Vicenin-2 Hinders Pro-Inflammatory Response via Targeting the CaMKKβ-AMPK-SIRT1 Axis in Lipopolysaccharide-Stressed THP-1 Cells
Abstract
1. Introduction
2. Results
2.1. Vicenin-2 Does Not Induce Cytotoxic Effects in THP-1 Cells
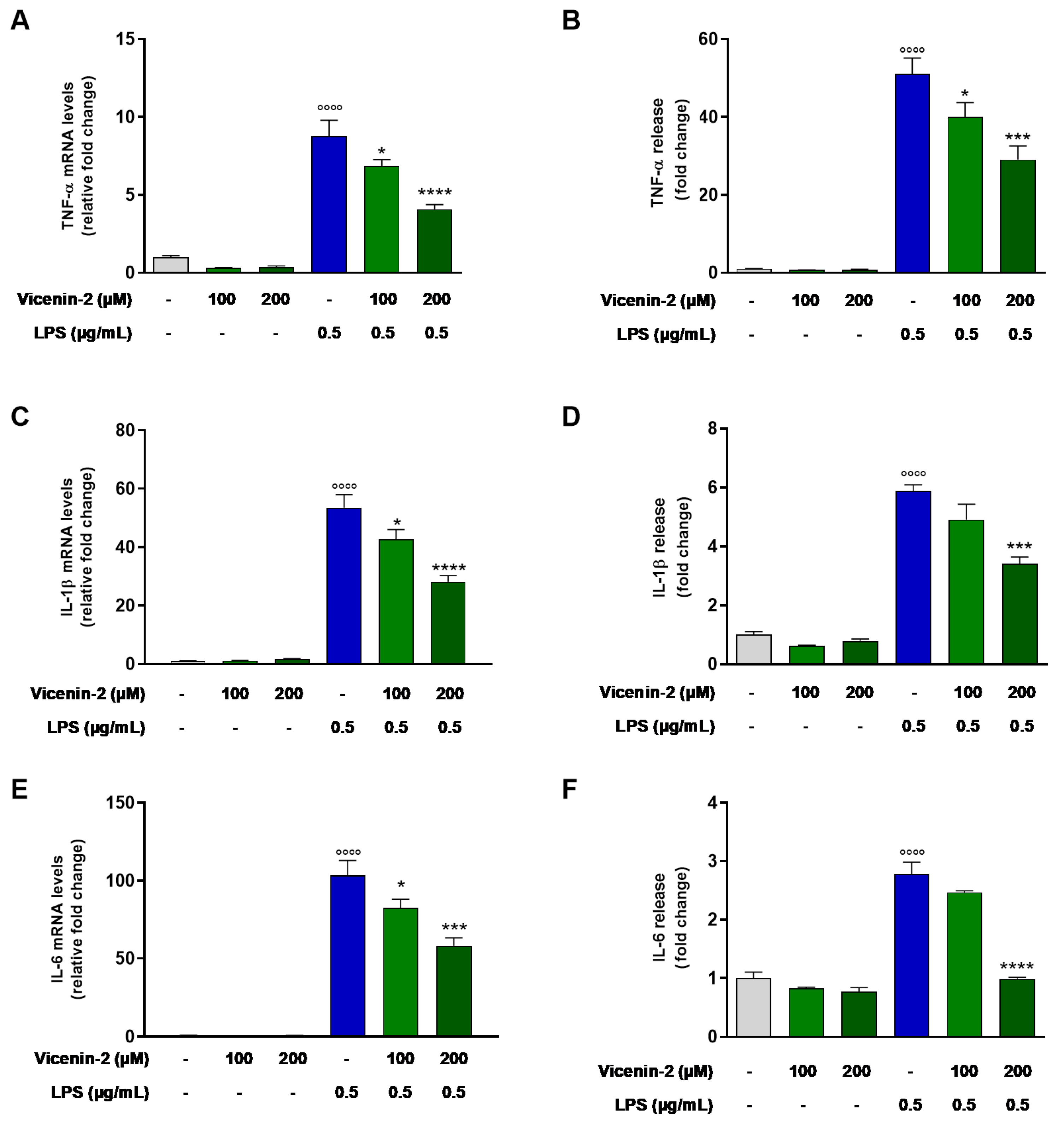
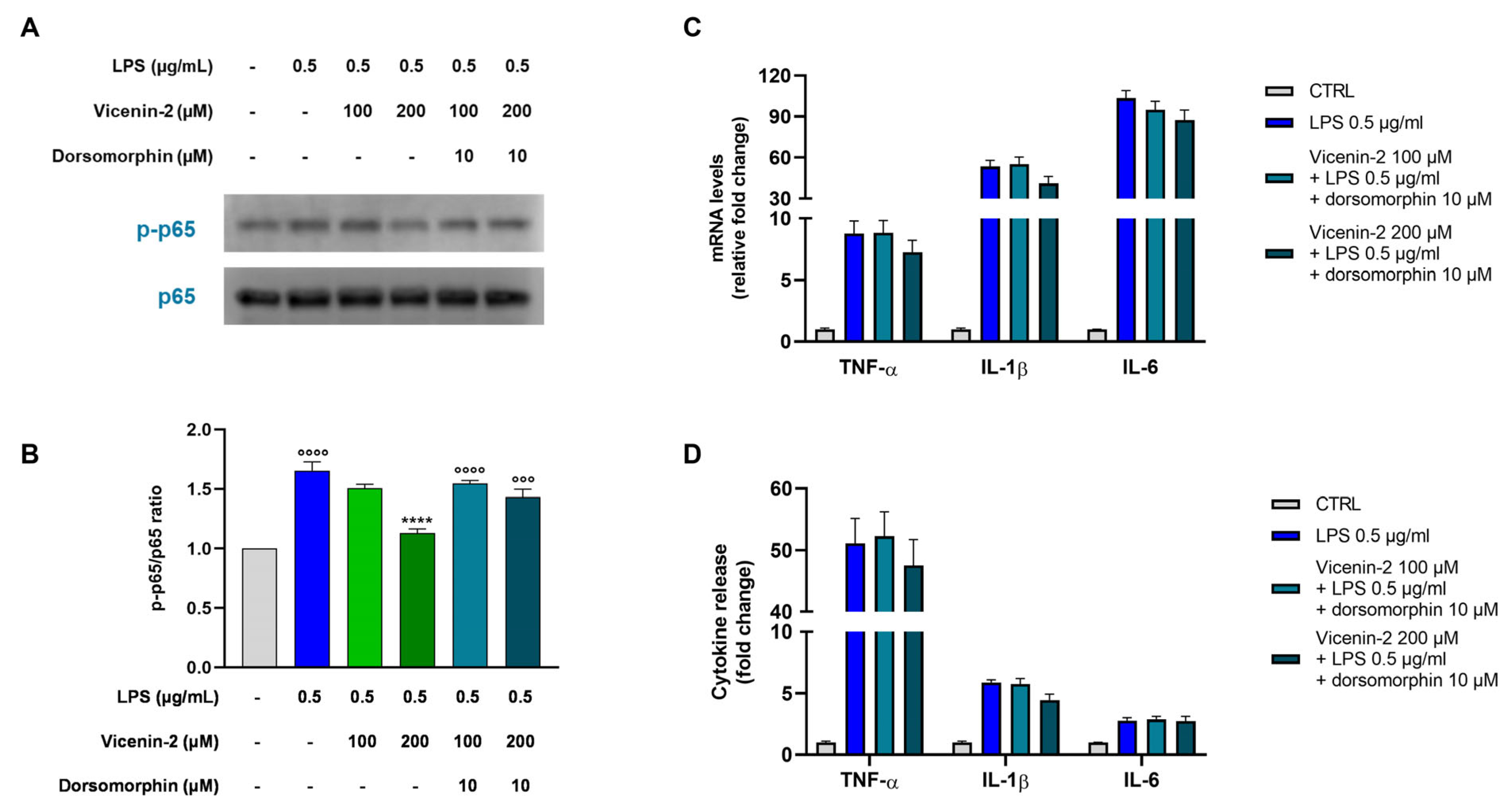
2.2. Vicenin-2 Reduces Gene Expression and Release of Pro-Inflammatory Cytokines in LPS-Stressed THP-1 Cells
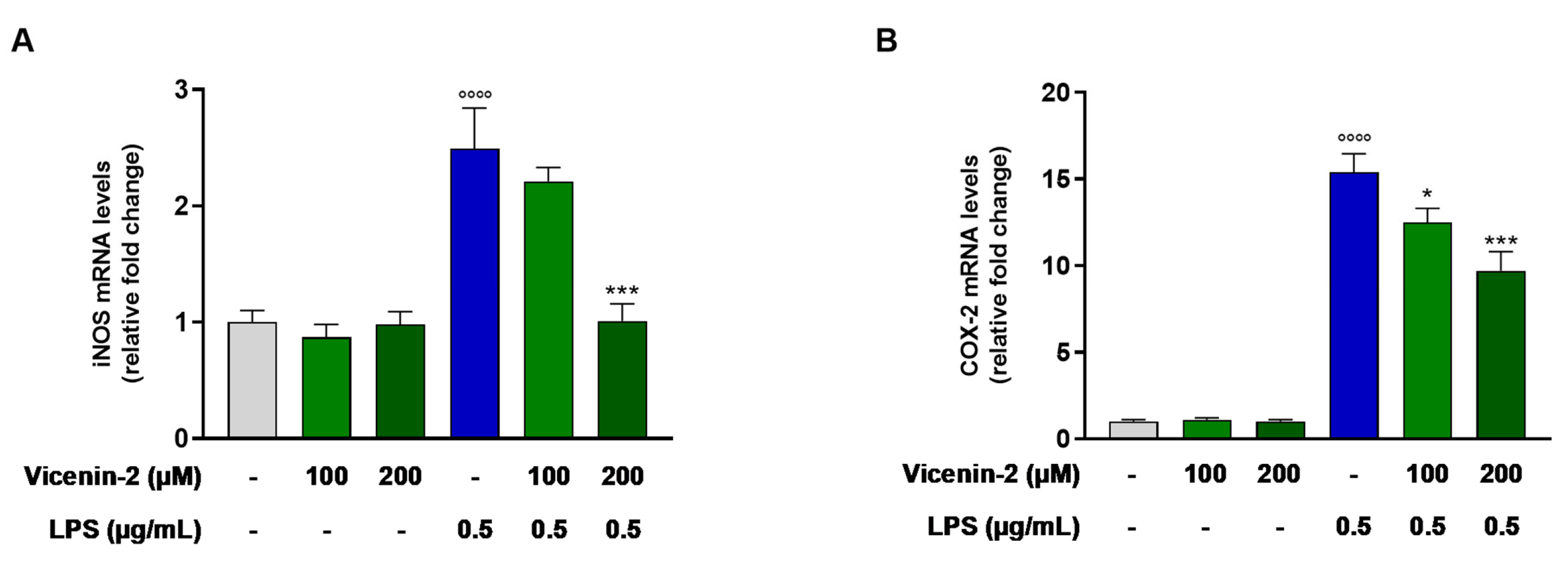
2.3. Vicenin-2 Hampers the mRNA Levels of iNOS and COX-2 in THP-1 Cells Exposed with LPS
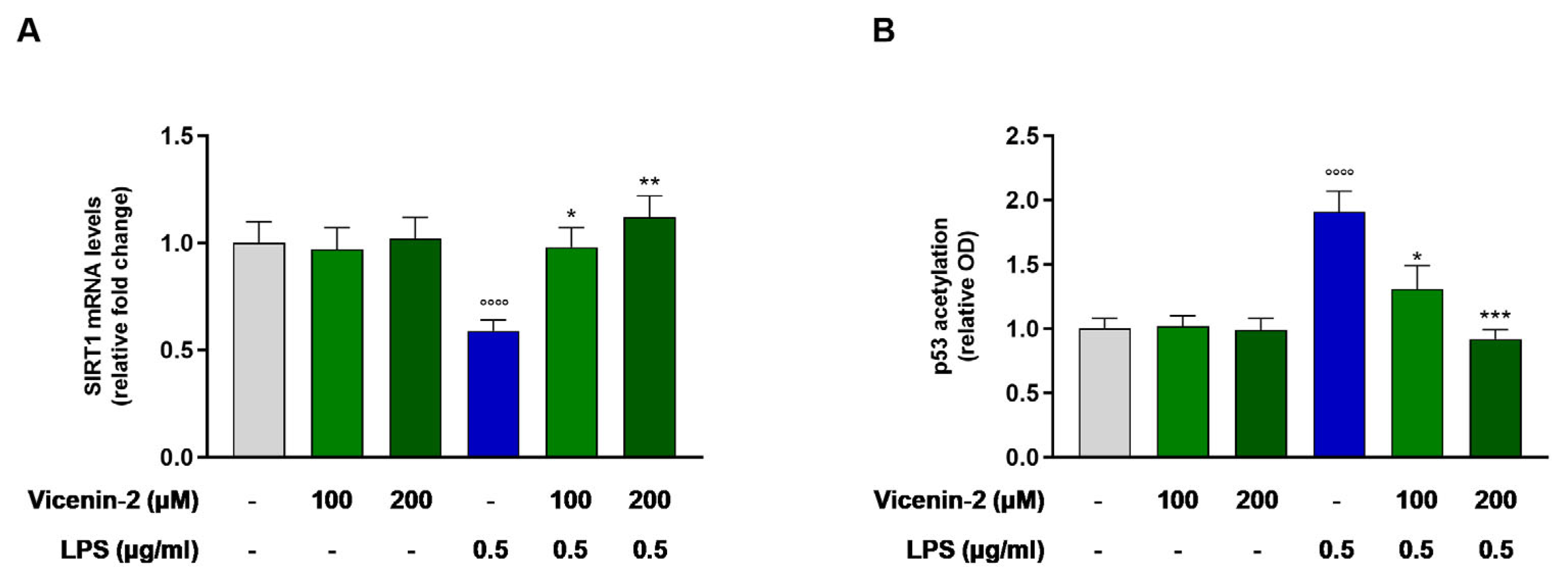
2.4. Vicenin-2 Restores the Gene Expression and Activity of SIRT1 After LPS Exposure in THP-1 Cells
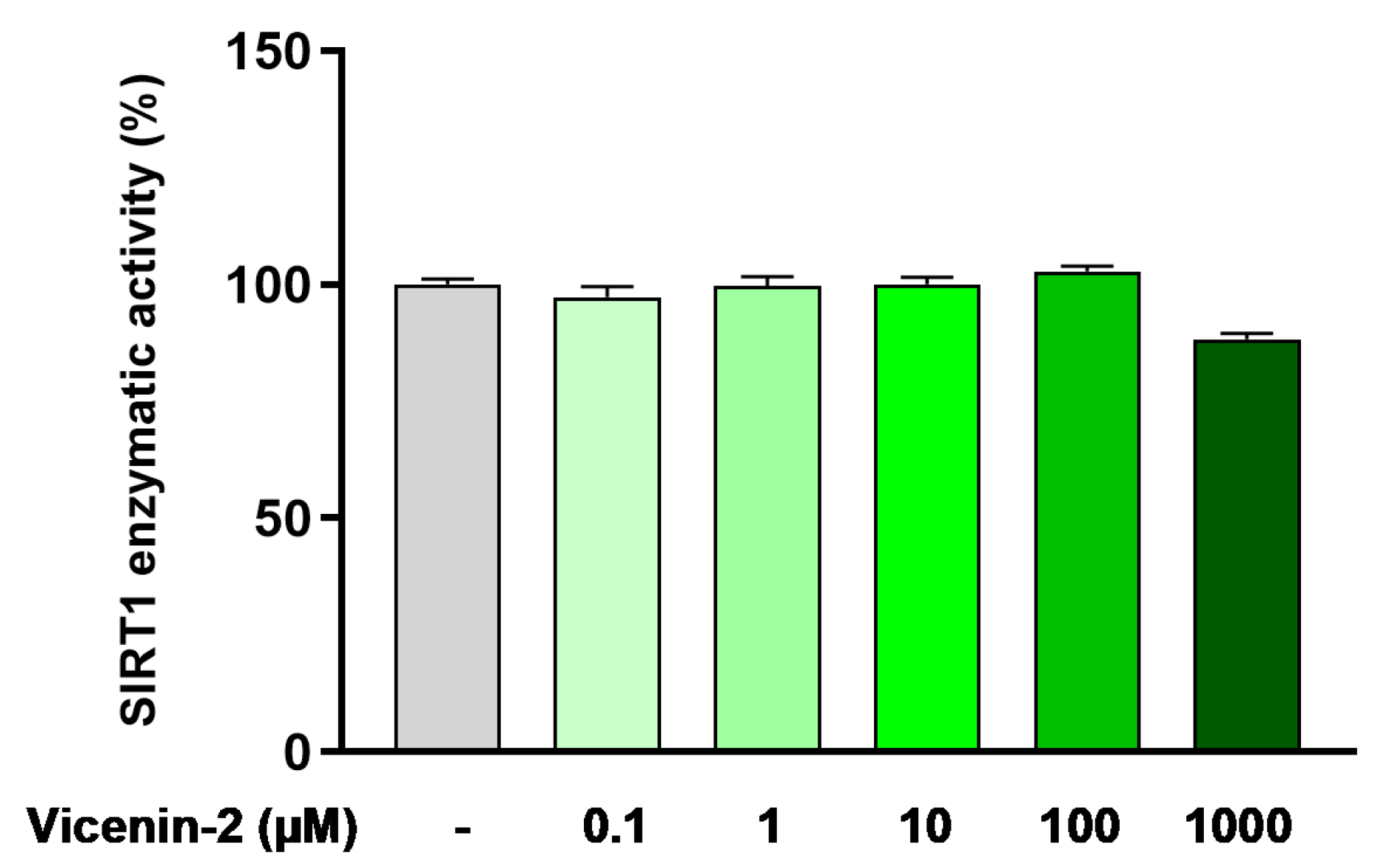
2.5. Vicenin-2 Does Not Directly Alter SIRT1 Deacetylase Activity
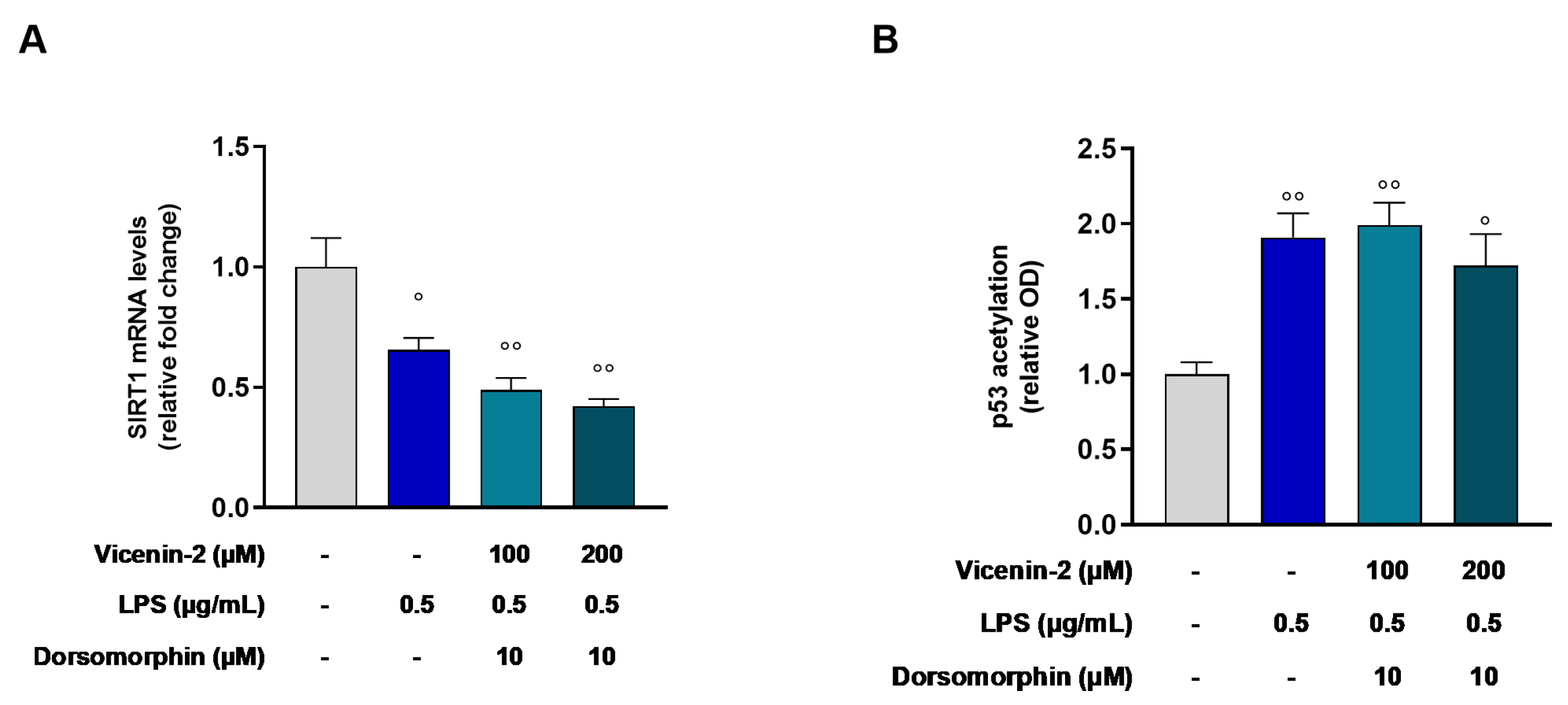
2.6. AMPK Is Involved in the Restoration of SIRT1 Gene Levels Elicited by Vicenin-2 in LPS-Treated THP-1 Cells
2.7. The Blockage of AMPK Hampers the Anti-Inflammatory Potential of Vicenin-2
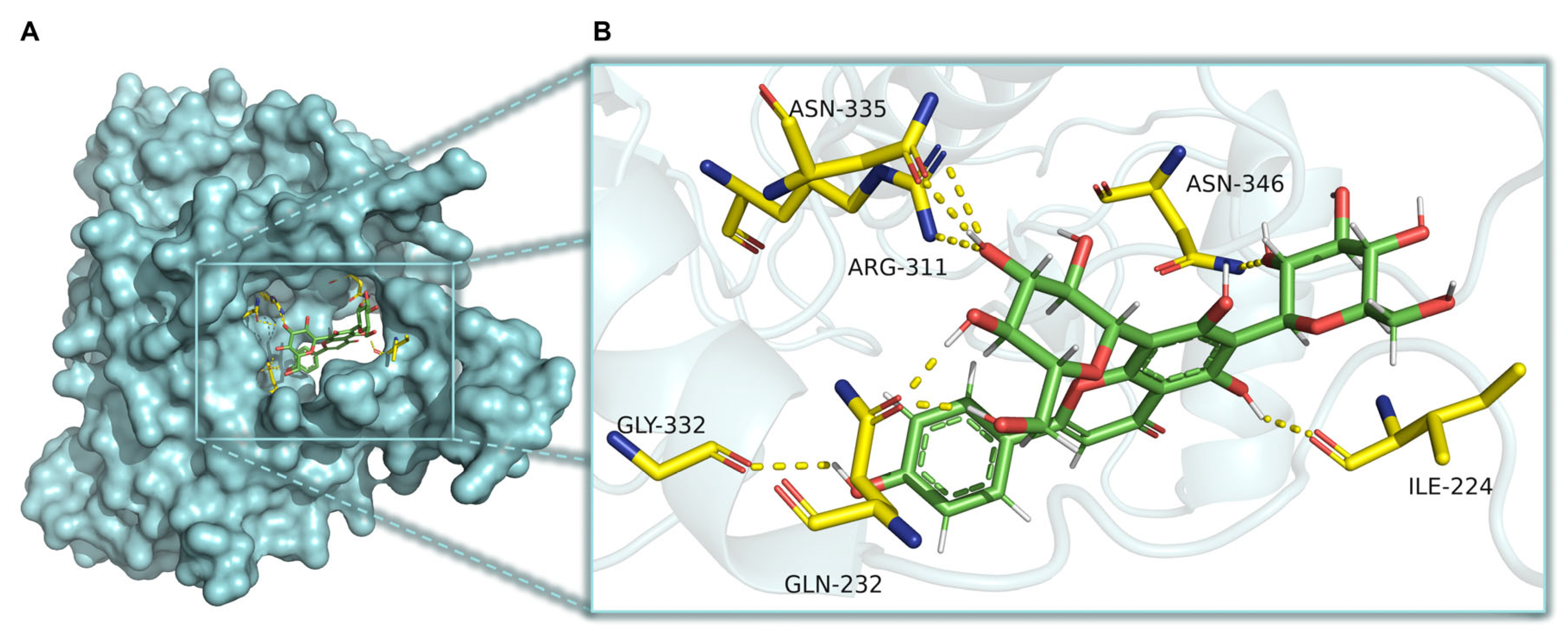
2.8. CaMKKβ Is a Potential Target of Vicenin-2 to Activate AMPK
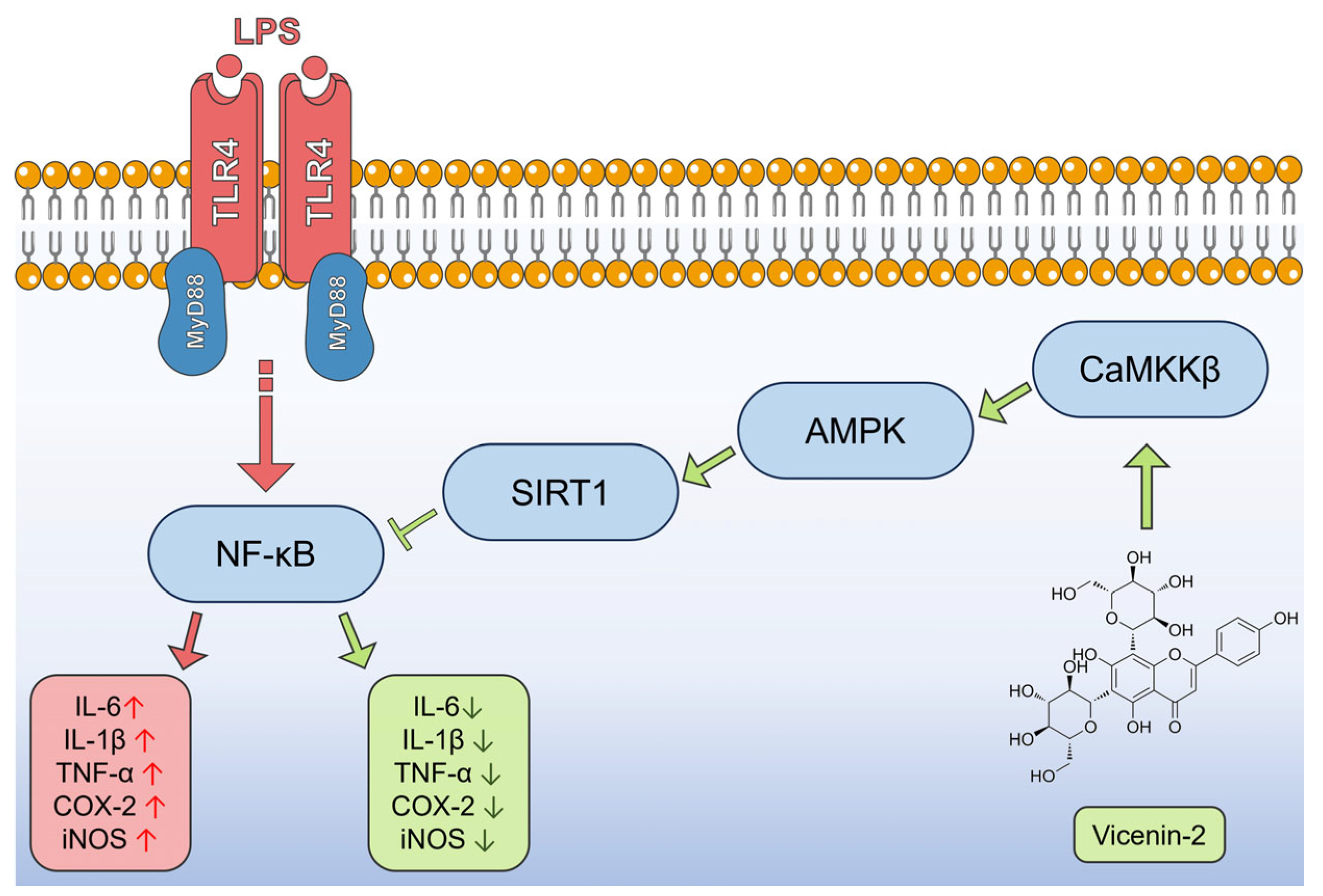
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Safety Profile of Vicenin-2
4.3. Evaluation of Cytokine Secretion
4.4. Real-Time PCR Analysis
4.5. Quantification of Acetylated p53 in THP-1-Treated Cells
4.6. SIRT1 Histone Deacetylase Activity Assay
4.7. Assessment of NF-κB Phosphorylation
4.8. Statistical Analysis
