Photodynamic Activation of Mammalian and Avian Cholecystokinin Type 1 Receptor Outside of the Pancreatic Acinar Cell Microenvironment
Abstract
1. Introduction
2. Results
2.1. Agonist-Stimulated Activation of Ectopically Expressed Human, Rat, Mouse and Peking Duck CCK1R
2.2. Photodynamic Activation of the Human, Rat, Mouse and Peking Duck CCK1R with Photosensitizer SALPC
2.3. Photodynamic Activation of the Human, Rat, Mouse and Peking Duck CCK1R with Tagged Protein Photosensitizer miniSOG
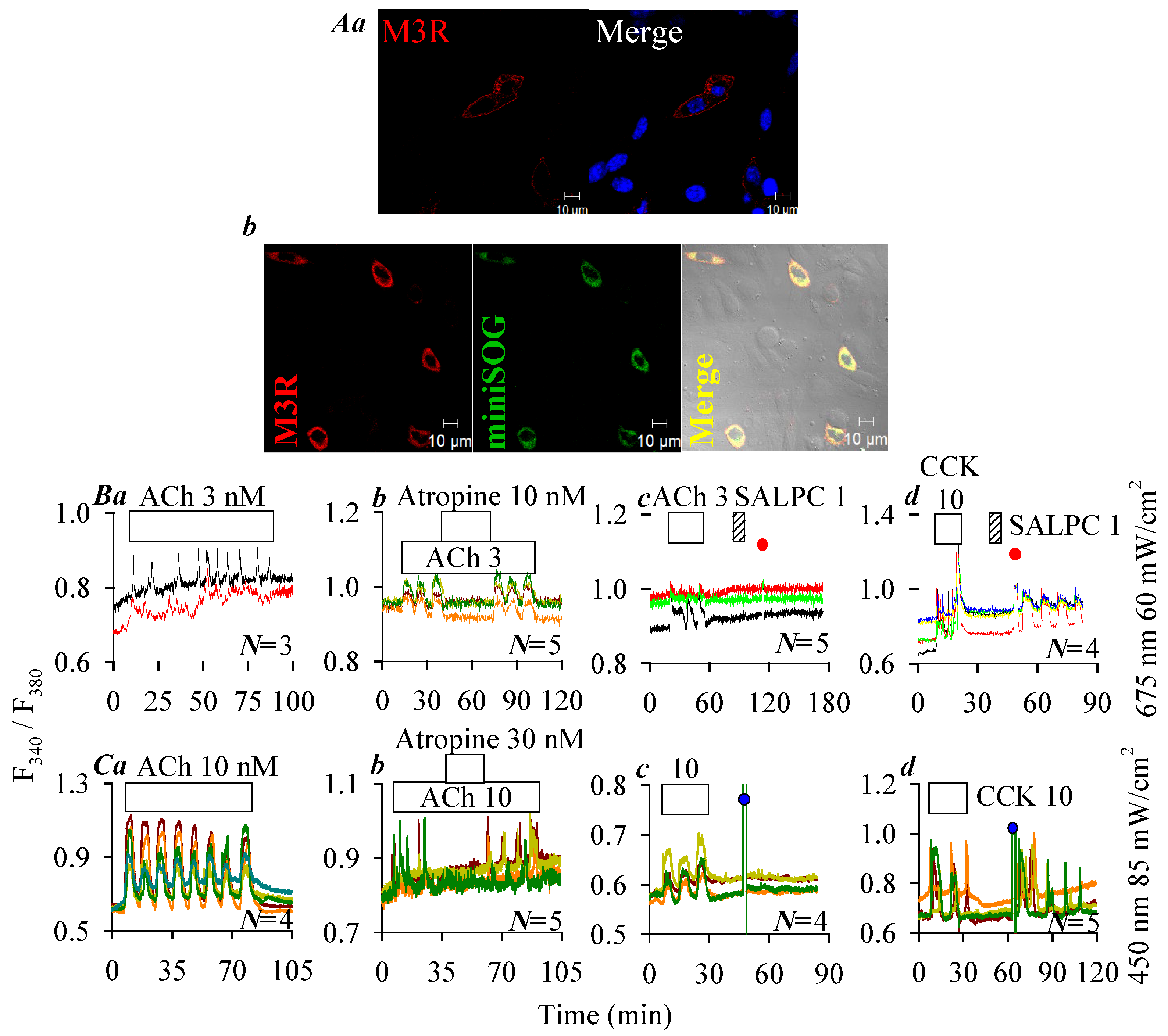
2.4. Lack of Photodynamic Effect on Human M3R
2.5. Structural Correlates of Permanent Photodynamic Activation of the Human, Rat, Mouse and Peking Duck CCK1R
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Vector Constructs and CHO-K1 Cell Transfection
4.4. Immunocytochemistry
4.5. Photodynamic Treatment
4.6. Calcium Imaging
4.7. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AlPcS4 | Sulphonated (4) aluminum phthalocyanine |
| CCD | Charge coupled device |
| CCK1R | Cholecystokinin 1 receptor |
| CHO | Chinese hamster ovary cells |
| dCCK1R | Duck cholecystokinin 1 receptor |
| ECL | Extracellular loop |
| ER | Endoplasmic reticulum |
| ERES | Endoplasmic reticulum exit sites |
| FBS | Fetal bovine serum |
| GPCR | G protein coupled receptor |
| GPCR-ABSO | G protein coupled receptor activated by singlet oxygen |
| GEPP | Genetically encoded protein photosensitizer |
| hCCK1R | Human cholecystokinin 1 receptor |
| HEK293 | Human embryonic kidney cell line 293 |
| hM3R | Human type 3 muscarinic acetylcholine receptor |
| ICL | Intracellular loop |
| LED | Light emission diode |
| M3R | Type 3 muscarinic acetylcholine receptor |
| miniSOG | mini singlet oxygen generator |
| mCCK1R | Mouse cholecystokinin 1 receptor |
| Msr | Methionine sulfoxide reductase |
| MEM | Minimum essential medium |
| PDB | Photodynamic biology; Protein Data Bank |
| PTI | Photon Technology International Inc. |
| 1O2 | Singlet oxygen |
| pCCK1R | Peking duck cholecystokinin 1 receptor |
| rCCK1R | Rat cholecystokinin 1 receptor |
| ROS | Reactive oxygen species |
| SALPC | Sulphonated aluminum phthalocyanine |
| SEM | Standard error of means |
| TM | Transmembrane domain |
| TRITC | Tetramethylrhodamine isothiocyanate |
References
- Alexander, S.P.H.; Kelly, E.; Mathie, A.A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Buneman, O.P.; Faccenda, E.; Harding, S.D.; Spedding, M.; et al. The concise guide to pharmacology 2023/24: Introduction and other protein targets. Br. J. Pharmacol. 2023, 180(SI 2), S1–S22. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D. The concise guide to pharmacology 2023/24: G protein-coupled receptors. Br. J. Pharmacol. 2023, 180, S23–S144. [Google Scholar] [CrossRef] [PubMed]
- Congreve, M.; de Graaf, C.; Swain, N.A.; Tate, C.G. Impact of GPCR structures on drug discovery. Cell 2020, 181, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR drug targets. Cell 2018, 172, 41–54.e19. [Google Scholar] [CrossRef]
- Morales, P.; Scharf, M.M.; Bermudez, M.; Egyed, A.; Franco, R.; Hansen, O.K.; Jagerovic, N.; Jakubík, J.; Keserű, G.M.; Kiss, D.J.; et al. Progress on the development of class A GPCR-biased ligands. Br. J. Pharmacol. 2025, 182, 3249–3300. [Google Scholar] [CrossRef]
- Cui, Z.J. To activate a G protein coupled receptor permanently by cell surface photodynamic action in the gastrointestinal tract. World J. Gastroenterol. 2025, 31, 102423. [Google Scholar] [CrossRef]
