Inflammatory Biomarkers and Oral Health Disorders as Predictors of Head and Neck Cancer: A Retrospective Longitudinal Study
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Survival Distributions and Log-Rank Test Results
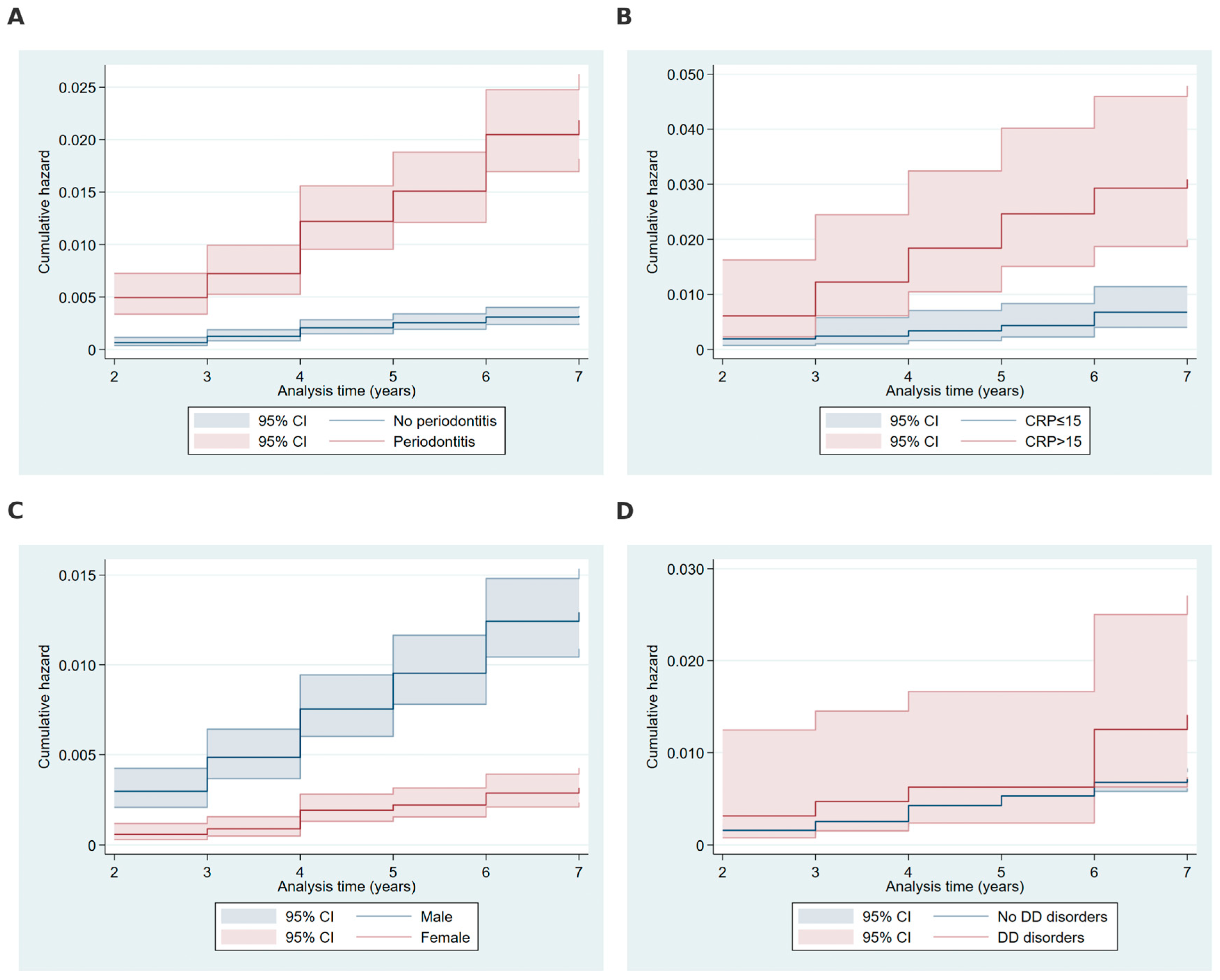
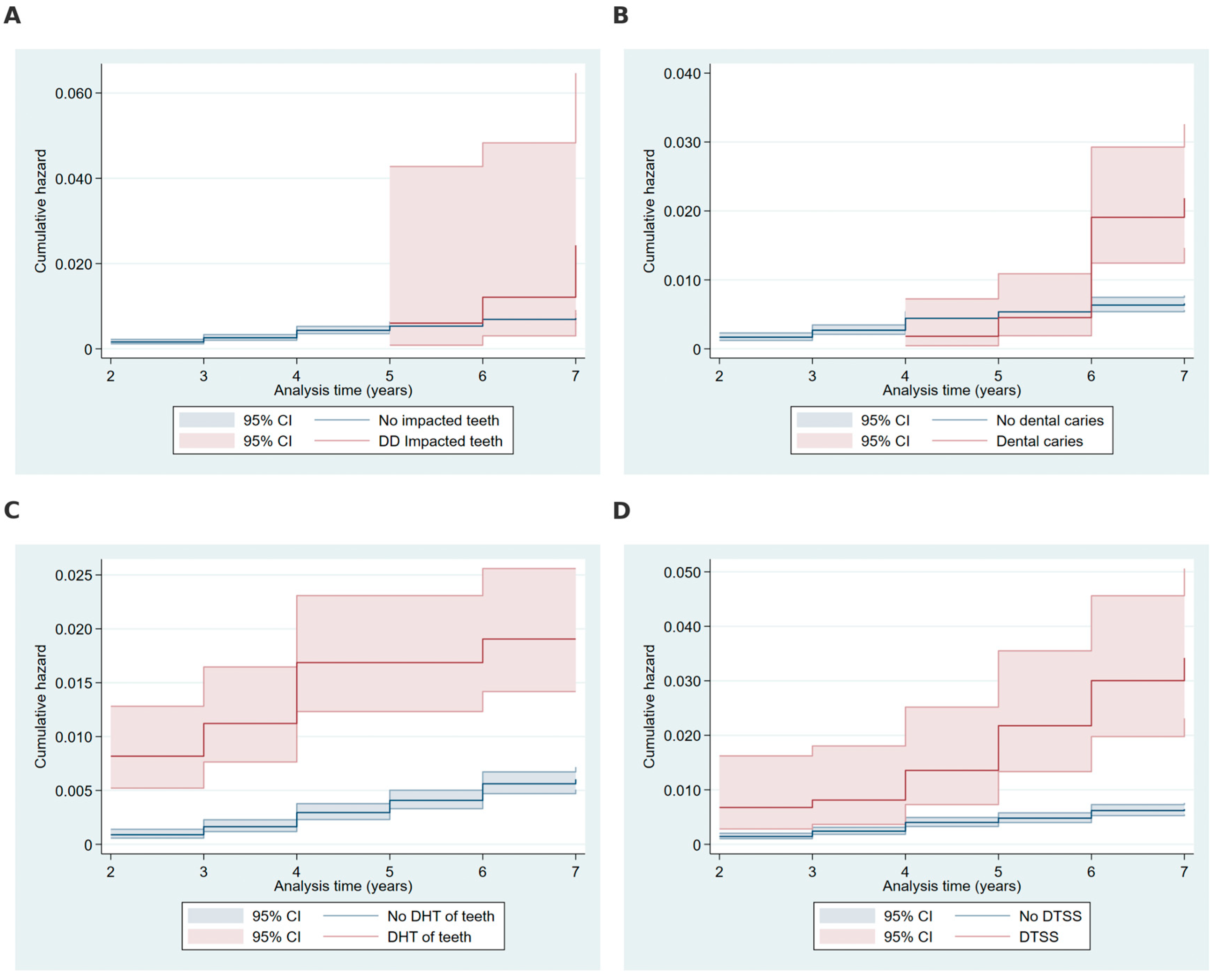
