NAD+ Promotes Superovulation of Huaxi Cattle Through Regulation of Cumulus Cell Proliferation and Oocyte Maturation
Abstract
1. Introduction
2. Results
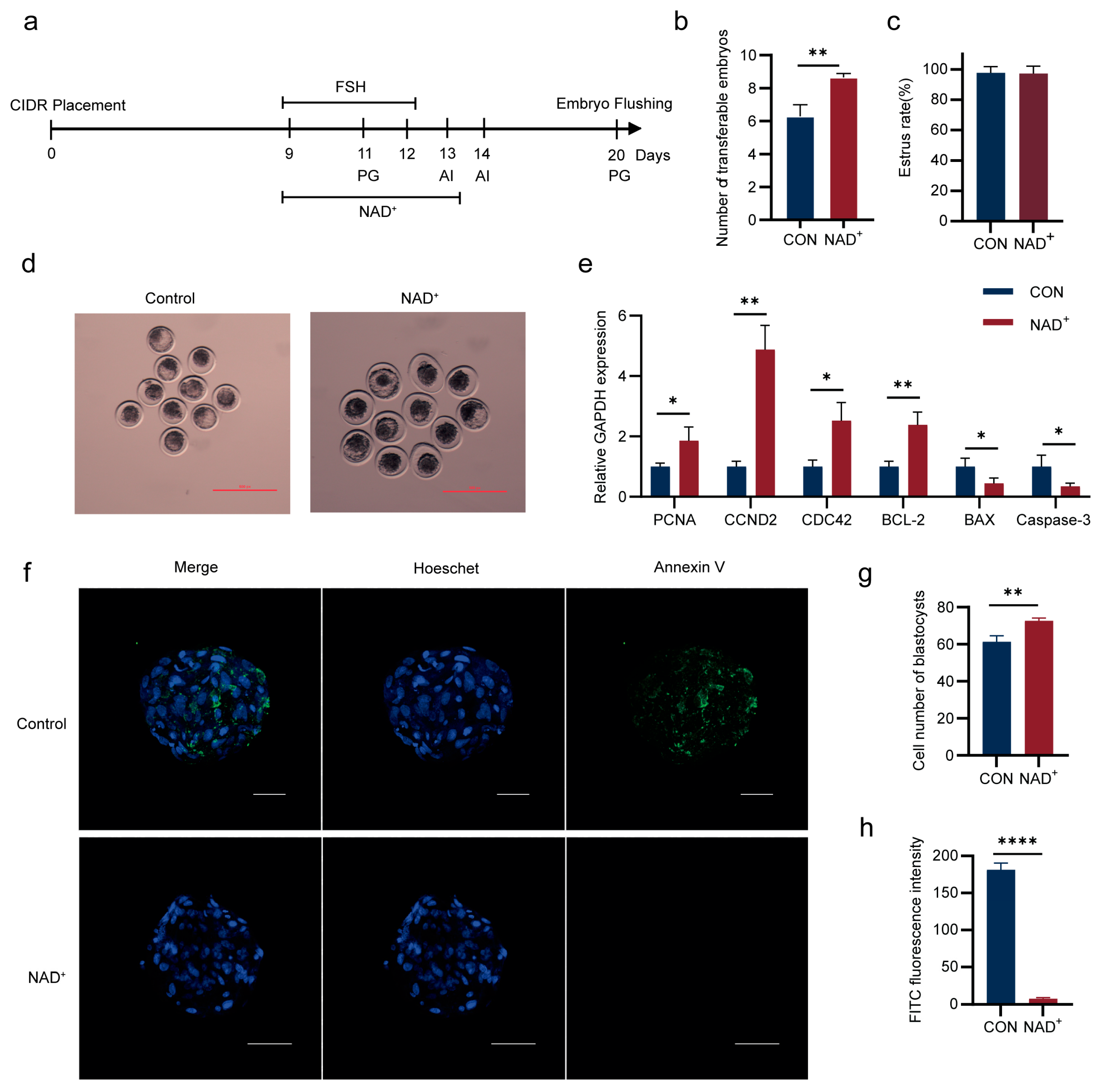
2.1. NAD+ Promotes Superovulation in Huaxi Cattle
2.2. NAD+ Promotes Lipid Metabolism During Superovulation in Huaxi Cattle
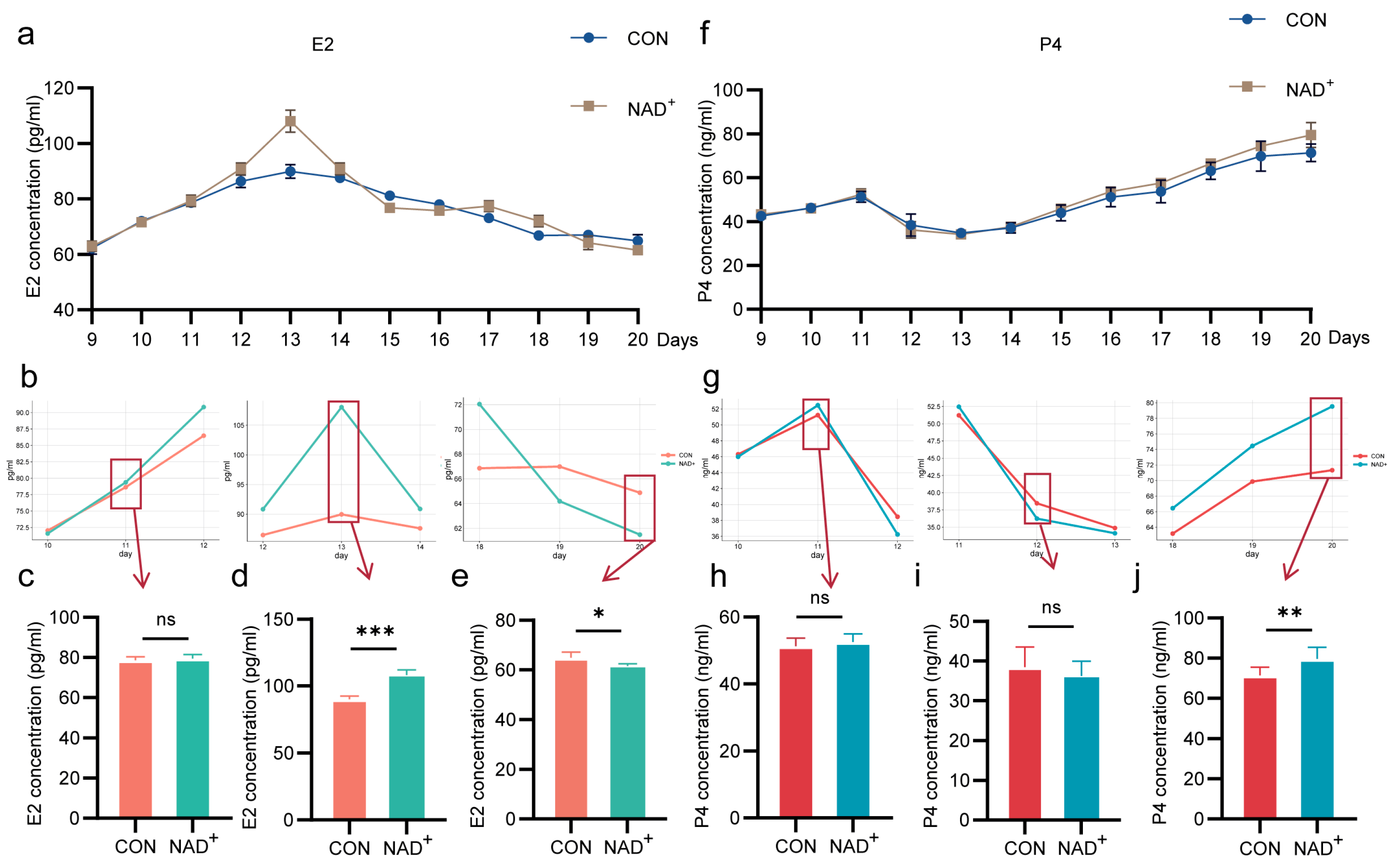
2.3. NAD+ Regulates Hormone Secretion During Superovulation in Huaxi Cattle
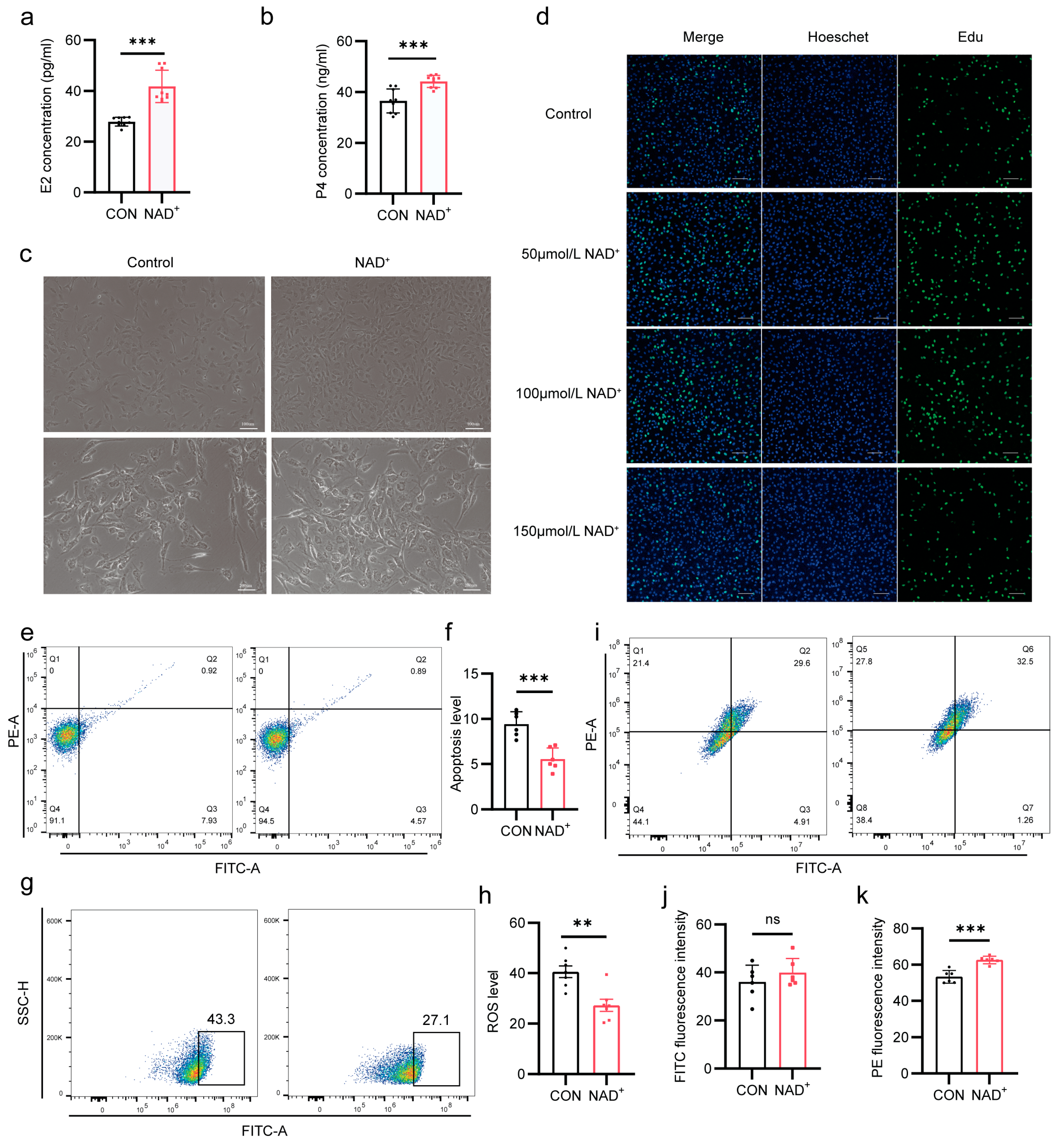
2.4. NAD+ Improves Hormone Synthesis by Promoting Proliferation of Cumulus Cells
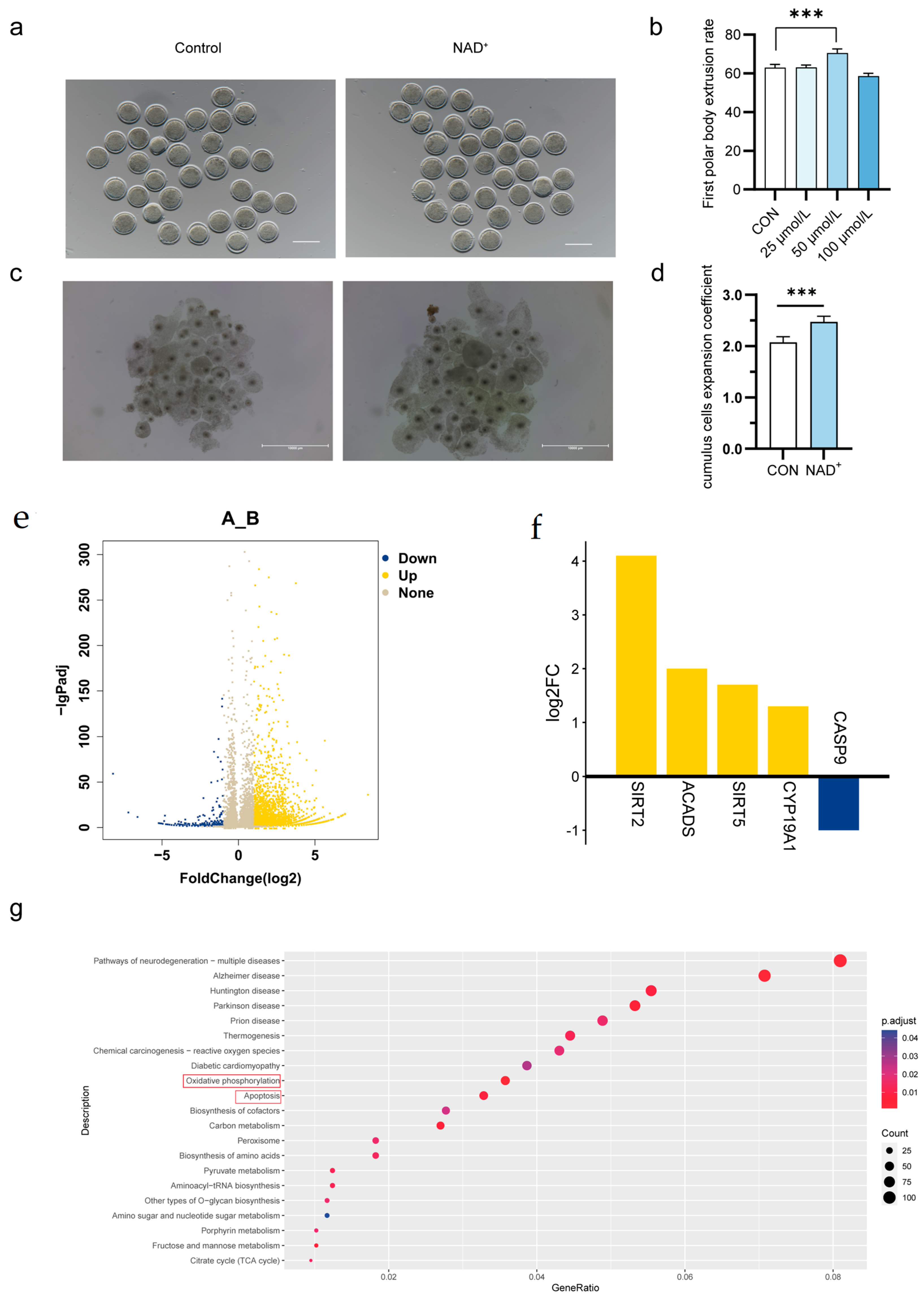
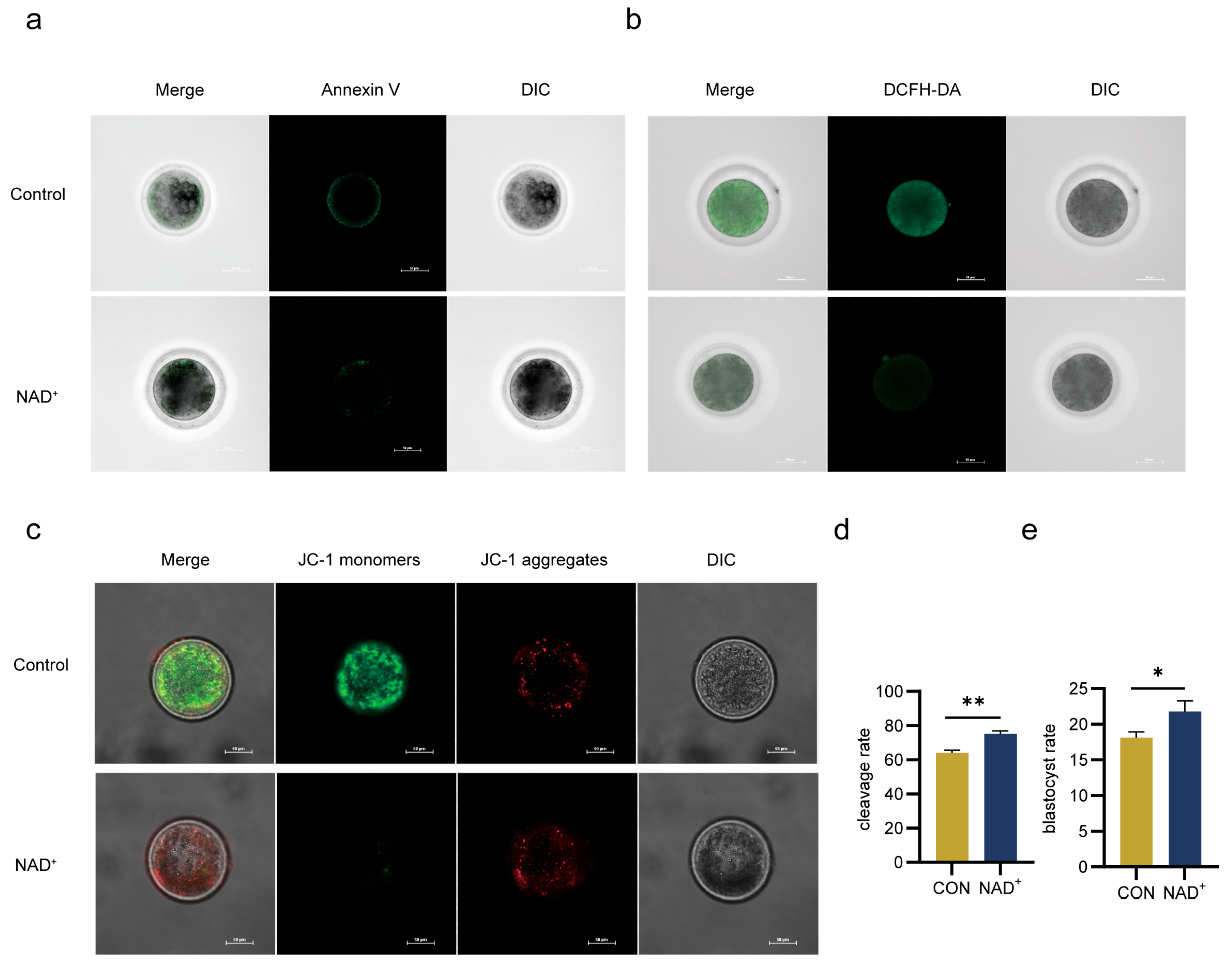
2.5. NAD+ Directly Promotes Oocytes Maturation
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Superovulation Treatment and Artificial Insemination
4.3. Embryo Flushing
4.4. Serum Collection
4.5. Evaluation of Oocyte Maturation After In Vitro Maturation (IVM)
4.6. Culture of Cumulus Cells
4.7. RNA Extraction and Quantitative Real-Time PCR (qPCR)
4.8. Apoptosis, ROS and Mitochondrial Membrane Potential Staining
4.9. Sample Preparation and Analysis of Metabolomics
4.10. Analysis of Single-Cell RNA-Seq
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1
| Time | Day9 | Day10 | Day11 | Day12 | Day13 | Day14 | Day20 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | PM | AM | PM | AM | PM | AM | PM | AM | PM | AM | PM | ||
| Hormone | FSH | FSH | FSH | FSH | FSH/PG | FSH/PG | FSH | FSH | AI | AI | PG | ||
| Dose | 100 | 100 | 75 | 75 | 50/4 | 50/4 | 25 | 25 | 4 | ||||
| (Unit) | μg | μg | μg | μg | μg/mg | μg/mg | μg | μg | mg | ||||
Appendix A.2
| Name | Primer Sequence (5′-3′) |
|---|---|
| PCNA | F: GTCCAGGGCTCCATCTTGAA |
| R: CAAGGAGACATGAGACGAGT | |
