The Role of Primary Cilia in Modulating the Luteinization Process of Ovarian Granulosa Cells in Mice
Abstract
1. Introduction
2. Results
2.1. Expression Changes of Primary Cilia During the In Vivo Induced Luteinization of Mouse Granulosa Cells
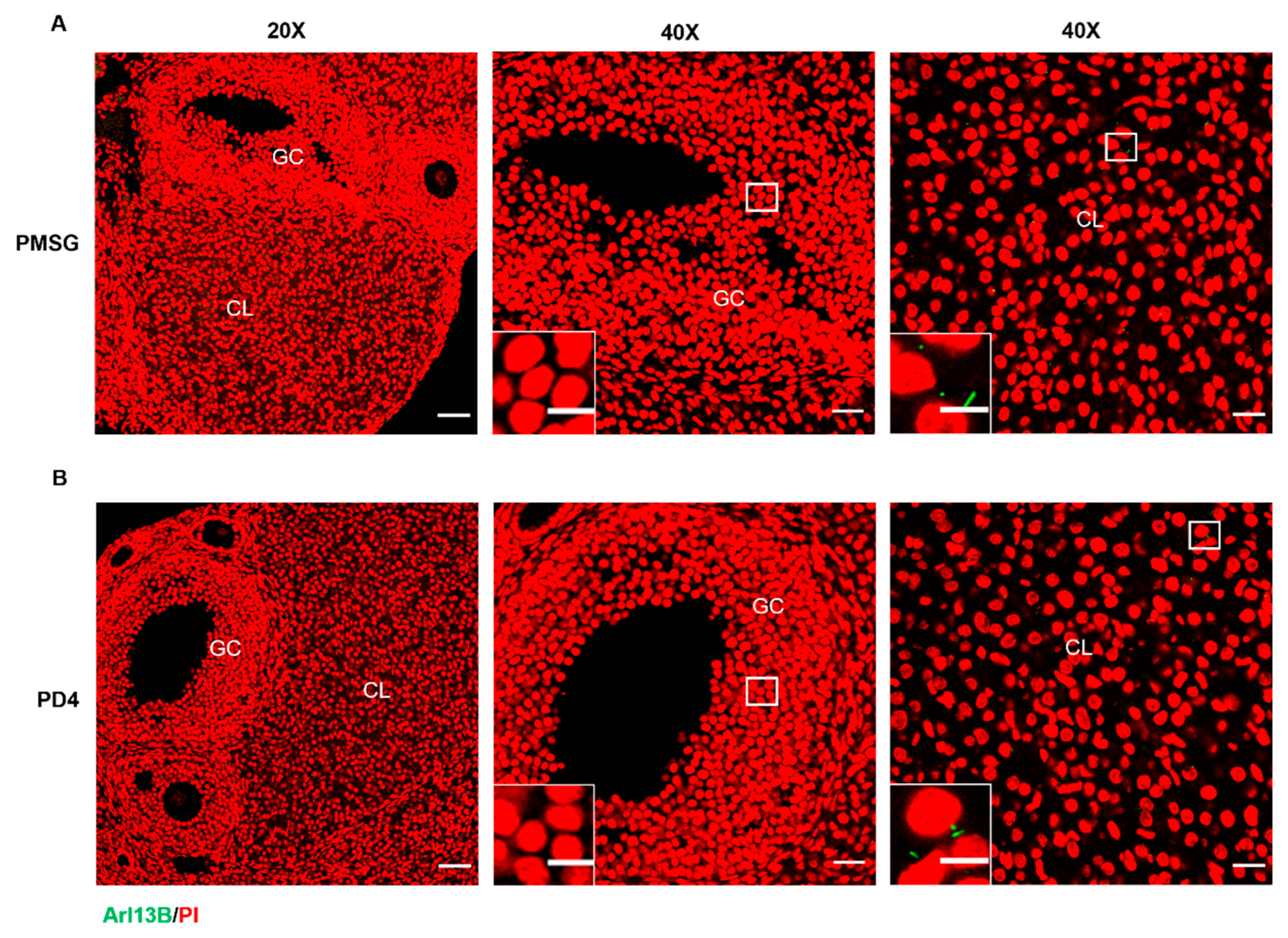
2.1.1. Primary Cilia Are Localized to Luteal Cells in Mice
2.1.2. Th Upregulation of Primary Cilia Expression During the In Vivo Induced Luteinization of Mouse Granulosa Cells
2.2. Patterns of Primary Cilia Formation During the Luteinization of Mouse Granulosa Cells
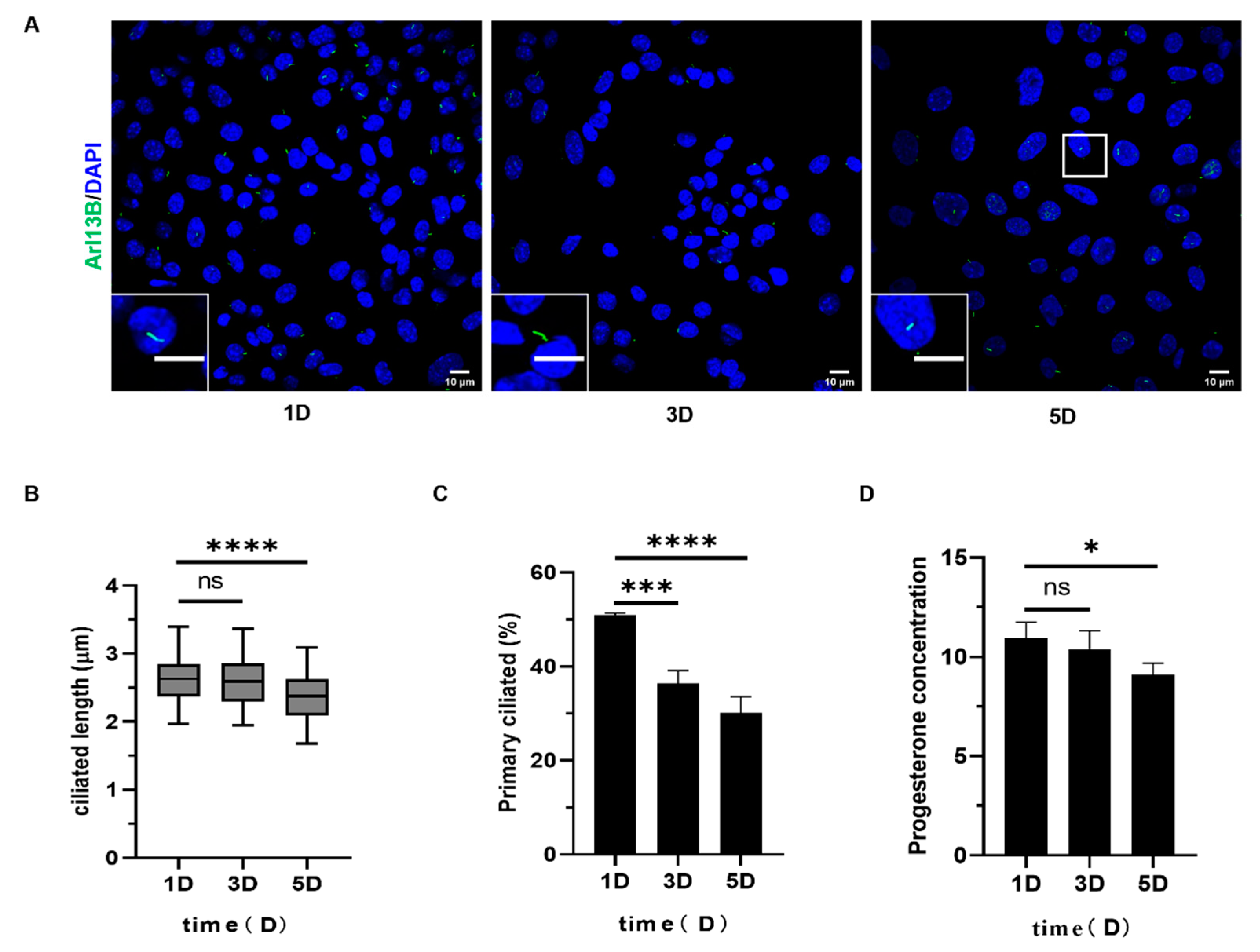
2.2.1. The Length and Number of Primary Cilia Increased in the Early Stage of Luteinization of Mouse Follicular Granulosa Cells
2.2.2. The Length and Number of Primary Cilia Decreased During the Late Luteinization of Mouse Follicular Granulosa Cells
2.3. Effect of Dynamic Changes of Primary Cilia on Luteinization of Follicular Granulosa Cells in Mice
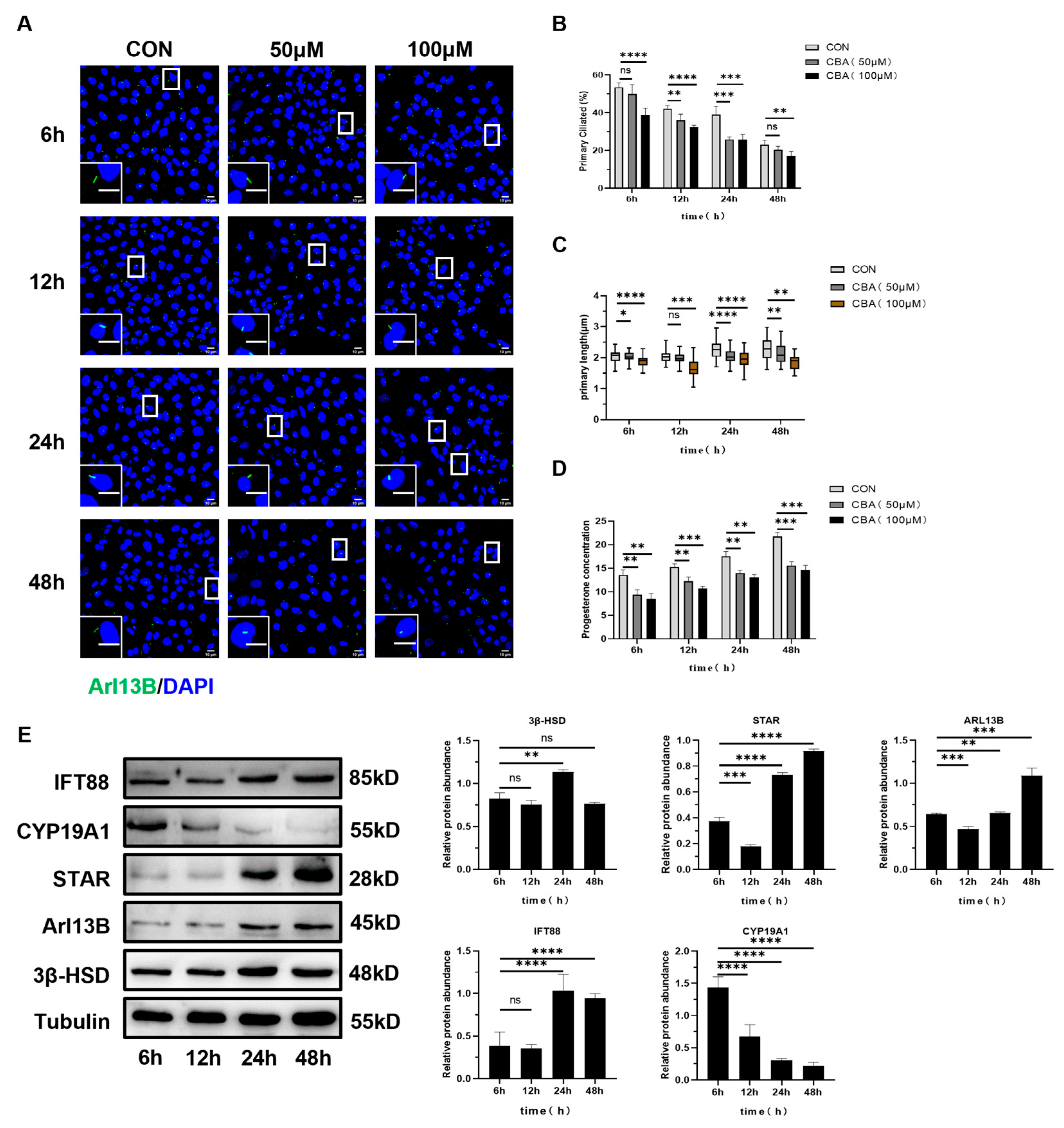
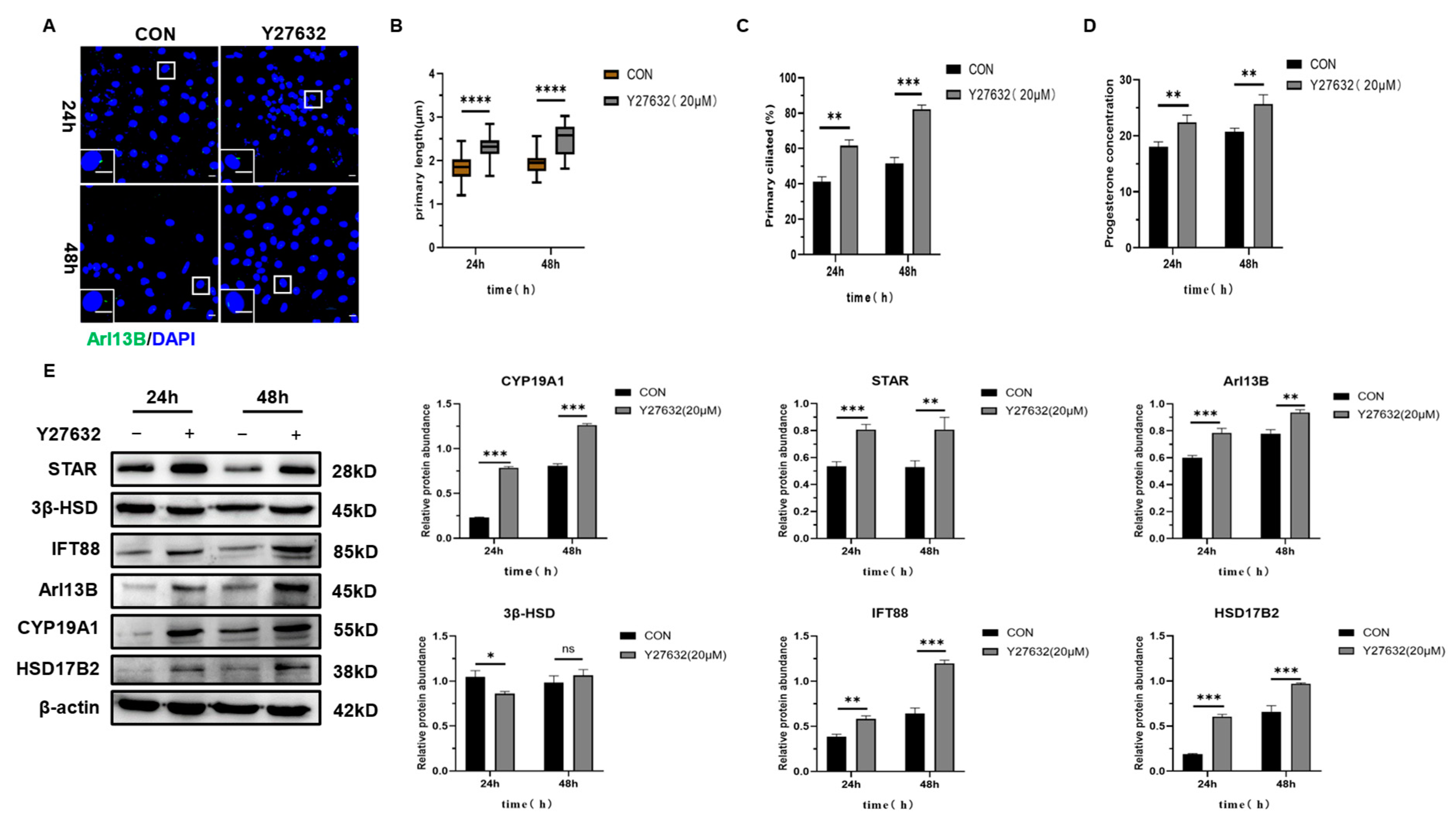
2.3.1. The Expression of Luteinizing Related Molecules and the Secretion of Progesterone in Mouse Follicular Granulosa Cells Decreased After Inhibiting the Occurrence of Primary Cilia
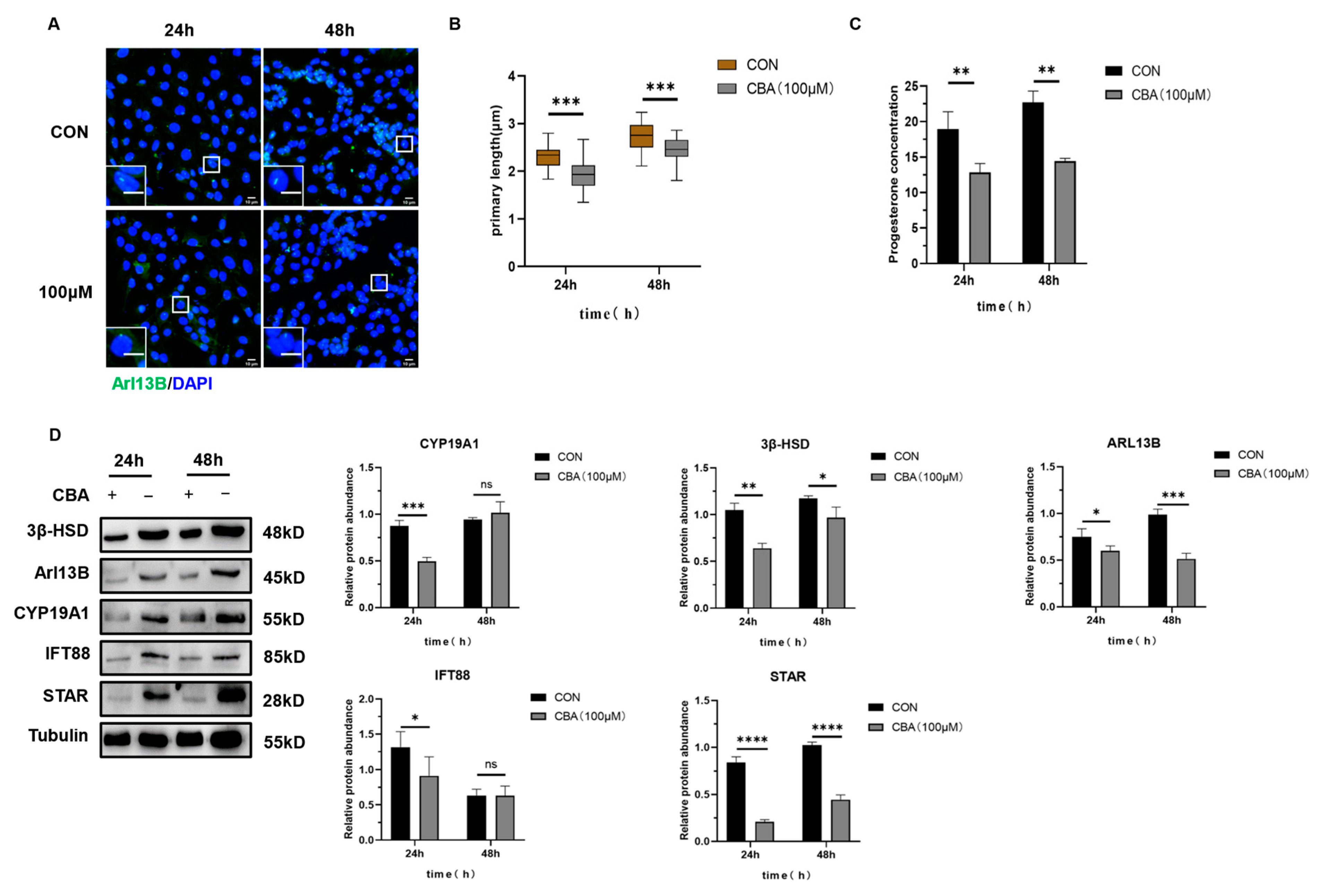
2.3.2. The Expression of Luteinizing Related Molecules and the Secretion of Progesterone in Mouse Follicular Granulosa Cells Were Increased After the Induction of Primary Cilia
3. Discussion
4. Materials and Methods
4.1. Animals and Ovarian Collection
4.2. Cell Isolation, Culture, and Treatment
4.3. ELISA Experiment
4.4. Western Blotting
