Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress
Abstract
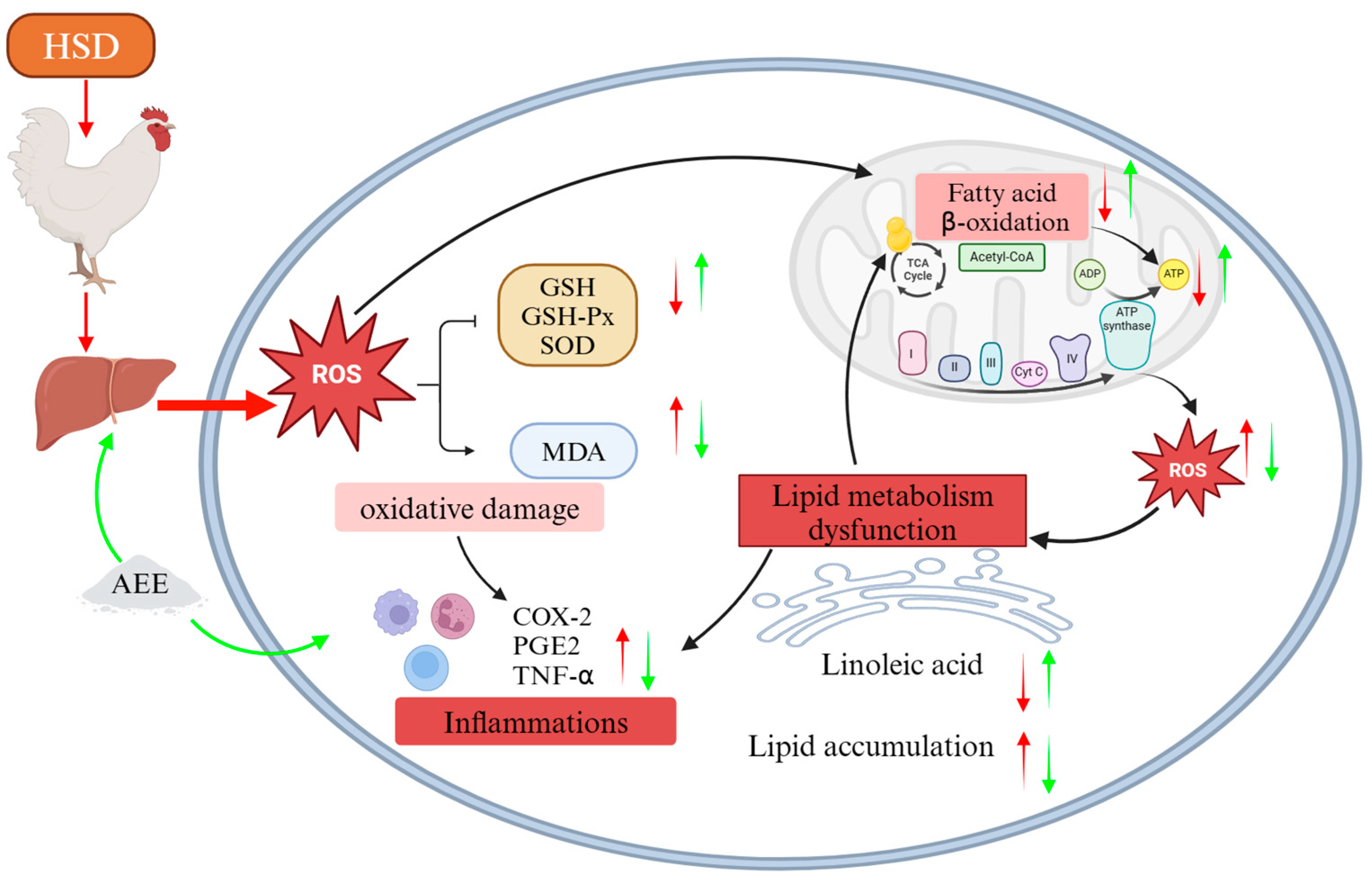
1. Introduction
2. Results
2.1. Effects of AEE on Growth Performance of HSD Broilers
2.2. Effects of AEE on Antioxidant Function in Liver from HSD Broilers
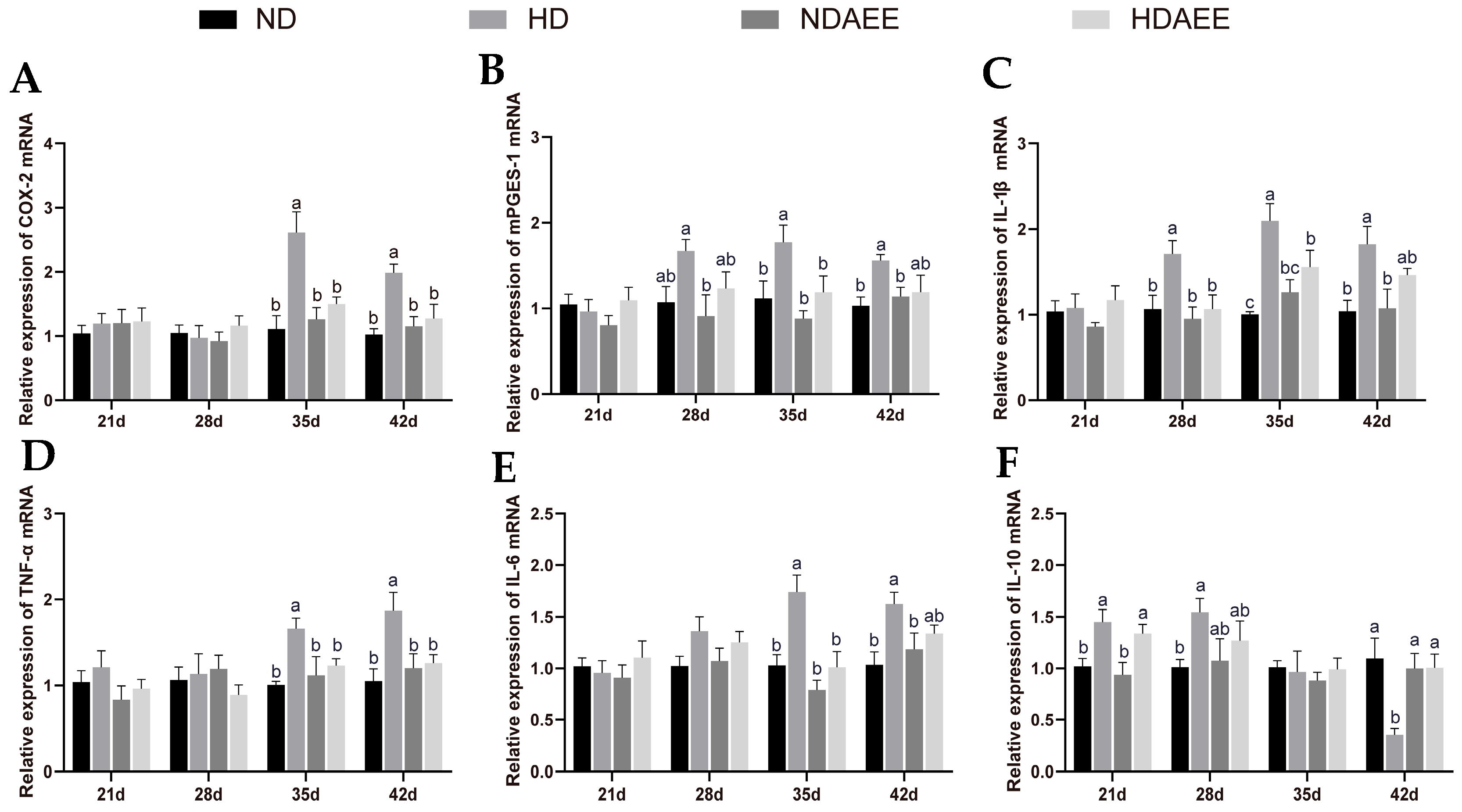
2.3. Effects of AEE on Inflammatory Gene Expression in Liver from HSD Broilers
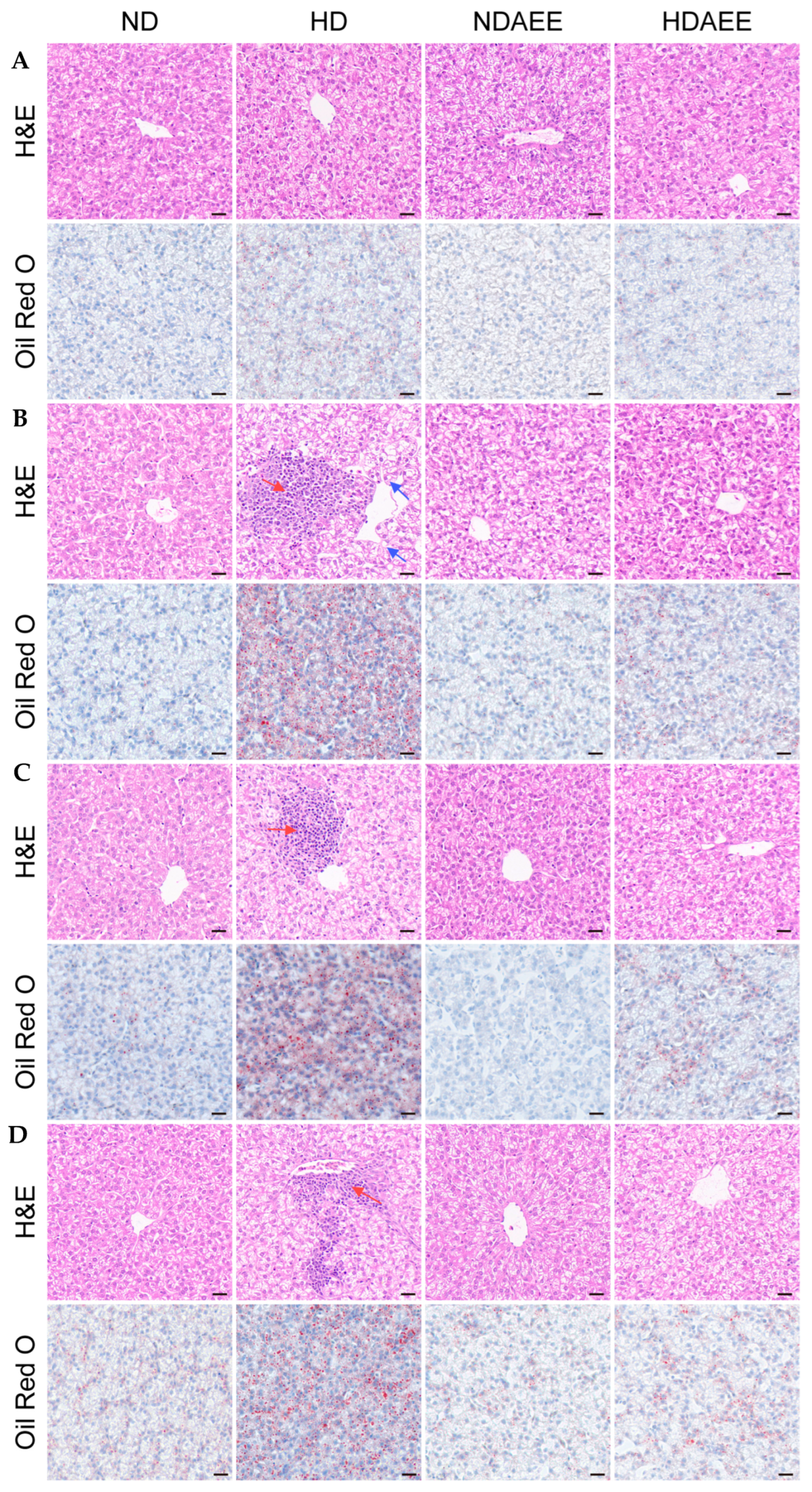
2.4. Effects of AEE on Pathological Liver Injury in HSD Broilers
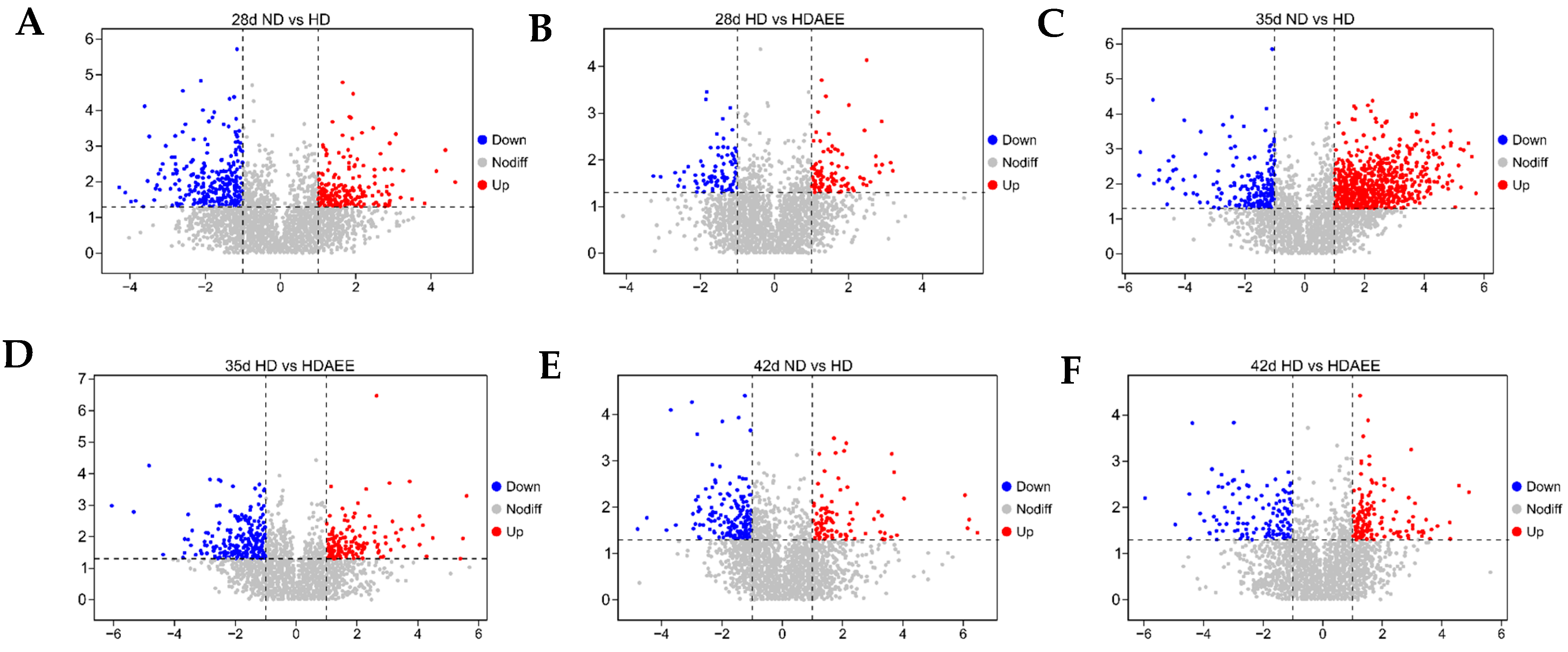
2.5. Identification of Differentially Expressed Metabolites
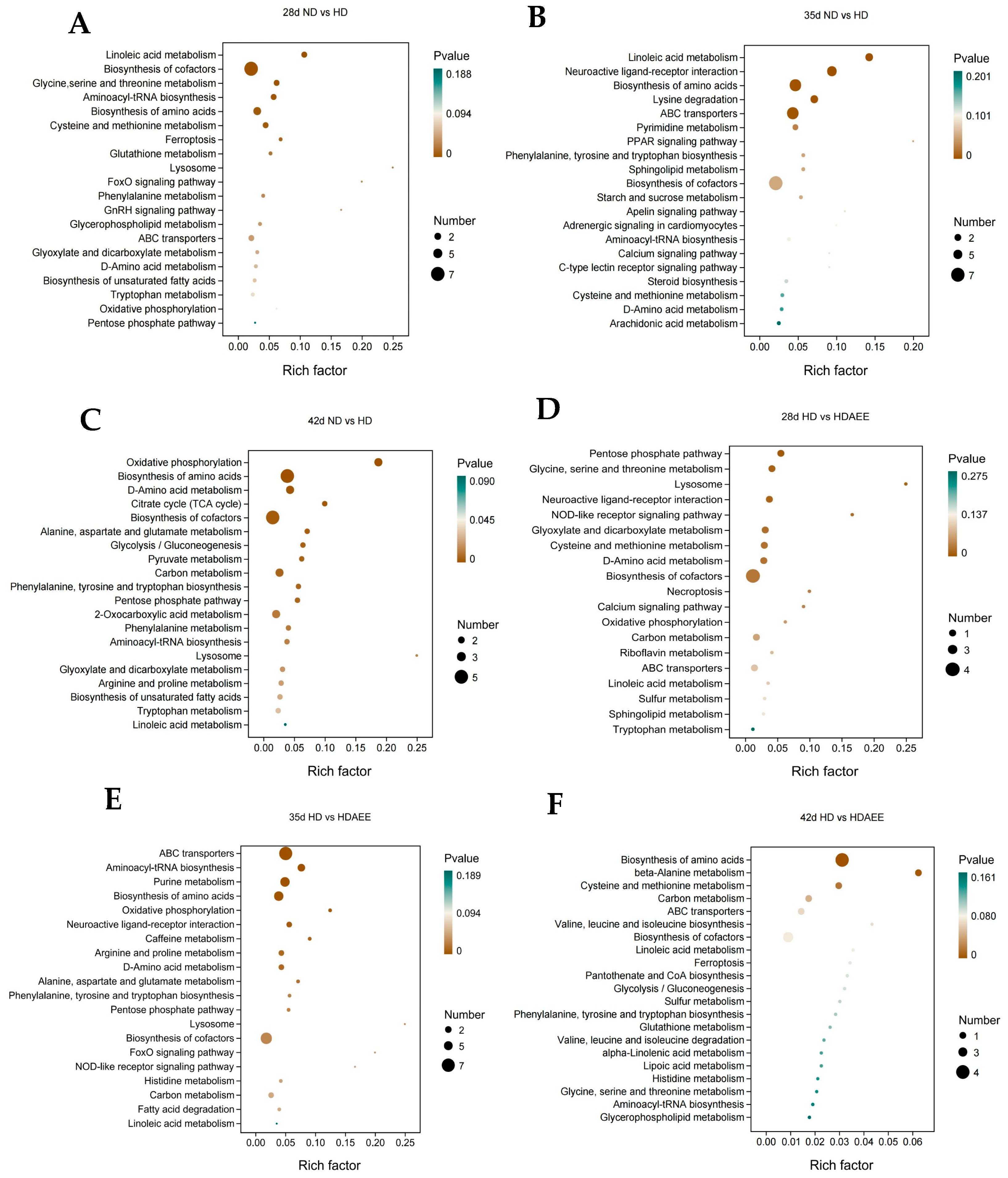
2.6. Pathway Analysis of Differential Metabolites
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Growth Performance
4.3. Sample Collection
4.4. Examination of Liver Histology
4.5. Detection of Oxidative Damage Indicators
4.6. Real-Time Quantitative PCR (RT-qPCR) Analysis of Liver mRNA Expression Levels
4.7. Liver Metabolomics
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, H.N.; Heins, B.J. Effects of Outdoor Stocking Density on Growth, Feather Damage and Behavior of Slow-Growing Free-Range Broilers. Animals 2021, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Yin, D.; Mahmood, T.; Yuan, J. Transcriptome analysis reveals a molecular understanding of nicotinamide and butyrate sodium on meat quality of broilers under high stocking density. BMC Genom. 2020, 21, 412. [Google Scholar] [CrossRef] [PubMed]
- Estevez, I. Density allowances for broilers: Where to set the limits? Poult. Sci. 2007, 86, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Kim, H.-J.; Hong, E.-C.; Kang, H.-K.J.A. Effects of stocking density on growth performance, antioxidant status, and meat quality of finisher broiler chickens under high temperature. Antioxidants 2022, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- European Union. Council directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union 2007, 182, 19–28. [Google Scholar]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.F.; Alkhedaide, A.Q.; Ramadan, A.A.I.; Hafez, A.S.E.; Hussein, M.A. Potential impact of stocking density on growth, carcass traits, indicators of biochemical and oxidative stress and meat quality of different broiler breeds. Poult. Sci. 2021, 100, 101442. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Bai, D.; Li, Y.; He, X.; Ito, K.; Liu, K.; Tan, H.; Zhen, W.; Zhang, B.; et al. Dietary Supplementation with Chlorogenic Acid Enhances Antioxidant Capacity, Which Promotes Growth, Jejunum Barrier Function, and Cecum Microbiota in Broilers under High Stocking Density Stress. Animals 2023, 13, 303. [Google Scholar] [CrossRef]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef]
- Dai, F.; Du, Y.T.; Zheng, Y.L.; Zhou, B. Inhibiting NF-κB-Mediated Inflammation by Catechol-Type Diphenylbutadiene via an Intracellular Copper- and Iron-Dependent Pro-Oxidative Role. J. Agric. Food Chem. 2020, 68, 10029–10035. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid. Med. Cell Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.L. Neuroendocrine-immune system interactions and autoimmunity. Annu. Rev. Immunol. 1995, 13, 307–338. [Google Scholar] [CrossRef]