| CCND2 | F: TGACCGCTGAGAAGTTATGC |
| R: CGCCAGGTTCCATTTCAACT | |
| CDC42 | F: GTTGTTGTGGGTGATGGTGC |
| R: TCCCCACCAATCATAACTGT | |
| BCL-2 | F: GAGTCGGATCGCAACTTGGA |
| R: CTCTCGGCTGCTGCATTGT | |
| BAX | F: GGCTGGACATTGGACTTCCTTC |
| R: TGGTCACTGTCTGCCATGTGG | |
| Caspase-3 | F: TACTTGGGAAGGTGTGAGAAAACTAA |
| R: AACCCGTCTCCCTTTATATTGCT | |
| GAPDH | F: GGGTCATCATCTCTGCACCT |
| R: GGTCATAAGTCCCTCCACGA |
References
- Ma, J.; Gao, X.; Li, J.; Gao, H.; Wang, Z.; Zhang, L.; Xu, L.; Gao, H.; Li, H.; Wang, Y.; et al. Assessing the genetic background and selection signatures of Huaxi Cattle using high-density SNP array. Animals 2021, 11, 3469. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Liang, M.; Du, L.; Li, K.; Li, J.; Qian, L.; Xue, Q.; Qiu, S.; Xu, L.; Zhang, L.; et al. Transcriptome analysis of compensatory growth and meat quality alteration after varied restricted feeding conditions in beef cattle. Int. J. Mol. Sci. 2024, 25, 2704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, B.; Wang, J.; Zhang, L.; Xu, L.; Chen, Y.; Wang, Z.; Gao, H.; Li, J.; Gao, X. Evaluation of genomic mating approach based on genetic algorithms for long-term selection in Huaxi cattle. BMC Genom. 2024, 25, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, X.; Yao, D.; Wang, B.; Li, Y.; Zhang, J.; Zhang, X. Insights into transcriptomic differences in ovaries between lambs and adult sheep after superovulation treatment. Animals 2023, 13, 665. [Google Scholar] [CrossRef]
- Mikkola, M.; Hasler, J.F.; Taponen, J. Factors affecting embryo production in superovulated Bos taurus cattle. Reprod. Fertil. Dev. 2019, 32, 104–124. [Google Scholar] [CrossRef]