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imaimatsu, K.; Uchida, A.; Hiramatsu, R.; Kanai, Y. Gonadal Sex Differentiation and Ovarian Organogenesis along the Cortical-Medullary Axis in Mammals. Int. J. Mol. Sci. 2022, 23, 13373. [Google Scholar] [CrossRef] [PubMed]
- Abedel-Majed, M.A.; Romereim, S.M.; Davis, J.S.; Cupp, A.S. Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Front. Endocrinol. 2019, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M. Short communication: Concentrations of the mammalian lignan enterolactone in preovulatory follicles and the correlation with intrafollicular estradiol in dairy cows fed extruded flaxseed. J. Dairy Sci. 2015, 98, 8814–8817. [Google Scholar] [CrossRef] [PubMed]
- Lalitkumar, P.G.L.; Lundström, E.; Byström, B.; Ujvari, D.; Murkes, D.; Tani, E.; Söderqvist, G. Effects of Estradiol/Micronized Progesterone vs. Conjugated Equine Estrogens/Medroxyprogesterone Acetate on Breast Cancer Gene Expression in Healthy Postmenopausal Women. Int. J. Mol. Sci. 2023, 24, 4123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mi, M.; Hao, W.; Fan, Q.; Gao, B. Progesterone down-regulates SLIT/ROBO expression in mouse corpus luteum. Acta Histochem. 2017, 119, 740–746. [Google Scholar] [CrossRef]
- DeWitt, N.A.; Whirledge, S.; Kallen, A.N. Updates on molecular and environmental determinants of luteal progesterone production. Mol. Cell Endocrinol. 2020, 515, 110930. [Google Scholar] [CrossRef] [PubMed]
- Przygrodzka, E.; Hou, X.; Zhang, P.; Plewes, M.R.; Franco, R.; Davis, J.S. PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis. Endocrinology 2021, 162, bqab015. [Google Scholar] [CrossRef]