2.3. Cumulative Hazard Analysis
2.4. Weibull Regression Results
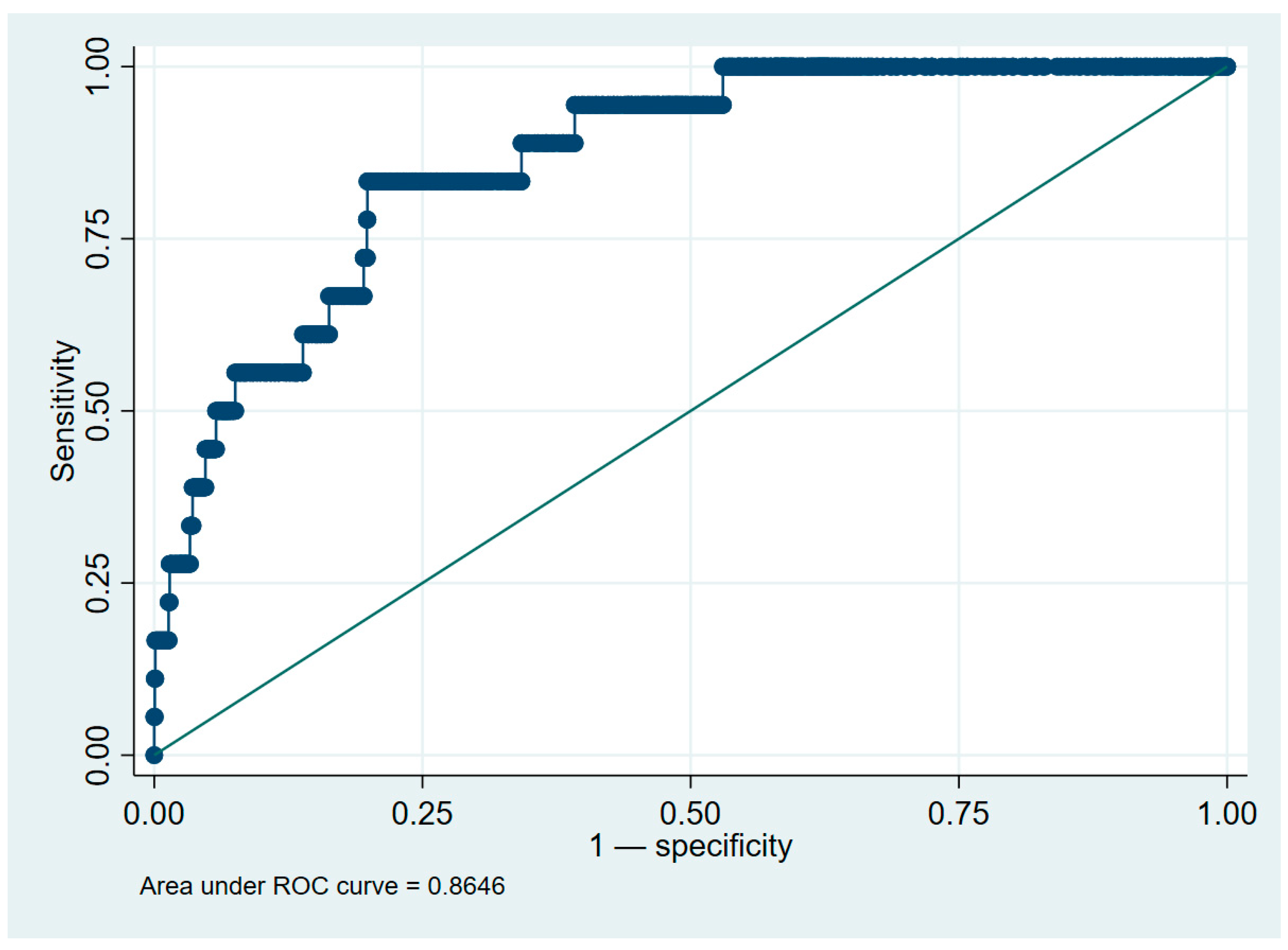
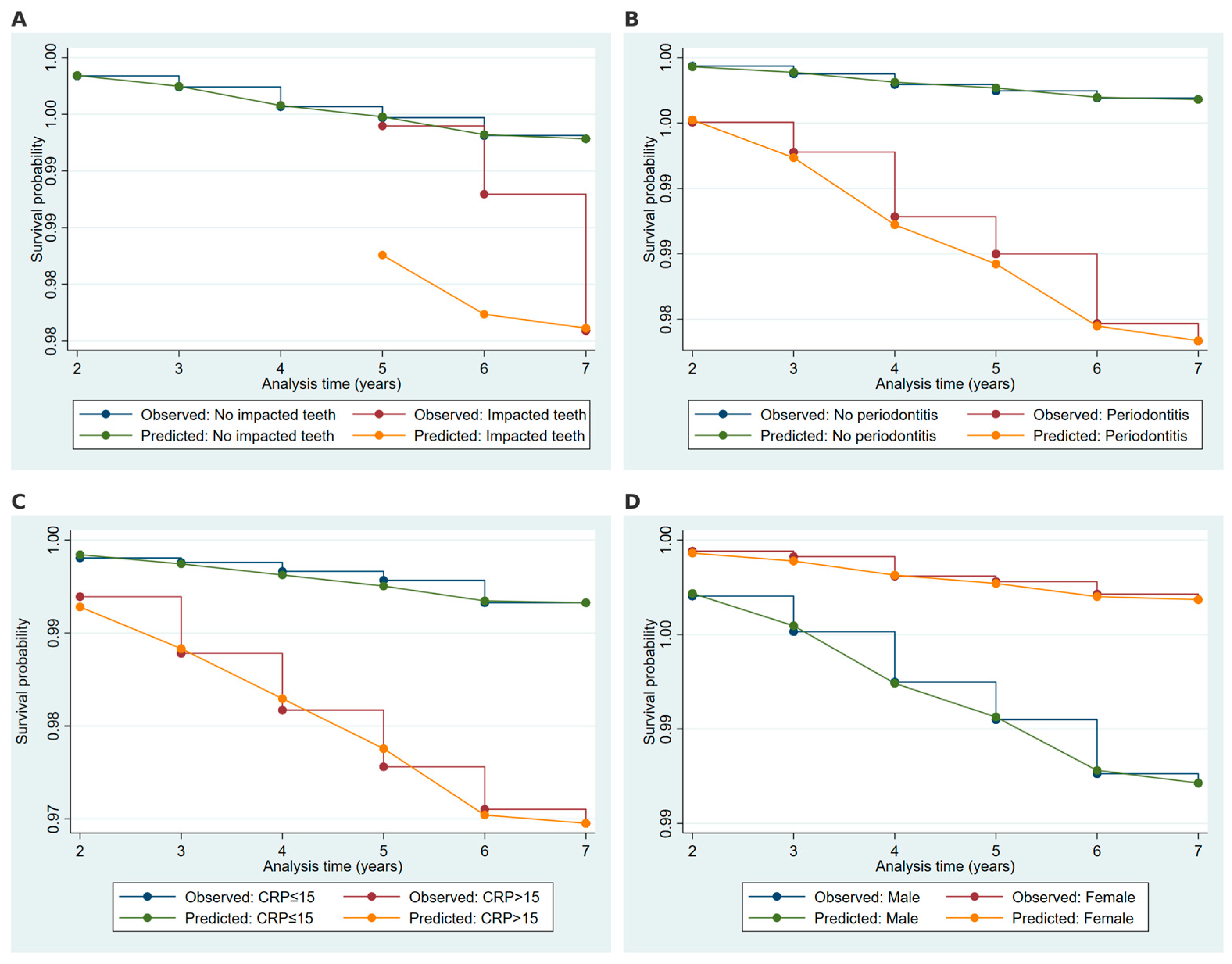
2.5. Model Validation and Discrimination Metrics
3. Discussion
3.1. Future Directions
3.2. Strengths and Limitations
4. Materials and Methods
4.1. Data Cleaning and Processing
4.2. Statistical Analysis
4.2.1. Baseline Characteristics
4.2.2. Log-Rank Tests and Kaplan–Meier Survival Analysis
4.2.3. Cumulative Hazard Estimation
4.2.4. Weibull Regression
4.2.5. Model Comparison
4.2.6. Model Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 21 February 2025).
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63, S91–S99. [Google Scholar] [CrossRef]
- Lip and Oral Cavity Cancer Treatment—NCI. Available online: https://www.cancer.gov/types/head-and-neck/patient/adult/lip-mouth-treatment-pdq (accessed on 21 February 2025).
