Peptide Fractions Extracted from the Hemolymph of Hermetia illucens Inhibit Growth and Motility and Enhance the Effects of Traditional Chemotherapeutics in Human Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
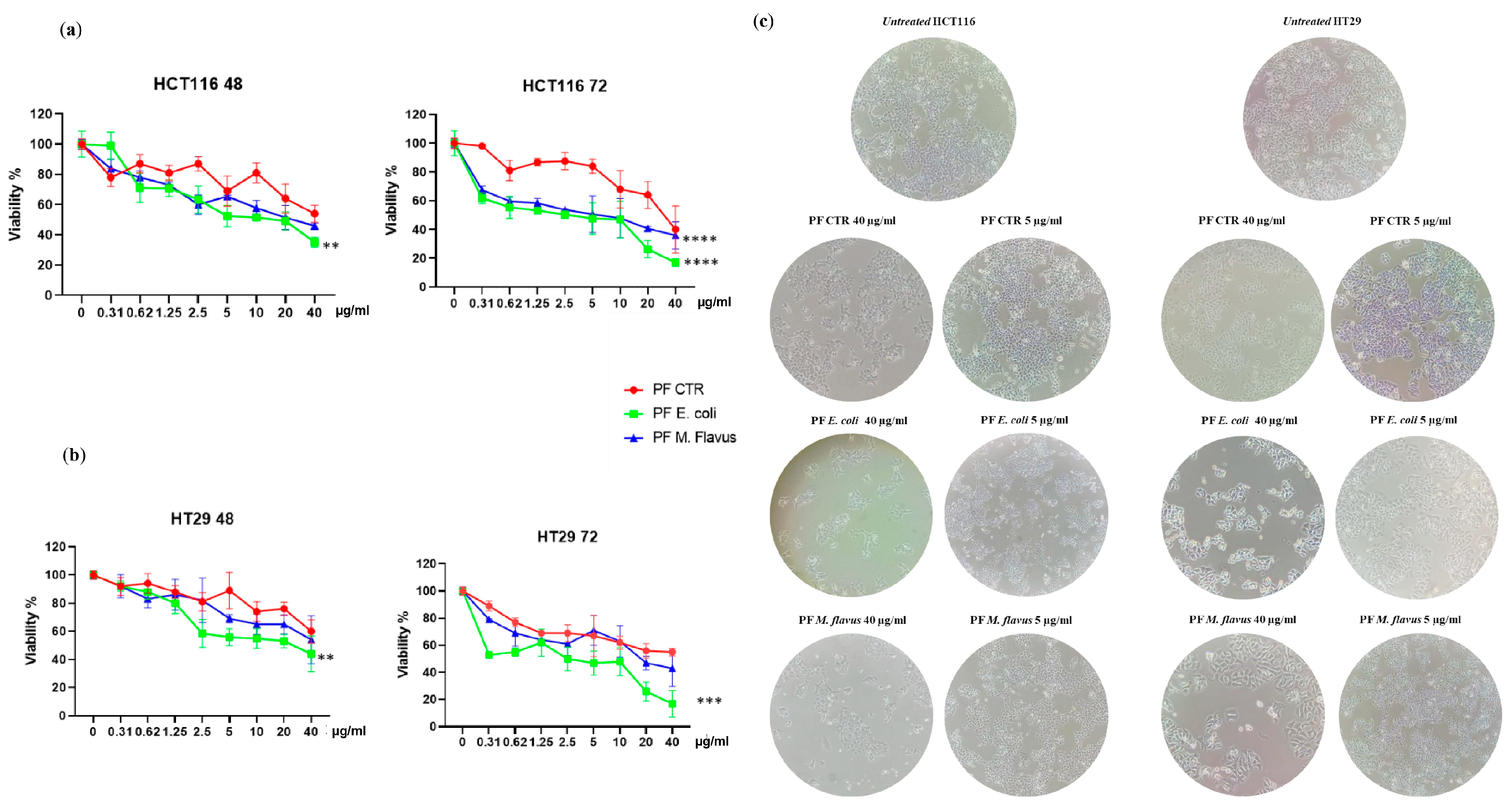
2.1. Inhibitory Effects of H. Illucens-Derived Peptide Fractions on CRC Cells’ Viability
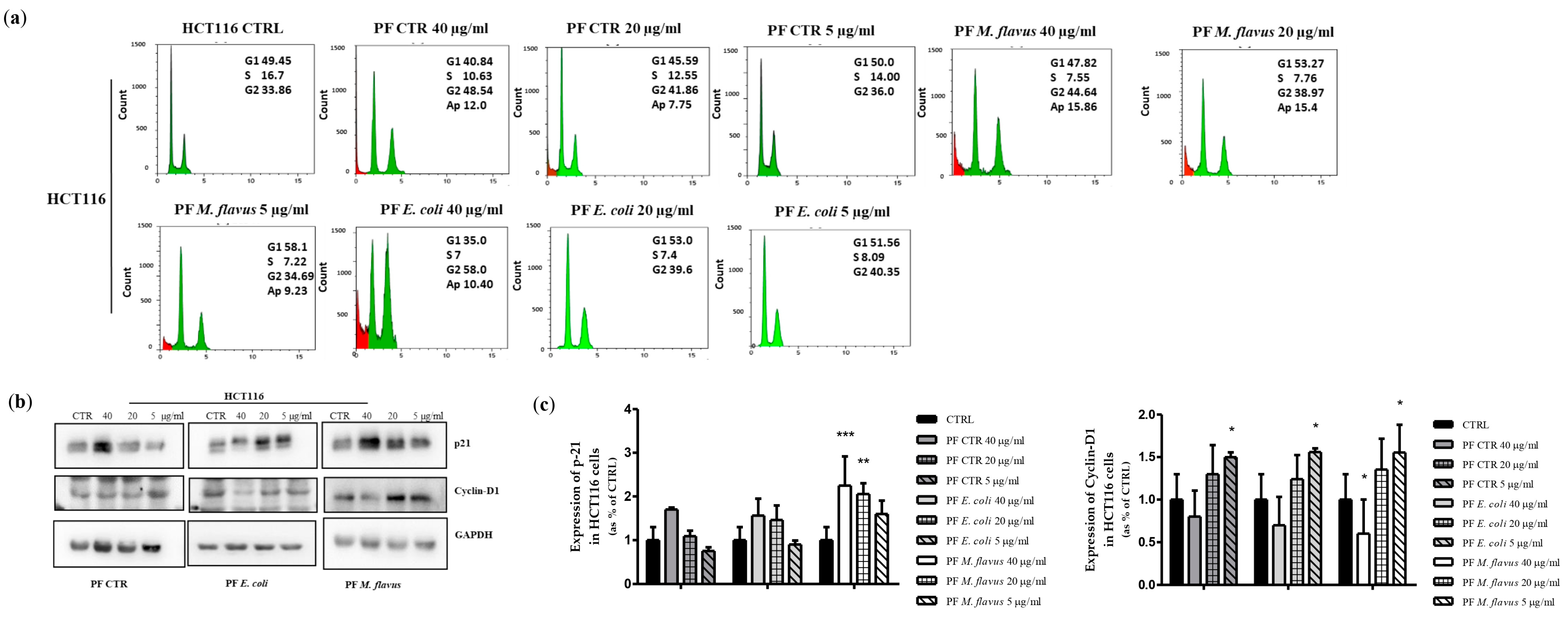
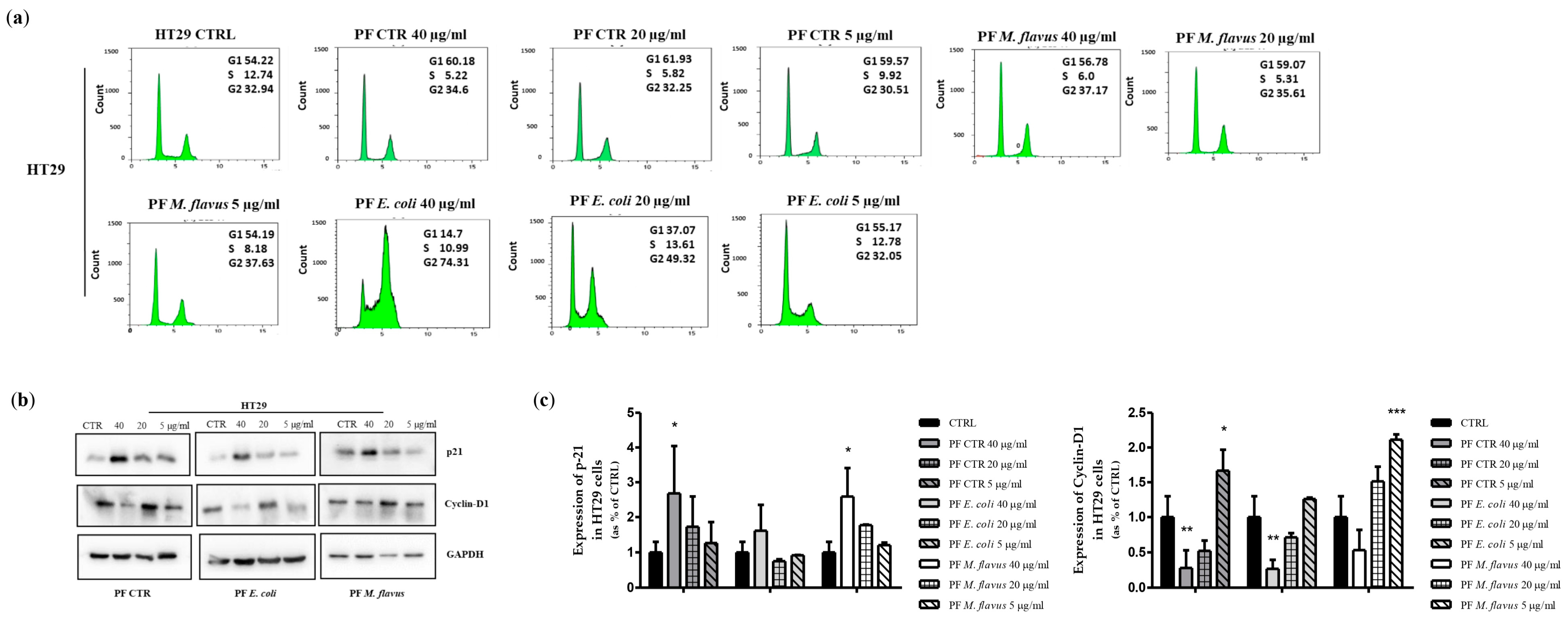
2.2. Peptide Fractions Induced G2/M Phase Arrest and Apoptosis in Human CRC Cells
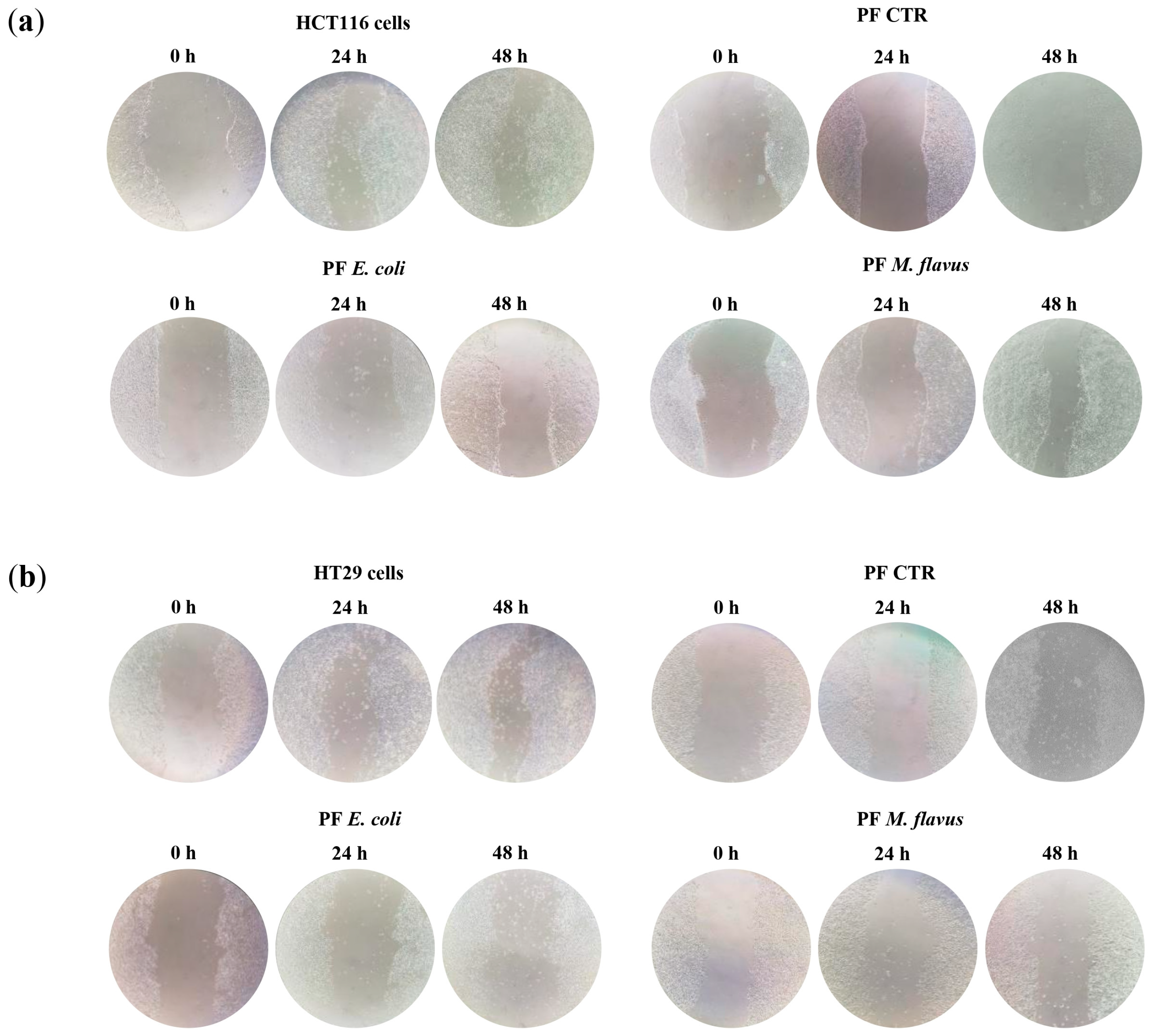
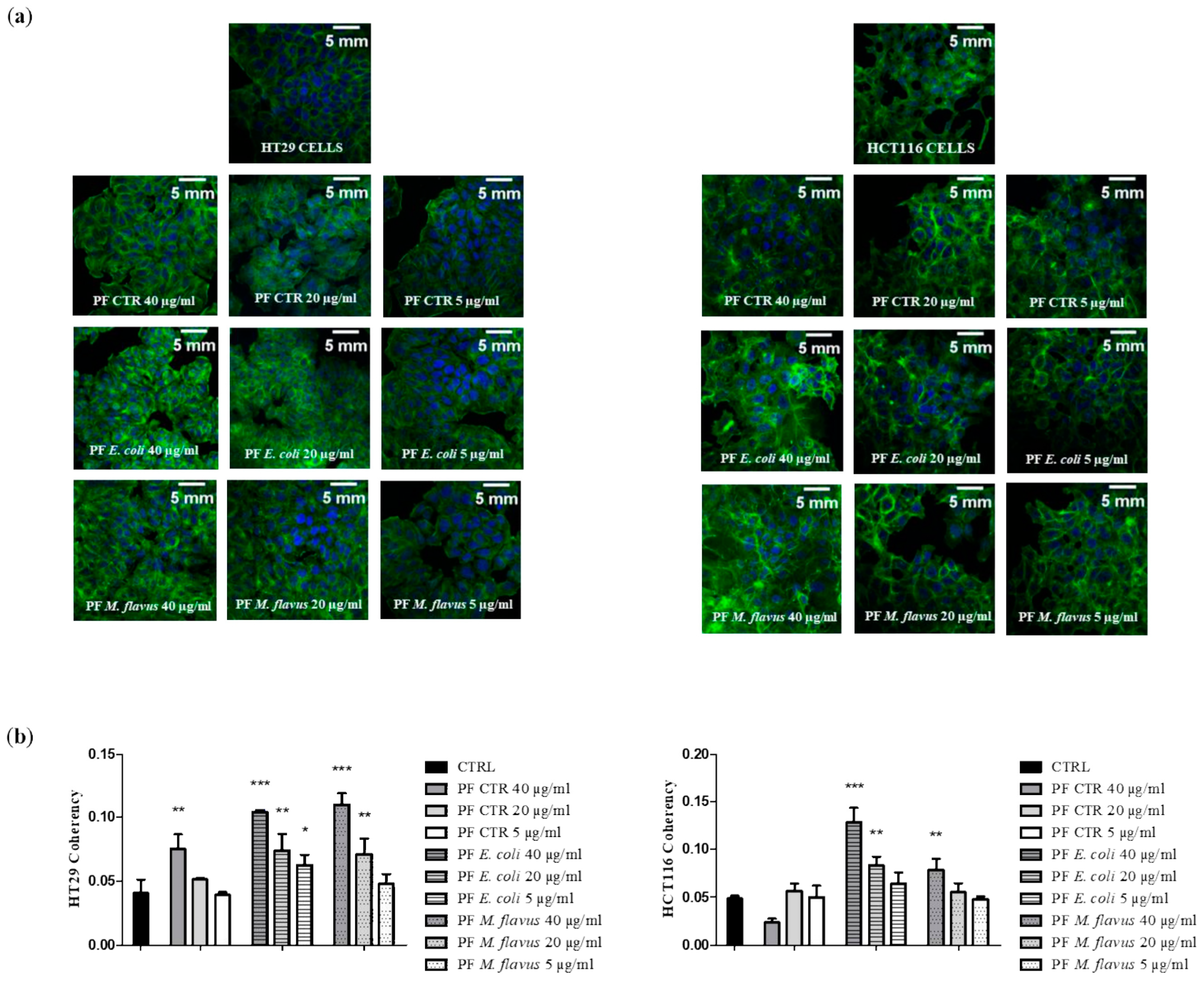
2.3. Peptide Fractions Increased Motility and Affected Cytoskeleton Organization in Human CRC Cells
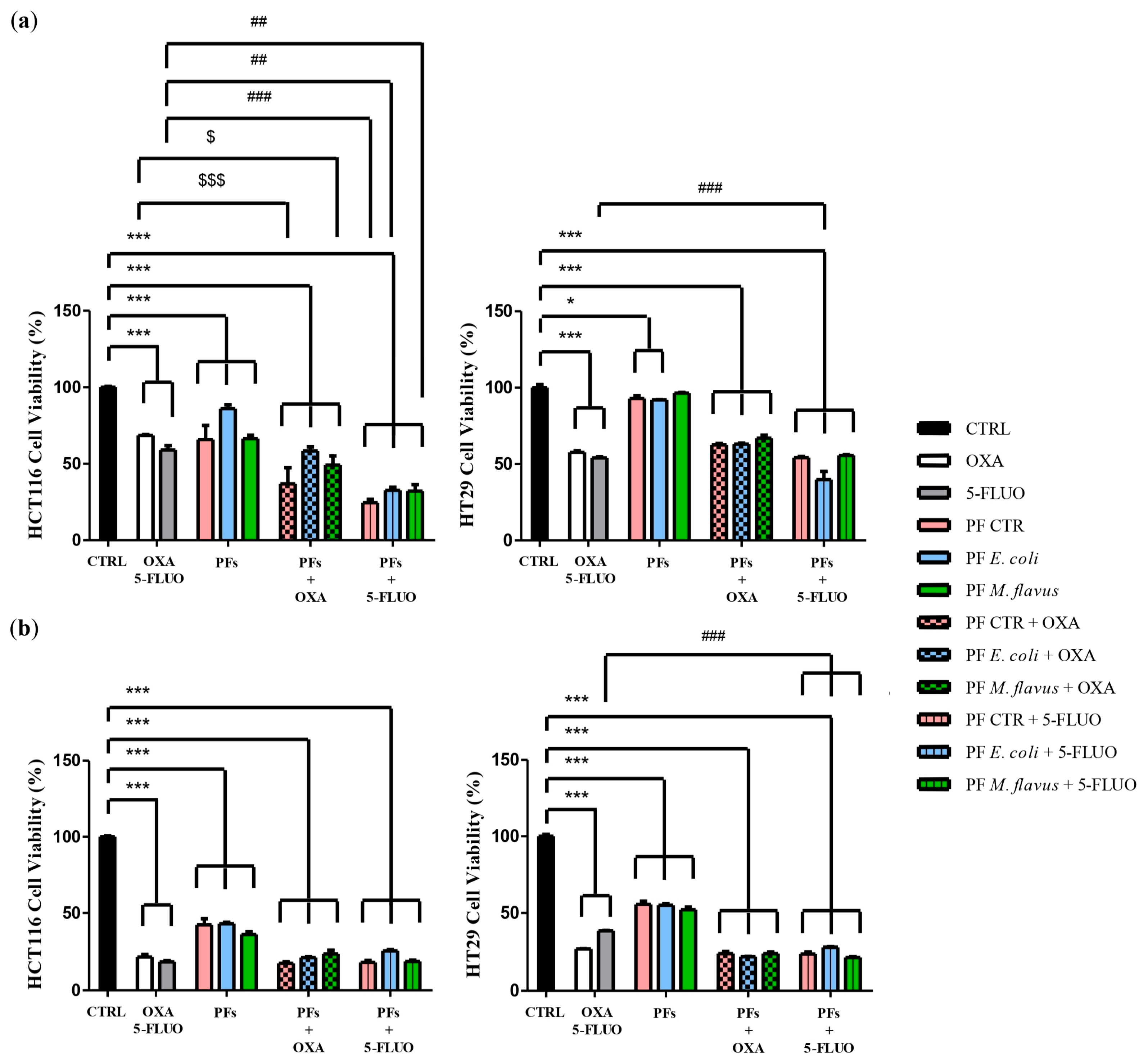
2.4. Peptide Fractions Enhanced Cytotoxicity of Chemotherapeutic Drugs in Human CRC Cells
3. Discussion
4. Materials and Methods
4.1. Infection of H. illucens Larvae, Hemolymph Extraction, and Precipitation by Organic Solvents
4.2. Cell Culture
4.3. Measurement of Cell Viability
4.4. Western Blot Analysis
4.5. Alkaline Phosphatase (AP) Assay
4.6. Apoptosis and Cell Cycle Analysis
4.7. Confocal Microscopy
4.8. Wound-Healing Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.H.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.; Zunino, F. Overview of Tumor Cell Chemoresistance Mechanisms. Explor. Target. Antitumor Ther. 2005, 111, 127–148. [Google Scholar]
- Vasan, N.; Baselga, J.; Hyman, D.M.A. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on Anticancer Activities of Antimicrobial Peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Qin, Y.; Qin, Z.D.; Chen, J.; Cai, C.G.; Li, L.; Feng, L.Y.; Wang, Z.; Duns, G.J.; He, N.Y.; Chen, Z.S.; et al. From Antimicrobial to Anticancer Peptides: The Transformation of Peptides. Recent Pat. Anticancer Drug Discov. 2019, 14, 70–84. [Google Scholar] [CrossRef]
- Gaspar, D.; Salomé Veiga, A.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Lei, C.; Li, W.; Han, J.; Zhang, J.; Zhang, Y. A Sweet Warning: Mucin-Type O-Glycans in Cancer. Cells 2022, 11, 3666. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Leuschner, C.; Hansel, W. Membrane Disrupting Lytic Peptides for Cancer Treatments. Curr. Pharm. Des. 2004, 10, 2299–2310. [Google Scholar] [CrossRef]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Alves, A.C.; Ribeiro, D.; Nunes, C.; Reis, S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2231–2244. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Santa-González, G.A.; Gallego, V. Bioactive cationic peptides as potential agents for breast cancer treatment. Biosci. Rep. 2021, 41, BSR20211218C. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Waseen, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef]
- Chernysh, S.; Kim, S.I.; Bekker, G.; Pleskach, V.A.; Filatova, N.A.; Anikin, V.B.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Salvia, R.; Somma, A.D.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Salvia, R.; Popovic, Z.D.; Sgambato, A.; Falabella, P. Tools in the Era of Multidrug Resistance in Bacteria: Applications for New Antimicrobial Peptides Discovery. Curr. Pharm. Des. 2022, 28, 2854–2866. [Google Scholar] [CrossRef]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 991–1010. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Mancini, I.M.; Canini, D.; Masi, S.; Falabella, P. Mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull. Insectology 2022, 75, 75–82. [Google Scholar]
- Scieuzo, C.; Giglio, F.; Rinaldi, R.; Lekka, M.E.; Cozzolino, F.; Monaco, V.; Monti, M.; Salvia, R.; Falabella, P. In Vitro Evaluation of the Antibacterial Activity of the Peptide Fractions Extracted from the Hemolymph of Hermetia illucens (Diptera: Stratiomyidae). Insects 2023, 14, 464. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Cane, C.; Scieuzo, C.; Salvia, R.; Falabella, P.; Duilio, A. Structural and functional characterization of a novel recombinant antimicrobial peptide from Hermetia illucens. Curr. Issues Mol. Biol. 2022, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Chemotherapy for colorectal cancer in the elderly. World J. Gastroenterol. 2015, 21, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, H.; Yan, D.; Cui, F.; Wang, X.; Yu, F.; Xue, Y.; Feng, X.; Wang, J.; Wang, X.; et al. PAK6 Increase Chemoresistance and Is a Prognostic Marker for Stage II and III Colon Cancer Patients Undergoing 5-FU Based Chemotherapy. Oncotarget 2014, 6, 355–367. [Google Scholar] [CrossRef]