- Lerner, S.P.; Thayne, W.V.; Baker, R.D.; Henschen, T.; Meredith, S.; Inskeep, E.K.; Dailey, R.A.; Lewis, P.E.; Butcher, R.L. Age, dose of FSH and other factors affecting superovulation in Holstein cows. J. Anim. Sci. 1986, 63, 176–183. [Google Scholar] [CrossRef]
- McCue, P.M. Superovulation. Vet. Clin. N. Am. Equine Pract. 1996, 12, 1–11. [Google Scholar] [CrossRef]
- Gutierrez-Reinoso, M.A.; Escribano, E.H.; Cabezas, I.; Hugues, F.; Parra, N.C.; Zúniga, R.; Sánchez, O.; Toledo, J.R.; Garcia-Herreros, M. Superovulation of dairy cows using recombinant FSH (bscrFSH): Effect of the number of FSH applications on ovarian response, hormone profiles, and in vivo embryo production. Theriogenology 2024, 234, 42–50. [Google Scholar] [CrossRef]
- Poddar, S.K.; Sifat, A.E.; Haque, S.; Nahid, N.A.; Chowdhury, S.; Mehedi, I. Nicotinamide mononucleotide: Exploration of diverse therapeutic applications of a potential molecule. Biomolecules 2019, 9, 34. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Zapata-Pérez, R.; Wanders, R.J.A.; van Karnebeek, C.D.M.; Houtkooper, R.H. NAD+ homeostasis in human health and disease. EMBO Mol. Med. 2021, 13, e13943. [Google Scholar] [CrossRef] [PubMed]
- Long, A.N.; Owens, K.; Schlappal, A.E.; Kristian, T.; Fishman, P.S.; Schuh, R.A. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, T.; Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Xu, H.; Mao, X.; Zhang, S.; Ren, J.; Jiang, S.; Cai, L.; Miao, X.; Tao, Y.; Peng, C.; Lv, M.; et al. Perfluorooctanoic acid triggers premature ovarian insufficiency by impairing NAD+ synthesis and mitochondrial function in adult zebrafish. Toxicol. Sci. 2024, 201, 118–128. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Therapeutic potential of nicotinamide adenine dinucleotide (NAD). Eur. J. Pharmacol. 2020, 879, 173158. [Google Scholar] [CrossRef]