- Bureau, C.; Hennequet-Antier, C.; Couty, M.; Guémené, D. Gene array analysis of adrenal glands in broiler chickens following ACTH treatment. BMC Genom. 2009, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Wang, J.; Li, K.; Heng, C.; Jiang, L.; Cai, C.; Zhan, X. Effects of different stocking densities on tracheal barrier function and its metabolic changes in finishing broilers. Poult. Sci. 2020, 99, 6307–6316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, S.H.; Li, J.Y. The Protective Effect of Aspirin Eugenol Ester on Paraquat-Induced Acute Liver Injury Rats. Front. Med. 2020, 7, 589011. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.R.; Liu, X.W.; Li, S.H.; Qin, Z.; Bai, L.X.; Ge, W.B.; Li, J.Y.; Yang, Y.J. Untargeted lipidomics and metagenomics reveal the mechanism of aspirin eugenol ester relieving hyperlipidemia in ApoE-/- mice. Front. Nutr. 2022, 9, 1030528. [Google Scholar] [CrossRef]
- Ma, N.; Yang, Y.; Liu, X.; Li, S.; Qin, Z.; Li, J. Plasma metabonomics and proteomics studies on the anti-thrombosis mechanism of aspirin eugenol ester in rat tail thrombosis model. J. Proteom. 2020, 215, 103631. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Z.D.; Qin, Z.; Liu, X.W.; Li, S.H.; Bai, L.X.; Ge, W.B.; Li, J.Y.; Yang, Y.J. Aspirin eugenol ester alleviates lipopolysaccharide-induced acute lung injury in rats while stabilizing serum metabolites levels. Front. Immunol. 2022, 13, 939106. [Google Scholar] [CrossRef]
- Huang, M.Z.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. Br. J. Pharmacol. 2019, 176, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Bai, D.; Zhong, J.; Hu, X.; Zhang, R.; Zhen, W.; Ito, K.; Zhang, B.; Yang, Y.; et al. Effect of dietary aspirin eugenol ester on the growth performance, antioxidant capacity, intestinal inflammation, and cecal microbiota of broilers under high stocking density. Poult. Sci. 2024, 103, 103825. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhen, W.; Bai, D.; Hu, X.; Zhang, H.; Zhang, R.; Ito, K.; Zhang, Y.; Zhang, B.; Ma, Y. Effects of Aspirin Eugenol Ester on Liver Oxidative Damage and Energy Metabolism in Immune-Stressed Broilers. Antioxidants 2024, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, Ö.; Köksal, B.H.; Tatlı, O.; Sevim, Ö.; Ahsan, U.; Üner, A.G.; Ulutaş, P.A.; Beyaz, D.; Büyükyörük, S.; Yakan, A.; et al. Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers. Poult. Sci. 2015, 94, 2395–2403. [Google Scholar] [CrossRef]
- Insawake, K.; Songserm, T.; Songserm, O.; Plaiboon, A.; Homwong, N.; Adeyemi, K.D.; Rassmidatta, K.; Ruangpanit, Y. Effects of isoquinoline alkaloids as an alternative to antibiotic on oxidative stress, inflammatory status, and cecal microbiome of broilers under high stocking density. Poult. Sci. 2025, 104, 104671. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, X.; Li, X.; Yang, Y.; Zhang, J. Genotoxic evaluation of aspirin eugenol ester using the Ames test and the mouse bone marrow micronucleus assay. Food Chem. Toxicol. 