A Comprehensive Analysis Revealing BUB1B as a Potential Prognostic and Immunological Biomarker in Lung Adenocarcinoma
Abstract
1. Introduction
2. Results
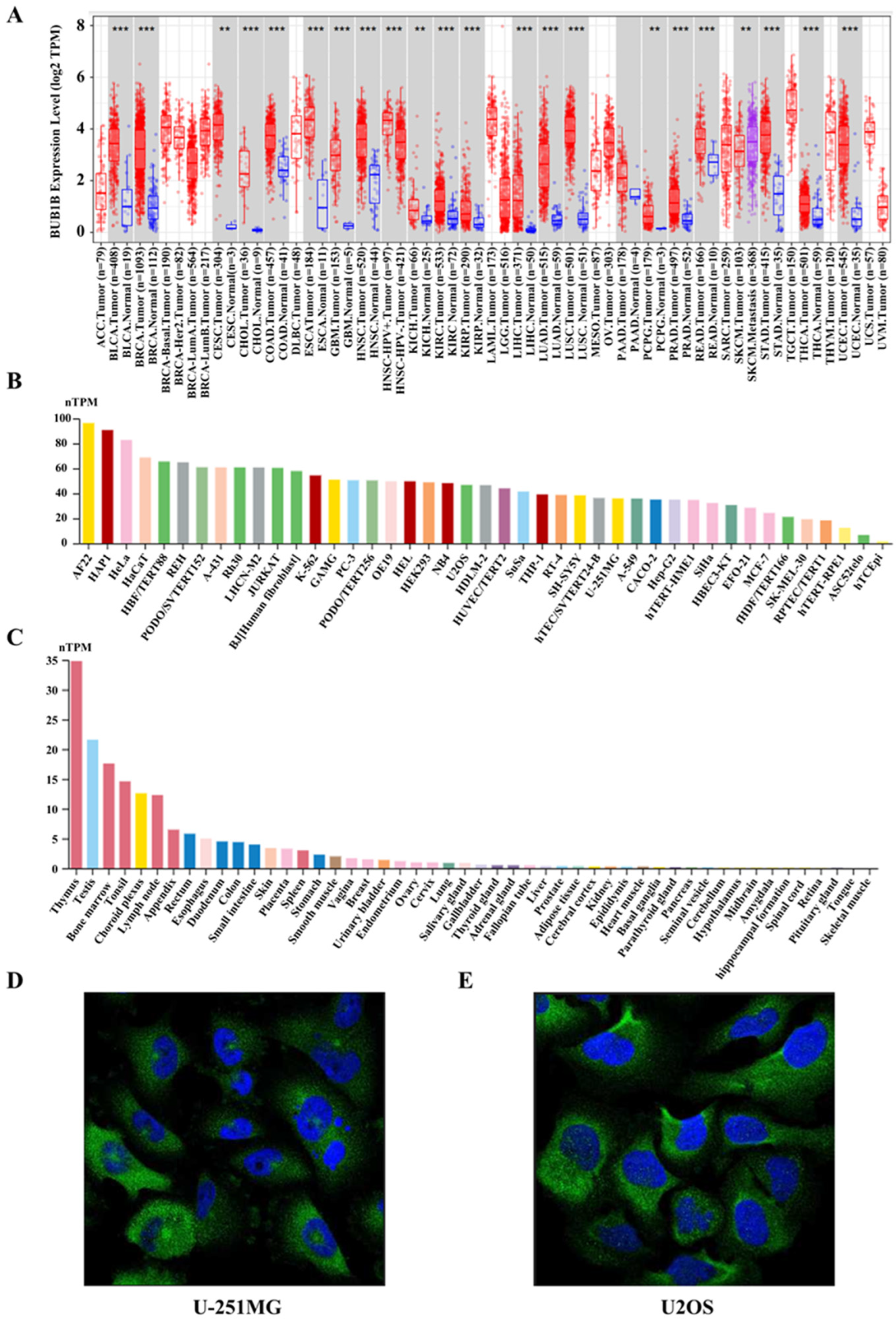
2.1. BUB1B Was Upregulated in Multiple Human Cancers
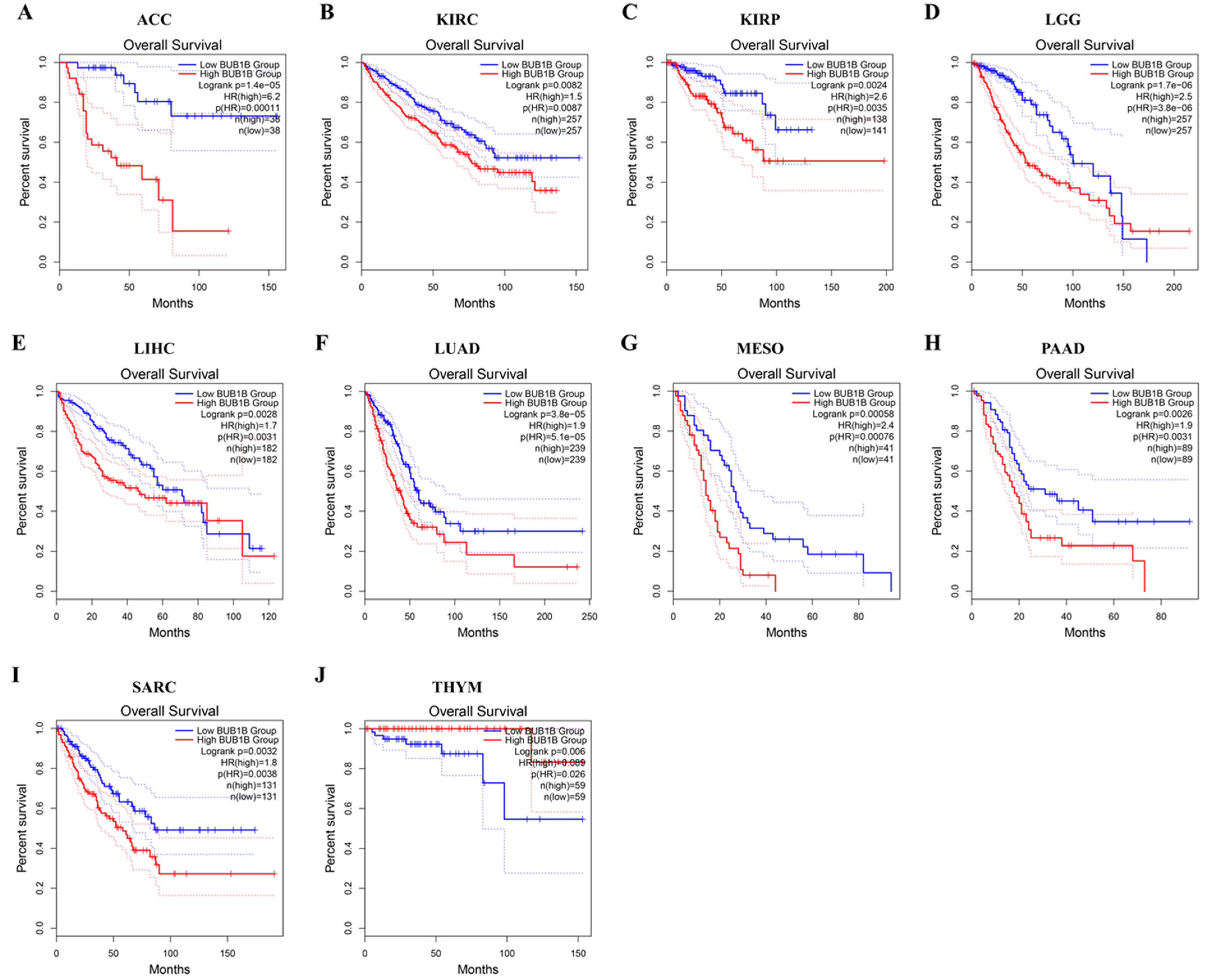
2.2. Pan-Cancer Analysis of the Diagnostic and Prognostic Value of BUB1B
2.3. The Characteristics of BUB1B Mutations in the TCGA Pan-Cancer Cohort
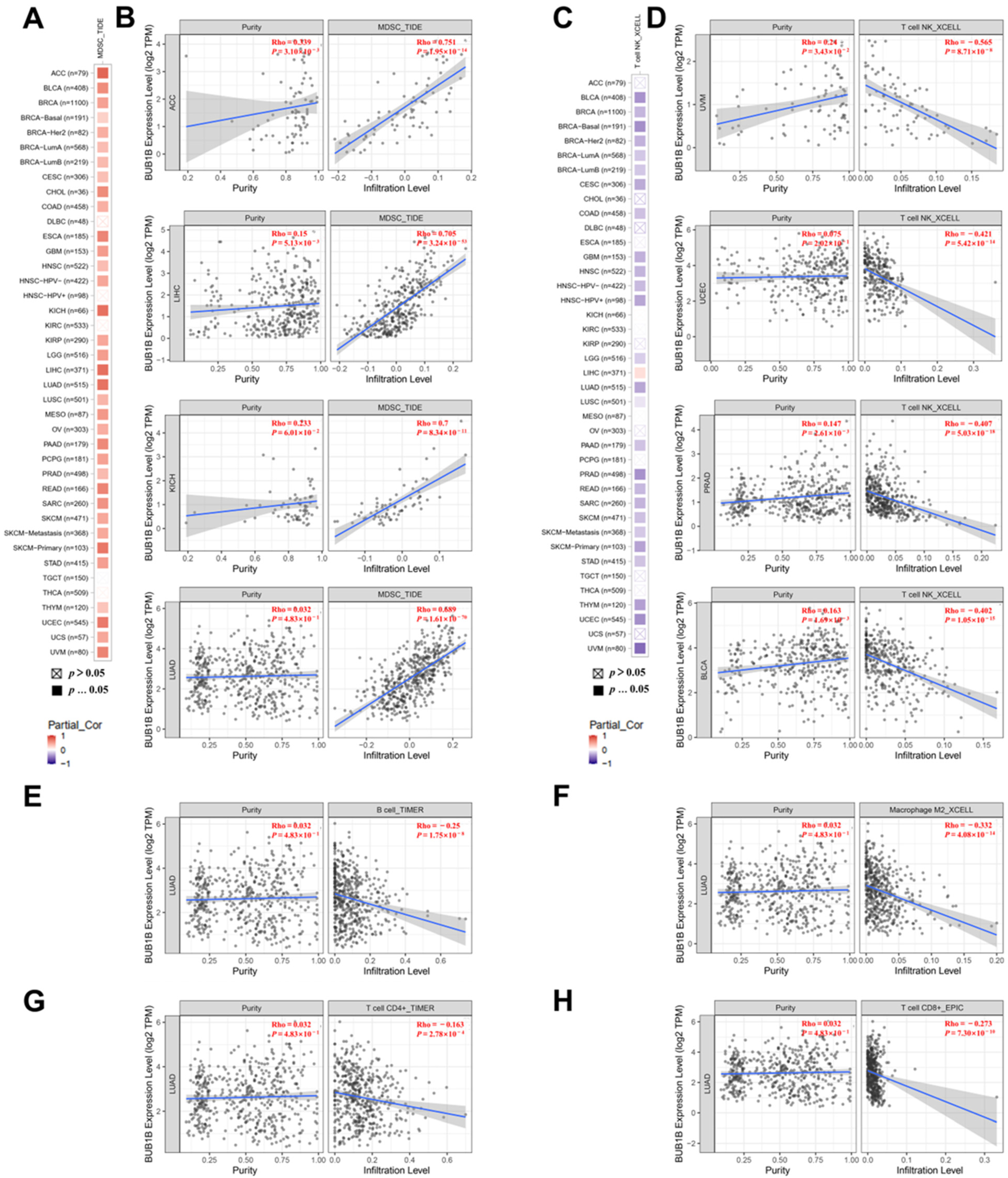
2.4. BUB1B Is Related to Immune Microenvironment in Pan-Cancer
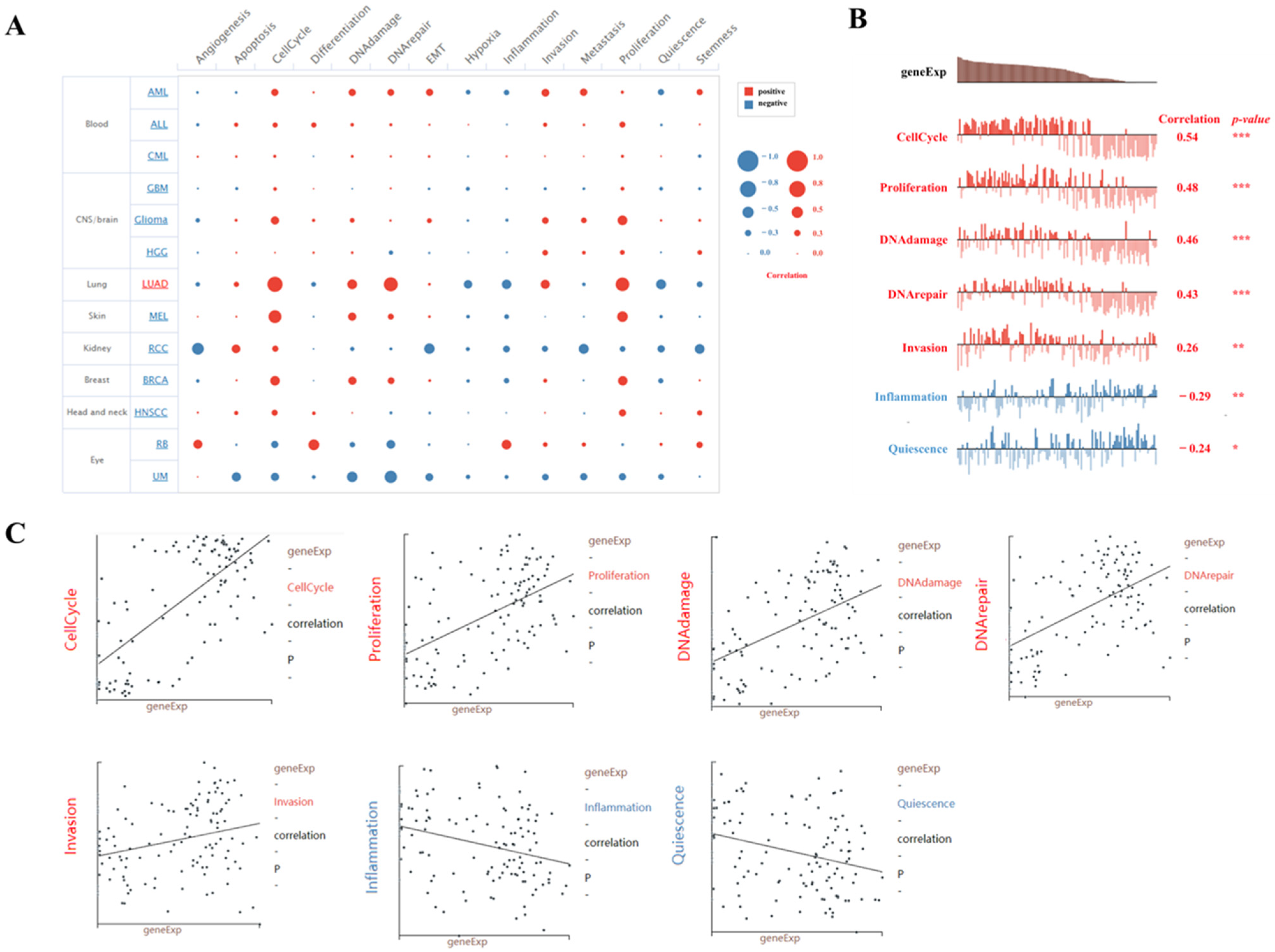
2.5. The Function Analysis of BUB1B in LUAD
2.6. Single-Cell Sequence Analysis of the Correlation Between BUB1B Expression and 14 Cancer Functional States in LUAD
2.7. BUB1B Knockdown Suppressed LUAD Cell Proliferation, Migration, and EMT In Vitro
3. Discussion
4. Materials and Methods
4.1. Analysis of the Expression Level of BUB1B in the Pan-Cancer Datasets
4.2. Identification of the Correlation Between BUB1B Expression Levels and Diagnosis and Prognosis Analysis in Human Cancers
4.3. Association Between BUB1B Expression and Mutation
4.4. Analysis of the Correlation Between BUB1B and Immune Infiltration
4.5. Gene Co-Expression Analysis
4.6. Single-Cell Sequence Analysis
4.7. Cell Culture and siRNA Transfection
4.8. RNA Isolation and qPCR
4.9. Western Blotting
4.10. Cell Proliferation
4.11. Wound-Healing Assay
4.12. Cell Migration Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duerinck, J.; Schwarze, J.K.; Awada, G.; Tijtgat, J.; Vaeyens, F.; Bertels, C.; Geens, W.; Klein, S.; Seynaeve, L.; Cras, L.; et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: A phase I clinical trial. J. Immunother. Cancer 2021, 9, e002296. [Google Scholar] [CrossRef]