- Punt, C.J.A.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Li, C.; Aidoo, O.F.; Fernando, I.; Haddad, M.A.; Pereira, J.A.M.; Blinov, A.; Golik, A.; Câmara, J.S. Unravelling the potential of insects for medicinal purposes—A comprehensive review. Heliyon 2023, 9, 15938. [Google Scholar] [CrossRef]
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Pucciarelli, V.; Borrelli, L.; Addeo, N.F.; Bovera, F.; Laginestra, A.; Schmitt, E.; Falabella, P. Antimicrobial activity of lipids extracted from Hermetia illucens reared on different substrates. Appl. Microbiol. Biotechnol. 2024, 108, 167. [Google Scholar] [CrossRef]
- Triunfo, M.; Guarnieri, A.; Ianniciello, D.; Coviello, L.; Vitti, A.; Nuzzaci, M.; Salvia, R.; Scieuzo, C.; Falabella, P. Hermetia illucens, an innovative and sustainable source of chitosan-based coating for postharvest preservation of strawberries. iScience 2023, 26, 108576. [Google Scholar] [CrossRef]
- Boschi, A.; Scieuzo, C.; Salvia, R.; Arias, C.F.; Peces Perez, R.; Bertocchini, F.; Falabella, P. Beyond Microbial Biodegradation: Plastic Degradation by Galleria mellonella. J. Polym. Environ. 2023, 32, 2158–2177. [Google Scholar] [CrossRef]
- Tafi, E.; Triunfo, M.; Guarnieri, A.; Ianniciello, D.; Salvia, R.; Scieuzo, C.; Ranieri, A.; Castagna, A.; Lepuri, S.; Hahn, T.; et al. Preliminary investigation on the effect of insect-based chitosan on preservation of coated fresh cherry tomatoes. Sci. Rep. 2023, 13, 7030. [Google Scholar] [CrossRef] [PubMed]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Ianniciello, D.; Scieuzo, C.; Hahn, T.; Zibek, S.; Falabella, P. Usage of chitosan from Hermetia illucens as a preservative for fresh Prunus species fruits: A preliminary analysis. Chem. Biol. Technol. Agric. 2023, 10, 101. [Google Scholar] [CrossRef]
- Salvia, R.; Nardiello, M.; Scieuzo, C.; Scala, A.; Bufo, S.A.; Rao, A.; Vogel, H.; Falabella, P. Novel factors of viral origin inhibit TOR pathway gene expression. Front. Physiol. 2018, 9, 1678. [Google Scholar] [CrossRef]