- Brady, K.; Liu, H.-C.; Hicks, J.; Long, J.A.; Porter, T.E. Global gene expression analysis of the turkey hen hypothalamo-pituitary-gonadal axis during the preovulatory hormonal surge. Poult. Sci. 2023, 102, 102547. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, H.; Bartram, S.; Charalambides, M.M.; Murthy, S.; Petitt, T.; Pradeep, A.; Vineall, O.; Abaraonye, I.; Lancaster, A.; Koysombat, K.; et al. Kisspeptin-neuron control of LH pulsatility and ovulation. Front. Endocrinol. 2022, 13, 951938. [Google Scholar] [CrossRef]
- Blitek, A.; Szymanska, M.; Pieczywek, M.; Morawska-Pucinska, E. Luteal P4 synthesis in early pregnant gilts after induction of estrus with PMSG/hCG. Anim. Reprod. Sci. 2016, 166, 28–35. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nishimura, R.; Yamashita, M.; Yamaguchi, T.; Hishinuma, M.; Okuda, K. Effect of hypoxia on progesterone production by cultured bovine early and mid luteal cells. J. Reprod. Dev. 2019, 65, 67–72. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cheung, G.; Porter, E.; Papadopoulos, V. The neurosteroid pregnenolone is synthesized by a mitochondrial P450 enzyme other than CYP11A1 in human glial cells. J. Biol. Chem. 2022, 298, 102110. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.; Raman, C.; Elmets, C.; Jetten, A.; Slominski, A.; Tuckey, R. The significance of CYP11A1 expression in skin physiology and pathology. Mol. Cell Endocrinol. 2021, 530, 111238. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, M.; Kaur, J.; Prasad, M.; Pawlak, K.J.; Marshall, B.; Perry, E.W.; Whittal, R.M.; Bose, H.S. An Outer Mitochondrial Translocase, Tom22, Is Crucial for Inner Mitochondrial Steroidogenic Regulation in Adrenal and Gonadal Tissues. Mol. Cell Biol. 2016, 36, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Orly, J.; Stocco, D.M. The role of the steroidogenic acute regulatory (StAR) protein in female reproductive tissues. Horm. Metab. Res. 1999, 31, 389–398. [Google Scholar] [CrossRef]
