The Proteome of Acute Muscle Pain: Observations from Acute Hypertonic-Saline-Induced Pain in Humans
Abstract
1. Introduction
2. Results
2.1. Cohort One—Molecular Data (Proteomics and Cytokines)
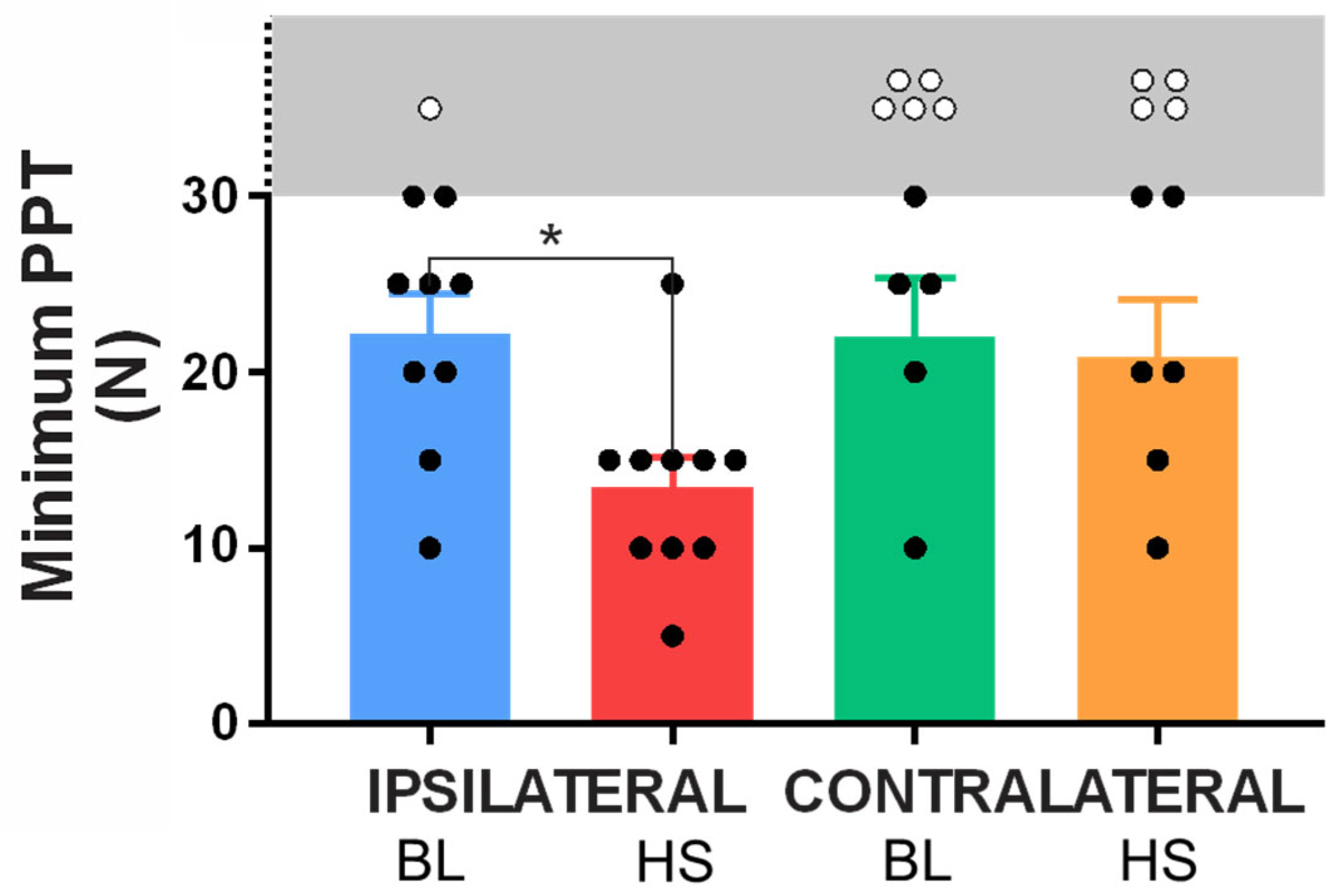
2.2. Cohort Two—Assessment of Mechanical Hyperalgesia
Minimum Pressure Pain Thresholds
3. Discussion
4. Materials and Methods
4.1. Cohort 1—Proteomic Changes Asscoiated with Acute Pain
4.1.1. Blood Sample Collection and Plasma Isolation
4.1.2. Proteomics
4.1.3. Cytokine Analysis
4.1.4. Identification, Enrichment, and Pathway Analysis
4.2. Cohort 2—Examination of Mechanical Hyperalgesia During Acute Muscle Pain
4.2.1. Pressure Pain Threshold (PPT) Testing
4.2.2. Hypertonic Saline (HS) Infusion
4.3. Statistical Analysis
4.3.1. Molecular Data
4.3.2. Sensory Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Hypertonic saline |
| PPT | Pressure pain threshold |
References
- Steinbrocker, O.; Isenberg, S.A.; Silver, M.; Neustadt, D.; Kuhn, P.; Schittone, M. Observations on pain produced by injection of hypertonic saline into muscles and other supportive tissues. J. Clin. Investig. 1953, 32, 1045–1051. [Google Scholar] [CrossRef]
- Kellgren, J.H. Observations on referred pain arising from muscle. Clin. Sci. 1938, 3, 175–190. [Google Scholar]
- Graven-Nielsen, T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand. J. Rheumatol. Suppl. 2006, 35, 1–43. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Arendt-Nielsen, L.; Svensson, P.; Jensen, T.S. Experimental muscle pain: A quantitative study of local and referred pain in humans following injection of hypertonic saline. J. Musculoskelet. Pain 1997, 5, 49–69. [Google Scholar] [CrossRef]
- Dunn, J.S.; Mahns, D.A.; Nagi, S.S. Modulation of muscle pain is not somatotopically restricted: An experimental model using concurrent hypertonic-normal saline infusions in humans. Front. Pain Res. 2020, 1, 601544. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Arendt-Nielsen, L. Induction and assessment of muscle pain, referred pain and muscular hyperalgesia. Curr. Pain Headache Rep. 2003, 7, 443–451. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Arendt-Nielsen, L.; Svensson, P.; Jensen, T.S. Quantification of local and referred muscle pain in humans after sequential i.m. injections of hypertonic saline. Pain 1997, 69, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mahns, D.A.; Nagi, S.S. An investigation into the peripheral substrates involved in the tactile modulation of cutaneous pain with emphasis on the C-tactile fibres. Exp. Brain Res. 2013, 227, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.S.; Mahns, D.A. Mechanical allodynia in human glabrous skin mediated by low-threshold cutaneous mechanoreceptors with unmyelinated fibres. Exp. Brain Res. 2013, 231, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.S.; Rubin, T.K.; Chelvanayagam, D.K.; Macefield, V.G.; Mahns, D.A. Allodynia mediated by C-tactile afferents in human hairy skin. J. Physiol. 2011, 589, 4065–4075. [Google Scholar] [CrossRef]
- Samour, M.S.; Nagi, S.S.; Shortland, P.J.; Mahns, D.A. Minocycline prevents muscular pain hypersensitivity and cutaneous allodynia produced by repeated intramusuclar injections of hypertonic saline in healthy human participants. J. Pain 2017, 18, 994–1005. [Google Scholar] [CrossRef]
- Rubin, T.K.; Henderson, L.A.; Macefield, V.G. Changes in the spatiotemporal expression of local and referred pain following repeated intramuscular injections of hypertonic saline: A longitudinal study. J. Pain 2010, 11, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Olausson, P.; Ghafouri, B.; Backryd, E.; Gerdle, B. Clear differences in cerebrospinal fluid proteome between women with chronic widespread pain and healthy women—A multivariate explorative cross-sectional study. J. Pain Res. 2017, 10, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, K.; Olausson, P.; Carlsson, A.; Ghafouri, N.; Gerdle, B.; Ghafouri, B. Systemic alterations in plasma proteins from women with chronic widespread pain compared to healthy controls: A proteomic study. J. Pain Res. 2017, 10, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Han, C.L.; Sheng, Y.C.; Wang, S.Y.; Chen, Y.H.; Kang, J.H. Serum proteome profiles revealed dysregulated proteins and mechanisms associated with fibromyalgia syndrome in women. Sci. Rep. 2020, 10, 12347, Erratum in Sci. Rep. 2021, 11, 8478. [Google Scholar] [CrossRef]
