The Loss of HJV Aggravates Muscle Atrophy by Promoting the Activation of the TβRII/Smad3 Pathway
Abstract
1. Introduction
2. Results
2.1. HJV Expression Was Decreased in Atrophied Muscles
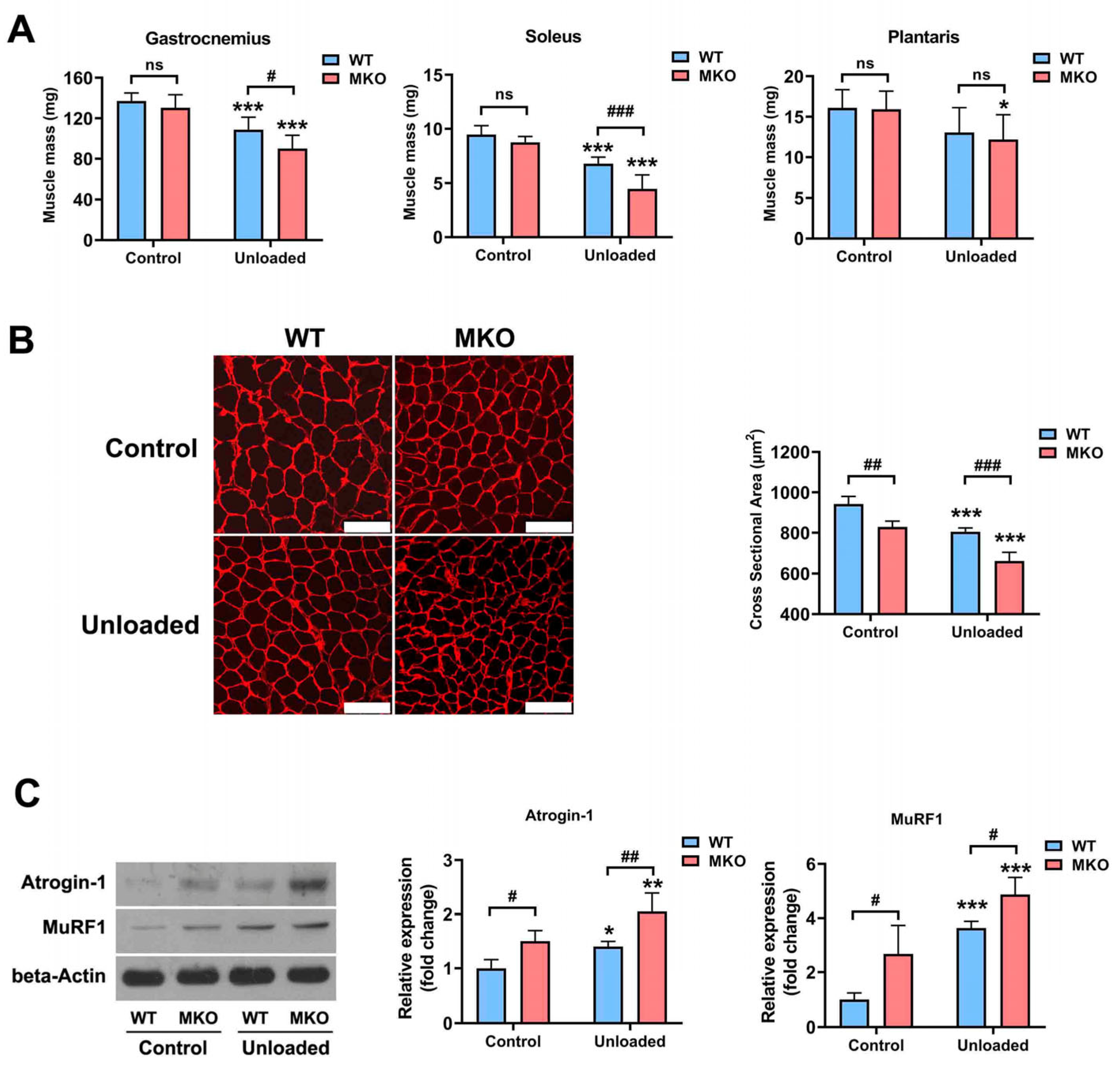
2.2. The Depletion of Hjv Exacerbated Disuse-Induced Muscle Atrophy
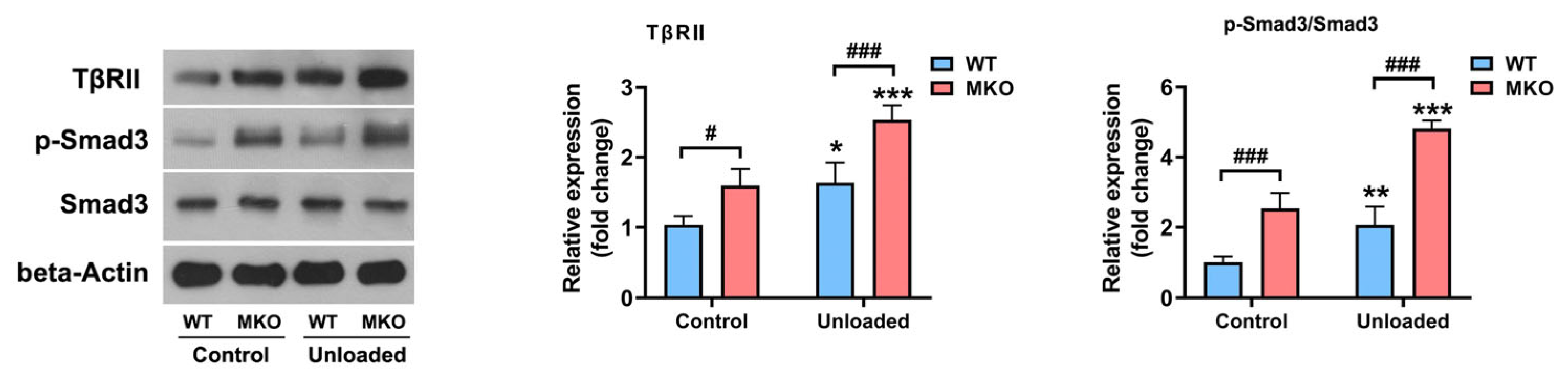
2.3. The Depletion of Hjv Enhanced Muscle Disuse-Induced Smad3 Activation by Upregulating TβRII Expression
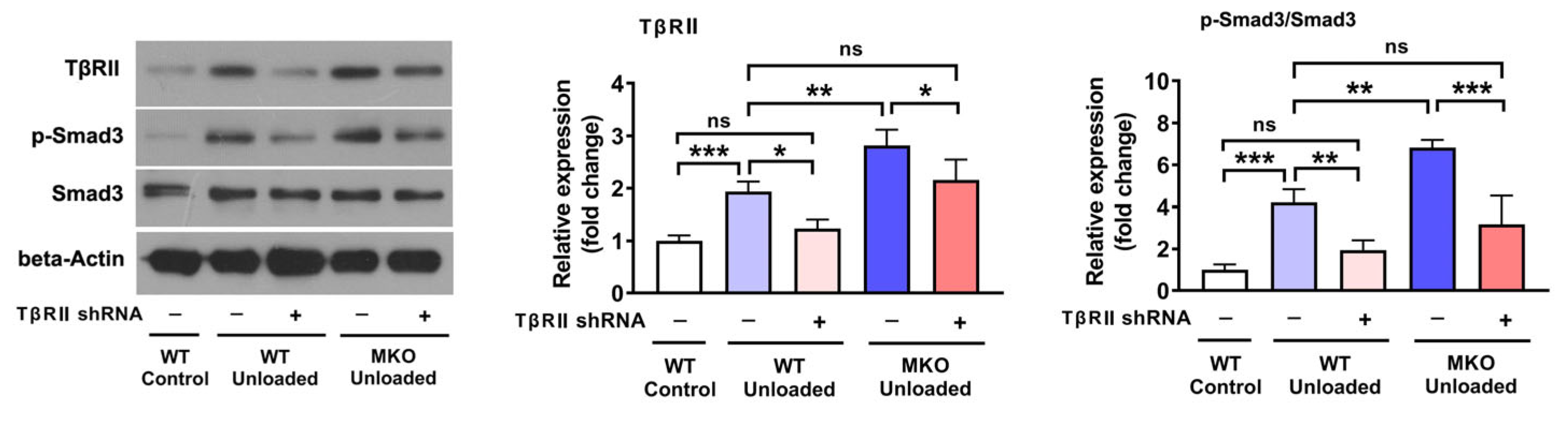
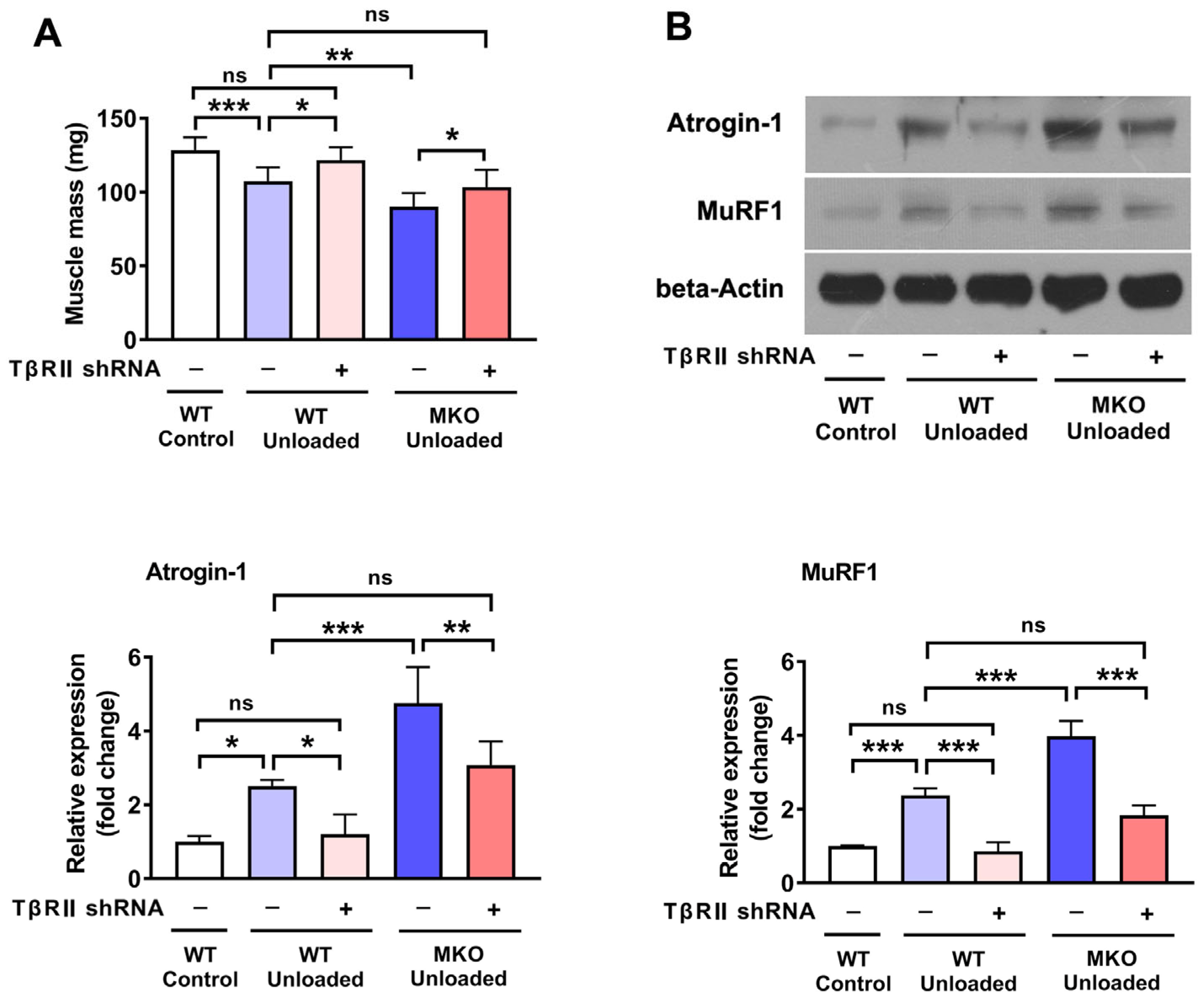
2.4. The Knockdown of TβRII Reversed the Pro-Atrophic Effects Observed in MKO Mice
3. Discussion
4. Materials and Methods
4.1. Mice and Hindlimb Unloading
4.2. RNA Extraction and Real-Time PCR (qPCR)
4.3. Adenoviral Vector Construction and In Vivo Injection
4.4. Western Blot Analysis
4.5. Immunohistochemical Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Adv | Adenovirus |
| Akt | Protein kinase B |
| ANOVA | Analysis of variance |
| Atrogin-1 | Muscle atrophy F-box |
| BMP | Bone morphogenetic protein |
| CSA | Cross-sectional area |
| DMD | Duchenne muscular dystrophy |
| HJV | Hemojuvelin |
| HU | Hindlimb unloading |
| FoxO | Forkhead box class O |
| MKO | Muscle-specific knockout mice |
| MuRF1 | Muscle ring finger1 |
| shRNA | Short hairpin RNA |
| RT | Real-time |
| Smad | Drosophila mothers against decapentaplegic protein |
| TGF-β | The transforming growth factor-beta |
| TβRII | Transforming growth factor-β type II receptor |
| WT | Wild type |
References
- Cao, R.Y.; Li, J.; Dai, Q.; Li, Q.; Yang, J. Muscle Atrophy: Present and Future. Adv. Exp. Med. Biol. 2018, 1088, 605–624. [Google Scholar] [CrossRef]
- Khalil, R. Ubiquitin-Proteasome Pathway and Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Protein Breakdown in Muscle Wasting: Role of Autophagy-Lysosome and Ubiquitin-Proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.V.; Waddell, D.S.; Siu, R.; Stein, M.; Dewey, S.; Furlow, J.D.; Bodine, S.C. Upregulation of Proteasome Activity in Muscle RING Finger 1-Null Mice Following Denervation. FASEB J. 2012, 26, 2986–2999. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-Proteasome Pathway in Skeletal Muscle Atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Lan, X.-Q.; Deng, C.-J.; Wang, Q.-Q.; Zhao, L.-M.; Jiao, B.-W.; Xiang, Y. The Role of TGF-β Signaling in Muscle Atrophy, Sarcopenia and Cancer Cachexia. Gen. Comp. Endocrinol. 2024, 353, 114513. [Google Scholar] [CrossRef]
- Chen, J.L.; Colgan, T.D.; Walton, K.L.; Gregorevic, P.; Harrison, C.A. The TGF-β Signalling Network in Muscle Development, Adaptation and Disease. Adv. Exp. Med. Biol. 2016, 900, 97–131. [Google Scholar] [CrossRef]
- Klein, G.L. Transforming Growth Factor-Beta in Skeletal Muscle Wasting. Int. J. Mol. Sci. 2022, 23, 1167. [Google Scholar] [CrossRef]
- Abrigo, J.; Simon, F.; Cabrera, D.; Cordova, G.; Trollet, C.; Cabello-Verrugio, C. Central Role of Transforming Growth Factor Type Beta 1 in Skeletal Muscle Dysfunctions: An Update on Therapeutic Strategies. Curr. Protein Pept. Sci. 2018, 19, 1189–1200. [Google Scholar] [CrossRef]
- Dasgupta, A.; Gibbard, D.F.; Schmitt, R.E.; Arneson-Wissink, P.C.; Ducharme, A.M.; Bruinsma, E.S.; Hawse, J.R.; Jatoi, A.; Doles, J.D. A TGF-β/KLF10 Signaling Axis Regulates Atrophy-Associated Genes to Induce Muscle Wasting in Pancreatic Cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2215095120. [Google Scholar] [CrossRef] [PubMed]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming Growth Factor-Beta Induces Skeletal Muscle Atrophy and Fibrosis through the Induction of Atrogin-1 and Scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 Transcription Factors Control Muscle Mass in Adulthood. Am. J. Physiol. Cell Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A.; McNally, R.M.; Hoffmann, F.M.; Hornberger, T.A. Smad3 Induces Atrogin-1, Inhibits mTOR and Protein Synthesis, and Promotes Muscle Atrophy in Vivo. Mol. Endocrinol. 