- Salvia, R.; Grimaldi, A.; Girardello, R.; Scieuzo, C.; Scala, A.; Bufo, S.A.; Vogel, H.; Falabella, P. Aphidius ervi teratocytes release enolase and fatty acid binding protein through exosomal vesicles. Front. Physiol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Salvia, R.; Scieuzo, C.; Grimaldi, A.; Fanti, P.; Moretta, A.; Franco, A.; Varricchio, P.; Bradleigh-Vinson, S.; Falabella, P. Role of ovarian proteins secreted by Toxoneuron nigriceps (Viereck) (hymenoptera, braconidae) in the early suppression of host immune response. Insects 2021, 12, 33. [Google Scholar] [CrossRef]
- Salvia, R.; Cozzolino, F.; Scieuzo, C.; Grimaldi, A.; Franco, A.; Bradleigh-Vinson, S.; Monti, M.; Falabella, P. Identification and Functional Characterization of Toxoneuron nigriceps Ovarian Proteins Involved in the Early Suppression of Host Immune Response. Insects 2022, 13, 144. [Google Scholar] [CrossRef]
- Salvia, R.; Scieuzo, C.; Boschi, A.; Pezzi, M.; Mistri, M.; Munari, C.; Chicca, M.; Vogel, H.; Cozzolino, F.; Monaco, V.; et al. An Overview of Ovarian Calyx Fluid Proteins of Toxoneuron nigriceps (Viereck) (Hymenoptera: Braconidae): An Integrated Transcriptomic and Proteomic Approach. Biomolecules 2023, 13, 1547. [Google Scholar] [CrossRef]
- Slocinska, M.; Marciniak, P.; Rosinski, G. Insects Antiviral and Anticancer Peptides: New Leads for the Future? Protein Pept. Lett. 2008, 15, 578–585. [Google Scholar] [CrossRef]
- Chowanski, S.; Adamski, Z.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Slocinska, M.; Spochacz, M.; Szymczak, M.; Urbanski, A.; Walkowiak-Nowicka, K.; et al. Insect Peptides—Perspectives in Human Diseases Treatment. Curr. Med. Chem. 2017, 24, 3116–3152. [Google Scholar] [CrossRef]
- Mohamed, N.T.; Salem El-Ebiarie, A.; Essam, W. Liquid Chromatography of Hemolymph of Adult Trachyderma philistina (Coleoptera: Tenebrionidae) and Antitumor Effect of Crude Hemolymph Against Different Cell Lines. Eur. J. Mol. Clin. Med. 2020, 7, 1–20. [Google Scholar]
- Büyükkiraz, E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Ishibashi, J.; Tanaka, H.; Sato, M.; Asoka, A.; Taylor, D.; Yamakawa, M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides 2009, 30, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Feng, Q.; Sun, H.; Liao, Y.; Du, L.; Yang, R.; Li, X.; Yang, Y.; Xia, Q. Isolation and Purification of Active Antimicrobial Peptides from Hermetia illucens L., and Its Effects on CNE2. bioRxiv 2018, 35336. [Google Scholar] [CrossRef]