- Ramirez-Tejero, J.A.; Martinez-Lara, E.; Rus, A.; Camacho, M.V.; Del Moral, M.L.; Siles, E. Insight into the biological pathways underlying fibromyalgia by a proteomic approach. J. Proteom. 2018, 186, 47–55. [Google Scholar] [CrossRef]
- Togha, M.; Rahimi, P.; Farajzadeh, A.; Ghorbani, Z.; Faridi, N.; Zahra Bathaie, S. Proteomics analysis revealed the presence of inflammatory and oxidative stress markers in the plasma of migraine patients during the pain period. Brain Res. 2022, 1797, 148100. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ma, Q.; Zhang, J.; Yin, R.Y.; You, J.; Hao, Q.Z.; Wu, X.R.; Kang, J.J.; Wang, L.B.; Deng, Y.T.; et al. Large-Scale Plasma Proteomics to Profile Pathways and Prognosis of Chronic Pain. Adv. Sci. 2025, 12, e2410160. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Khoonsari, P.E.; Musunri, S.; Herman, S.; Svensson, C.I.; Tanum, L.; Gordh, T.; Kultima, K. Systematic analysis of the cerebrospinal fluid proteome of fibromyalgia patients. J. Proteom. 2019, 190, 35–43. [Google Scholar] [CrossRef]
- Khoonsari, P.E.; Ossipova, E.; Lengqvist, J.; Svensson, C.I.; Kosek, E.; Kadetoff, D.; Jakobsson, P.J.; Kultima, K.; Lampa, J. The human CSF pain proteome. J. Proteom. 2019, 190, 67–76. [Google Scholar] [CrossRef]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef]
- Fliniaux, I.; Germain, E.; Farfariello, V.; Prevarskaya, N. TRPs and Ca2+ in cell death and survival. Cell Calcium 2018, 69, 4–18. [Google Scholar] [CrossRef]
- Theilgaard-Monch, K.; Jacobsen, L.C.; Nielsen, M.J.; Rasmussen, T.; Udby, L.; Gharib, M.; Arkwright, P.D.; Gombart, A.F.; Calafat, J.; Moestrup, S.K.; et al. Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood 2006, 108, 353–361. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Verrastro, I.; Pasha, S.; Jensen, K.T.; Pitt, A.R.; Spickett, C.M. Mass spectrometry-based methods for identifying oxidized proteins in disease: Advances and challenges. Biomolecules 2015, 5, 378–411. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Asgar, J.; Shou, H.; Pak, J.; Da Silva, J.T.; Ro, J.Y. Intraganglionic reactive oxygen species mediate inflammatory pain and hyperalgesia through TRPA1 in the rat. Front. Pain Res. 2023, 4, 1204057. [Google Scholar] [CrossRef]