- Hajializadeh, Z.; Khaksari, M. The protective effects of 17-β estradiol and SIRT1 against cardiac hypertrophy: A review. Heart Fail. Rev. 2022, 27, 725–738. [Google Scholar] [CrossRef]
- Song, M.; Li, Y.; Zhou, Y.; Yan, J.; Zhou, X.; Gao, Q.; Miao, Y.; Xiong, B. Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress. J. Anim. Sci. Biotechnol. 2022, 13, 68. [Google Scholar] [CrossRef]
- Dölle, C.; Skoge, R.H.; Vanlinden, M.R.; Ziegler, M. NAD biosynthesis in humans--enzymes, metabolites and therapeutic aspects. Curr. Top. Med. Chem. 2013, 13, 2907–2917. [Google Scholar] [CrossRef]
- Cambronne, X.A.; Stewart, M.L.; Kim, D.; Jones-Brunette, A.M.; Morgan, R.K.; Farrens, D.L.; Cohen, M.S.; Goodman, R.H. Biosensor reveals multiple sources for mitochondrial NAD+. Science 2016, 352, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Lubusky, M.; Prochazka, M.; Dhaifalah, I.; Horak, D.; Geierova, M.; Santavy, J. Fetal enterolithiasis: Prenatal sonographic and MRI diagnosis in two cases of urorectal septum malformation (URSM) sequence. Prenat. Diagn. 2006, 26, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nagai, T.; Hamano, S.; Kuwayama, M.; Okamura, N.; Okano, A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol. Reprod. 1993, 49, 228–232. [Google Scholar] [CrossRef]
- Geshi, M.; Takenouchi, N.; Yamauchi, N.; Nagai, T. Effects of sodium pyruvate in nonserum maturation medium on maturation, fertilization, and subsequent development of bovine oocytes with or without cumulus cells. Biol. Reprod. 2000, 63, 1730–1734. [Google Scholar] [CrossRef]