- Nwajiaku, O.L.; de Jong-Hoogland, D.; Ulmschneider, M.B. BPS2025-Design of membrane-lytic peptides for the treatment of individual cancer types. Biophys. J. 2025, 124, 98a–99a. [Google Scholar] [CrossRef]
- Ntwasa, M.; Goto, A.; Kurata, S. Coleopteran antimicrobial peptides: Prospects for clinical applications. Int. J. Microbiol. 2012, 2012, 101989. [Google Scholar] [CrossRef]
- Robles-Fort, A.; García-Robles, I.; Fernando, W.; Hoskin, D.W.; Rausell, C.; Real, M.D. Dual antimicrobial and antiproliferative activity of TcPaSK peptide derived from a Tribolium castaneum insect defensin. Microorganisms 2021, 9, 222. [Google Scholar] [CrossRef]
- Abd El-Aal, A.A.A.; Jayakumar, F.A.; Lahiri, C.; Tan, K.O.; Reginald, K. Novel cationic cryptides in Penaeus vannamei demonstrate antimicrobial and anti-cancer activities. Sci. Rep. 2023, 13, 14673. [Google Scholar] [CrossRef]
- Koszałka, P.; Kamysz, E.; Wejda, M.; Kamysz, W.; Bigda, J. Antitumor Activity of Antimicrobial Peptides against U937. Acta Biochim. Pol. 2011, 58, 111–117. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, X.; Zhang, Q.; Tangthianchaichana, J.; Guo, M.; Du, S.; Lu, Y. Anticancer mechanisms and potential anticancer applications of antimicrobial peptides and their nano agents. Int. J. Nanomed. 2024, 19, 1017–1039. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.M.; Gandhi, S.C.; Wilding, J.L.; Muschel, R.; Bodmer, W.F. Cancer stem cells from colorectal cancer-derived cell lines. Proc. Natl. Acad. Sci. USA 2010, 107, 3722–3727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Babaei, E.; Neri, F.; Feizi, M.A.H. Anti-proliferative and apoptotic effect of gemini curcumin in p53-wild type and p53-mutant colorectal cancer cell lines. Int. J. Pharm. 2021, 601, 120592. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, W.; Lei, S.; Xiong, L.; Zhao, H.; Liang, D.; Lei, Z.; Zhou, N.; Yao, H.; Liang, Y. Wild-type and mutant p53 differentially modulate miR-124/iASPP feedback following photodynamic therapy in human colon cancer cell line. Cell Death Dis. 2017, 8, e3096. [Google Scholar] [CrossRef]