- Joseph, E.K.; Levine, J.D. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur. J. Neurosci. 2004, 20, 2896–2902. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, Z.; Li, Y.; Yu, Y.; Zhang, L. The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 898574. [Google Scholar] [CrossRef]
- Anandasabapathy, N.; Feder, R.; Mollah, S.; Tse, S.W.; Longhi, M.P.; Mehandru, S.; Matos, I.; Cheong, C.; Ruane, D.; Brane, L.; et al. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J. Exp. Med. 2014, 211, 1875–1891. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Fevang, B.; Yndestad, A.; Damas, J.K.; Halvorsen, B.; Holm, A.M.; Beiske, K.; Aukrust, P.; Froland, S.S. Chemokines and common variable immunodeficiency; possible contribution of CCL19, CCL21 and CCR7 to immune dysregulation. Clin. Exp. Immunol. 2009, 158, 237–245. [Google Scholar] [CrossRef]
- Lin, S.G.; Ba, Z.; Alt, F.W.; Zhang, Y. RAG Chromatin Scanning During V(D)J Recombination and Chromatin Loop Extrusion are Related Processes. Adv. Immunol. 2018, 139, 93–135. [Google Scholar] [CrossRef]
- Eipper, B.A.; Bloomquist, B.T.; Husten, E.J.; Milgram, S.L.; Mains, R.E. Peptidylglycine alpha-amidating monooxygenase and other processing enzymes in the neurointermediate pituitary. Ann. N. Y Acad. Sci. 1993, 680, 147–160. [Google Scholar] [CrossRef]
- Newman, L.S.; McKeever, M.O.; Okano, H.J.; Darnell, R.B. Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell 1995, 82, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.C.; Sim, R.B.; Lea, S.M.; Fremeaux-Bacchi, V.; Blom, A.M. Complement factor I in health and disease. Mol. Immunol. 2011, 48, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Shutov, L.P.; Warwick, C.A.; Shi, X.; Gnanasekaran, A.; Shepherd, A.J.; Mohapatra, D.P.; Woodruff, T.M.; Clark, J.D.; Usachev, Y.M. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. J. Neurosci. 2016, 36, 5055–5070. [Google Scholar] [CrossRef]
- Didiasova, M.; Wujak, L.; Schaefer, L.; Wygrecka, M. Factor XII in coagulation, inflammation and beyond. Cell Signal 2018, 51, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, W.; Yamagata, R.; Nakagawasai, O.; Tan-No, K. Angiotensin-Related Peptides and Their Role in Pain Regulation. Biology 2023, 12, 755. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Mechanisms of Chromatin Remodeling and Repurposing During Extracellular Translocation. Adv. Protein Chem. Struct. Biol. 2017, 106, 113–137. [Google Scholar] [CrossRef]
