Acid Sphingomyelinase Regulates AdipoRon-Induced Differentiation of Arterial Smooth Muscle Cells via TFEB Activation
Abstract
1. Introduction
2. Results
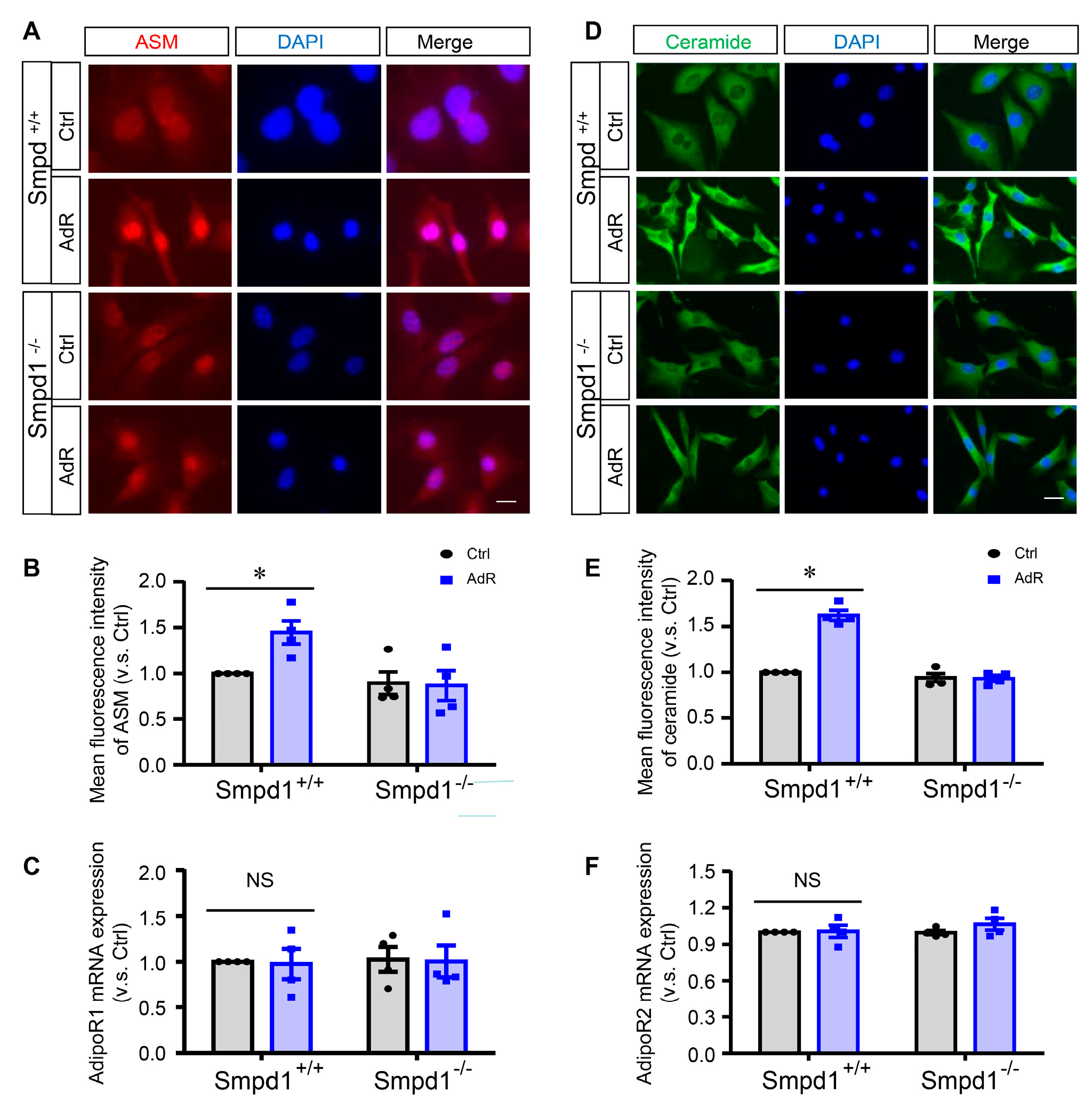
2.1. AdipoRon Induces ASM-Mediated Ceramide Signaling in SMCs
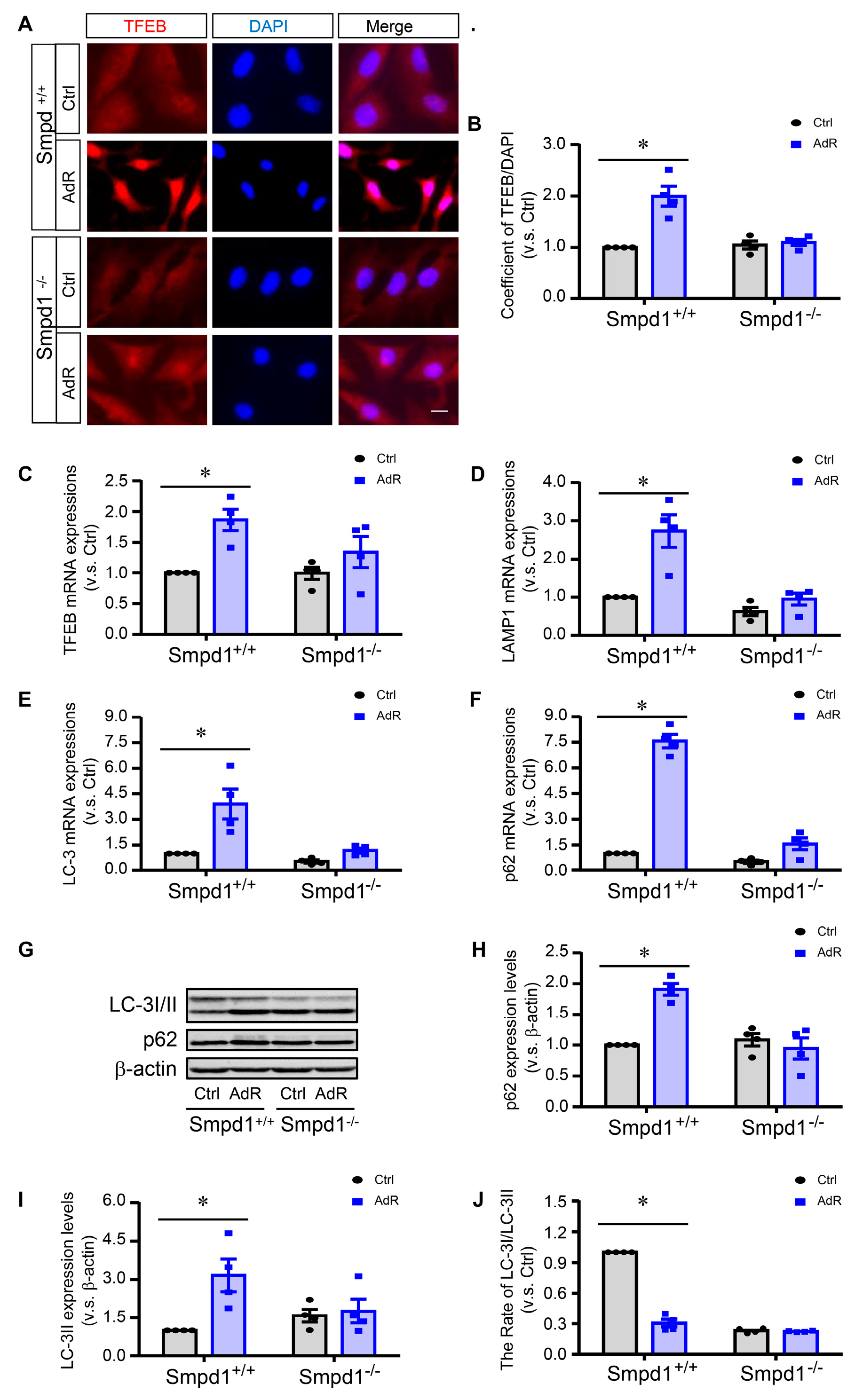
2.2. ASM Deficiency Inhibits adipoRon-Induced TFEB Activation and Autophagy in SMCs
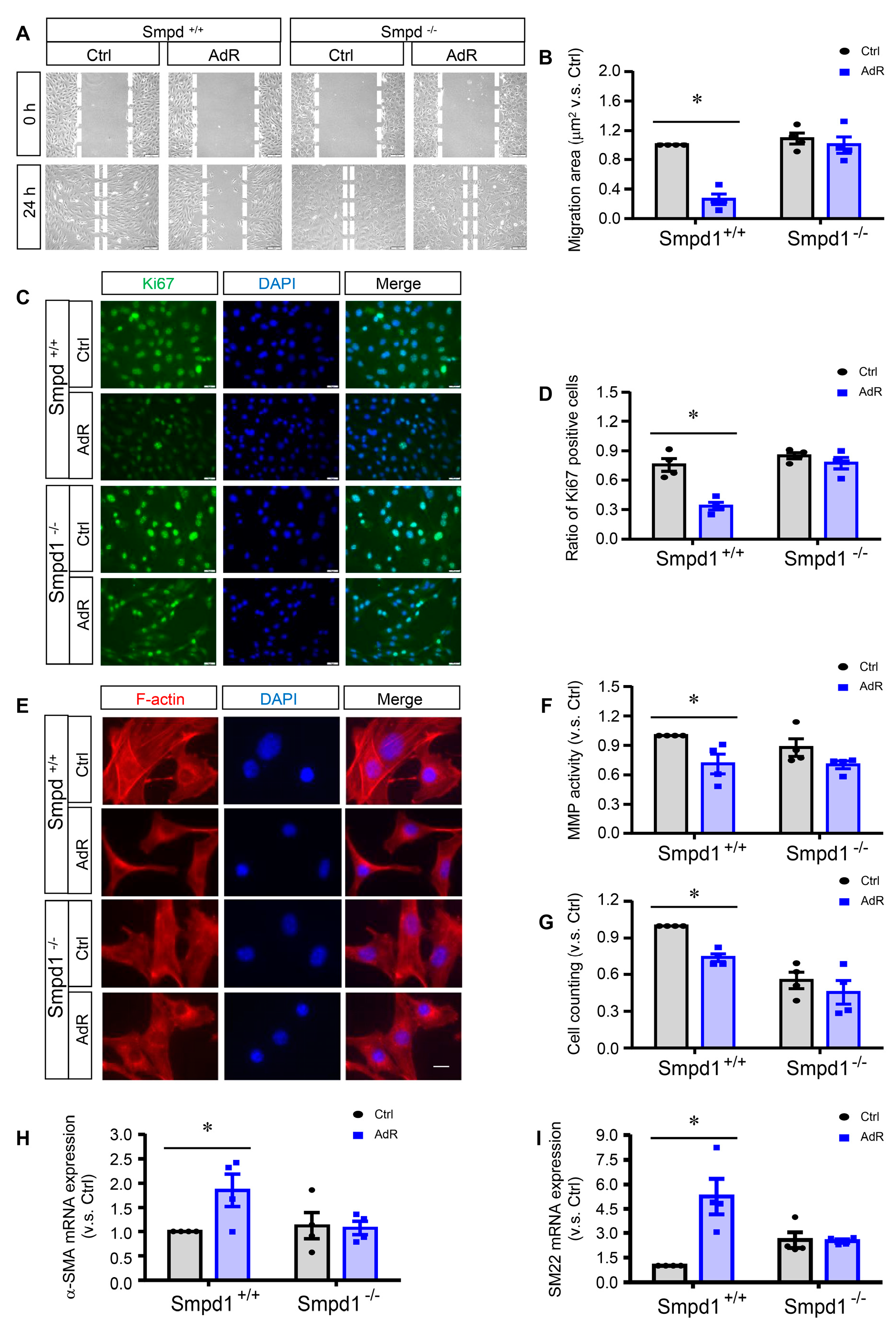
2.3. ASM Deficiency Prevents the Inhibitory Effects of adipoRon on SMC Proliferation and Migration
2.4. Effect of Calcineurin Inhibition on adipoRon-Induced TFEB–Autophagy in SMCs
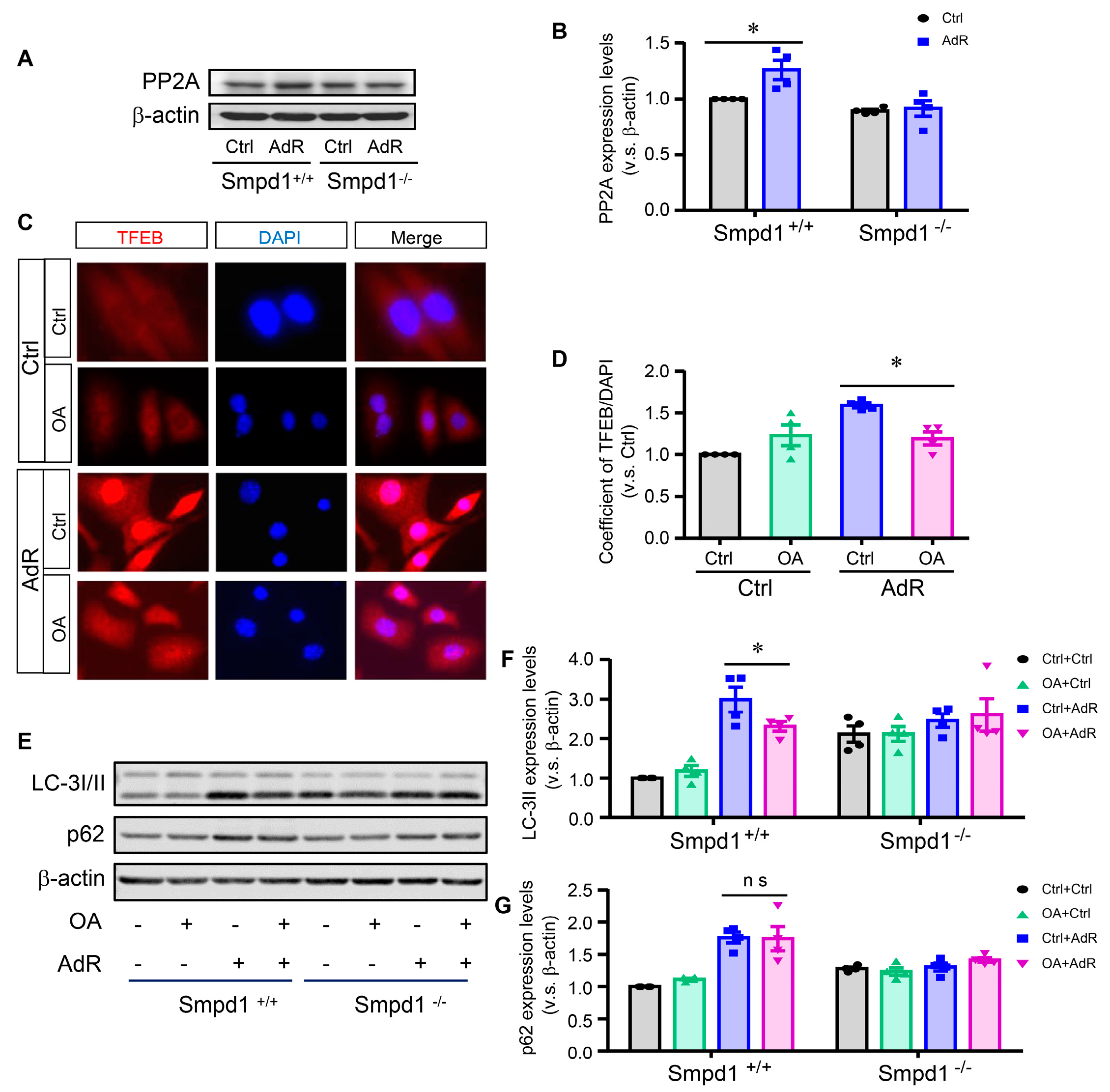
2.5. Effect of PP2A Inhibition on adipoRon-Induced TFEB–Autophagy in SMCs
2.6. Lysosomal Ca2+ Release by ML-SA1 Rescues adipoRon-Induced Activation of Calcineurin and TFEB in Smpd1−/− SMCs
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Mice
4.3. Primary Culture of Arterial SMCs from Mice
4.4. Immunoblotting
4.5. Real-Time Quantitative PCR
4.6. Immunofluorescence Staining
4.7. Wound Scratch Assay
4.8. MMP Activity Assay
4.9. Statistics Analysis
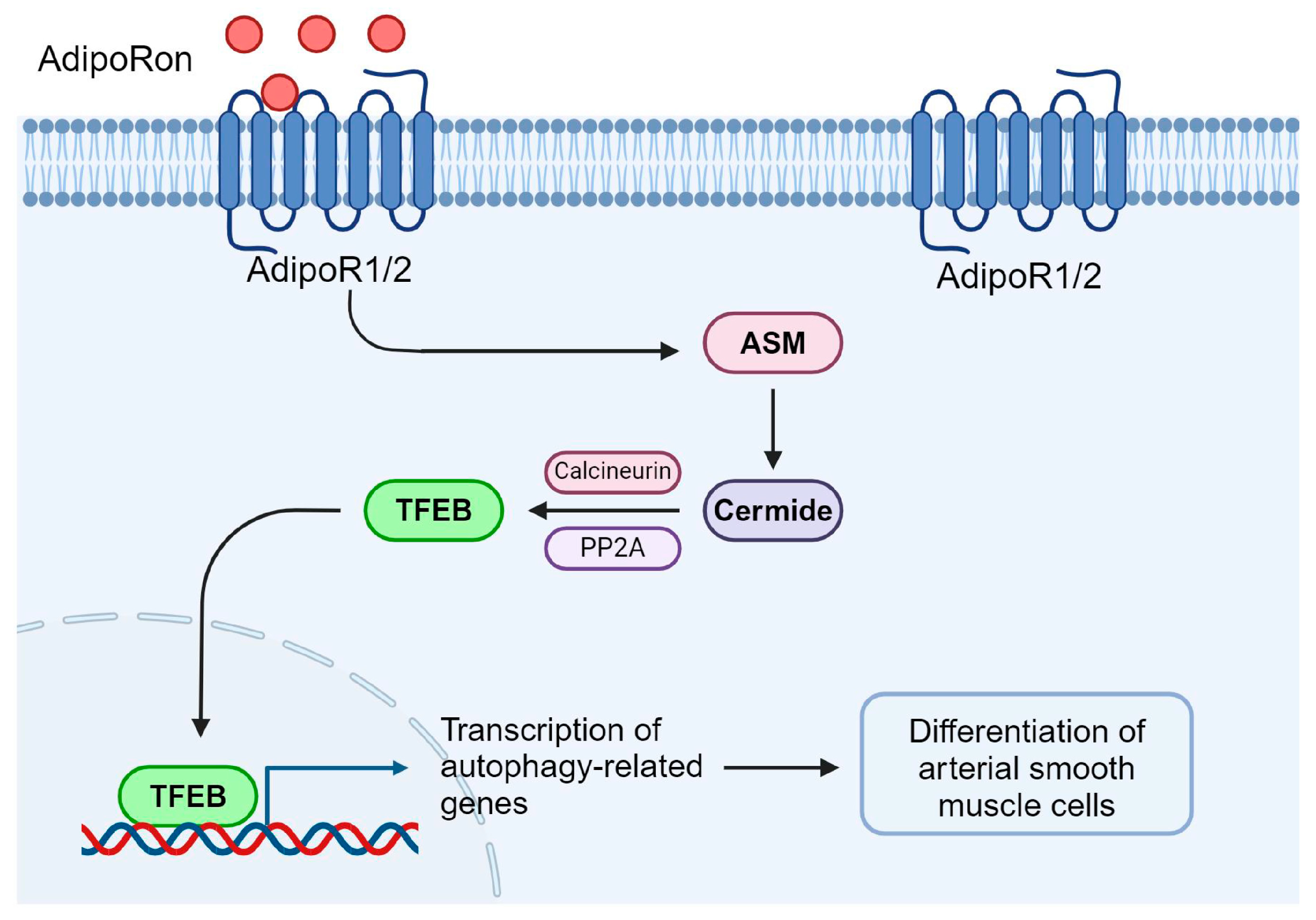
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Elmarasi, M.; Elmakaty, I.; Elsayed, B.; Elsayed, A.; Zein, J.A.; Boudaka, A.; Eid, A.H. Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection. J. Cell. Physiol. 2024, 239, e31200. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gomez, D. Smooth muscle cell phenotypic diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.M.; Hall, I.F.; Serio, S.; Zani, S.; Climent, M.; Salvarani, N.; Carullo, P.; Civilini, E.; Condorelli, G.; Elia, L.; et al. miR-128-3p Is a Novel Regulator of Vascular Smooth Muscle Cell Phenotypic Switch and Vascular Diseases. Circ. Res. 2020, 126, e120–e135. [Google Scholar] [CrossRef] [PubMed]