- Jin, X.; Mei, H.; Li, X.; Ma, Y.; Zeng, A.; Wang, Y.; Lu, X.; Chu, F.; Wu, Q.; Zhu, J. Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim. Biophys. Sin. 2010, 42, 259–265. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, I.; Kim, S.; Yun, E.; Nam, S.; Ahn, M.; Kang, D.; Hwang, J.S. Anticancer activity of CopA3 dimer peptide in human gastric cancer cells. BMB Rep. 2015, 48, 324–329. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, H.; Nam, S.; Yun, E.; Kim, S.; Ahn, M.; Chang, J.S.; Hwang, J.S. CopA3 peptide from Copris tripartitus induces apoptosis in human leukemia cells via a caspase-independent pathway. BMB Rep. 2012, 45, 85–90. [Google Scholar] [CrossRef]
- Li, H.; Niu, J.; Wang, X.; Niu, M.; Liao, C. The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances. Pharmaceutics 2023, 15, 2278. [Google Scholar] [CrossRef]
- Ruan, Y.; Shen, T.; Wang, Y.; Hou, M.; Li, J.; Sun, T. Antimicrobial peptide LL-37 attenuates LTA induced inflam-matory effect in macrophages. Int. Immunopharmacol. 2013, 15, 575–580. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Sabe-rian, E.; Jenča, A.; Ghanipour-Meybodi, R.; Jenča, A.; Petrášová, A.; Jenčová, J. From defense to offense: Antimicrobial peptides as promising therapeutics for cancer. Front. Oncol. 2024, 14, 1463088. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Yamazaki, T.; He, T.; Alam, M.M.; Liu, J.; Trivett, A.L.; Sveinbjørnsson, B.; Rekdal, Ø.; Galluzzi, L.; Oppenheim, J.J.; et al. LTX-315 triggers anticancer immunity by inducing MyD88-dependent maturation of dendritic cells. Front. Immunol. 2024, 15, 1332922. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Vishwanatha, J.K. Role of Anti-Cancer Peptides as Immunomodu-latory Agents: Potential and Design Strategy. Pharmaceutics 2022, 14, 2686. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.; Rumble, R.B.; Maroun, J. Role of oxaliplatin combined with 5-fluorouracil and folinic acid in the first- and second-line treatment of advanced colorectal cancer. Curr. Oncol. 2006, 13, 173–184. [Google Scholar] [CrossRef]
- Aghamiri, S.; Zandsalimi, F.; Raee, P.; Abdollahifar, M.A.; Tan, S.C.; Low, T.Y.; Najafi, S.; Ashrafizadeh, M.; Zarrabi, A.; Ghanbarian, H.; et al. Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol. Res. 2021, 171, 105777. [Google Scholar] [CrossRef]