4.9. Docking Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.; Peskar, B.A. Anti-inflammatory effects of aspirin and sodium salicylate. Eur. J. Pharmacol. 2002, 447, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Meng, N.; Guo, X.; Niu, X.; Zhao, Z.; Wang, W.; Xie, X.; Lv, P. Dl-3-n-Butylphthalide Promotes Remyelination and Suppresses Inflammation by Regulating AMPK/SIRT1 and STAT3/NF-kappaB Signaling in Chronic Cerebral Hypoperfusion. Front. Aging Neurosci. 2020, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARgamma Pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Ishfaq, M.; Han, Y.; Zhang, X.; Li, X.; Wang, B.; Lu, X.; Gao, B. Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-kappaB Pathway. Molecules 2023, 28, 7388. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. Inflammation and Obesity: The Pharmacological Role of Flavonoids in the Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 2899. [Google Scholar] [CrossRef]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apcam1137). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Marrassini, C.; Davicino, R.; Acevedo, C.; Anesini, C.; Gorzalczany, S.; Ferraro, G. Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J. Nat. Prod. 2011, 74, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can. J. Physiol. Pharmacol. 2016, 94, 287–295. [Google Scholar] [CrossRef]
- Lee, I.C.; Bae, J.S. Anti-inflammatory effects of vicenin-2 and scolymoside on polyphosphate-mediated vascular inflammatory responses. Inflamm. Res. 2016, 65, 203–212. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Zhang, W.; Rengarajan, T. Vicenin-2 inhibits Wnt/beta-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des. Devel Ther. 2018, 12, 1303–1310. [Google Scholar] [CrossRef]