2013, 62, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.; Yang, Y.; Liu, X.; Zhang, J.; Li, B.; Zhou, X.; Niu, J.; Wei, X.; Liu, Z. A 15-day oral dose toxicity study of aspirin eugenol ester in Wistar rats. Food Chem. Toxicol. 2012, 50, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, X.; Yang, Y.; Li, J.; Ma, N.; Li, B. In vivo and in vitro metabolism of aspirin eugenol ester in dog by liquid chromatography tandem mass spectrometry. Biomed. Chromatogr. 2015, 29, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Feddes, J.J.; Emmanuel, E.J.; Zuidhoft, M.J. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 2002, 81, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.M.; El-Sonousy, N.K.; Arafa, A.S.; Sallam, M.G.; Ateya, A.; Abdelhady, A.Y. Oregano essential oil and Bacillus subtilis role in enhancing broiler’s growth, stress indicators, intestinal integrity, and gene expression under high stocking density. Sci. Rep. 2024, 14, 25411. [Google Scholar] [CrossRef]
- Xiao, C.; Zhu, Q.; Comer, L.; Pan, X.; Everaert, N.; Schroyen, M.; Song, B.; Song, Z. Dietary 25-hydroxy-cholecalciferol and additional vitamin E improve bone development and antioxidant capacity in high-density stocking broilers. J. Anim. Sci. 2023, 101, skad369. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.; Su, G.; Kestin, S.C. Effects of age and stocking density on leg weakness in broiler chickens. Poult. Sci. 2000, 79, 864–870. [Google Scholar] [CrossRef]
- Kridtayopas, C.; Rakangtong, C.; Bunchasak, C.; Loongyai, W. Effect of prebiotic and synbiotic supplementation in diet on growth performance, small intestinal morphology, stress, and bacterial population under high stocking density condition of broiler chickens. Poult. Sci. 2019, 98, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Qian, B.; Cai, T.; Chen, Y.; Li, T.; Cheng, Y.; Wu, Z.; Liu, C.; Ye, M.; Du, Y.; et al. Aloin Attenuates Oxidative Stress, Inflammation, and CCl(4)-Induced Liver Fibrosis in Mice: Possible Role of TGF-β/Smad Signaling. J. Agric. Food Chem. 2023, 71, 19475–19487. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zeng, J.; Chen, L.; Wang, M.; He, S.; Muhmood, A.; Chen, X.; Huang, K.; Gan, F. Farnesoid X Receptor Plays a Key Role in Ochratoxin A-Induced Nephrotoxicity by Targeting Ferroptosis In Vivo and In Vitro. J. Agric. Food Chem. 2023, 71, 14365–14378. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Shah, R.; Muriel, P. Oxidative Stress and Inflammation in Hepatic Diseases: Current and Future Therapy. Oxid. Med. Cell Longev. 2017, 2017, 3140673. [Google Scholar] [CrossRef]
- Larsson, K.; Kock, A.; Idborg, H.; Arsenian Henriksson, M.; Martinsson, T.; Johnsen, J.I.; Korotkova, M.; Kogner, P.; Jakobsson, P.J. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc. Natl. Acad. Sci. USA 2015, 112, 8070–8075. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Borchert, G.L.; Surazynski, A.; Phang, J.M. Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene 2008, 27, 6729–6737. [Google Scholar] [CrossRef]