- Bakare, O.O.; Gokul, A.; Wu, R.; Niekerk, L.-A.; Klein, A.; Keyster, M. Biomedical Relevance of Novel Anticancer Peptides in the Sensitive Treatment of Cancer. Biomolecules 2021, 11, 1120. [Google Scholar] [CrossRef]
- Bauso, L.V.; La Fauci, V.; Munaò, S.; Bonfiglio, D.; Armeli, A.; Maimone, N.; Longo, C.; Calabrese, G. Biological Activity of Natural and Synthetic Peptides as Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 7264. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Maiti, T.K. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008, 26, 210–217. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.L.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef]
- Chinnadurai, R.K.; Khan, N.; Meghwanshi, G.K.; Ponne, S.; Althobiti, M.; Kumar, R. Current research status of anti-cancer peptides: Mechanism of action, production, and clinical applications. Biomed. Pharmacother. 2023, 164, 114996. [Google Scholar] [CrossRef]
- Ghaly, G.; Tallima, H.; Dabbish, E.; Badr ElDin, N.; Abd El-Rahman, M.K.; Ibrahim, M.A.A.; Shoeib, T. Anti-Cancer Peptides: Status and Future Prospects. Molecules 2023, 28, 1148. [Google Scholar] [CrossRef] [PubMed]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides–challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.R.; Yan, J.X.; Zhang, B.Z.; Song, J.J.; Jia, P.F.; Wang, R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 2009, 278, 65–72. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014, 351, 13–22. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, L.; Yu, L.; Huang, J.; Huang, S.; Yao, Y. A review for antimicrobial peptides with anticancer properties: Re-purposing of potential anticancer agents. BIO Integr. 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Parchebafi, A.; Tamanaee, F.; Ehteram, H.; Ahmad, E.; Nikzad, H.; Kashani, H.H. The dual interaction of antimicrobial peptides on bacteria and cancer cells; mechanism of action and therapeutic strategies of nanostructures. Microb. Cell Factories 2022, 21, 118. [Google Scholar] [CrossRef]
- Piktel, E.; Wnorowska, U.; Gorbacz-Konończuk, J.; Sienkiewicz, J.; Głuszek, K.; Okła, S.; Bucki, R. From antimicrobial to anticancer: Unraveling the potential of pleurocidin and pleurocidin-derived peptides in the treatment of cancers. Front. Pharmacol. 2024, 15, 1340029. [Google Scholar] [CrossRef]