- Matthews, E.K.; Cui, Z.J. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on isolated rat pancreatic acini. Biochem. Pharmacol. 1990, 39, 1445–1457. [Google Scholar] [CrossRef]
- Brennan, S.C.; Mun, H.C.; Delbridge, L.; Kuchel, P.W.; Conigrave, A.D. Temperature sensing by the calcium-sensing receptor. Front. Physiol. 2023, 14, 1117352. [Google Scholar] [CrossRef]
- Ohnishi, K.; Sokabe, T.; Miura, T.; Tominaga, M.; Ohta, A.; Kuhara, A. G protein-coupled receptor-based thermosensation determines temperature acclimatization of Caenorhabditis elegans. Nat. Commun. 2024, 15, 1660. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Brandis, A.; Nevo, R.; Kiss, V.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5, 16146. [Google Scholar] [CrossRef]
- Zhang, C.; Tong, F.; Zhou, B.; He, M.D.; Liu, S.; Zhou, X.M.; Ma, Q.; Feng, T.Y.; Du, W.J.; Yang, H.; et al. TMC6 functions as a GPCR-like receptor to sense noxious heat via Gαq signaling. Cell Discov. 2025, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.G.; Puri, V.; Singh, R.D.; Hanada, K.; Pagano, R.E.; Miller, L.J. Differential effects of modification of membrane cholesterol and sphingolipids on the conformation, function, and trafficking of the G protein-coupled cholecystokinin receptor. J. Biol. Chem. 2005, 280, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.G.; Zhao, P.; Cary, B.P.; Xu, X.; Desai, A.J.; Dong, M.; Mobbs, J.I.; Toufaily, C.; Furness, S.G.B.; Christopoulos, A.; et al. Cholesterol-dependent dynamic changes in the conformation of the type 1 cholecystokinin receptor affect ligand binding and G protein coupling. PLoS Biol. 2024, 22, e3002673. [Google Scholar] [CrossRef] [PubMed]
- Christofidi, M.; Tzortzini, E.; Mavromoustakos, T.; Kolocouris, A. Effects of membrane cholesterol on the structure and function of selected class A GPCRs—Challenges and future perspectives. Biochemsitry 2025, 64, 4011–4049. [Google Scholar] [CrossRef]
- David, D.; Bentulila, Z.; Tauber, M.; Ben-Chaim, Y. G protein-coupled receptors regulated by membrane potential. Int. J. Mol. Sci. 2022, 23, 13988. [Google Scholar] [CrossRef]
- Tauber, M.; Ben-Chaim, Y. Voltage sensors embedded in G protein-coupled receptors. Int. J. Mol. Sci. 2024, 25, 5295. [Google Scholar] [CrossRef]
- Zhang, X.J.C.; Zhou, Y.; Cao, C. Thermodynamics of GPCR activation. Biophys. Rep. 2015, 1, 115–119. [Google Scholar] [CrossRef]
- Zhang, X.J.C.; Zhou, Y.; Cao, C. Proton transfer during class-A GPCR activation: Do the CWxP motif and the membrane potential act in concert? Biophys. Rep. 2018, 4, 115–122. [Google Scholar] [CrossRef]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef]
- Liang, H.Y.; Song, Z.M.; Cui, Z.J. Lasting inhibition of receptor-mediated calcium oscillations in pancreatic acini by neutrophil respiratory burst--a novel mechanism for secretory blockade in acute pancreatitis? Biochem. Biophys. Res. Commun. 2013, 437, 361–367. [Google Scholar] [CrossRef]
- An, Y.P.; Xiao, R.; Cui, H.; Cui, Z.J. Selective activation by photodynamic action of cholecystokinin receptor in the freshly isolated rat pancreatic acini. Br. J. Pharmacol. 2003, 139, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Photodynamic physiology-photonanomanipulations in cellular physiology with protein photosensitisers. Front. Physiol. 2017, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.N.; Li, Y.; Jiang, W.Y.; Cui, Z.J. Cholecystokinin 1 receptor—A unique G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO). Front. Physiol. 