- Hassan, N.; Ali, A.; Withycombe, C.; Ahluwalia, M.; Al-Nasseri, R.H.; Tonks, A.; Morris, K. TET-2 up-regulation is associated with the anti-inflammatory action of Vicenin-2. Cytokine 2018, 108, 37–42. [Google Scholar] [CrossRef]
- Kang, H.; Ku, S.K.; Jung, B.; Bae, J.S. Anti-inflammatory effects of vicenin-2 and scolymoside in vitro and in vivo. Inflamm. Res. 2015, 64, 1005–1021. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, B.P.; Westerheide, S.D.; Baldwin, A.S., Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell Biol. 2001, 21, 7065–7077. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Cai, G.Y.; Chen, X.M. Energy restriction in renal protection. Br. J. Nutr. 2018, 120, 1149–1158. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, X.; Liu, F.; Zhang, Q.; Ding, L.; Li, G.; Qiu, F. Potential of natural products in inflammation: Biological activities, structure-activity relationships, and mechanistic targets. Arch. Pharm. Res. 2024, 47, 377–409. [Google Scholar] [CrossRef]
- Hybiak, J.; Broniarek, I.; Kiryczynski, G.; Los, L.D.; Rosik, J.; Machaj, F.; Slawinski, H.; Jankowska, K.; Urasinska, E. Aspirin and its pleiotropic application. Eur. J. Pharmacol. 2020, 866, 172762. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.Y. Anti-CD14 antibody reduces LPS responsiveness via TLR4 internalization in human monocytes. Mol. Immunol. 2014, 57, 210–215. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Xie, C.L.; Li, J.L.; Xue, E.X.; Dou, H.C.; Lin, J.T.; Chen, K.; Wu, H.Q.; Wu, L.; Xuan, J.; Huang, Q.S. Vitexin alleviates ER-stress-activated apoptosis and the related inflammation in chondrocytes and inhibits the degeneration of cartilage in rats. Food Funct. 