2013, 27, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, L.M.; Witczak, C.A.; Houmard, J.A.; Brault, J.J. SMAD3 Augments FoxO3-Induced MuRF-1 Promoter Activity in a DNA-Binding-Dependent Manner. Am. J. Physiol. Cell Physiol. 2014, 307, C278–C287. [Google Scholar] [CrossRef] [PubMed]
- Tando, T.; Hirayama, A.; Furukawa, M.; Sato, Y.; Kobayashi, T.; Funayama, A.; Kanaji, A.; Hao, W.; Watanabe, R.; Morita, M.; et al. Smad2/3 Proteins Are Required for Immobilization-Induced Skeletal Muscle Atrophy. J. Biol. Chem. 2016, 291, 12184–12194. [Google Scholar] [CrossRef]
- Umezu, T.; Nakamura, S.; Sato, Y.; Kobayashi, T.; Ito, E.; Abe, T.; Kaneko, M.; Nomura, M.; Yoshimura, A.; Oya, A.; et al. Smad2 and Smad3 Expressed in Skeletal Muscle Promote Immobilization-Induced Bone Atrophy in Mice. Biochem. Biophys. Res. Commun. 2021, 582, 111–117. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Wagner, J.; Papanikolaou, G.; Sebastiani, G.; Pantopoulos, K. Conditional Disruption of Mouse HFE2 Gene: Maintenance of Systemic Iron Homeostasis Requires Hepatic but Not Skeletal Muscle Hemojuvelin. Hepatology 2011, 54, 1800–1807. [Google Scholar] [CrossRef]
- Wallace, D.F.; Dixon, J.L.; Ramm, G.A.; Anderson, G.J.; Powell, L.W.; Subramaniam, N. Hemojuvelin (HJV)-Associated Hemochromatosis: Analysis of HJV and HFE Mutations and Iron Overload in Three Families. Haematologica 2005, 90, 254–255. [Google Scholar]
- Babitt, J.L.; Huang, F.W.; Wrighting, D.M.; Xia, Y.; Sidis, Y.; Samad, T.A.; Campagna, J.A.; Chung, R.T.; Schneyer, A.L.; Woolf, C.J.; et al. Bone Morphogenetic Protein Signaling by Hemojuvelin Regulates Hepcidin Expression. Nat. Genet. 2006, 38, 531–539. [Google Scholar] [CrossRef]