- McDonald, O.G.; Owens, G.K. Programming smooth muscle plasticity with chromatin dynamics. Circ. Res. 2007, 100, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jacquet, L.; Karamariti, E.; Xu, Q. Origin and differentiation of vascular smooth muscle cells. J. Physiol. 2015, 593, 3013–3030. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Adiponectin and cardiovascular health: An update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Kim, Y.; Choi, B.S.; Kim, Y.S.; Kim, H.W.; Lim, K.M.; Kim, M.J.; et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 2018, 85, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kwon, Y.; Byeon, S.; Haam, C.E.; Lee, Y.H. AdipoRon, adiponectin receptor agonist, improves vascular function in the mesenteric arteries of type 2 diabetic mice. PLoS ONE 2020, 15, e0230227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Li, R.; Lau, W.B.; Yuan, Y.X.; Liang, B.; Li, R.; Gao, E.H.; Koch, W.J.; Ma, X.L.; et al. AdipoRon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E275–E282. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; Xu, X.; Terasawa, T.; Dong, L.Q. Anti-inflammatory and anti-proliferative action of adiponectin mediated by insulin signaling cascade in human vascular smooth muscle cells. Mol. Biol. Rep. 2020, 47, 6561–6572. [Google Scholar] [CrossRef] [PubMed]
- Fairaq, A.; Shawky, N.M.; Osman, I.; Pichavaram, P.; Segar, L. AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: Implications toward suppression of neointimal hyperplasia. Pharmacol. Res. 2017, 119, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Chen, J.; Li, X.; Umetani, M.; Chen, Y.; Li, P.L.; Zhang, Y. Contribution of transcription factor EB to adipoRon-induced inhibition of arterial smooth muscle cell proliferation and migration. Am. J. Physiol. Cell. Physiol. 