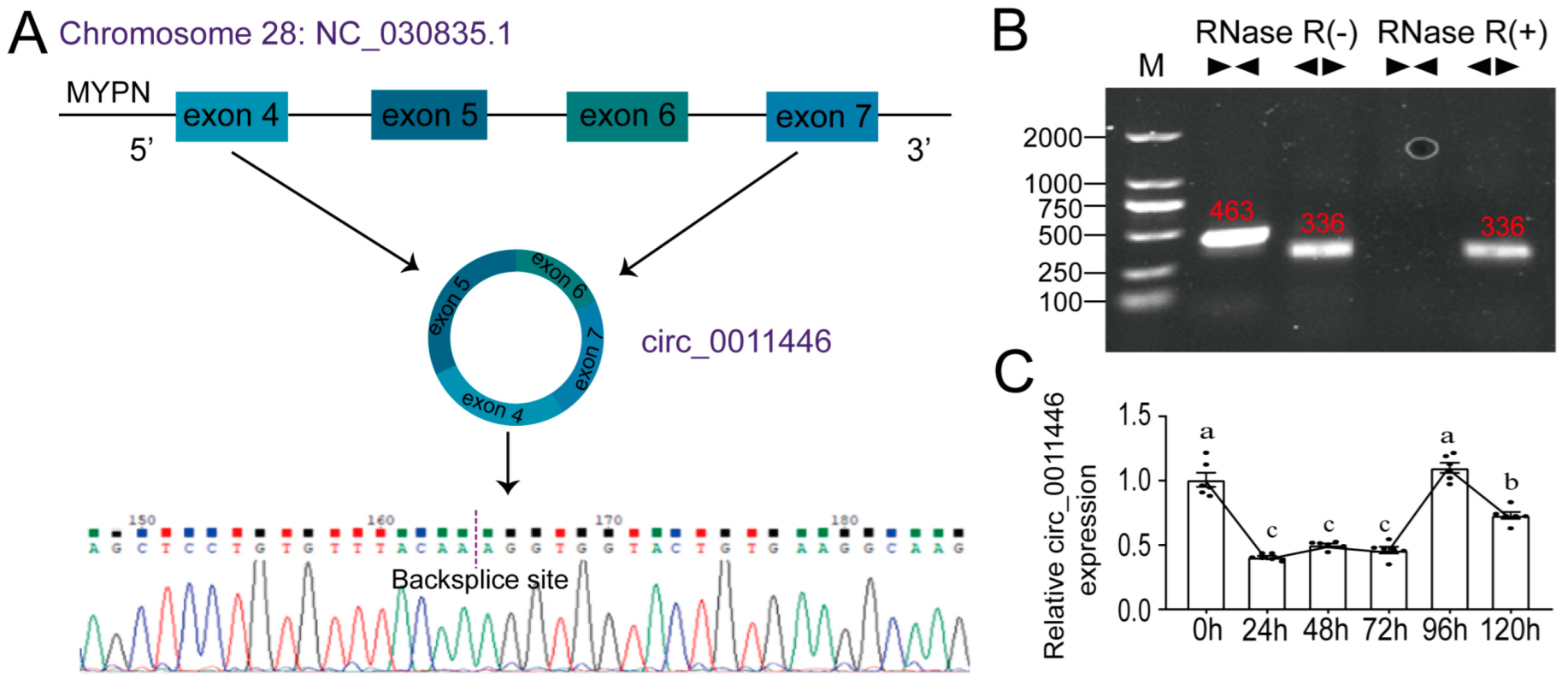

| Name | Sequence (5′-3′) |
|---|---|
| mimics NC | S: UUCUCCGAACGUGUCACGUTT |
| A: ACGUGACACGUUCGGAGAATT | |
| miR-27a-5p mimics | S: AGGGCUUAGCUGCUUGUGAGCA |
| A: CUCACAAGCAGCUAAGCCCUUU | |
| inhibitor NC | CAGUACUUUUGUGUAGUACAA |
| miR-27a-5p inhibitor | UGCUCACAAGCAGCUAAGCCCU |
| si-NC | S: UUCUCCGAACGUGUCACGUTT |
| A: ACGUGACACGUUCGGAGAATT | |
| FAM49B-Si1 | S: GCAUGAGGAUUAAUAAUGUTT |
| A: ACAUUAUUAAUCCUCAUGCTT | |
| FAM49B-Si2 | S: GCUACAACAAAGUUUGUAUTT |
| A: AUACAAACUUUGUUGUAGCTT | |
| FAM49B-Si3 | S: GCGUCAUAAUACUCUAUGATT |
| A: UCAUAGAGUAUUAUGACGCTT |
| Primer Name | Primer Sequence (5′-3′) | Product Length |
|---|---|---|
| divergent primer | S: GAATCGAATCCAGAAGCCAAAT A: TCCCATAGATGTTAGAAGCAAAGC | 336 |
| convergent primer | S: GCCTCCTCATTCAGCCAGC A: CAGCCCTCCATCTCTCCCA | 463 |
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| U6 | S: TGGAACGCTTCACGAATTTGCG A: GGAACGATACAGAGAAGATTAGC |
| GAPDH | S: GCAAGTTCCACGGCACAG A: TCAGCACCAGCATCACCC |
| UXT | S: GCAAGTGGATTTGGGCTGTAAC A: ATGGAGTCCTTGGTGAGGTTGT |
| PPARƳ | S: AAGCGTCAGGGTTCCACTATG A: GAACCTGATGGCGTTATGAGAC |
| C/EBPα | S: AAGCGTCAGGGTTCCACTATG A: GAACCTGATGGCGTTATGAGAC |
| C/EBPβ | S: AAGCGTCAGGGTTCCACTATG A: GAACCTGATGGCGTTATGAGAC |
| SREBP1 | S: AAGCGTCAGGGTTCCACTATG A: GAACCTGATGGCGTTATGAGAC |
| AP2 | S: AAGCGTCAGGGTTCCACTATG A: GAACCTGATGGCGTTATGAGAC |
| miR-27a-5p | S: AGTCTAAGGGCTTAGCTGCTTG |
| A: GTGCAGGGTCCGAGGT | |