- Rodriguez, A.; Pan, P.; Parkkila, S. Expression Studies of Neogenin and Its Ligand Hemojuvelin in Mouse Tissues. J. Histochem. Cytochem. 2007, 55, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, J.; Wang, F.; Gong, J.; Wang, L.; Wu, Q.; Li, W.; Liu, H.; Wang, J.; Zhang, K.; et al. Hemojuvelin Is a Novel Suppressor for Duchenne Muscular Dystrophy and Age-Related Muscle Wasting. J. Cachexia Sarcopenia Muscle 2019, 10, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Severyn, C.J.; Rotwein, P. Conserved Proximal Promoter Elements Control Repulsive Guidance Molecule c/Hemojuvelin (Hfe2) Gene Transcription in Skeletal Muscle. Genomics 2010, 96, 342–351. [Google Scholar] [CrossRef][Green Version]
- Kuninger, D.; Kuns-Hashimoto, R.; Kuzmickas, R.; Rotwein, P. Complex Biosynthesis of the Muscle-Enriched Iron Regulator RGMc. J. Cell Sci. 2006, 119, 3273–3283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.L.; Walton, K.L.; Hagg, A.; Colgan, T.D.; Johnson, K.; Qian, H.; Gregorevic, P.; Harrison, C.A. Specific Targeting of TGF-β Family Ligands Demonstrates Distinct Roles in the Regulation of Muscle Mass in Health and Disease. Proc. Natl. Acad. Sci. USA 2017, 114, E5266–E5275. [Google Scholar] [CrossRef] [PubMed]
- Chia, Z.-J.; Cao, Y.-N.; Little, P.J.; Kamato, D. Transforming Growth Factor-β Receptors: Versatile Mechanisms of Ligand Activation. Acta Pharmacol. Sin. 2024, 45, 1337–1348. [Google Scholar] [CrossRef]
- Criswell, D.S.; Booth, F.W.; DeMayo, F.; Schwartz, R.J.; Gordon, S.E.; Fiorotto, M.L. Overexpression of IGF-I in Skeletal Muscle of Transgenic Mice Does Not Prevent Unloading-Induced Atrophy. Am. J. Physiol. 1998, 275, E373–E379. [Google Scholar] [CrossRef]
- Mavalli, M.D.; DiGirolamo, D.J.; Fan, Y.; Riddle, R.C.; Campbell, K.S.; van Groen, T.; Frank, S.J.; Sperling, M.A.; Esser, K.A.; Bamman, M.M.; et al. Distinct Growth Hormone Receptor Signaling Modes Regulate Skeletal Muscle Development and Insulin Sensitivity in Mice. J. Clin. Investig. 2010, 120, 4007–4020. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Zhang, P.; Liu, H.; He, J.; Zhang, C.; Fan, M.; Chen, X. PGC-1α over-Expression Suppresses the Skeletal Muscle Atrophy and Myofiber-Type Composition during Hindlimb Unloading. Biosci. Biotechnol. Biochem. 