2019, 317, C1034–C1047. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Acid sphingomyelinase, autophagy, and atherosclerosis. J. Mol. Med. 2014, 92, 429–431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Xu, M.; Pitzer, A.L.; Xia, M.; Boini, K.M.; Li, P.L.; Zhang, Y. Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: Protective role in atherosclerosis. J. Mol. Med. 2014, 92, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guan, Y.; Chen, J.; Li, X.; McConnell, B.K.; Zhou, W.; Boini, K.M.; Zhang, Y. Contribution of p62/SQSTM1 to PDGF-BB-induced myofibroblast-like phenotypic transition in vascular smooth muscle cells lacking Smpd1 gene. Cell. Death. Dis. 2018, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Q.; Li, P.L.; Nguyen, T.; Li, X.; Zhang, Y. Regulation of dynein-mediated autophagosomes trafficking by ASM in CASMCs. Front. Biosci. (Landmark Ed.) 2016, 21, 696–706. [Google Scholar] [PubMed]
- Justice, M.J.; Bronova, I.; Schweitzer, K.S.; Poirier, C.; Blum, J.S.; Berdyshev, E.V.; Petrache, I. Inhibition of acid sphingomyelinase disrupts LYNUS signaling and triggers autophagy. J. Lipid. Res. 2018, 59, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Oaks, J.; Ogretmen, B. Regulation of PP2A by sphingolipid metabolism and signaling. Front. Oncol. 2015, 4, 388. [Google Scholar] [CrossRef] [PubMed]