- Chaurasia, A.; Alam, S.I.; Singh, N. Oral cancer diagnostics. Natl. J. Maxillofac. Surg. 2021, 12, 324–332. [Google Scholar] [CrossRef]
- Lestón, J.S.; Dios, P.D. Diagnostic clinical aids in oral cancer. Oral Oncol. 2010, 46, 418–422. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef]
- Heller, M.A.; Nyirjesy, S.C.; Balsiger, R.; Talbot, N.; VanKoevering, K.K.; Haring, C.T.; Old, M.O.; Kang, S.Y.; Seim, N.B. Modifiable risk factors for oral cavity cancer in non-smokers: A systematic review and meta-analysis. Oral Oncol. 2023, 137, 106300. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Gasmi, A.; Benahmed, A.G.; Noor, S.; Mujawdiya, P. Porphyromonas Gingivalis in the Development of Periodontitis: Impact on Dysbiosis and Inflammation. Arch. Razi Inst. 2022, 77, 1539–1551. [Google Scholar] [CrossRef]
- Ghanem, A.S.; Németh, O.; Móré, M.; Nagy, A.C. Role of oral health in heart and vascular health: A population-based study. PLoS ONE 2024, 19, e0301466. [Google Scholar] [CrossRef]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer-current concepts of the etiopathogenesis. Oncol. Rev. 2020, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, K.; Vandana, K.L. Indices for measuring periodontitis: A literature review. Int. Dent. J. 2020, 61, 76–84. [Google Scholar] [CrossRef]
- Flemmig, T.F. Periodontitis. Ann. Periodontol. 1999, 4, 32–37. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Su, S.-C.; Chang, L.-C.; Huang, H.-D.; Peng, C.-Y.; Chuang, C.-Y.; Chen, Y.-T.; Lu, M.-Y.; Chiu, Y.-W.; Chen, P.-Y.; Yang, S.-F. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 2020, 42, 127–135. [Google Scholar] [CrossRef]
- Knight, E.T.; Liu, J.; Seymour, G.J.; Faggion, C.M.; Cullinan, M.P. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontology 2000 2016, 71, 22–51. [Google Scholar] [CrossRef]
- Yasuno, T.; Tada, K.; Takahashi, K.; Watanabe, M.; Ito, K.; Arima, H.; Masutani, K. Dysbiosis of oral bacteria in patients with chronic kidney disease. Ren. Replace. Ther. 2024, 10, 63. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’addona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef]
- Asteriou, E.; Gkoutzourelas, A.; Mavropoulos, A.; Katsiari, C.; Sakkas, L.I.; Bogdanos, D.P. Curcumin for the Management of Periodontitis and Early ACPA-Positive Rheumatoid Arthritis: Killing Two Birds with One Stone. Nutrients 2018, 10, 908. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2020, 88, 116–126. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Nuclear factor-κB. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef]
- Ma, Q.; Hao, S.; Hong, W.; Tergaonkar, V.; Sethi, G.; Tian, Y.; Duan, C. Versatile function of NF-ĸB in inflammation and cancer. Exp. Hematol. Oncol. 2024, 13, 68. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’aiuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef]
- Pandey, A. C-Reactive Protein (CRP) and its Association with Periodontal Disease: A Brief Review. J. Clin. Diagn. Res. 2014, 8, ZE21. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, M.; Samols, D.; Kushner, I. STAT3 Participates in Transcriptional Activation of the C-reactive Protein Gene by Interleukin-6. J. Biol. Chem. 1996, 271, 9503–9509. [Google Scholar] [CrossRef]
- Ngwa, D.N.; Pathak, A.; Agrawal, A. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Mol. Immunol. 2022, 146, 50–56. [Google Scholar] [CrossRef]
- Kramer, F.; Torzewski, J.; Kamenz, J.; Veit, K.; Hombach, V.; Dedio, J.; Ivashchenko, Y. Interleukin-1β stimulates acute phase response and C-reactive protein synthesis by inducing an NFκB- and C/EBPβ-dependent autocrine interleukin-6 loop. Mol. Immunol. 2008, 45, 2678–2689. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer—Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]
- Antonelli, A.; Battaglia, A.M.; Sacco, A.; Petriaggi, L.; Giorgio, E.; Barone, S.; Biamonte, F.; Giudice, A. Ferroptosis and oral squamous cell carcinoma: Connecting the dots to move forward. Front. Oral Health 2024, 5, 1461022. [Google Scholar] [CrossRef]