2018, 9, 5740–5749. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1beta-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1alpha pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef]
- Yuan, J.; Hou, K.; Yao, Y.; Du, Z.; Lu, C.; Yuan, Q.; Gao, X. Gold Clusters Attenuate Inflammation in Rat Mesangial Cells via Inhibiting the Activation of NF-kappaB Pathway. Nanomaterials 2020, 10, 712. [Google Scholar] [CrossRef]
- Tang, T.; Scambler, T.E.; Smallie, T.; Cunliffe, H.E.; Ross, E.A.; Rosner, D.R.; O’Neil, J.D.; Clark, A.R. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E2, dual specificity phosphatase 1 and tristetraprolin. Sci. Rep. 2017, 7, 4350. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Cui, Y.; Zhao, Y.; Liu, L.; Wang, H.; Yang, L. Orientin relieves lipopolysaccharide-induced acute lung injury in mice: The involvement of its anti-inflammatory and anti-oxidant properties. Int. Immunopharmacol. 2021, 90, 107189. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Zou, Y.; Chen, J.; Cui, C.; Song, J.; Yang, M.M.; Gao, J.; Hu, H.Q.; Xia, L.Q.; Wang, L.M.; et al. Didymin alleviates metabolic dysfunction-associated fatty liver disease (MAFLD) via the stimulation of Sirt1-mediated lipophagy and mitochondrial biogenesis. J. Transl. Med. 2023, 21, 921. [Google Scholar] [CrossRef]
- Lee, J.J.; Ng, S.C.; Hsu, J.Y.; Liu, H.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Galangin Reverses H2O2-Induced Dermal Fibroblast Senescence via SIRT1-PGC-1alpha/Nrf2 Signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Xu, X.L. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe−/−mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef]