2018, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.; Li, Y.; Li, Z.Y.; Cui, Z.J. Permanent photodynamic cholecystokinin 1 receptor activation: Dimer-to-monomer conversion. Cell Mol. Neurobiol. 2018, 38, 1283–1292. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.J. NanoLuc bioluminescence-driven photodynamic activation of cholecystokinin 1 receptor with genetically-encoded protein photosensitizer miniSOG. Int. J. Mol. Sci. 2020, 21, 3763. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.J. Photogenetical activation of cholecystokinin 1 receptor with different genetically encoded protein photosensitizers and from varied subcellular sites. Biomolecules 2020, 10, 1423. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.J. Photodynamic activation of the cholecystokinin 1 receptor with tagged genetically encoded protein photosensitizers: Optimizing the tagging patterns. Photochem. Photobiol. 2022, 98, 1215–1228. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.J. Transmembrane domain 3 is a transplantable pharmacophore in the photodynamic activation of cholecystokinin 1 receptor. ACS Pharmacol. Transl. Sci. 2022, 5, 539–547. [Google Scholar] [CrossRef]
- Cui, Z.J. Photodynamic vision at low light level—what is more important, the prethetic retinal or the apo-rhodopsin moiety? FASEB J 2025, 39, e70470. [Google Scholar] [CrossRef]
- Cui, Z.J.; Kanno, T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J. Physiol. 1997, 504, 47–55. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Z.J. Photodynamic activation of cholecystokinin 1 receptor is conserved in mammalian and avian pancreatic acini. Biomedicines 2023, 11, 885. [Google Scholar] [CrossRef]
- Cui, Z.J.; Matthews, E.K. Photodynamic modulation of cellular function. Acta Pharmacol. Sin. 1998, 19, 297–303. [Google Scholar] [PubMed]
- Cui, Z.J.; Han, Z.Q.; Li, Z.Y. Modulating protein activity and cellular function by methionine residue oxidation. Amino Acids 2012, 43, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J. GPCR-ABSO—G protein coupled receptor activated by singlet oxygen. J. Beijing Normal Univ. (Nat. Sci.) 2020, 56, 1–8. [Google Scholar]
- Tang, W.Z.; Cui, Z.J. Permanent photodynamic activation of the cholecystokinin 2 receptor. Biomolecules 2020, 10, 236. [Google Scholar] [CrossRef]
- Wang, B.J.; Cui, Z.J. How does cholecystokinin stimulate exocrine pancreatic secretion? From birds, rodents, to humans. Am. J. Physiol. Reg. Integr. Physiol. 2007, 292, R666–R678. [Google Scholar] [CrossRef]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef]
- South, E.H.; Ritter, R.C. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 1988, 9, 601–612. [Google Scholar] [CrossRef]
- Ritter, R.C.; Brenner, L.A.; Tamura, C.S. Endogenous CCK and the peripheral neural substrates of intestinal satiety. Ann. N. Y. Acad. Sci. 1994, 713, 255–267. [Google Scholar] [CrossRef]
- Degen, L.; Matzinger, D.; Drewe, J.; Beglinger, C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides 2001, 22, 1265–1269. [Google Scholar] [CrossRef]
- Berna, M.J.; Jensen, R.T. Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr. Top. Med. Chem. 2007, 7, 1211–1231. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Bilgüvar, K.; Ishigame, K.; Sestan, N.; Günel, M.; Louvi, A.; Lei, S. Functional synergy between cholecystokinin receptors CCKAR and CCKBR in mammalian brain development. PLoS ONE 2015, 10, e0124295. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Dolai, S.; Xie, L.; Winter, E.; Orabi, A.I.; Karimian, N.; Cosen-Binker, L.I.; Huang, Y.C.; Thorn, P.; Cattral, M.S.; et al. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J. Biol. Chem. 2017, 292, 5957–5969. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Flatt, P.R.; Irwin, N. Cholecystokinin (CCK) and related adjunct peptide therapies for the treatment of obesity and type 2 diabetes. Peptides 2018, 100, 229–235. [Google Scholar] [CrossRef]