- Li, M.; Jin, S.; Zhang, Z.; Ma, H.; Yang, X. Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer Lett. 2021, 527, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, Y.; Ying, Y.; Ding, Y. Current knowledge of ferroptosis in the pathogenesis and prognosis of oral squamous cell carcinoma. Cell. Signal. 2024, 119, 111176. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Gao, B.; Chen, X. Baicalin induces ferroptosis in oral squamous cell carcinoma by suppressing the activity of FTH1. J. Gene Med. 2024, 26, e3669. [Google Scholar] [CrossRef] [PubMed]
- Kenessey, I.; Nagy, P.; Polgár, C. The Hungarian situation of cancer epidemiology in the second decade of the 21st century. Magy Onkol 2022, 66, 175–184. [Google Scholar]
- O’sullivan, A.; Kabir, Z.; Harding, M. Lip, Oral Cavity and Pharyngeal Cancer Burden in the European Union from 1990–2019 Using the 2019 Global Burden of Disease Study. Int. J. Environ. Res. Public Health 2022, 19, 6532. [Google Scholar] [CrossRef]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef]
- Irani, S. New Insights into Oral Cancer—Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- OECD. EU Country Cancer Profile: Hungary 2023. In EU Country Cancer Profiles; OECD: Paris, France, 2023. [Google Scholar] [CrossRef]
- Hussein, A.A.; Helder, M.N.; de Visscher, J.G.; Leemans, C.R.; Braakhuis, B.J.; de Vet, H.C.; Forouzanfar, T. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur. J. Cancer 2017, 82, 115–127. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ghanem, A.S.; Móré, M.; Nagy, A.C. Assessing the impact of sociodemographic and lifestyle factors on oral health: A cross-sectional study in the Hungarian population. Front. Public Health 2023, 11, 1276758. [Google Scholar] [CrossRef]
- Nemes, J.A.; Redl, P.; Boda, R.; Kiss, C.; Márton, I.J. Oral Cancer Report from Northeastern Hungary. Pathol. Oncol. Res. 2008, 14, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tezal, M.; Scannapieco, F.A.; Wactawski-Wende, J.; Meurman, J.H.; Marshall, J.R.; Rojas, I.G.; Stoler, D.L.; Genco, R.J. Dental Caries and Head and Neck Cancers. Arch. Otolaryngol. Neck Surg. 2013, 139, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Mensch, K.; Nagy, G.; Nagy, Á.; Bródy, A. A szájüreg leggyakoribb bakteriális eredetű kórképeinek jellegzetességei, diagnosztikája és kezelése. Orvosi Hetil. 2019, 160, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.; Hajishengallis, G. A new inflammatory cytokine on the block: Re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dent. Res. 2008, 87, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Singh, A.K.; Shukla, N.K.; Das, S.N. Inter-relation of Th1, Th2, Th17 and Treg cytokines in oral cancer patients and their clinical significance. Hum. Immunol. 2014, 75, 330–337. [Google Scholar] [CrossRef]
- Anvar, M.T.; Rashidan, K.; Arsam, N.; Rasouli-Saravani, A.; Yadegari, H.; Ahmadi, A.; Asgari, Z.; Vanan, A.G.; Ghorbaninezhad, F.; Tahmasebi, S. Th17 cell function in cancers: Immunosuppressive agents or anti-tumor allies? Cancer Cell Int. 2024, 24, 355. [Google Scholar] [CrossRef]
- Huang, N.; Dong, H.; Luo, Y.; Shao, B. Th17 Cells in Periodontitis and Its Regulation by A20. Front. Immunol. 2021, 12, 742925. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ouyang, Y.; Zhang, Z.; Wen, S.; Pi, Y.; Chen, D.; Su, Z.; Liang, Z.; Guo, L.; Wang, Y. The role of Th17 cells: Explanation of relationship between periodontitis and COPD? Inflamm. Res. 2022, 71, 1011–1024. [Google Scholar] [CrossRef]
- Gaur, P.; Shukla, N.K.; Das, S.N. Phenotypic and Functional Characteristics of Th17 (CD4+IL17A+) Cells in Human Oral Squamous Cell Carcinoma and Its Clinical Relevance. Immunol. Investig. 2017, 46, 689–702. [Google Scholar] [CrossRef]
- Brauer, M.; A Roth, G.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; et al. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef]
- Komlós, G.; Csurgay, K.; Horváth, F.; Pelyhe, L.; Németh, Z. Periodontitis as a risk for oral cancer: A case–control study. BMC Oral Health 2021, 21, 640. [Google Scholar] [CrossRef]
- Surlari, Z.; Virvescu, D.I.; Baciu, E.-R.; Vasluianu, R.-I.; Budală, D.G. The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma? Appl. Sci. 2021, 11, 12100. [Google Scholar] [CrossRef]