- Yuan, H.; Duan, S.; Guan, T.; Yuan, X.; Lin, J.; Hou, S.; Lai, X.; Huang, S.; Du, X.; Chen, S. Vitexin protects against ethanol-induced liver injury through Sirt1/p53 signaling pathway. Eur. J. Pharmacol. 2020, 873, 173007. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, L.; Yang, X.; Huang, J.; Wang, J.; Wu, X.; Shi, J. Recent advances in anti-inflammation via AMPK activation. Heliyon 2024, 10, e33670. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Teng, C.; Wang, J.; Yu, J.; Jin, C.; Wang, L.; Wu, L.; Lin, Z.; Yu, Z.; et al. Orientin downregulating oxidative stress-mediated endoplasmic reticulum stress and mitochondrial dysfunction through AMPK/SIRT1 pathway in rat nucleus pulposus cells in vitro and attenuated intervertebral disc degeneration in vivo. Apoptosis 2022, 27, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, X.; Liu, Z.; Ming, X.; Han, B.; Cai, W.; Yang, X.; Huang, Z.; Shi, Z.; Wu, J.; et al. Orientin Promotes Antioxidant Capacity, Mitochondrial Biogenesis, and Fiber Transformation in Skeletal Muscles through the AMPK Pathway. J. Agric. Food Chem. 2024, 72, 6226–6235. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O. Plant-Derived Flavonoids as AMPK Activators: Unveiling Their Potential in Type 2 Diabetes Management through Mechanistic Insights, Docking Studies, and Pharmacokinetics. Appl. Sci. 2024, 14, 8607. [Google Scholar] [CrossRef]