- VanderWalde, A.; Bellasea, S.L.; Kendra, K.L.; Khushalani, N.I.; Campbell, K.M.; Scumpia, P.O.; Kuklinski, L.F.; Collichio, F.; Sosman, J.A.; Ikeguchi, A.; et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: A randomized phase 2 trial. Nat. Med. 2023, 29, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Yang, X.; Long, J.; Bai, Y.; Yang, X.; Mao, Y.; Sang, X.; Seery, S.; Zhao, H. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J. Hematol. Oncol. 2019, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Nakamura, M.; Andersson, P.; Yang, X.; Zhang, Y.; Seki, T.; Scherzer, M.; Dubey, O.; Wang, X.; et al. Dual roles of endothelial FGF-2–FGFR1–PDGF-BB and perivascular FGF-2–FGFR2–PDGFRβ signaling pathways in tumor vascular remodeling. Cell Discov. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pamplona, R.; Berenguer, A.; Cordero, D.; Mollevi, D.G.; Crous-Bou, M.; Sole, X.; Pare-Brunet, L.; Guino, E.; Salazar, R.; Santos, C.; et al. Aberrant gene expression in mucosa adjacent to tumor reveals a molecular crosstalk in colon cancer. Mol. Cancer 2014, 13, 46. [Google Scholar] [CrossRef]
- Grabovska, Y.; Mackay, A.; O Hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J.; et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, Z.; Zhou, Q.; Zeng, H.; Zhang, H.; Liu, Z.; Huang, Q.; Wang, J.; Chang, Y.; Xia, Y.; et al. Identification and validation of dichotomous immune subtypes based on intratumoral immune cells infiltration in clear cell renal cell carcinoma patients. J. Immunother. Cancer 2020, 8, e000447. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.J.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhu, G.; He, J.; Xu, X.; Zhu, W.; Jiang, W.; He, G. CircMAPK1 promoted CD8 + T cell infiltration in LUAD by improving the IGF2BP1 dependent CCL5 upregulation. Int. Immunopharmacol. 2024, 127, 111267. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, H.; Wu, C.; Peng, Y. Molecular markers that predict response to combined radiotherapy and immunotherapy in patients with lung adenocarcinoma: A bioinformatics analysis. Transl. Cancer Res. 2023, 12, 2646–2659. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, Y.; Liang, L.; Lin, J. Significance of cuproptosis-related lncRNA signature in LUAD prognosis and immunotherapy: A machine learning approach. Thorac. Cancer 2023, 14, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Martinez, L.A.; Tian, W.; Li, B.; Warrington, R.; Jia, L.; Brautigam, C.A.; Luo, X.; Yu, H. The Cdc20-binding Phe Box of the Spindle Checkpoint Protein BubR1 Maintains the Mitotic Checkpoint Complex During Mitosis. J. Biol. Chem. 2015, 290, 2431–2443. [Google Scholar] [CrossRef] [PubMed]
- Overlack, K.; Bange, T.; Weissmann, F.; Faesen, A.C.; Maffini, S.; Primorac, I.; Müller, F.; Peters, J.; Musacchio, A. BubR1 Promotes Bub3-Dependent APC/C Inhibition during Spindle Assembly Checkpoint Signaling. Curr. Biol. 2017, 27, 2915–2927. [Google Scholar] [CrossRef]