- Nyborg, N.C.B.; Kirk, R.K.; de Boer, A.S.; Andersen, D.W.; Bugge, A.; Wulff, B.S.; Thorup Inger Clausen, T.R. Cholecystokinin-1 receptor agonist induced pathological findings in the exocrine pancreas of non-human primates. Toxicol. Appl. Pharmacol. 2020, 399, 115035. [Google Scholar] [CrossRef]
- Lo, C.C.; Davidson, W.S.; Hibbard, S.K.; Georgievsky, M.; Lee, A.; Tso, P.; Woods, S.C. Intraperitoneal CCK and fourth-intraventricular Apo AIV require both peripheral and NTS CCK1R to reduce food intake in male rats. Endocrinology 2014, 155, 1700–1707. [Google Scholar] [CrossRef]
- Kageyama, H.; Kita, T.; Horie, S.; Takenoya, F.; Funahashi, H.; Kato, S.; Hirayama, M.; Lee, E.Y.; Sakurai, J.; Inoue, S.; et al. Immunohistochemical analysis of cholecystokinin A receptor distribution in the rat pancreas. Regul. Pept. 2005, 126, 137–143. [Google Scholar] [CrossRef]
- Moralejo, D.H.; Ogino, T.; Kose, H.; Yamada, T.; Matsumoto, K. Genetic verification of the role of CCK-AR in pancreatic proliferation and blood glucose and insulin regulation using a congenic rat carrying CCK-AR null allele. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 259–274. [Google Scholar] [PubMed]
- Peitl, B.; Dobronte, R.; Drimba, L.; Sari, R.; Varga, A.; Nemeth, J.; Pazmany, T.; Szilvassy, Z. Involvement of cholecystokinin in baseline and post-prandial whole body insulin sensitivity in rats. Eur. J. Pharmacol. 2010, 644, 251–256. [Google Scholar] [CrossRef]
- Plaza, A.; Merino, B.; Cano, V.; Dominguez, G.; Perez-Castells, J.; Fernandez-Alfonso, M.S.; Sengenes, C.; Chowen, J.A.; RuizGayo, M. Cholecystokinin is involved in triglyceride fatty acid uptake by rat adipose tissue. J. Endocrinol. 2018, 236, 137–150. [Google Scholar] [CrossRef]
- Avirineni, B.S.; Singh, A.; Zapata, R.C.; Phillips, C.D.; Chelikani, P.K. Dietary whey and egg proteins interact with inulin fiber to modulate energy balance and gut microbiota in obese rats. J. Nutr. Biochem. 2022, 99, 108860. [Google Scholar] [CrossRef] [PubMed]
- Shimazoe, T.; Morita, M.; Ogiwara, S.; Kojiya, T.; Goto, J.; Kamakura, M.; Moriya, T.; Shinohara, K.; Takiguchi, S.; Kono, A.; et al. Cholecystokinin-A receptors regulate photic input pathways to the circadian clock. FASEB J. 2008, 22, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Yoshimichi, G.; Lo, C.C.; Tamashiro, K.L.; Ma, L.; Lee, D.M.; Begg, D.P.; Liu, M.; Sakai, R.R.; Woods, S.C.; Yoshimatsu, H.; et al. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1336–G1342. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ohta, H.; Izumi, H.; Matsuda, Y.; Seki, M.; Toda, T.; Akiyama, M.; Matsushima, Y.; Goto, Y.; Kaga, M.; et al. Behavioral and cortical EEG evaluations confirm the roles of both CCKA and CCKB receptors in mouse CCK induced anxiety. Behav. Brain Res. 2013, 237, 325–332. [Google Scholar] [CrossRef]
- Zhang, J.G.; Liu, J.X.; Jia, X.X.; Geng, J.; Yu, F.; Cong, B. Cholecystokinin octapeptide regulates the differentiation and effector cytokine production of CD4+ T cells in vitro. Int. Immunopharmacol. 2014, 20, 307–315. [Google Scholar] [CrossRef]