2017, 81, 500–513. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Liu, H.; Li, J.; Wang, J.; Li, Y.; Chen, X.; Yang, Z.; Fan, M. Dystrophin Involved in the Susceptibility of Slow Muscles to Hindlimb Unloading via Concomitant Activation of TGF-Β1/Smad3 Signaling and Ubiquitin-Proteasome Degradation in Mice. Cell Biochem. Biophys. 2014, 70, 1057–1067. [Google Scholar] [CrossRef]
- Sun, C.-C.; Xiao, J.-L.; Sun, C.; Tang, C.-F. Ferroptosis and Its Potential Role in the Physiopathology of Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2024, 25, 12463. [Google Scholar] [CrossRef] [PubMed]
- Vinke, J.S.J.; Gorter, A.R.; Eisenga, M.F.; Dam, W.A.; van der Meer, P.; van den Born, J.; Bakker, S.J.L.; Hoes, M.F.; de Borst, M.H. Iron Deficiency Is Related to Lower Muscle Mass in Community-Dwelling Individuals and Impairs Myoblast Proliferation. J. Cachexia Sarcopenia Muscle 2023, 14, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Chen, H.; Yin, H.; Zhang, Z.; Zhao, G.; Luo, C.; Zhuang, R.; Chen, A.; Han, T. Association of Serum Iron Metabolism with Muscle Mass and Frailty in Older Adults: A Cross-Sectional Study of Community-Dwelling Older Adults. Medicine 2024, 103, e39348. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.K.; Lee, W.-Y.; Yeo, W.J.; Kyung, B.S.; Jung, K.W.; Seo, H.K.; Lee, Y.-S.; Suh, D.W. A Novel Muscle Atrophy Mechanism: Myocyte Degeneration Due to Intracellular Iron Deprivation. Cells 2022, 11, 2853. [Google Scholar] [CrossRef]
- Alves, F.M.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Age-Related Changes in Skeletal Muscle Iron Homeostasis. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 16–24. [Google Scholar] [CrossRef]
- Wyart, E.; Hsu, M.Y.; Sartori, R.; Mina, E.; Rausch, V.; Pierobon, E.S.; Mezzanotte, M.; Pezzini, C.; Bindels, L.B.; Lauria, A.; et al. Iron Supplementation Is Sufficient to Rescue Skeletal Muscle Mass and Function in Cancer Cachexia. EMBO Rep. 2022, 23, e53746. [Google Scholar] [CrossRef]