- Sood, A.; Jothiswaran, V.V.; Singh, A.; Sharma, A. Anticancer peptides as novel immunomodulatory therapeutic candidates for cancer treatment. Explor. Target. Anti-Tumor Ther. 2024, 5, 1074. [Google Scholar] [CrossRef]
- Tonk, M.; Vilcinskas, A.; Rahnamaeian, M. Insect antimicrobial peptides: Potential tools for the prevention of skin cancer. Appl. Microbiol. Biotechnol. 2016, 100, 7397–7405. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucchetti, D.; Rinaldi, R.; Artemi, G.; Salvia, R.; De Stefano, F.; Scieuzo, C.; Falabella, P.; Sgambato, A. Peptide Fractions Extracted from the Hemolymph of Hermetia illucens Inhibit Growth and Motility and Enhance the Effects of Traditional Chemotherapeutics in Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2025, 26, 1891. https://doi.org/10.3390/ijms26051891
Lucchetti D, Rinaldi R, Artemi G, Salvia R, De Stefano F, Scieuzo C, Falabella P, Sgambato A. Peptide Fractions Extracted from the Hemolymph of Hermetia illucens Inhibit Growth and Motility and Enhance the Effects of Traditional Chemotherapeutics in Human Colorectal Cancer Cells. International Journal of Molecular Sciences. 2025; 26(5):1891. https://doi.org/10.3390/ijms26051891
Chicago/Turabian StyleLucchetti, Donatella, Roberta Rinaldi, Giulia Artemi, Rosanna Salvia, Federica De Stefano, Carmen Scieuzo, Patrizia Falabella, and Alessandro Sgambato. 2025. "Peptide Fractions Extracted from the Hemolymph of Hermetia illucens Inhibit Growth and Motility and Enhance the Effects of Traditional Chemotherapeutics in Human Colorectal Cancer Cells" International Journal of Molecular Sciences 26, no. 5: 1891. https://doi.org/10.3390/ijms26051891
APA StyleLucchetti, D., Rinaldi, R., Artemi, G., Salvia, R., De Stefano, F., Scieuzo, C., Falabella, P., & Sgambato, A. (2025). Peptide Fractions Extracted from the Hemolymph of Hermetia illucens Inhibit Growth and Motility and Enhance the Effects of Traditional Chemotherapeutics in Human Colorectal Cancer Cells. International Journal of Molecular Sciences, 26(5), 1891. https://doi.org/10.3390/ijms26051891