- Martina, J.A.; Puertollano, R. Protein phosphatase 2A stimulates activation of TFEB and TFE3 transcription factors in response to oxidative stress. J. Biol. Chem. 2018, 293, 12525–12534. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell. Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A review of obesity, physical activity, and cardiovascular disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An overview of the role of adipokines in cardiometabolic diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Frisbee, J.C. Obesity and vascular dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Fang, J.; Xing, X.; Wang, H.; Shi, X.; Liu, X.; Niu, T.; Liu, K. Small-molecule agonist AdipoRon alleviates diabetic retinopathy through the AdipoR1/AMPK/EGR4 pathway. J. Transl. Med. 2024, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr. Potential adiponectin receptor response modifier therapeutics. Front. Endocrinol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gulbins, E.; Zhang, Y. Oxidative stress triggers Ca-dependent lysosome trafficking and activation of acid sphingomyelinase. Cell. Physiol. Biochem. 2012, 30, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Idone, V.; Devlin, C.; Fernandes, M.C.; Flannery, A.; He, X.; Schuchman, E.; Tabas, I.; Andrews, N.W. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell. Biol. 2010, 189, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Yi, F.; Jin, S.; Xia, M.; Chen, Q.Z.; Gulbins, E.; Li, P.L. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid. Redox. Signal. 2007, 9, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Y.H.; Hannun, Y.A. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J. Biol. Chem. 2007, 282, 11549–11561. [Google Scholar] [CrossRef] [PubMed]