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 2015, 60, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Lu, H. Correlation of BUB1 and BUB1B with the development and prognosis of endometrial cancer. Sci. Rep. 2024, 14, 17084. [Google Scholar] [CrossRef] [PubMed]
- Rio, F.T.; Lavoie, J.; Hamel, N.; Geyer, F.C.; Kushner, Y.B.; Novak, D.J.; Wark, L.; Capelli, C.; Reis-Filho, J.S.; Mai, S.; et al. Homozygous BUB1B mutation and susceptibility to gastrointestinal neoplasia. N. Engl. J. Med. 2010, 363, 2628–2637. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gu, C.; Luo, C.; Li, F.; Wang, M. BUB1B promotes multiple myeloma cell proliferation through CDC20/CCNB axis. Med. Oncol. 2015, 32, 81. [Google Scholar] [CrossRef]
- Tang, X.; Guo, M.; Ding, P.; Deng, Z.; Ke, M.; Yuan, Y.; Zhou, Y.; Lin, Z.; Li, M.; Gu, C.; et al. BUB1B and circBUB1B_544aa aggravate multiple myeloma malignancy through evoking chromosomal instability. Signal Transduct. Target. Ther. 2021, 6, 361. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, S.; Wang, P.; Wang, H.; Sha, B.; Peng, H.; Ju, Z.; Rao, J.; Lu, L. BUB1B promotes hepatocellular carcinoma progression via activation of the mTORC1 signaling pathway. Cancer Med. 2020, 9, 8159–8172. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, F.; Zhang, H.; Chen, Q. Overexpression of BUB1B, CCNA2, CDC20, and CDK1 in tumor tissues predicts poor survival in pancreatic ductal adenocarcinoma. Biosci. Rep. 2019, 39, BSR20182306. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xiang, C. Aberrant Expression of BUB1B Contributes to the Progression of Thyroid Carcinoma and Predicts Poor Outcomes for Patients. J. Cancer 2022, 13, 2336–2351. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, G.; Cai, Z.; Wang, C.; Liu, Z.; Lin, Z.; Wu, Y.; Liang, Y.; Han, Z.; Liu, J.; et al. Overexpression of BUB1B contributes to progression of prostate cancer and predicts poor outcome in patients with prostate cancer. OncoTargets Ther. 2016, 9, 2211–2220. [Google Scholar] [CrossRef]
- Sekino, Y.; Han, X.; Kobayashi, G.; Babasaki, T.; Miyamoto, S.; Kobatake, K.; Kitano, H.; Ikeda, K.; Goto, K.; Inoue, S.; et al. BUB1B Overexpression Is an Independent Prognostic Marker and Associated with CD44, p53, and PD-L1 in Renal Cell Carcinoma. Oncology 2021, 99, 240–250. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Shang, L.; Yu, J.; Qu, Q. The FOXM1/BUB1B signaling pathway is essential for the tumorigenicity and radioresistance of glioblastoma. Oncol. Rep. 2017, 38, 3367–3375. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Fancello, L.; Gandini, S.; Pelicci, P.G.; Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: Major advancements and challenges. J. Immunother. Cancer 2019, 7, 183. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients with Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef]
- Kim, T.; Laird, P.W.; Park, P.J. The Landscape of Microsatellite Instability in Colorectal and Endometrial Cancer Genomes. Cell 2013, 155, 858–868. [Google Scholar] [CrossRef]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Duffy, M.J.; Crown, J. Biomarkers for Predicting Response to Immunotherapy with Immune Checkpoint Inhibitors in Cancer Patients. Clin. Chem. 