- Dozier, W.A., 3rd; Thaxton, J.P.; Purswell, J.L.; Olanrewaju, H.A.; Branton, S.L.; Roush, W.B. Stocking density effects on male broilers grown to 1.8 kilograms of body weight. Poult. Sci. 2006, 85, 344–351. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity. 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Xu, P.B.; Lou, J.S.; Ren, Y.; Miao, C.H.; Deng, X.M. Gene expression profiling reveals the defining features of monocytes from septic patients with compensatory anti-inflammatory response syndrome. J. Infect. 2012, 65, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Simsek, U.; Cerci, I.; Dalkilic, B.; Yilmaz, O.; Ciftci, M. Impact of stocking density and feeding regimen on broilers: Chicken meat composition, fatty acids, and serum cholesterol levels. J. Appl. Poult. Res. 2009, 18, 514–520. [Google Scholar] [CrossRef]
- Ioannou, G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2016, 27, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Watford, M.; Wu, G. Protein. Adv. Nutr. 2018, 9, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Luo, L.; Bian, X.; Liu, R.H.; Zhao, S.; Chen, Y.; Sun, K.; Jiang, J.; Liu, Z.; Zeng, L. Pu-erh Tea Restored Circadian Rhythm Disruption by Regulating Tryptophan Metabolism. J. Agric. Food Chem. 2022, 70, 5610–5623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, J.M.; Zhang, L.S.; Zhang, Y.R.; Cai, S.M.; Yu, J.H.; Xia, Z.F. Effects of tryptophan supplementation on growth performance, antioxidative activity, and meat quality of ducks under high stocking density. Poult. Sci. 2015, 94, 1894–1901. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Ducker, G.S.; Billingham, L.K.; Martinez, C.A.; Mainolfi, N.; Suri, V.; Friedman, A.; Manfredi, M.G.; Weinberg, S.E.; Rabinowitz, J.D.; et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metab. 2019, 29, 1003–1011.e4. [Google Scholar] [CrossRef]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef]
- Miller, C.G.; Holmgren, A.; Arnér, E.S.J.; Schmidt, E.E. NADPH-dependent and -independent disulfide reductase systems. Free Radic. Biol. Med. 2018, 127, 248–261. [Google Scholar] [CrossRef]
- Liu, N.; Si, X.; Ji, Y.; Yang, Q.; Bai, J.; He, Y.; Jia, H.; Song, Z.; Chen, J.; Yang, L.; et al. l-Proline improves the cytoplasmic maturation of mouse oocyte by regulating glutathione-related redox homeostasis. Theriogenology 2023, 195, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shende, V.V.; Bauman, K.D.; Moore, B.S. The shikimate pathway: Gateway to metabolic diversity. Nat. Prod. Rep. 2024, 41, 604–648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Tan, Y.Q.; Chen, Z.H.; Zhao, W.; Liu, T. Adenosine triphosphate can act as a determinant of lysine acetylation of non-native and native substrates. Microbiol. Res. 2023, 268, 127296. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, A.; Delezie, E.; Parmentier, H.K.; Buyse, J.; Everaert, N. Limited evidence for trans-generational effects of maternal dietary supplementation with ω-3 fatty acids on immunity in broiler chickens. Vet. J. 2015, 203, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Kishawy, A.T.; Amer, S.A.; Abd El-Hack, M.E.; Saadeldin, I.M.; Swelum, A.A. The impact of dietary linseed oil and pomegranate peel extract on broiler growth, carcass traits, serum lipid profile, and meat fatty acid, phenol, and flavonoid contents. Asian-Australas. J. Anim. Sci. 2019, 32, 1161–1171. [Google Scholar] [CrossRef]