- Ermini, L.; Farrell, A.; Alahari, S.; Ausman, J.; Park, C.; Sallais, J.; Melland-Smith, M.; Porter, T.; Edson, M.; Nevo, O.; et al. Ceramide-induced lysosomal biogenesis and exocytosis in early-onset preeclampsia promotes exosomal release of SMPD1 causing endothelial dysfunction. Front. Cell. Dev. Biol. 2021, 9, 652651. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, P.; Pivari, F.; Barcella, M.; Merelli, I.; Zulueta, A.; Dei Cas, M.; Rosso, L.; Ghidoni, R.; Caretti, A.; Paroni, R.; et al. Myriocin modulates the altered lipid metabolism and storage in cystic fibrosis. Cell. Signal. 2021, 81, 109928. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, B.; Rhein, C.; Mileva, I.; Ahmad, R.; Clarke, C.J.; Snider, J.; Obeid, L.M.; Hannun, Y.A. Identification of an acid sphingomyelinase ceramide kinase pathway in the regulation of the chemokine CCL5. J. Lipid. Res. 2018, 59, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Y.H.; Hannun, Y.A. The acid sphingomyelinase/ceramide pathway: Biomedical significance and mechanisms of regulation. Curr. Mol. Med. 2010, 10, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Nicoletti, A.; Li, Z.; Michel, J.B. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012, 95, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Bhat, O.M.; Li, P.L. Lysosome function in cardiovascular diseases. Cell. Physiol. Biochem. 2021, 55, 277–300. [Google Scholar] [PubMed]
- Wang, Y.T.; Li, X.; Chen, J.; McConnell, B.K.; Chen, L.; Li, P.L.; Chen, Y.; Zhang, Y. Activation of TFEB ameliorates dedifferentiation of arterial smooth muscle cells and neointima formation in mice with high-fat diet. Cell. Death. Dis. 2019, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Thompson, M.D.; Cohen, R.A.; Tong, X. Autophagy and oxidative stress in cardiovascular diseases. Biochim. Biophys. Acta. 2015, 1852, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Nussenzweig, S.C.; Verma, S.; Finkel, T. The role of autophagy in vascular biology. Circ. Res. 2015, 116, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Lavandero, S.; Chiong, M.; Rothermel, B.A.; Hill, J.A. Autophagy in cardiovascular biology. J. Clin. Investig. 