- Bose, C.; Megyesi, J.; Karaduta, O.; Singh, S.P.; Swaminathan, S.; Shah, S.V. Iron Chelation Prevents Age-Related Skeletal Muscle Sarcopenia in Klotho Gene Mutant Mice, a Genetic Model of Aging. J. Cachexia Sarcopenia Muscle 2025, 16, e13678. [Google Scholar] [CrossRef]
- Horeau, M.; Delalande, M.; Ropert, M.; Leroyer, P.; Martin, B.; Orfila, L.; Loréal, O.; Derbré, F. Sex Similarities and Divergences in Systemic and Muscle Iron Metabolism Adaptations to Extreme Physical Inactivity in Rats. J. Cachexia Sarcopenia Muscle 2024, 15, 1989–1998. [Google Scholar] [CrossRef]
- Chen, W.; Huang, F.W.; de Renshaw, T.B.; Andrews, N.C. Skeletal Muscle Hemojuvelin Is Dispensable for Systemic Iron Homeostasis. Blood 2011, 117, 6319–6325. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tao, W.; Jia, J.; Yuan, M.; Li, W.; Zhang, P.; Chen, X. The Loss of HJV Aggravates Muscle Atrophy by Promoting the Activation of the TβRII/Smad3 Pathway. Int. J. Mol. Sci. 2025, 26, 2016. https://doi.org/10.3390/ijms26052016
Wang L, Tao W, Jia J, Yuan M, Li W, Zhang P, Chen X. The Loss of HJV Aggravates Muscle Atrophy by Promoting the Activation of the TβRII/Smad3 Pathway. International Journal of Molecular Sciences. 2025; 26(5):2016. https://doi.org/10.3390/ijms26052016
Chicago/Turabian StyleWang, Lu, Wuchen Tao, Jiajie Jia, Min Yuan, Wenjiong Li, Peng Zhang, and Xiaoping Chen. 2025. "The Loss of HJV Aggravates Muscle Atrophy by Promoting the Activation of the TβRII/Smad3 Pathway" International Journal of Molecular Sciences 26, no. 5: 2016. https://doi.org/10.3390/ijms26052016
APA StyleWang, L., Tao, W., Jia, J., Yuan, M., Li, W., Zhang, P., & Chen, X. (2025). The Loss of HJV Aggravates Muscle Atrophy by Promoting the Activation of the TβRII/Smad3 Pathway. International Journal of Molecular Sciences, 26(5), 2016. https://doi.org/10.3390/ijms26052016