- Ma, C.; Rice, I.; Pen, D.; Li, H.; Cao, G.; Du, C. The changes and signaling pathways of luteinization-related genes in the cyclic variation of sheep ovarian function. Chin. J. Vet. Sci. Anim. Husb. 2016, 36, 343–347. [Google Scholar]
- Johnson, A.L.; Lee, J. Granulosa cell responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poult. Sci. 2016, 95, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ronen-Fuhrmann, T.; Timberg, R.; King, S.R.; Hales, K.H.; Hales, D.B.; Stocco, D.M.; Orly, J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 1998, 139, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Hickey, G.J.; Chen, S.; Besman, M.J.; Shively, J.E.; Hall, P.F.; Gaddy-Kurten, D.; Richards, J.S. Hormonal regulation, tissue distribution, and content of aromatase cytochrome P450 messenger ribonucleic acid and enzyme in rat ovarian follicles and corpora lutea: Relationship to estradiol biosynthesis. Endocrinology 1988, 122, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Asada, H.; Kizuka, F.; Tamura, I.; Maekawa, R.; Taketani, T.; Sato, S.; Yamagata, Y.; Tamura, H.; Sugino, N. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology 2013, 154, 458–470. [Google Scholar] [CrossRef]
- Meng, B.; Cao, Z.; Gai, Y.; Liu, M.; Gao, M.; Chen, M.; Chen, M.; Ning, Z.; Luan, X. Effects of recombinant goose adiponectin on steroid hormone secretion in Huoyan geese ovarian granulosa cells. Anim. Reprod. Sci. 2019, 205, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, L.; Xiao, X.; Min, X.; Wu, Y.; Yang, Z.; Wen, X. Structure, function, and research progress of primary cilia in reproductive physiology and reproductive diseases. Front. Cell Dev. Biol. 2024, 12, 1418928. [Google Scholar] [CrossRef]