- Meister, A. Transport and metabolism of glutathione and gamma-glutamyl amino acids. Biochem. Soc. Trans. 1983, 11, 793–794. [Google Scholar] [CrossRef]
- Lim, J.M.; Liou, S.S.; Hansel, W. Intracytoplasmic glutathione concentration and the role of beta-mercaptoethanol in preimplantation development of bovine embryos. Theriogenology 1996, 46, 429–439. [Google Scholar] [CrossRef]
- de Matos, D.G.; Furnus, C.C.; Moses, D.F. Glutathione synthesis during in vitro maturation of bovine oocytes: Role of cumulus cells. Biol. Reprod. 1997, 57, 1420–1425. [Google Scholar] [CrossRef]
- Mori, T.; Amano, T.; Shimizu, H. Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured in vitro. Biol. Reprod. 2000, 62, 913–919. [Google Scholar] [CrossRef][Green Version]
- Downs, S.M.; Utecht, A.M. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol. Reprod. 1999, 60, 1446–1452. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Cocero, M.J.; Dominguez, V.; McNeilly, A.S.; Gonzalez-Bulnes, A. Follicular wave status at the beginning of the FSH treatment modifies reproductive features in superovulated sheep. Reprod. Biol. 2006, 6, 243–264. [Google Scholar] [PubMed]
- Gradela, A.; Esper, C.R.; Rosa, E.S.A.A. Plasma concentrations of progesterone, 17 beta-estradiol and androstenedione and superovulatory response of Nelore cows (Bos indicus) treated with FSH. Theriogenology 1996, 45, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.-H.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef] [PubMed]
- Moor, R.M.; Polge, C.; Willadsen, S.M. Effect of follicular steroids on the maturation and fertilization of mammalian oocytes. J. Embryol. Exp. Morphol. 1980, 56, 319–335. [Google Scholar] [CrossRef]