- Qi, K.K.; Chen, J.L.; Zhao, G.P.; Zheng, M.Q.; Wen, J. Effect of dietary omega6/omega3 on growth performance, carcass traits, meat quality and fatty acid profiles of Beijing-you chicken. J. Anim. Physiol. Anim. Nutr. 2010, 94, 474–485. [Google Scholar]
- Couëdelo, L.; Buaud, B.; Abrous, H.; Chamekh-Coelho, I.; Majou, D.; Boué-Vaysse, C. Effect of increased levels of dietary α-linolenic acid on the n-3 PUFA bioavailability and oxidative stress in rat. Br. J. Nutr. 2022, 127, 1320–1333. [Google Scholar] [CrossRef]
- Andres Contreras, G.; De Koster, J.; de Souza, J.; Laguna, J.; Mavangira, V.; Nelli, R.K.; Gandy, J.; Lock, A.L.; Sordillo, L.M. Lipolysis modulates the biosynthesis of inflammatory lipid mediators derived from linoleic acid in adipose tissue of periparturient dairy cows. J. Dairy. Sci. 2020, 103, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Shimamura, A.; Ishikawa, T.; Fujiwara, Y.; Ichi, I. FADS2 inhibition in essential fatty acid deficiency induces hepatic lipid accumulation via impairment of very low-density lipoprotein (VLDL) secretion. Biochem. Biophys. Res. Commun. 2018, 496, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X. The Effects of a Low Linoleic Acid/α-Linolenic Acid Ratio on Lipid Metabolism and Endogenous Fatty Acid Distribution in Obese Mice. Int. J. Mol. Sci. 2023, 24, 12117. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Heine, K.B.; Hood, W.R. Mitochondrial behaviour, morphology, and animal performance. Biol. Rev. Camb. Philos. Soc. 2020, 95, 730–737. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Indo, H.P.; Davidson, M.; Yen, H.C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef] [PubMed]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef]
- Koo, B.; Choi, J.; Holanda, D.M.; Yang, C.; Nyachoti, C.M. Comparative effects of dietary methionine and cysteine supplementation on redox status and intestinal integrity in immunologically challenged-weaned pigs. Amino Acids 2023, 55, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Nur Mahendra, M.Y.; Kamaludeen, J.; Pertiwi, H. Omega-6: Its Pharmacology, Effect on the Broiler Production, and Health. Vet. Med. Int. 2023, 2023, 3220344. [Google Scholar] [CrossRef]
- Farstad, M.; Berge, R. On the capacity of the beta-oxidation of palmitate and palmitoyl-esters in rat liver mitochondria. Acta Physiol. Scand. 1978, 104, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Glass-Marmor, L.; Morgenstern, H.; Beitner, R. Calmodulin antagonists decrease glucose 1,6-bisphosphate, fructose 1,6-bisphosphate, ATP and viability of melanoma cells. Eur. J. Pharmacol. 1996, 313, 265–271. [Google Scholar] [CrossRef]