- Wang, B.; Liang, Z.; Liu, P. Functional aspects of primary cilium in signaling, assembly and microenvironment in cancer. J. Cell Physiol. 2021, 236, 3207–3219. [Google Scholar] [CrossRef]
- Delforge, J.P.; Thomas, K.; Roux, F.; de Siqueira, J.C.; Ferin, J. Time relationships between granulosa cells growth and luteinization, and plasma luteinizing hormone discharge in human. 1. A morphometric analysis. Fertil. Steril. 1972, 23, 1–11. [Google Scholar] [CrossRef]
- Yan, Z.; Neulen, J.; Raczek, S.; Weich, H.A.; Keck, C.; Grunwald, K.; Breckwoldt, M. Vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) production by luteinized human granulosa cells in vitro; a paracrine signal in corpus luteum formation. Gynecol. Endocrinol. 1998, 12, 149–153. [Google Scholar] [CrossRef]
- Zhan, D.; Xiang, W.; Guo, F.; Ma, Y. Basic fibroblast growth factor increases IFT88 expression in chondrocytes. Mol. Med. Rep. 2017, 16, 6590–6599. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Toh, M.T.; Narasimhan, V.; Thamilselvam, S.K.; Choksi, S.P.; Roy, S. A function for the Joubert syndrome protein Arl13b in ciliary membrane extension and ciliary length regulation. Dev. Biol. 2015, 397, 225–236. [Google Scholar] [CrossRef]
- Shim, S.; Goyal, R.; Panoutsopoulos, A.A.; Balashova, O.A.; Lee, D.; Borodinsky, L.N. Calcium dynamics at the neural cell primary cilium regulate Hedgehog signaling-dependent neurogenesis in the embryonic neural tube. Proc. Natl. Acad. Sci. USA 2023, 120, e2074930176. [Google Scholar] [CrossRef]