- Yibcharoenporn, C.; Chusuth, P.; Jakakul, C.; Rungrotmongkol, T.; Chavasiri, W.; Muanprasat, C. Discovery of a novel chalcone derivative inhibiting CFTR chloride channel via AMPK activation and its anti-diarrheal application. J. Pharmacol. Sci. 2019, 140, 273–283. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Hu, G.; He, D.; Li, Y.; Ran, X.; Du, J.; Fu, S.; Liu, D. Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKbeta/AMPK-PGC-1alpha Signaling Axis. Front. Immunol. 2019, 10, 2650. [Google Scholar] [CrossRef]
- Sharma, P.; Venkatachalam, K.; Binesh, A. Decades Long Involvement of THP-1 Cells as a Model for Macrophage Research: A Comprehensive Review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2024, 23, 85–104. [Google Scholar] [CrossRef]
- Yu, P.B.; Hong, C.C.; Sachidanandan, C.; Babitt, J.L.; Deng, D.Y.; Hoyng, S.A.; Lin, H.Y.; Bloch, K.D.; Peterson, R.T. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 2008, 4, 33–41. [Google Scholar] [CrossRef]
- Askari, B.; Rudbari, H.A.; Micale, N.; Schirmeister, T.; Maugeri, A.; Navarra, M. Anticancer study of heterobimetallic platinum(II)-ruthenium(II) and platinum(II)-rhodium(III) complexes with bridging dithiooxamide ligand. J. Organomet. Chem. 2019, 900, 120918. [Google Scholar] [CrossRef]
- Maugeri, A.; Russo, C.; Patane, G.T.; Barreca, D.; Mandalari, G.; Navarra, M. The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-kappaB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Abeta-Exposed THP-1 Cells. Molecules 2023, 28, 7949. [Google Scholar] [CrossRef]
- Maugeri, A.; Russo, C.; Musumeci, L.; Lombardo, G.E.; De Sarro, G.; Barreca, D.; Cirmi, S.; Navarra, M. The Anticancer Effect of a Flavonoid-Rich Extract of Bergamot Juice in THP-1 Cells Engages the SIRT2/AKT/p53 Pathway. Pharmaceutics 2022, 14, 2168. [Google Scholar] [CrossRef]