| miR-27a-5p stem-loop | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGCTCACA |
| FAM49B | S: GAGGGACACGAACAGAATCACC |
| A: GAGGGAGAGGAACAGAGGAAAG | |
| FAM49B 3’UTR | S: GGAAAAGCACCTGCTGTAGAC |
| A: TTTTATCATCAACAGCCATTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-M.; Lv, J.-S.; Liu, K.-H.; Li, Y.-Y.; Zhu, J.-J.; Xiong, Y.; Wang, Y.; Lin, Y.-Q. Circ_0011446 Regulates Intramuscular Adipocyte Differentiation in Goats via the miR-27a-5p/FAM49B Axis. Int. J. Mol. Sci. 2025, 26, 2294. https://doi.org/10.3390/ijms26052294
Wang J-M, Lv J-S, Liu K-H, Li Y-Y, Zhu J-J, Xiong Y, Wang Y, Lin Y-Q. Circ_0011446 Regulates Intramuscular Adipocyte Differentiation in Goats via the miR-27a-5p/FAM49B Axis. International Journal of Molecular Sciences. 2025; 26(5):2294. https://doi.org/10.3390/ijms26052294
Chicago/Turabian StyleWang, Jian-Mei, Jin-Shi Lv, Ke-Han Liu, Yan-Yan Li, Jiang-Jiang Zhu, Yan Xiong, Yong Wang, and Ya-Qiu Lin. 2025. "Circ_0011446 Regulates Intramuscular Adipocyte Differentiation in Goats via the miR-27a-5p/FAM49B Axis" International Journal of Molecular Sciences 26, no. 5: 2294. https://doi.org/10.3390/ijms26052294
APA StyleWang, J.-M., Lv, J.-S., Liu, K.-H., Li, Y.-Y., Zhu, J.-J., Xiong, Y., Wang, Y., & Lin, Y.-Q. (2025). Circ_0011446 Regulates Intramuscular Adipocyte Differentiation in Goats via the miR-27a-5p/FAM49B Axis. International Journal of Molecular Sciences, 26(5), 2294. https://doi.org/10.3390/ijms26052294