- Miceli, C.; Roccio, F.; Penalva-Mousset, L.; Burtin, M.; Leroy, C.; Nemazanyy, I.; Kuperwasser, N.; Pontoglio, M.; Friedlander, G.; Morel, E.; et al. The primary cilium and lipophagy translate mechanical forces to direct metabolic adaptation of kidney epithelial cells. Nat. Cell Biol. 2020, 22, 1091–1102. [Google Scholar] [CrossRef]
- Meier-Vismara, E.; Walker, N.; Vogel, A. Single cilia in the articular cartilage of the cat. Exp. Cell Biol. 1979, 47, 161–171. [Google Scholar] [CrossRef]
- Johnson, E.T.; Nicola, T.; Roarty, K.; Yoder, B.K.; Haycraft, C.J.; Serra, R. Role for primary cilia in the regulation of mouse ovarian function. Dev. Dyn. 2008, 237, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yan, Y.-P.; Liang, C.; He, Y.-Y.; Wang, Y.; Li, M.-Y.; Chen, S.-T.; Li, Y.; Liu, A.-X.; Yan, G.-J.; et al. Primary Cilia Restrain PI3K-AKT Signaling to Orchestrate Human Decidualization. Int. J. Mol. Sci. 2022, 23, 15573. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, I.P.; McNamara, L.M.; Hoey, D.A. Estrogen withdrawal alters cytoskeletal and primary ciliary dynamics resulting in increased Hedgehog and osteoclastogenic paracrine signalling in osteocytes. Sci. Rep. 2021, 11, 9272. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [PubMed]
- Lv, J. Effect of Insulin on Luteinization of Porcine Granule Cells. Ph.D. Thesis, Guangxi University, Nanning, China, 2022. [Google Scholar]
- Lv, J.; Cheng, J.; Zhang, R.; Zou, C.; An, Q.; Li, P.; Wei, Y.; Wei, Y. Comparative study on methods of inducing luteinization of porcine granule cells in vitro. Chin. J. Anim. Sci. 2022, 58, 175–179. [Google Scholar]
- Guerra, J.; Chiodelli, P.; Tobia, C.; Gerri, C.; Presta, M. Long-Pentraxin 3 Affects Primary Cilium in Zebrafish Embryo and Cancer Cells via the FGF System. Cancers 2020, 12, 1756. [Google Scholar] [CrossRef]