- Jing, J.; Zeng, H.; Shao, Q.; Tang, J.; Wang, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; et al. Selenomethionine alleviates environmental heat stress induced hepatic lipid accumulation and glycogen infiltration of broilers via maintaining mitochondrial and endoplasmic reticulum homeostasis. Redox Biol. 2023, 67, 102912. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Han, M.; Wu, Y.; Dong, Y.; Miao, Z.; Zhang, J.; Song, X.; Jia, R.; Su, Y.; Liu, C.; et al. Dietary Supplemental Chromium Yeast Improved the Antioxidant Capacity, Immunity and Liver Health in Broilers under High Stocking Density. Animals 2022, 12, 2216. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; Ribeiro de Araujo, D.; Padula, C. Validation of a HPLC-UV method for the quantification of budesonide in skin layers. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2021, 1164, 122512. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Edmands, W.M.; Barupal, D.K.; Scalbert, A. MetMSLine: An automated and fully integrated pipeline for rapid processing of high-resolution LC-MS metabolomic datasets. Bioinformatics 2015, 31, 788–790. [Google Scholar] [CrossRef]

| Items 1 | ND | HD | ND-AEE | HD-AEE | SEM | p-Value |
|---|---|---|---|---|---|---|
| 1–14 d | ||||||
| ADFI, g | 35.44 | 35.27 | 35.71 | 35.43 | 0.42 | 0.78 |
| ADG, g | 29.86 | 29.67 | 29.77 | 29.75 | 0.3 | 0.94 |
| FCR | 1.19 | 1.19 | 1.2 | 1.19 | 0.01 | 0.53 |
| 15–28 d | ||||||
| ADFI, g | 109.27 a | 105.04 b | 108.41 ab | 106.61 ab | 1.35 | 0.03 |
| ADG, g | 82.86 | 79.5 | 82.67 | 81.65 | 1.7 | 0.22 |
| FCR | 1.32 | 1.32 | 1.31 | 1.31 | 0.02 | 0.89 |
| 29–42 d | ||||||
| ADFI, g | 160.66 a | 145.9 b | 165.43 a | 155.63 a | 4.46 | <0.01 |
| ADG, g | 94.63 a | 68.49 c | 95.12 a | 79.94 b | 4.37 | <0.01 |
| FCR | 1.7 c | 2.13 a | 1.75 c | 1.95 b | 0.05 | <0.01 |
| Items 1 | ND | HD | ND-AEE | HD-AEE | SEM | p-Value |
|---|---|---|---|---|---|---|
| T-AOC (mmol/g) | ||||||
| 21 d | 0.14 | 0.13 | 0.14 | 0.12 | 0.008 | 0.26 |
| 28 d | 0.13 a | 0.11 b | 0.13 a | 0.13 a | 0.005 | 0.02 |
| 35 d | 0.13 a | 0.11 b | 0.13 a | 0.13 a | 0.007 | 0.01 |
| 42 d | 0.13 a | 0.11 b | 0.13 ab | 0.12 ab | 0.008 | 0.15 |
| GSH-Px (U/mgprot) | ||||||
| 21 d | 59.47 | 58.36 | 58.17 | 58.99 | 4.023 | 0.99 |
| 28 d | 46.62 a | 36.63 b | 48.55 a | 47.65 a | 3.184 | <0.01 |
| 35 d | 62.03 a | 47.11 b | 58.13 a | 58.57 a | 3.78 | 0.01 |
| 42 d | 66.43 a | 54.72 b | 65.1 a | 66.58 a | 3.693 | 0.01 |
| CAT (U/mgprot) | ||||||
| 21 d | 37.51 | 34.12 | 34.44 | 34.71 | 1.791 | 0.24 |
| 28 d | 32.21 | 35.75 | 35.17 | 36.74 | 2.679 | 0.39 |
| 35 d | 37.3 a | 31.5 b | 36.54 a | 36.88 a | 2.13 | 0.04 |
| 42 d | 49.44 a | 37.25 b | 47.07 a | 46.2 a | 3.106 | 0.03 |
| SOD (U/mgprot) | ||||||
| 21 d | 31.3 | 30.68 | 30.33 | 29.62 | 1.538 | 0.75 |
| 28 d | 27.37 ab | 24.99 c | 28.73 a | 26.61 b | 0.764 | <0.01 |
| 35 d | 27.2 a | 24.52 b | 29.09 a | 28.73 a | 1.011 | <0.01 |
| 42 d | 34.98 a | 32.78 ab | 34.36 ab | 32.64 b | 1.014 | 0.08 |
| MDA (nmol/mgprot) | ||||||
| 21 d | 0.33 | 0.36 | 0.35 | 0.35 | 0.034 | 0.82 |
| 28 d | 0.31 b | 0.4 a | 0.34 b | 0.31 b | 0.026 | <0.01 |
| 35 d | 0.28 b | 0.35 a | 0.28 b | 0.3 ab | 0.029 | 0.07 |
| 42 d | 0.44 b | 0.52 a | 0.4 b | 0.39 b | 0.027 | <0.01 |
| Metabolite | p-Value | log2FC | Variation | Pathway | |
|---|---|---|---|---|---|
| 28 d ND vs. HD | Arachidonic acid | 0.014 | −0.659 | Down | Linoleic acid metabolism |