- Takiguchi, S.; Suzuki, S.; Sato, Y.; Kanai, S.; Miyasaka, K.; Jimi, A.; Shinozaki, H.; Takata, Y.; Funakoshi, A.; Kono, A.; et al. Role of CCK-A receptor for pancreatic function in mice: A study in CCK-A receptor knockout mice. Pancreas 2002, 24, 276–283. [Google Scholar] [CrossRef]
- Rodríguez-Sinovas, A.; Fernández, E.; Manteca, X.; Fernández, A.G.; Goñalons, E. CCK is involved in both peripheral and central mechanisms controlling food intake in chickens. Am. J. Physiol. 1997, 272, R334–R340. [Google Scholar] [CrossRef]
- Degolier, T.F.; Brown, D.R.; Duke, G.E.; Palmer, M.M.; Swenson, J.R.; Carraway, R.E. Neurotensin and cholecystokinin contract gallbladder circular muscle in chickens. Poult. Sci. 2013, 92, 2156–2162. [Google Scholar] [CrossRef]
- El-Kassas, S.; Odemuyiwa, S.; Hajishengallis, G.; Connell, T.D.; Nashar, T.O. Expression and regulation of cholecystokinin receptor in the chicken’s immune organs and cells. J. Clin. Cell Immunol. 2016, 7, 471. [Google Scholar] [CrossRef]
- Dunn, I.C.; Meddle, S.L.; Wilson, P.W.; Wardle, C.A.; Law, A.S.; Bishop, V.R.; Hindar, C.; Robertson, G.W.; Burt, D.W.; Ellison, S.J.; et al. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E909–E921. [Google Scholar] [CrossRef]
- Xiao, R.; Cui, Z.J. Mutual dependence of VIP/PACAP and CCK receptor signaling for a physiological role in duck exocrine pancreatic secretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 286, R189–R198. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.; Zhang, W.; Wang, P. Photosensitizers for photodynamic therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.K.; Cui, Z.J. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on AR4-2J cells, a carcinoma cell line of rat exocrine pancreas. Br. J. Cancer 1990, 61, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Al-Laith, M.; Matthews, E.K.; Cui, Z.J. Photodynamicdrug action on isolated rat pancreatic acini. Mobilization of arachidonic acid and prostaglandin production. Biochem. Pharmacol. 1993, 46, 567–573. [Google Scholar] [CrossRef]
- Westberg, M.; Bregnhøj, M.; Etzerodt, M.; Ogilby, P.R. Temperature sensitive singlet oxygen photosensitization by LOV-derived fluorescent flavoproteins. J. Phys. Chem. B 2017, 121, 2561–2574. [Google Scholar] [CrossRef]
- Kitasaka, S.; Yagi, M.; Kikuchi, A. Suppression of menthyl anthranilate (UV-A sunscreen)-sensitized singlet oxygen generation by Trolox and α-tocopherol. Photochem. Photobiol. Sci. 2020, 19, 913–919. [Google Scholar] [CrossRef]
- Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration dependence of the antioxidant and prooxidant activity of Trolox in HeLa cells: Involvement in the induction of apoptotic volume decrease. Antioxidants 2020, 9, 1058. [Google Scholar] [CrossRef]
- Wei, F.S.; Wang, Q.; Han, J.Z.; Goswamee, P.; Gupta, A.; McQuiston, A.R.; Liu, Q.L.; Zhou, L. Photodynamic modification of native HCN channels expressed in thalamocortical neurons. ACS Chem. Neurosci. 2020, 11, 851–863. [Google Scholar] [CrossRef]
- Meza, U.; Delgado-Ramírez, M.; Romero-Méndez, C.; Sánchez-Armass, S.; Rodríguez-Menchaca, A.A. Functional marriage in plasma membrane: Critical cholesterol level-optimal protein activity. Br. J. Pharmacol. 2020, 177, 2456–2465. [Google Scholar] [CrossRef]