2015, 125, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, J.; Liu, Q.; Su, J.; Zhao, Y.; Zheng, G.; Yang, Z.; Zhuo, D.; Ma, C.; Fan, G. The induction of endothelial autophagy and its role in the development of atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 831847. [Google Scholar] [CrossRef] [PubMed]
- Salabei, J.K.; Hill, B.G. Implications of autophagy for vascular smooth muscle cell function and plasticity. Free. Radic. Biol. Med. 2013, 65, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, X.; Cui, X.; Wang, J.; Liu, S.; Li, H.; Yang, J.; Zhang, C.; Zhang, W. Smooth muscle-specific HuR knockout induces defective autophagy and atherosclerosis. Cell. Death. Dis. 2021, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.D.; Jeong, S.J.; Zhang, X.; Sergin, I.; Razani, B. TFEB and trehalose drive the macrophage autophagy-lysosome system to protect against atherosclerosis. Autophagy 2018, 14, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Willett, R.; Martina, J.A.; Zewe, J.P.; Wills, R.; Hammond, G.R.V.; Puertollano, R. TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes. Nat. Commun. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rydzewski, N.; Hider, A.; Zhang, X.; Yang, J.; Wang, W.; Gao, Q.; Cheng, X.; Xu, H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell. Biol. 2016, 18, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, D.; Li, P.; Yuan, X.; Yarotskyy, V.; Li, P.L. Regulation of exosome release by lysosomal acid ceramidase in coronary arterial endothelial cells: Role of TRPML1 channel. Curr. Top. Membr. 2022, 90, 37–63. [Google Scholar] [PubMed]