- Elebyary, O.; Barbour, A.; Fine, N.; Tenenbaum, H.C.; Glogauer, M. The Crossroads of Periodontitis and Oral Squamous Cell Carcinoma: Immune Implications and Tumor Promoting Capacities. Front. Oral Health 2021, 1, 584705. [Google Scholar] [CrossRef] [PubMed]
- Gonde, N.; Rathod, S.; Kolte, A.; Lathiya, V.; Ughade, S. Association between tooth loss and risk of occurrence of oral cancer—A systematic review and meta-analysis. Dent. Res. J. 2023, 20, 4. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Adeyemo, W.L. Do pathologies associated with impacted lower third molars justify prophylactic removal? A critical review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 448–452. [Google Scholar] [CrossRef]
- Araújo, J.P.; Kowalski, L.P.; Rodrigues, M.L.; de Almeida, O.P.; Pinto, C.A.L.; Alves, F.A. Malignant transformation of an odontogenic cyst in a period of 10 years. Case Rep. Dent. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Salema, H.; Joshi, S.; Pawar, S.; Nair, V.S.; Deo, V.V.; Sanghai, M.M. Evaluation of the Role of C-reactive Protein as a Prognostic Indicator in Oral Pre-malignant and Malignant Lesions. Cureus 2024, 16, e60812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, D. Prognostic Impact of Serum CRP Level in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 889844. [Google Scholar] [CrossRef]
- Volpato, S.; Pahor, M.; Ferrucci, L.; Simonsick, E.M.; Guralnik, J.M.; Kritchevsky, S.B.; Fellin, R.; Harris, T.B. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitior-1 in well-functioning older adults. Circulation 2004, 109, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette smoking and variations in systemic immune and inflammation markers. JNCI J. Natl. Cancer Inst. 2014, 106, dju294. [Google Scholar] [CrossRef]
- AlShammari, A.; AlSaleh, S.; AlKandari, A.; AlSaqabi, S.; AlJalahmah, D.; AlSulimmani, W.; AlDosari, M.; AlHazmi, H.; AlQaderi, H. The association between dental caries and serum crp in the us adult population: Evidence from NHANES 2015–2018. BMC Public Health 2024, 24, dju294. [Google Scholar] [CrossRef]
- Malyszko, J.; Tesarova, P.; Capasso, G.; Capasso, A. The link between kidney disease and cancer: Complications and treatment. Lancet 2020, 396, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, Q.; Liu, B.; Ma, Q.; Zhang, T.; Huang, T.; Lv, Z.; Wang, R. Chronic Kidney Disease and Cancer: Inter-Relationships and Mechanisms. Front. Cell Dev. Biol. 2022, 10, 868715. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Staplin, N.; Emberson, J.; Baigent, C.; Turner, R.; Chalmers, J.; Zoungas, S.; Pollock, C.; Cooper, B.; Harris, D.; et al. Chronic kidney disease and the risk of cancer: An individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016, 16, 488. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Nelson, W. Hazard Plotting for Incomplete Failure Data. J. Qual. Technol. 1969, 1, 27–52. [Google Scholar] [CrossRef]
- McKeague, I.W. Introduction to Aalen (1978) Nonparametric Inference for a Family of Counting Processes. In Breakthroughs in Statistics; Springer Series in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1997; pp. 347–367. [Google Scholar] [CrossRef]
- Weibull, W. A Statistical Distribution Function of Wide Applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar] [CrossRef]
- Collett, D. Modelling Survival Data in Medical Research; Taylor & Francis: London, UK, 2015. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Treisman, M. Book Review: Signal Detection Theory and ROC Analysis. Q. J. Exp. Psychol. 1977, 29, 745–746. [Google Scholar] [CrossRef]
- Harrell, F.E.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the Yield of Medical Tests. JAMA J. Am. Med. Assoc. 1982, 247, 2543. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
| Variable | Category | N (%) |
|---|---|---|
| Age, years | Mean (SD) | 50.43 (15.40) |
| Median (IQR) | 51 (40–62) | |
| Sex | Male | 2193 (45.40) |
| Female | 2637 (54.60) | |
| Periodontitis | No | 3319 (68.67) |
| Yes | 1514 (31.33) | |
| C-reactive protein | Normal (≤15) | 295 (74.31) |
| High (>15) | 102 (25.69) | |