- Girotti, A.W.; Korytowski, W. Cholesterol as a singlet oxygen detector in biological systems. Methods Enzymol. 2000, 319, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Oasa, S.; Mak, W.H.; Maddox, A.L.; Lima, C.; Saftics, A.; Terenius, L.; Jovanović-Talisman, T.; Vukojević, V. Effects of ethanol and opioid receptor antagonists naltrexone and LY2444296 on the organization of cholesterol- and sphingomyelin-enriched plasma membrane domains. ACS Chem. Neurosci. 2025, 16, 4133–4146. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rojo, N.; Feng, S.H.; Morstein, J.; Pritzl, S.D.; Asaro, A.; López, S.; Xu, Y.; Harayama, T.; Vepřek, N.A.; Arp, C.J.; et al. Optical control of membrane viscosity modulates ER-to-Golgi trafficking. ACS Cent. Sci. 2025, 11, 1736–1752. [Google Scholar] [CrossRef] [PubMed]
- Archer-Lahlou, E.; Escrieut, C.; Clerc, P.; Martinez, J.; Moroder, L.; Logsdon, C.; Kopin, A.; Seva, C.; Dufresne, M.; Pradayrol, L.; et al. Molecular mechanism underlying partial and full agonism mediated by the human cholecystokinin-1 receptor. J. Biol. Chem. 2005, 280, 10664–10674. [Google Scholar] [CrossRef]
- Yu, N.; Smagghe, G. CCK(-like) and receptors: Structure and phylogeny in a comparative perspective. Gen. Comp. Endocrinol. 2014, 209, 74–81. [Google Scholar] [CrossRef]
- Liu, Q.F.; Yang, D.H.; Zhuang, Y.W.; Croll, T.I.; Cai, X.Q.; Dai, A.T.; He, X.H.; Duan, J.; Yin, W.C.; Ye, C.Y.; et al. Ligand recognition and G-protein coupling selectivity of cholecystokinin A receptor. Nat. Chem. Biol. 2021, 17, 1238–1244. [Google Scholar] [CrossRef]
- Wess, J.; Blin, N.; Mutschler, E.; BlumL, K. Muscarinic acetylcholine receptors: Structural basis of ligand binding and G protein coupling. Life Sci. 1995, 56, 915–922. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar]
- Martinez-Archundia, M.; Cordomi, A.; Garriga, P.; Perez, J.J. Molecular modeling of the M3 acetylcholine muscarinic receptor and its binding site. J. Biomed. Biotechnol. 2012, 2012, 789741. [Google Scholar] [CrossRef]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef]
- Kruse, A.C.; Li, J.; Hu, J.; Kobilka, B.K.; Wess, J. Novel insights into M3 muscarinic acetylcholine receptor physiology and structure. J. Mol. Neurosci. 2014, 53, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Foucaud, M.; Archer-Lahlou, E.; Marco, E.; Tikhonova, I.G.; Maigret, B.; Escrieut, C.; Langer, I.; Fourmy, D. Insights into the binding and activation sites of the receptors for cholecystokinin and gastrin. Regul. Pept. 2008, 145, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Gao, F. Structural basis of cholecystokinin receptor binding and regulation. Pharmacol. Ther. 2008, 119, 83–95. [Google Scholar] [CrossRef]
- Gigoux, V.; Escrieut, C.; Fehrentz, J.A.; Poirot, S.; Maigret, B.; Moroder, L.; Gully, D.; Martinez, J.; Vaysse, N.; Fourmy, D. Arginine 336 and asparagine 333 of the human cholecystokinin-A receptor binding site interact with the penultimate aspartic acid and the C-terminal amide of cholecystokinin. J. Biol. Chem. 1999, 274, 20457–20464. [Google Scholar] [CrossRef]
- Escrieut, C.; Gigoux, V.; Archer, E.; Verrier, S.; Maigret, B.; Behrendt, R.; Moroder, L.; Bignon, E.; Silvente-Poirot, S.; Pradayrol, L.; et al. The biologically crucial C terminus of cholecystokinin and the non-peptide agonist SR-146,131 share a common binding site in the human CCK1 receptor. Evidence for a crucial role of Met-121 in the activation process. J. Biol. Chem. 2002, 277, 7546–7555. [Google Scholar] [CrossRef]
- Archer-Lahlou, E.; Tikhonova, I.; Escrieut, C.; Dufresne, M.; Seva, C.; Pradayrol, L.; Moroder, L.; Maigret, B.; Fourmy, D. Modeled structure of a G-protein-coupled receptor: The cholecystokinin-1 receptor. J. Med. Chem. 2005, 48, 180–191. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Wu, M.; Guo, Y.; Guo, W.; Zhong, L.; Cai, X.; Dai, A.; Jang, W.; Shakhnovich, E.I.; et al. Common activation mechanism of class A GPCRs. eLife 2019, 8, e50279. [Google Scholar] [CrossRef]