- Huang, Z.; Wells, D. The human oocyte and cumulus cells relationship: New insights from the cumulus cell transcriptome. Mol. Hum. Reprod. 2010, 16, 715–725. [Google Scholar] [CrossRef]
- Boucret, L.; Chao de la Barca, J.M.; Morinière, C.; Desquiret, V.; Ferré-L’Hôtellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef]
- Hasegawa, J.; Yanaihara, A.; Iwasaki, S.; Otsuka, Y.; Negishi, M.; Akahane, T.; Okai, T. Reduction of progesterone receptor expression in human cumulus cells at the time of oocyte collection during IVF is associated with good embryo quality. Hum. Reprod. 2005, 20, 2194–2200. [Google Scholar] [CrossRef][Green Version]
- Yamashita, Y.; Shimada, M.; Okazaki, T.; Maeda, T.; Terada, T. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol. Reprod. 2003, 68, 1193–1198. [Google Scholar] [CrossRef]
- Liang, J.; Huang, F.; Song, Z.; Tang, R.; Zhang, P.; Chen, R. Impact of NAD+ metabolism on ovarian aging. Immun. Ageing 2023, 20, 70. [Google Scholar] [CrossRef]
- Peluso, J.J. Progesterone as a regulator of granulosa cell viability. J. Steroid Biochem. Mol. Biol. 2003, 85, 167–173. [Google Scholar] [CrossRef]
- Almada, M.; Oliveira, A.; Amaral, C.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.; Correia-da-Silva, G.; Teixeira, N. Anandamide targets aromatase: A breakthrough on human decidualization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158512. [Google Scholar] [CrossRef] [PubMed]
- Vega, W.H.O.; Quirino, C.R.; Bartholazzi-Junior, A.; Rua, M.A.S.; Serapião, R.V.; Oliveira, C.S. Variants in the CYP19A1 gene can affect in vitro embryo production traits in cattle. J. Assist. Reprod. Genet. 