| 10E,12Z-Octadecadienoic acid | 0.032 | −0.373 | Down | Linoleic acid metabolism | |
| 13(S)-HPODE | 0.032 | −1.311 | Down | Linoleic acid metabolism | |
| L-Serine | 0.001 | −2.799 | Down | Glycine, serine, and threonine metabolism | |
| L-Tryptophan | 0.037 | −0.419 | Down | Glycine, serine, and threonine metabolism | |
| 3-Phosphoglycerate | 0.009 | 2.428 | Up | Glycine, serine, and threonine metabolism | |
| L-Proline | 0.012 | −0.938 | Down | Biosynthesis of amino acids | |
| Glutathione | 0.013 | −0.666 | Down | Glutathione metabolism | |
| NADPH | 0 | −1.378 | Down | Glutathione metabolism | |
| ADP | 0.044 | −0.92 | Down | Oxidative phosphorylation | |
| 35 d ND vs. HD | γ-Linolenic acid | 0.047 | −0.336 | Down | Linoleic acid metabolism |
| 10E,12Z-Octadecadienoic acid | 0.038 | −0.523 | Down | Linoleic acid metabolism | |
| 9-OxoODE | 0.043 | 1.648 | Up | Linoleic acid metabolism | |
| Prostaglandin E2 | 0.037 | 2.756 | Up | Arachidonic acid metabolism | |
| Leukotriene B4 | 0.038 | 1.043 | Up | Arachidonic acid metabolism | |
| L-Proline | 0.022 | −0.971 | Down | Biosynthesis of amino acids | |
| (3S,5S)-3,5-Diaminohexanoate | 0.038 | 0.671 | Up | Lysine degradation | |
| N6-Acetyl-L-lysine | 0.036 | 0.964 | Up | Lysine degradation | |
| 2-Keto-6-acetamidocaproate | 0.034 | 1.722 | Up | Lysine degradation | |
| Phosphoenolpyruvic acid | 0.018 | 0.446 | Up | Phenylalanine, tyrosine, and tryptophan biosynthesis | |
| L-Phenylalanine | 0.046 | 0.726 | Up | Phenylalanine, tyrosine, and tryptophan biosynthesis | |
| Epinephrine | 0 | 3.736 | Up | Neuroactive ligand–receptor interaction | |
| 42 d ND vs. HD | ATP | 0.035 | −2.251 | Down | Oxidative phosphorylation |
| Pyrophosphate | 0.047 | −0.206 | Down | Oxidative phosphorylation | |
| Succinic acid | 0.047 | −1.668 | Down | Oxidative phosphorylation | |
| Oxalacetic acid | 0.024 | −0.415 | Down | Citrate cycle (TCA cycle) | |
| L-Tryptophan | 0.024 | −0.525 | Down | Biosynthesis of amino acids | |
| L-Proline | 0.008 | −1.19 | Down | Biosynthesis of amino acids | |
| Linoleic acid | 0.035 | −0.372 | Down | Linoleic acid metabolism | |
| β-D-Fructose 6-phosphate | 0.027 | −1.381 | Down | Pentose phosphate pathway | |
| 2-Keto-D-gluconic acid | 0.011 | −0.146 | Down | Pentose phosphate pathway |
| Metabolite | p-Value | log2FC | Variation | Pathway | |
|---|---|---|---|---|---|
| 28 d HD vs. HDAEE | Glyceric acid | 0.038 | 0.296 | Up | Pentose phosphate pathway |
| Ribose 1,5-bisphosphate | 0.001 | 3.332 | Up | Pentose phosphate pathway | |
| L-Serine | 0.024 | 2.319 | Up | Cysteine and methionine metabolism | |
| 4-Methylthio-2-oxobutanoate | 0.042 | 1.51 | Up | Cysteine and methionine metabolism | |
| ATP | 0.05 | 1.407 | Up | Oxidative phosphorylation | |
| γ-Linolenic acid | 0.047 | 0.133 | Up | Linoleic acid metabolism | |
| 35 d HD vs. HDAEE | ATP | 0.022 | 1.213 | Up | Oxidative phosphorylation |
| Pyrophosphate | 0.031 | −0.29 | Down | Oxidative phosphorylation | |
| Adenosine | 0.048 | −0.924 | Down | Neuroactive ligand–receptor interaction | |
| 6-Phosphogluconic acid | 0.022 | 1.101 | Up | Pentose phosphate pathway | |