2019, 65, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Sammut, S.; Galson, J.D.; Minter, R.; Sun, B.; Chin, S.; De Mattos-Arruda, L.; Finch, D.K.; Schätzle, S.; Dias, J.; Rueda, O.M.; et al. Predictability of B cell clonal persistence and immunosurveillance in breast cancer. Nat. Immunol. 2024, 25, 916–924. [Google Scholar] [CrossRef]
- Ferris, S.T.; Durai, V.; Wu, R.; Theisen, D.J.; Ward, J.P.; Bern, M.D.; Davidson, J.T.; Bagadia, P.; Liu, T.; Briseño, C.G.; et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature 2020, 584, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Salehi-Rad, R.; Crosson, W.; Momcilovic, M.; Lim, R.J.; Ong, S.L.; Huang, Z.L.; Zhang, T.; Abascal, J.; Dumitras, C.; et al. Inhibition of Granulocytic Myeloid-Derived Suppressor Cells Overcomes Resistance to Immune Checkpoint Inhibition in LKB1-Deficient Non–Small Cell Lung Cancer. Cancer Res. 2021, 81, 3295–3308. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chhatar, S.; Mishra, A.; Lal, G. Natural killer T cell activation increases iNOS+CD206− M1 macrophage and controls the growth of solid tumor. J. Immunother. Cancer 2019, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Motohashi, S.; Okamoto, Y.; Yoshino, I.; Nakayama, T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin. Immunol. 2011, 140, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, J.; Weng, L.; Tang, W.; Jin, S.; Ma, W. Myeloid-derived suppressor cells—New and exciting players in lung cancer. J. Hematol. Oncol. 2020, 13, 10. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic. Acids. Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic. Acids. Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor–immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic. Acids. Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yan, M.; Zhang, G.; Liu, W.; Deng, C.; Liao, G.; Xu, L.; Luo, T.; Yan, H.; Long, Z.; et al. CancerSEA: A cancer single-cell state atlas. Nucleic. Acids. Res. 2019, 47, D900–D908. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; An, F.; Zhang, W.; Zhu, X.; Meng, S.; Zhao, B. A Comprehensive Analysis Revealing BUB1B as a Potential Prognostic and Immunological Biomarker in Lung Adenocarcinoma. Int. J. Mol. Sci. 2025, 26, 2061. https://doi.org/10.3390/ijms26052061
Hao Z, An F, Zhang W, Zhu X, Meng S, Zhao B. A Comprehensive Analysis Revealing BUB1B as a Potential Prognostic and Immunological Biomarker in Lung Adenocarcinoma. International Journal of Molecular Sciences. 2025; 26(5):2061. https://doi.org/10.3390/ijms26052061
Chicago/Turabian StyleHao, Zhenzhen, Fei An, Wanting Zhang, Xiaoshuang Zhu, Shihao Meng, and Bo Zhao. 2025. "A Comprehensive Analysis Revealing BUB1B as a Potential Prognostic and Immunological Biomarker in Lung Adenocarcinoma" International Journal of Molecular Sciences 26, no. 5: 2061. https://doi.org/10.3390/ijms26052061
APA StyleHao, Z., An, F., Zhang, W., Zhu, X., Meng, S., & Zhao, B. (2025). A Comprehensive Analysis Revealing BUB1B as a Potential Prognostic and Immunological Biomarker in Lung Adenocarcinoma. International Journal of Molecular Sciences, 26(5), 2061. https://doi.org/10.3390/ijms26052061