- Atkins, M.; Wurmser, M.; Darmon, M.; Roche, F.; Nicol, X.; Métin, C. CXCL12 targets the primary cilium cAMP/cGMP ratio to regulate cell polarity during migration. Nat. Commun. 2023, 14, 8003. [Google Scholar] [CrossRef]
- Toriyama, M.; Rizaldy, D.; Nakamura, M.; Atsumi, Y.; Toriyama, M.; Fujita, F.; Okada, F.; Morita, A.; Itoh, H.; Ishii, K.J. Corrigendum: Dendritic cell proliferation by primary cilium in atopic dermatitis. Front. Mol. Biosci. 2023, 10, 1215185. [Google Scholar] [CrossRef] [PubMed]
- Alzarka, B.; Charnaya, O.; Gunay-Aygun, M. Diseases of the primary cilia: A clinical characteristics review. Pediatr. Nephrol. 2024, 40, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, X. Research progress of insulin-like growth factor I regulating follicle development. Chin. J. Eugen. Genet. 2017, 25, 1–2. [Google Scholar]
- Sekulovski, N.; Whorton, A.E.; Shi, M.; Hayashi, K.; MacLean, J.A., II. Periovulatory insulin signaling is essential for ovulation, granulosa cell differentiation, and female fertility. FASEB J. 2020, 34, 2376–2391. [Google Scholar] [CrossRef]
- Pescador, N.; Stocco, D.M.; Murphy, B.D. Growth factor modulation of steroidogenic acute regulatory protein and luteinization in the pig ovary. Biol. Reprod. 1999, 60, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Kim, J.H.; Lee, S.R.; Kim, S.H.; Chae, H.D. Influence of pretreatment of insulin on the phosphorylation of extracellular receptor kinase by gonadotropin-releasing hormone and gonadotropins in cultured human granulosa cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 113–117. [Google Scholar] [CrossRef]