- Mobbs, J.I.; Belousoff, M.J.; Harikumar, K.G.; Piper, S.J.; Xu, X.M.; Furness, S.G.B.; Venugopal, H.; Christopoulos, A.; Danev, R.; Wootten, D.; et al. Structures of the human cholecystokinin 1 (CCK1) receptor bound to Gs and Gq mimetic proteins provide insight into mechanisms of G protein selectivity. PLoS Biol. 2021, 19, e3001295. [Google Scholar] [CrossRef]
- Zhang, X.F.; He, C.L.; Wang, M.; Zhou, Q.T.; Yang, D.H.; Zhu, Y.; Feng, W.B.; Zhang, H.; Dai, A.T.; Chu, X.J.; et al. Structures of the human cholecystokinin receptors bound to agonists and antagonists. Nat. Chem. Biol. 2021, 17, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Valley, C.C.; Cembran, A.; Perlmutter, J.D.; Lewis, A.K.; Labello, N.P.; Gao, J.; Sachs, J.N. The methionine-aromatic motif plays a unique role in stabilizing protein structure. J. Biol. Chem. 2012, 287, 34979–34991. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.S.; Warren, J.J. A survey of methionine-aromatic interaction geometries in the oxidoreductase class of enzymes: What could Met-aromatic interactions be doing near metal sites? J. Inorg. Biochem. 2018, 186, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.S.; Warren, J.J. The interaction between methionine and two aromatic amino acids is an abundant and multifunctional motif in proteins. Arch. Biochem. Biophys. 2019, 672, 108053. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Hole hopping through tyrosine/tryptophan chains protects proteins from oxidative damage. Proc. Natl. Acad. Sci. USA 2015, 112, 10920–10925. [Google Scholar] [CrossRef]
- Lewis, A.K.; Dunleavy, K.M.; Senkow, T.L.; Her, C.; Horn, B.T.; Jersett, M.A.; Mahling, R.; McCarthy, M.R.; Perell, G.T.; Valley, C.C.; et al. Oxidation increases the strength of the methionine-aromatic interaction. Nat. Chem. Biol. 2016, 12, 860–866. [Google Scholar] [CrossRef]
- Chatterjee, K.S.; Das, R. An “up” oriented methionine-aromatic structural motif in SUMO is critical for its stability and activity. J. Biol. Chem. 2021, 297, 100970. [Google Scholar] [CrossRef]
- Walgenbach, D.G.; Gregory, A.J.; Klein, J.C. Unique methionine-aromatic interactions govern the calmodulin redox sensor. Biochem. Biophys. Res. Commun. 2018, 505, 236–241. [Google Scholar] [CrossRef]
- Harikumar, K.G.; Dong, M.; Cheng, Z.; Pinon, D.I.; Lybrand, T.P.; Miller, L.J. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry 2006, 45, 14706–14716. [Google Scholar] [CrossRef]
- Ibrahim, A.Y.; Khaodeuanepheng, N.P.; Amarasekara, D.L.; Correia, J.J.; Lewis, K.A.; Fitzkee, N.C.; Hough, L.E.; Whitten, S.T. Intrinsically disordered regions that drive phase separation form a robustly distinct protein class. J. Biol. Chem. 2023, 299, 102801. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Ward, R.J.; Pediani, J.D.; Milligan, G. Ligand regulation of the quaternary organization of cell surface M3 muscarinic acetylcholine receptors analyzed by fluorescence resonance energy transfer (FRET) imaging and homogeneous time-resolved FRET. J. Biol. Chem. 2010, 285, 23318–23330. [Google Scholar] [CrossRef]