- Li, G.; Huang, D.; Zou, Y.; Kidd, J.; Gehr, T.W.B.; Li, N.; Ritter, J.K.; Li, P.L. Impaired autophagic flux and dedifferentiation in podocytes lacking Asah1 gene: Role of lysosomal TRPML1 channel. Biochim. Biophys. Acta. Mol. Cell. Res. 2023, 1870, 119386. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, X.; Li, X.; Zhang, X.; Yao, Z.; Dibble, S.; Dong, X.P.; Yu, T.; Lieberman, A.P.; Showalter, H.D.; et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012, 3, 731. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Ghazvini Zadeh, E.; Xu, P.; Gordillo, R.; Guo, Z.; Joffin, N.; Yu, B.; Scherer, P.E.; Li, W.H. PEGylated AdipoRon derivatives improve glucose and lipid metabolism under insulinopenic and high-fat diet conditions. J. Lipid. Res. 2021, 62, 100095. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Li, B.; Xu, H.; Wang, C.; Liu, Y.; Li, Y.; Li, C.; Gao, N.; Li, L. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca(2+) homeostasis in human adenoid cystic carcinoma cells. Cell. Biosci. 2014, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.; Gjoerup, O.V.; Kean, J.A.; Roberts, T.M.; Schaffhausen, B. Protein phosphatase 2A regulates life and death decisions via Akt in a context-dependent manner. Proc. Natl. Acad. Sci. USA 2007, 104, 19011–19016. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Leblebici, F.; Kip, S.N.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S.; Sieck, G.C. Regulation of sarcoplasmic reticulum Ca2+ reuptake in porcine airway smooth muscle. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2008, 294, L787–L796. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, X.; Ke, Y.; Solaro, R.J. Regulation of Ca(2+) transient by PP2A in normal and failing heart. Front. Physiol. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Bhat, O.M.; Li, G.; Yuan, X.; Huang, D.; Gulbins, E.; Kukreja, R.C.; Li, P.L. Arterial medial calcification through enhanced small extracellular vesicle release in smooth muscle-specific asah1 gene knockout mice. Sci. Rep. 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, W.; Wang, Z.; Moura, A.K.; Roudbari, K.; Zuo, R.; Hu, J.Z.; Wang, Y.-T.; Li, P.-L.; Zhang, Y. Acid Sphingomyelinase Regulates AdipoRon-Induced Differentiation of Arterial Smooth Muscle Cells via TFEB Activation. Int. J. Mol. Sci. 2025, 26, 2147. https://doi.org/10.3390/ijms26052147
Li X, Zhao W, Wang Z, Moura AK, Roudbari K, Zuo R, Hu JZ, Wang Y-T, Li P-L, Zhang Y. Acid Sphingomyelinase Regulates AdipoRon-Induced Differentiation of Arterial Smooth Muscle Cells via TFEB Activation. International Journal of Molecular Sciences. 2025; 26(5):2147. https://doi.org/10.3390/ijms26052147
Chicago/Turabian StyleLi, Xiang, Wei Zhao, Zhengchao Wang, Alexandra K. Moura, Kiana Roudbari, Rui Zuo, Jenny Z. Hu, Yun-Ting Wang, Pin-Lan Li, and Yang Zhang. 2025. "Acid Sphingomyelinase Regulates AdipoRon-Induced Differentiation of Arterial Smooth Muscle Cells via TFEB Activation" International Journal of Molecular Sciences 26, no. 5: 2147. https://doi.org/10.3390/ijms26052147
APA StyleLi, X., Zhao, W., Wang, Z., Moura, A. K., Roudbari, K., Zuo, R., Hu, J. Z., Wang, Y.-T., Li, P.-L., & Zhang, Y. (2025). Acid Sphingomyelinase Regulates AdipoRon-Induced Differentiation of Arterial Smooth Muscle Cells via TFEB Activation. International Journal of Molecular Sciences, 26(5), 2147. https://doi.org/10.3390/ijms26052147