- Maugeri, A.; Ferlazzo, N.; De Luca, L.; Gitto, R.; Navarra, M. The link between the AMPK/SIRT1 axis and a flavonoid-rich extract of Citrus bergamia juice: A cell-free, in silico, and in vitro study. Phytother. Res. 2019, 33, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, L.; Russo, C.; Schumacher, U.; Lombardo, G.E.; Maugeri, A.; Navarra, M. The pro-differentiating capability of a flavonoid-rich extract of Citrus bergamia juice prompts autophagic death in THP-1 cells. Sci. Rep. 2024, 14, 19971. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, M.; Rohrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; De Luca, L.; Gitto, R.; Lombardo, G.E.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. The SIRT2 Pathway Is Involved in the Antiproliferative Effect of Flavanones in Human Leukemia Monocytic THP-1 Cells. Biomedicines 2022, 10, 2383. [Google Scholar] [CrossRef]

| Gene | Name | NCBI Reference Sequence | Primer Sequence |
|---|---|---|---|
| ACTB | Actin beta | NM_001101.5 | Forward: 5-TTGTTACAGGAAGTCCCTTGCC-3′ Reverse: 5′-ATGCTATCACCTCCCCTGTGTG-3′ |
| IL1B | Interleukin 1 beta | NM_000576.3 | Forward: 5′-AGCCATGGCAGAAGTACCTG-3′ Reverse: 5′-TGAAGCCCTTGCTGTAGTGG-3′ |
| IL6 | Interleukin 6 | NM_000600.5 | Forward: 5′-CCACCGGGAACGAAAGAGAA-3′ Reverse: 5′-GAGAAGGCAACTGGACCGAA-3′ |
| NOS2 | Nitric oxide synthase 2 | NM_000625.4 | Forward: 5′-GGATGACAACCGATACCA-3′ Reverse: 5′-GAAGGCAATGGACTCAGA-3′ |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | NM_000963.4 | Forward: 5′-GCCTGGTCTGATGATGTA-3′ Reverse: 5′-TCTGGAACAACTGCTCAT-3′ |