- Torres-Perez, J.V.; Irfan, J.; Febrianto, M.R.; Di Giovanni, S.; Nagy, I. Histone post-translational modifications as potential therapeutic targets for pain management. Trends Pharmacol. Sci. 2021, 42, 897–911. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015, 36, 103–112. [Google Scholar] [CrossRef]
- Kalpachidou, T.; Spiecker, L.; Kress, M.; Quarta, S. Rho GTPases in the Physiology and Pathophysiology of Peripheral Sensory Neurons. Cells 2019, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Dipankar, P.; Kumar, P.; Dash, S.P.; Sarangi, P.P. Functional and Therapeutic Relevance of Rho GTPases in Innate Immune Cell Migration and Function during Inflammation: An In Silico Perspective. Mediat. Inflamm. 2021, 2021, 6655412. [Google Scholar] [CrossRef] [PubMed]
- Ringer, P.; Colo, G.; Fassler, R.; Grashoff, C. Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biol. 2017, 64, 6–16. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Henriksson, K.G. Pathophysiological mechanisms in chronic musculoskeletal pain (fibromyalgia): The role of central and peripheral sensitization and pain disinhibition. Best Pract. Res. Clin. Rheumatol. 2007, 21, 465–480. [Google Scholar] [CrossRef]
- Yunus, M.B. Role of central senitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract. Res. Clin. Rheumatol. 2007, 21, 481–497. [Google Scholar] [CrossRef]
- Ablin, J.; Neumann, L.; Buskila, D. Pathogenesis of fibromyalgia—A review. Jt. Bone Spine 2008, 75, 273–279. [Google Scholar] [CrossRef]
- Samour, M.S.; Nagi, S.S.; Mahns, D.A. Cav3.2-expressing low-threshold C fibres in human hairy skin contribute to cold allodynia—A non-TRPV1 and non-TRPM8-dependent phenomenon. Pain 2015, 156, 1566–1575. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Whitehead, N.P.; Canny, B.J.; Gregory, J.E.; Proske, U. Large-fiber mechanoreceptors contribute to muscle soreness after eccentric exercise. J. Pain 2001, 2, 209–219. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.S.; Mahns, D.A. C-tactile fibers contribute to cutaneous allodynia after eccentric exercise. J. Pain 2013, 14, 538–548. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Barlas, P.; Foster, N.E.; Baxter, G.D.; Wright, C.C. Gender differences in pressure pain threshold in healthy humans. Pain 2003, 101, 259–266. [Google Scholar] [CrossRef]
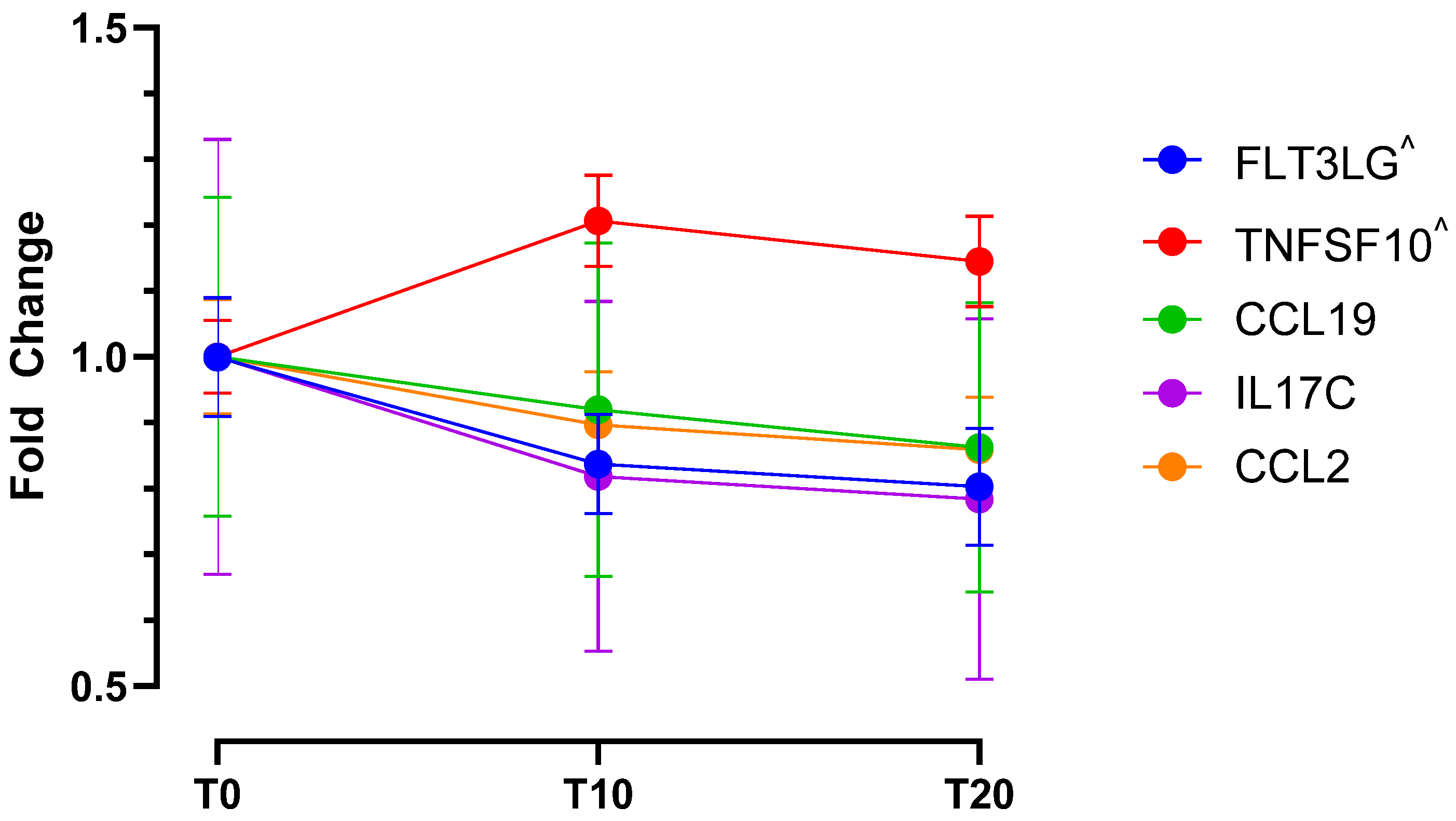
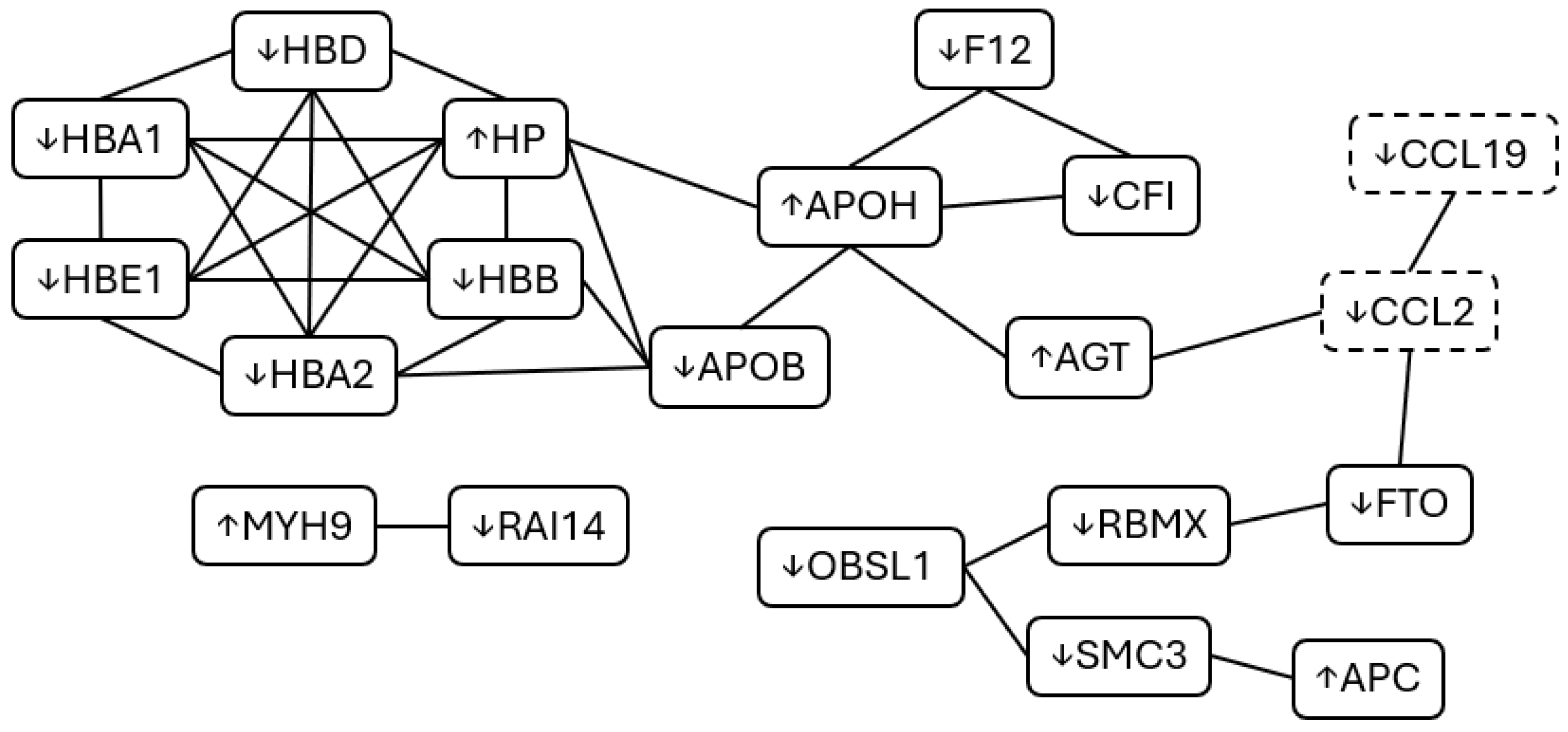
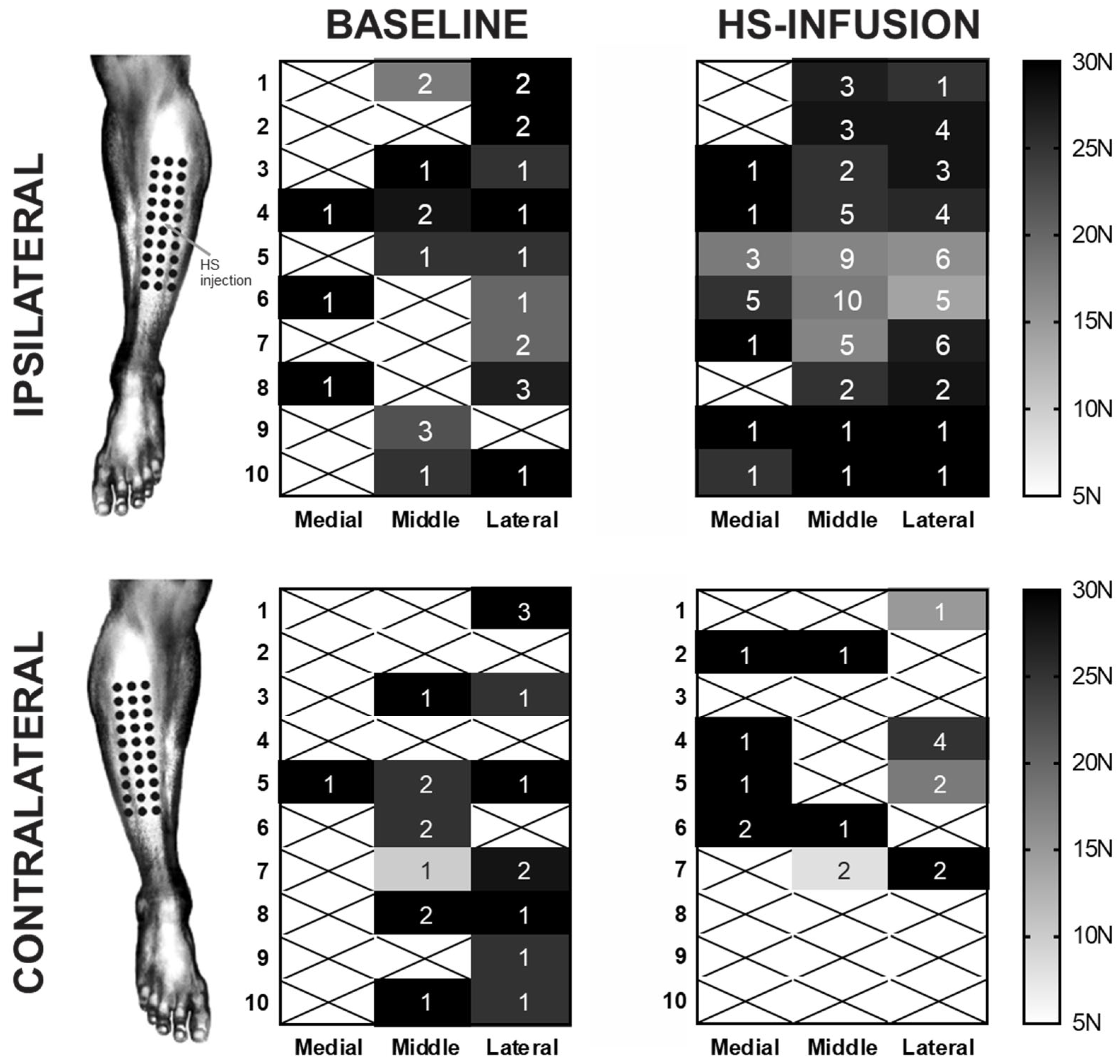
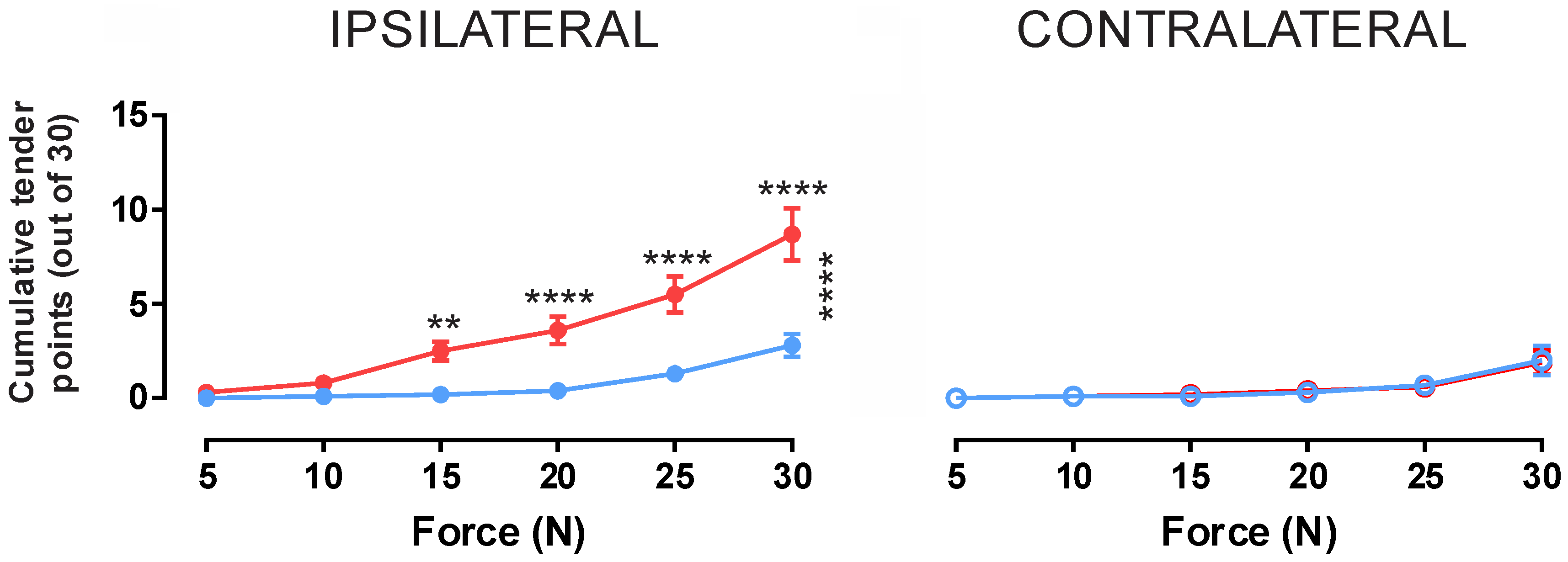
| Functional Group | Protein | Fold Change | ||
|---|---|---|---|---|
| T10 | T20 | T30 | ||
| Gene expression regulation | APC Adenomatous Polyposis Coli | 1.162 ± 0.100 | 1.156 ± 0.090 | 1.248 * ± 0.073 |
| ATF7IP Activating Transcription Factor 7 Interacting Protein | 0.859 * ± 0.062 | 0.967 ± 0.047 | 0.995 ± 0.051 | |
| CHD2 Chromodomain Helicase DNA Binding Protein 2 | 0.883 * ± 0.049 | 0.939 ± 0.030 | 0.939 ± 0.029 | |
| CNTLN Centlein | 0.779 * ± 0.069 | 0.784 ± 0.069 | 0.909 ± 0.006 | |
| DDX42 DEAD-Box Helicase 42 | 0.741 *** ± 0.038 | 0.845 * ± 0.040 | 0.831 ± 0.043 | |
| ERCC6L2 ERCC Excision Repair 6 Like 2 | 0.593 * ± 0.029 | 0.708 ± 0.037 | 0.678 ± 0.041 | |
| FTO Fat Mass and Obesity Associated Protein | 0.883 * ± 0.092 | 0.990 ± 0.072 | 1.041 ± 0.005 | |
| GTF3C1 General Transcription Factor 3C Subunit 1 | 0.909 * ± 0.038 | 0.946 ± 0.030 | 0.958 ± 0.031 | |
| LRRC47 Leucine Rich Repeat Containing 47 | 0.813 ± 0.172 | 0.807 * ± 1.059 | 0.954 ± 0.088 | |
| RBMX RNA Binding Motif Protein X-Linked | 0.803 * ± 0.064 | 0.913 ± 0.054 | 0.966 ± 0.065 | |
| SMC3 Structural Maintenance of Chromosomes 3 | 0.876 ± 0.081 | 0.854 * ± 0.059 | 0.841 ± 0.058 | |
| RANBP3 RAN Binding Protein 3 | 1.238 * ± 0.102 | 1.108 ± 1.069 | 1.036 ± 0.047 | |
| TOPAZ1 Testis and Ovary Specific PAZ Domain Containing 1 | 0.903 * ± 0.041 | 0.953 ± 0.042 | 0.987 ± 0.042 | |
| Coagulation and immunity | AGT Angiotensinogen | 1.911 * ± 0.374 | 1.297 ± 0.169 | 1.414 ± 0.067 |
| CFI Complement Factor I | 0.868 ** ± 0.049 | 0.946 ± 0.042 | 0.967 ± 0.037 | |
| F12 Coagulation Factor XII | 0.877 * ± 0.081 | 0.961 ± 0.065 | 0.984 ± 0.079 | |
| HP Haptoglobin | 1.059 ± 0.161 | 1.310 ±1.056 | 1.490 * ± 0.073 | |