| eGFR | Normal (≥60) | 317 (80.25) |
| Kidney Disease (<60) | 78 (19.75) | |
| Disorders of tooth development and eruption (K00) | No | 4767 (98.63) |
| Yes | 66 (1.37) | |
| Embedded and impacted teeth (K01) | No | 4793 (99.17) |
| Yes | 40 (0.83) | |
| Dental caries (K02) | No | 4698 (97.21) |
| Yes | 135 (2.79) | |
| Other diseases of hard tissues of teeth (K03) | No | 4063 (84.07) |
| Yes | 770 (15.93) | |
| Diseases of pulp and periapical tissues (K04) | No | 4686 (96.96) |
| Yes | 147 (3.04) | |
| Other disorders of gingiva and edentulous alveolar ridge (K06) | No | 4756 (98.41) |
| Yes | 77 (1.59) | |
| Other specified disorders of teeth and supporting structures (K08) | No | 4666 (96.54) |
| Yes | 167 (3.46) |
| Variable | Category | Observed Events | Expected Events | p-Value |
|---|---|---|---|---|
| Periodontitis | No | 59 | 134.85 | <0.001 |
| Yes | 114 | 38.15 | ||
| C-reactive protein | Normal (≤15) | 14 | 25.9 | 0.007 |
| High (>15) | 20 | 8.1 | ||
| Gender | Male | 130 | 73.5 | <0.001 |
| Female | 43 | 99.5 | ||
| Disorders of tooth development and eruption (K00) | No | 164 | 168.33 | 0.042 |
| Yes | 9 | 4.67 | ||
| Embedded and impacted teeth (K01) | No | 169 | 171.79 | 0.011 |
| Yes | 4 | 1.21 | ||
| Dental caries (K02) | No | 149 | 164.94 | <0.001 |
| Yes | 24 | 8.06 | ||
| Other diseases of hard tissues of teeth (K03) | No | 129 | 156.19 | <0.001 |
| Yes | 44 | 16.81 | ||
| Diseases of pulp and periapical tissues (K04) | No | 168 | 167.22 | 0.74 |
| Yes | 5 | 5.78 | ||
| Other disorders of gingiva and edentulous alveolar ridge (K06) | No | 171 | 170.95 | 0.971 |
| Yes | 2 | 2.05 | ||
| Other specified disorders of teeth and supporting structures (K08) | No | 148 | 167.64 | <0.001 |
| Yes | 25 | 5.36 | ||
| eGFR | Normal (≥60) | 13 | 14.53 | 0.489 |
| Kidney Disease (<60) | 9 | 7.47 |
| Variable | Category | HR (95% CI) | p-Value |
|---|---|---|---|
| Periodontitis | Yes vs. No (ref) | 5.99 [1.96–18.30] | 0.002 |
| C-reactive protein | High (>15) vs. Normal (≤15, ref) | 4.16 [1.45–12.00] | 0.008 |
| eGFR | Kidney Disease (<60) vs. Normal (≥60, ref) | 0.95 [0.35–2.61] | 0.919 |
| Age | Continuous (per year) | 0.98 [0.95–1.02] | 0.347 |
| Gender | Female vs. Male (ref) | 0.06 [0.01–0.50] | 0.009 |
| Disorders of tooth development and eruption (K00) | Yes vs. No (ref) | 3.97 [0.88–17.92] | 0.073 |
| Embedded and impacted teeth (K01) | Yes vs. No (ref) | 12.52 [2.48–63.18] | 0.002 |
| Dental caries (K02) | Yes vs. No (ref) | 2.21 [0.56–8.75] | 0.259 |
| Other diseases of hard tissues of teeth (K03) | Yes vs. No (ref) | 0.60 [0.13–2.86] | 0.524 |
| Diseases of pulp and periapical tissues (K04) | Yes vs. No (ref) | 0.52 [0.06–4.61] | 0.558 |
| Other specified disorders of teeth and supporting structures (K08) | Yes vs. No (ref) | 0.76 [0.18–3.17] | 0.704 |
| Parameter | Estimate (95% CI) | Interpretation |
|---|---|---|
| p | 2.20 [1.39–3.49] | Hazard increases over time (positive ageing effect) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanem, A.S.; Sipos, K.; Tóth, Á.; Nagy, A.C. Inflammatory Biomarkers and Oral Health Disorders as Predictors of Head and Neck Cancer: A Retrospective Longitudinal Study. Int. J. Mol. Sci. 2025, 26, 2279. https://doi.org/10.3390/ijms26052279
Ghanem AS, Sipos K, Tóth Á, Nagy AC. Inflammatory Biomarkers and Oral Health Disorders as Predictors of Head and Neck Cancer: A Retrospective Longitudinal Study. International Journal of Molecular Sciences. 2025; 26(5):2279. https://doi.org/10.3390/ijms26052279
Chicago/Turabian StyleGhanem, Amr Sayed, Kitti Sipos, Ágnes Tóth, and Attila Csaba Nagy. 2025. "Inflammatory Biomarkers and Oral Health Disorders as Predictors of Head and Neck Cancer: A Retrospective Longitudinal Study" International Journal of Molecular Sciences 26, no. 5: 2279. https://doi.org/10.3390/ijms26052279
APA StyleGhanem, A. S., Sipos, K., Tóth, Á., & Nagy, A. C. (2025). Inflammatory Biomarkers and Oral Health Disorders as Predictors of Head and Neck Cancer: A Retrospective Longitudinal Study. International Journal of Molecular Sciences, 26(5), 2279. https://doi.org/10.3390/ijms26052279