| SIRT1 | Sirtuin 1 | NM_012238.5 | Forward: 5’-AACTACTTCGCAACTATACC-3′ Reverse: 5′- ACCATGACACTGAATTATCC-3′ |
| TNFA | Tumor necrosis factor alpha | NM_000594.4 | Forward: 5′-CACAGTGAAGTGCTGGCAAC-3′ Reverse: 5′-ACATTGGGTCCCCCAGGATA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, A.; Russo, C.; Patanè, G.T.; Farina, M.; Rapisarda, A.; Masullo, M.; Navarra, M. Vicenin-2 Hinders Pro-Inflammatory Response via Targeting the CaMKKβ-AMPK-SIRT1 Axis in Lipopolysaccharide-Stressed THP-1 Cells. Int. J. Mol. Sci. 2025, 26, 2077. https://doi.org/10.3390/ijms26052077
Maugeri A, Russo C, Patanè GT, Farina M, Rapisarda A, Masullo M, Navarra M. Vicenin-2 Hinders Pro-Inflammatory Response via Targeting the CaMKKβ-AMPK-SIRT1 Axis in Lipopolysaccharide-Stressed THP-1 Cells. International Journal of Molecular Sciences. 2025; 26(5):2077. https://doi.org/10.3390/ijms26052077
Chicago/Turabian StyleMaugeri, Alessandro, Caterina Russo, Giuseppe Tancredi Patanè, Martina Farina, Antonio Rapisarda, Mariorosario Masullo, and Michele Navarra. 2025. "Vicenin-2 Hinders Pro-Inflammatory Response via Targeting the CaMKKβ-AMPK-SIRT1 Axis in Lipopolysaccharide-Stressed THP-1 Cells" International Journal of Molecular Sciences 26, no. 5: 2077. https://doi.org/10.3390/ijms26052077
APA StyleMaugeri, A., Russo, C., Patanè, G. T., Farina, M., Rapisarda, A., Masullo, M., & Navarra, M. (2025). Vicenin-2 Hinders Pro-Inflammatory Response via Targeting the CaMKKβ-AMPK-SIRT1 Axis in Lipopolysaccharide-Stressed THP-1 Cells. International Journal of Molecular Sciences, 26(5), 2077. https://doi.org/10.3390/ijms26052077