| IGHV3-35 Immunoglobulin Heavy Variable 3–35 | 0.711 * ± 0.069 | 0.761 * ± 0.049 | 0.675 ** ± 0.055 | |
| ITIH1 Inter-Alpha-Trypsin Inhibitor Heavy Chain 1 | 1.654 * ± 0.286 | 1.272 ± 2.001 | 1.449 ± 0.069 | |
| LTF Lactoferrin | 0.855 ± 0.059 | 0.887 * ± 0.042 | 0.876 ± 0.048 | |
| Vesicle trafficking | AP3B2 Adaptor Related Protein Complex 3 Subunit Beta 2 | 0.811 * ± 0.079 | 0.904 ** ± 0.057 | 0.921 ± 0.072 |
| RABEP1 Rabaptin, RAB GTPase Binding Effector Protein 1 | 1.371 * ± 0.146 | 1.116 ± 1.077 | 1.268 ± 0.075 | |
| SYTL2 Synaptotagmin-Like 2 | 1.096 ± 0.071 | 1.155 * ± 0.048 | 1.186 ** ± 0.077 | |
| Actin cytoskeleton and axon guidance regulation | CLIP1 CAP-Gly Domain Containing Linker Protein 1 | 0.834 ** ± 0.045 | 0.942 ± 0.051 | 0.979 ± 0.062 |
| DNAAF1 Dynein Axonemal Assembly Factor 1 | 0.590 ** ± 0.047 | 0.725 ± 0.053 | 0.683 ± 0.051 | |
| MAST1 Microtubule Associated Serine/Threonine Kinase 1 | 1.213 ± 0.058 | 1.258 * ± 0.068 | 1.220 ± 0.072 | |
| MYH9 Myosin Heavy Chain 9 | # | 1.717 **** ± 0.411 | # | |
| OBSL1 Obscurin Like Cytoskeletal Adaptor 1 | 0.954 ± 0.113 | 0.795 * ± 1.098 | 0.801 * ± 0.091 | |
| PLXNC1 Plexin C1 | 1.615 ± 0.310 | 2.056 ** ± 3.016 | 1.476 ± 0.051 | |
| PTPRS Protein Tyrosine Phosphatase Receptor Type S | 0.867 ** ± 0.045 | 0.911 ± 0.028 | 0.826 ** ± 0.034 | |
| RAI14 Retinoic Acid Induced 14 | 0.514 * ± 0.036 | 0.630 ± 0.030 | 0.598 * ± 0.037 | |
| Lipoprotein metabolism and transport | APOB Apolipoprotein B | 0.673 * ± 0.035 | 0.773 ± 0.039 | 0.764 ± 0.037 |
| APOH Apolipoprotein H | # | 1.293 ** ± 0.142 | # | |
| Peptide/protein metabolism | DPP3 Dipeptidyl Peptidase 3 | 1.319 * ± 0.152 | 1.107 ± 1.015 | 1.183 ± 0.040 |
| PAM Peptidylglycine Alpha-Amidating Monooxygenase | 0.904 ± 0.065 | 0.939 ** ± 0.060 | 0.938 ± 0.054 | |
| PARG Poly(ADP-Ribose) Glycohydrolase | 1.132 ** ± 0.062 | 1.072 ± 0.055 | 1.021 ± 0.064 | |
| TG Thyroglobulin | 1.255 ± 0.136 | 1.122 ± 1.070 | 1.246 * ± 0.011 | |
| Ca2+ channel/release | ANKRD36C Ankyrin Repeat Domain 36C | 0.953 ± 0.078 | 0.901 * ± 0.061 | 0.933 ± 0.042 |
| TRPM1 Transient Receptor Potential Cation Channel Subfamily M Member 1 | 0.977 ± 0.051 | 0.980 ± 0.041 | 0.928 * ± 0.030 | |