| 2-Keto-D-gluconic acid | 0 | 0.241 | Up | Pentose phosphate pathway | |
| L-Proline | 0.021 | 1.084 | Up | Biosynthesis of amino acids | |
| Phosphoenolpyruvic acid | 0.007 | −0.56 | Down | Biosynthesis of amino acids | |
| 1-Hexadecanol | 0.027 | −0.528 | Down | Fatty acid degradation | |
| Palmitoyl-L-carnitine | 0.034 | −0.821 | Down | Fatty acid degradation | |
| 10E,12Z-Octadecadienoic acid | 0.016 | 0.762 | Up | Linoleic acid metabolism | |
| 42 d HD vs. HDAEE | L-Proline | 0.013 | 1.172 | Up | Biosynthesis of amino acids |
| Glyceric acid 1,3-biphosphate | 0.004 | 1.425 | Up | Carbon metabolism | |
| Fructose 1,6-bisphosphate | 0.001 | 1.874 | Up | Carbon metabolism | |
| Glutathione | 0.043 | 0.336 | Up | Cysteine and methionine metabolism | |
| Phosphatidylcholine | 0.047 | 1.171 | Up | Linoleic acid metabolism |
| Ingredient 1 | Content (%) | |
|---|---|---|
| 1–21 d | 22–42 d | |
| Corn | 52.79 | 57.78 |
| Soybean meal | 36.89 | 30 |
| Zea gluten meal | 0 | 2.43 |
| Soybean oil | 4 | 4 |
| Sodium chloride | 0.3 | 0.3 |
| Choline chloride | 0.3 | 0.26 |
| Vitamin premix | 0.03 | 0.03 |
| Trace element premix | 0.2 | 0.2 |
| Stone powder | 1.222 | 1.171 |
| Dicalcium phosphate | 1.912 | 1.623 |
| DL-Methionine | 0.265 | 0.106 |
| L-Lysine | 0.038 | 0.045 |
| Wheat bran | 2 | 2 |
| Gene 1 | Primer Sequence (5′–3′) | Length (nt) | GenBank Number |
|---|---|---|---|
| COX-2 | F: CCGAATCGCAGCTGAATTCA R: GAAAGGCCATGTTCCAGCAT | 116 | NM_001277664.2 |
| mPGES-1 | F: AGGCTCAGGAAGAAGGCATT R: CACAGCTCCAAGGAAGAGGA | 153 | NM_001194983.1 |
| IL-1β | F: ACTGGGCATCAAGGGCTA R: GGTAGAAGATGAAGCGGGTC | 154 | NM_214005.1 |
| IL-6 | F: GCTGCGCTTCTACACAGA R: TCCCGTTCTCATCCATCTTCTC | 203 | NM_204628.1 |
| TNF-α | F: GAGCGTTGACTTGGCTGTC R: AAGCAACAACCAGCTA TGCAC | 176 | NM_214022.1 |
| IL-10 | F: AGAAATCCCTCCTCGCCAAT R: AAATAGCGAACGGCCCTCA | 121 | NM_001004414.2 |
| GAPDH | F: TGCTGCCCAGAACATCATCC R: ACGGCAGGTCAGGTCAACAA | 142 | NM_204305.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhang, Y.; Bai, D.; Zhen, W.; Ma, P.; Wang, Z.; Zhao, X.; Ma, X.; Xie, X.; Ito, K.; et al. Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress. Int. J. Mol. Sci. 2025, 26, 1877. https://doi.org/10.3390/ijms26051877
Guo C, Zhang Y, Bai D, Zhen W, Ma P, Wang Z, Zhao X, Ma X, Xie X, Ito K, et al. Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress. International Journal of Molecular Sciences. 2025; 26(5):1877. https://doi.org/10.3390/ijms26051877
Chicago/Turabian StyleGuo, Caifang, Yi Zhang, Dongying Bai, Wenrui Zhen, Penghui Ma, Ziwei Wang, Xiaodie Zhao, Xiqiang Ma, Xiaolin Xie, Koichi Ito, and et al. 2025. "Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress" International Journal of Molecular Sciences 26, no. 5: 1877. https://doi.org/10.3390/ijms26051877
APA StyleGuo, C., Zhang, Y., Bai, D., Zhen, W., Ma, P., Wang, Z., Zhao, X., Ma, X., Xie, X., Ito, K., Zhang, B., Yang, Y., Li, J., & Ma, Y. (2025). Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress. International Journal of Molecular Sciences, 26(5), 1877. https://doi.org/10.3390/ijms26051877