- Hu, J.X.; Hu, K.; Liu, T.; Stern, M.K.; Mistry, R.; Challiss, R.A.J.; Costanzi, S.; Wess, J. Novel structural and functional insights into M3 muscarinic receptor dimer/oligomer formation. J. Biol. Chem. 2013, 288, 34777–34790. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Q.; Li, S.S.; Li, H.L.; Zhong, L.L.; Tian, G.B.; Zhao, X.Y.; Wang, Q.P. A rapid and reproducible method for generating germ-free Drosophila melanogaster. Biophys. Rep. 2024, 10, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Heng, J.; Liu, F.; Zhang, S.; Liu, P. Isolation and proteomic study of fish liver lipid droplets. Biophys. Rep. 2023, 9, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ronan, E.A.; Iliff, A.J.; Al-Ebidi, R.; Kitsopoulos, P.; Grosh, K.; Liu, J.; Xu, X.Z.S. Characterization of auditory sensation in C. elegans. Biophys. Rep. 2024, 10, 351–363. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Z.; Liu, J. Generation and characterization of nanobodies targeting GPCR. Biophys. Rep. 2024, 10, 22–30. [Google Scholar] [CrossRef]
- Yang, C.G.; Ma, D.F.; Hu, S.X.; Li, M.; Lu, Y. Real-time analysis of nanoscale dynamics in membrane protein insertion via single-molecule imaging. Biophys. Rep. 2024, 10, 369–376. [Google Scholar] [CrossRef]
- Chen, X.M.; Guo, Q.H.; Guan, J.X.; Zhang, L.; Jiang, T.; Xie, L.P.; Fan, J. Single-molecule tracking in living microbial cells. Biophys. Rep. 2025, 11, 1–11. [Google Scholar] [CrossRef]
- Wang, P.; Tian, B.Y.; Xu, X.J.; Luan, H.Q.; Zhang, Y.; Sun, W.H.; Hu, L.Q.; Li, Y.Y.; Yao, Y.C.; Li, W.X.; et al. Neuronal synaptic architecture revealed by cryo-correlative light and electron microscopy. Biophys. Rep. 2025, 11, 198–208. [Google Scholar] [CrossRef]
- Sweiry, J.H.; Shibuya, I.; Asada, N.; Niwa, K.; Doolabh, K.; Habara, Y.; Kanno, T.; Mann, G.E. Acute oxidative stress modulates secretion and repetitive Ca2+ spiking in rat exocrine pancreas. Biochim. Biophys. Acta 1999, 1454, 19–30. [Google Scholar] [CrossRef]
- Oomori, Y.; Habara, Y.; Kanno, T. Muscarinic and nicotinic receptor-mediated Ca2+ dynamics in rat adrenal chromaffin cells during development. Cell Tissue Res. 1998, 294, 109–123. [Google Scholar] [CrossRef]
- Satoh, Y.; Sano, K.; Habara, Y.; Kanno, T. Effects of carbachol and catecholamines on ultrastructure and intracellular calcium-ion dynamics of acinar and myoepithelial cells of lacrimal glands. Cell Tissue Res. 1997, 289, 473–485. [Google Scholar] [CrossRef]
- Okada, N.; Ohshio, G.; Manabe, T.; Imamura, M.; Habara, Y.; Kanno, T. Intracellular Ca2+ dynamics and in vitro secretory response in acute pancreatitis induced by a choline-deficient, ethionine-supplemented diet in mice. Digestion 1995, 56, 502–508. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cui, Z.-J. Photodynamic Activation of Mammalian and Avian Cholecystokinin Type 1 Receptor Outside of the Pancreatic Acinar Cell Microenvironment. Int. J. Mol. Sci. 2025, 26, 12011. https://doi.org/10.3390/ijms262412011
Wang J, Cui Z-J. Photodynamic Activation of Mammalian and Avian Cholecystokinin Type 1 Receptor Outside of the Pancreatic Acinar Cell Microenvironment. International Journal of Molecular Sciences. 2025; 26(24):12011. https://doi.org/10.3390/ijms262412011
Chicago/Turabian StyleWang, Jie, and Zong-Jie Cui. 2025. "Photodynamic Activation of Mammalian and Avian Cholecystokinin Type 1 Receptor Outside of the Pancreatic Acinar Cell Microenvironment" International Journal of Molecular Sciences 26, no. 24: 12011. https://doi.org/10.3390/ijms262412011
APA StyleWang, J., & Cui, Z.-J. (2025). Photodynamic Activation of Mammalian and Avian Cholecystokinin Type 1 Receptor Outside of the Pancreatic Acinar Cell Microenvironment. International Journal of Molecular Sciences, 26(24), 12011. https://doi.org/10.3390/ijms262412011