| Erythrocytes gas exchange | HBA1 Hemoglobin Subunit Alpha 1 | 0.546 * ± 0.035 | 0.675 ± 0.033 | 0.613 * ± 0.042 |
| HBA2 Hemoglobin Subunit Alpha 2 | 0.629 * ± 0.045 | 0.741 ± 0.037 | 0.700 * ± 0.041 | |
| HBA2 Hemoglobin Subunit Alpha 2 | # | 0.620 * ± 0.056 | # | |
| HBB Hemoglobin Subunit Beta | 0.647 * ± 0.053 | 0.758 ± 0.047 | 0.738 ± 0.044 | |
| HBB Hemoglobin Subunit Beta | # | 0.597 * ± 0.033 | # | |
| HBD Hemoglobin Subunit Delta | 0.605 * ± 0.042 | 0.744 ± 0.052 | 0.695 * ± 0.046 | |
| HBD Hemoglobin Subunit Delta | # | 0.545 ** ± 0.033 | # | |
| HBE1 Hemoglobin Subunit Epsilon1 | 0.604 * ± 0.037 | 0.749 ± 0.039 | 0.691 ± 0.044 | |
| Force | Baseline | Infusion | p Value | Significance |
|---|---|---|---|---|
| 5 N | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.9976 | ns |
| 10 N | 0.1 ± 0.1 | 0.8 ± 0.4 | 0.8440 | ns |
| 15 N | 0.2 ± 0.1 | 2.5 ± 0.5 | 0.0022 | ** |
| 20 N | 0.4 ± 0.2 | 3.6 ± 0.7 | <0.0001 | **** |
| 25 N | 1.3 ± 0.4 | 5.5 ± 1.0 | <0.0001 | **** |
| 30 N | 2.7 ± 0.6 | 8.7 ± 1.4 | <0.0001 | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jubin, P.; Amigo, M.; Boulton, D.; Mahns, D.A.; Nagi, S.S.; Dunn, J.S. The Proteome of Acute Muscle Pain: Observations from Acute Hypertonic-Saline-Induced Pain in Humans. Int. J. Mol. Sci. 2025, 26, 11922. https://doi.org/10.3390/ijms262411922
Jubin P, Amigo M, Boulton D, Mahns DA, Nagi SS, Dunn JS. The Proteome of Acute Muscle Pain: Observations from Acute Hypertonic-Saline-Induced Pain in Humans. International Journal of Molecular Sciences. 2025; 26(24):11922. https://doi.org/10.3390/ijms262411922
Chicago/Turabian StyleJubin, Pauline, Marie Amigo, Daniel Boulton, David A. Mahns, Saad S. Nagi, and James S. Dunn. 2025. "The Proteome of Acute Muscle Pain: Observations from Acute Hypertonic-Saline-Induced Pain in Humans" International Journal of Molecular Sciences 26, no. 24: 11922. https://doi.org/10.3390/ijms262411922
APA StyleJubin, P., Amigo, M., Boulton, D., Mahns, D. A., Nagi, S. S., & Dunn, J. S. (2025). The Proteome of Acute Muscle Pain: Observations from Acute Hypertonic-Saline-Induced Pain in Humans. International Journal of Molecular Sciences, 26(24), 11922. https://doi.org/10.3390/ijms262411922