- Fitzsimons, L.A.; Tasouri, E.; Willaredt, M.A.; Stetson, D.; Gojak, C.; Kirsch, J.; Gardner, H.A.R.; Gorgas, K.; Tucker, K.L. Primary cilia are critical for tracheoesophageal septation. Dev. Dyn. 2024, 253, 312–332. [Google Scholar] [CrossRef]
- Sugino, N. Molecular mechanisms of luteinization. Obstet. Gynecol. Sci. 2014, 57, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.F.; Christenson, L.K.; Devoto, L.; Martinez, F. Providing progesterone for pregnancy: Control of cholesterol flux to the side-chain cleavage system. J. Reprod. Fertil. Suppl. 2000, 55, 3–12. [Google Scholar]
- Yan, Y. Dynamic Changes and Regulation of Primary Cilia During Implantation and Decidualization of Mouse Embryos. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Min, X.; Xiao, X.; Wu, Y.; Yang, Z.; Wen, X. The Role of Primary Cilia in Modulating the Luteinization Process of Ovarian Granulosa Cells in Mice. Int. J. Mol. Sci. 2025, 26, 2138. https://doi.org/10.3390/ijms26052138
Long X, Min X, Xiao X, Wu Y, Yang Z, Wen X. The Role of Primary Cilia in Modulating the Luteinization Process of Ovarian Granulosa Cells in Mice. International Journal of Molecular Sciences. 2025; 26(5):2138. https://doi.org/10.3390/ijms26052138
Chicago/Turabian StyleLong, Xiaochuan, Xiayu Min, Xinyao Xiao, Yao Wu, Zengming Yang, and Xin Wen. 2025. "The Role of Primary Cilia in Modulating the Luteinization Process of Ovarian Granulosa Cells in Mice" International Journal of Molecular Sciences 26, no. 5: 2138. https://doi.org/10.3390/ijms26052138
APA StyleLong, X., Min, X., Xiao, X., Wu, Y., Yang, Z., & Wen, X. (2025). The Role of Primary Cilia in Modulating the Luteinization Process of Ovarian Granulosa Cells in Mice. International Journal of Molecular Sciences, 26(5), 2138. https://doi.org/10.3390/ijms26052138