2018, 35, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Jessop, E.; Young, N.; Garcia-Del-Valle, B.; Crusher, J.T.; Obara, B.; Karakesisoglou, I. SIRT2 inhibition by AGK2 promotes perinuclear cytoskeletal organisation and reduces invasiveness of MDA-MB-231 triple-negative breast cancer cells in confined in vitro models. Cells 2024, 13, 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Tian, M.X.; Sun, R.Q.; Zhang, M.L.; Zhou, L.S.; Jin, L.; Chen, L.L.; Zhou, W.J.; Duan, K.L.; Chen, Y.J.; et al. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 2018, 19, 64. [Google Scholar] [CrossRef]
- Sever, A.I.M.; Alderson, T.R.; Rennella, E.; Aramini, J.M.; Liu, Z.H.; Harkness, R.W.; Kay, L.E. Activation of caspase-9 on the apoptosome as studied by methyl-TROSY NMR. Proc. Natl. Acad. Sci. USA 2023, 120, e2310944120. [Google Scholar] [CrossRef]
- Lisyová, J.; Chandoga, J.; Jungová, P.; Repiský, M.; Knapková, M.; Machková, M.; Dluholucký, S.; Behúlová, D.; Šaligová, J.; Potočňáková, Ľ.; et al. An unusually high frequency of SCAD deficiency caused by two pathogenic variants in the ACADS gene and its relationship to the ethnic structure in Slovakia. BMC Med. Genet. 2018, 19, 64. [Google Scholar] [CrossRef]
- Yang, H.; Woscholski, R. A novel high-throughput assay reveals that the temperature induced increases in transphosphatidylation of phospholipase D are dependent on the alcohol acceptor concentration. Biomolecules 2022, 12, 632. [Google Scholar] [CrossRef]
- Peppino Margutti, M.; Reyna, M.; Meringer, M.V.; Racagni, G.E.; Villasuso, A.L. Lipid signalling mediated by PLD/PA modulates proline and H2O2 levels in barley seedlings exposed to short- and long-term chilling stress. Plant Physiol. Biochem. 2017, 113, 149–160. [Google Scholar] [CrossRef]
- Zeng, X.-X.I.; Zheng, X.; Xiang, Y.; Cho, H.P.; Jessen, J.R.; Zhong, T.P.; Solnica-Krezel, L.; Brown, H.A. Phospholipase D1 is required for angiogenesis of intersegmental blood vessels in zebrafish. Dev. Biol. 2009, 328, 363–376. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Song, J.; Deng, J.; Sun, Z. Study on follicular fluid metabolomics components at different ages based on lipid metabolism. Reprod. Biol. Endocrinol. 2020, 18, 42. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, M.; Di, A.; Jiang, X.; Wu, J.; Zhang, J.; Liu, X.; Bai, C.; Su, G.; Song, L.; et al. NAD+ Promotes Superovulation of Huaxi Cattle Through Regulation of Cumulus Cell Proliferation and Oocyte Maturation. Int. J. Mol. Sci. 2025, 26, 2276. https://doi.org/10.3390/ijms26052276
Wang S, Liu M, Di A, Jiang X, Wu J, Zhang J, Liu X, Bai C, Su G, Song L, et al. NAD+ Promotes Superovulation of Huaxi Cattle Through Regulation of Cumulus Cell Proliferation and Oocyte Maturation. International Journal of Molecular Sciences. 2025; 26(5):2276. https://doi.org/10.3390/ijms26052276
Chicago/Turabian StyleWang, Song, Mingcheng Liu, Anqi Di, Xiqing Jiang, Junjia Wu, Jiandong Zhang, Xuefei Liu, Chunling Bai, Guanghua Su, Lishuang Song, and et al. 2025. "NAD+ Promotes Superovulation of Huaxi Cattle Through Regulation of Cumulus Cell Proliferation and Oocyte Maturation" International Journal of Molecular Sciences 26, no. 5: 2276. https://doi.org/10.3390/ijms26052276
APA StyleWang, S., Liu, M., Di, A., Jiang, X., Wu, J., Zhang, J., Liu, X., Bai, C., Su, G., Song, L., Li, G., Liu, Z., & Yang, L. (2025). NAD+ Promotes Superovulation of Huaxi Cattle Through Regulation of Cumulus Cell Proliferation and Oocyte Maturation. International Journal of Molecular Sciences, 26(5), 2276. https://doi.org/10.3390/ijms26052276





