Aqueous Extract of Bacopa procumbens and the NAPEL Formulation Mitigate MPTP-Induced Neurotoxicity via Nrf2/HSF1/HIF-1α Signaling in a Parkinson’s Disease Model
Abstract
1. Introduction
2. Results
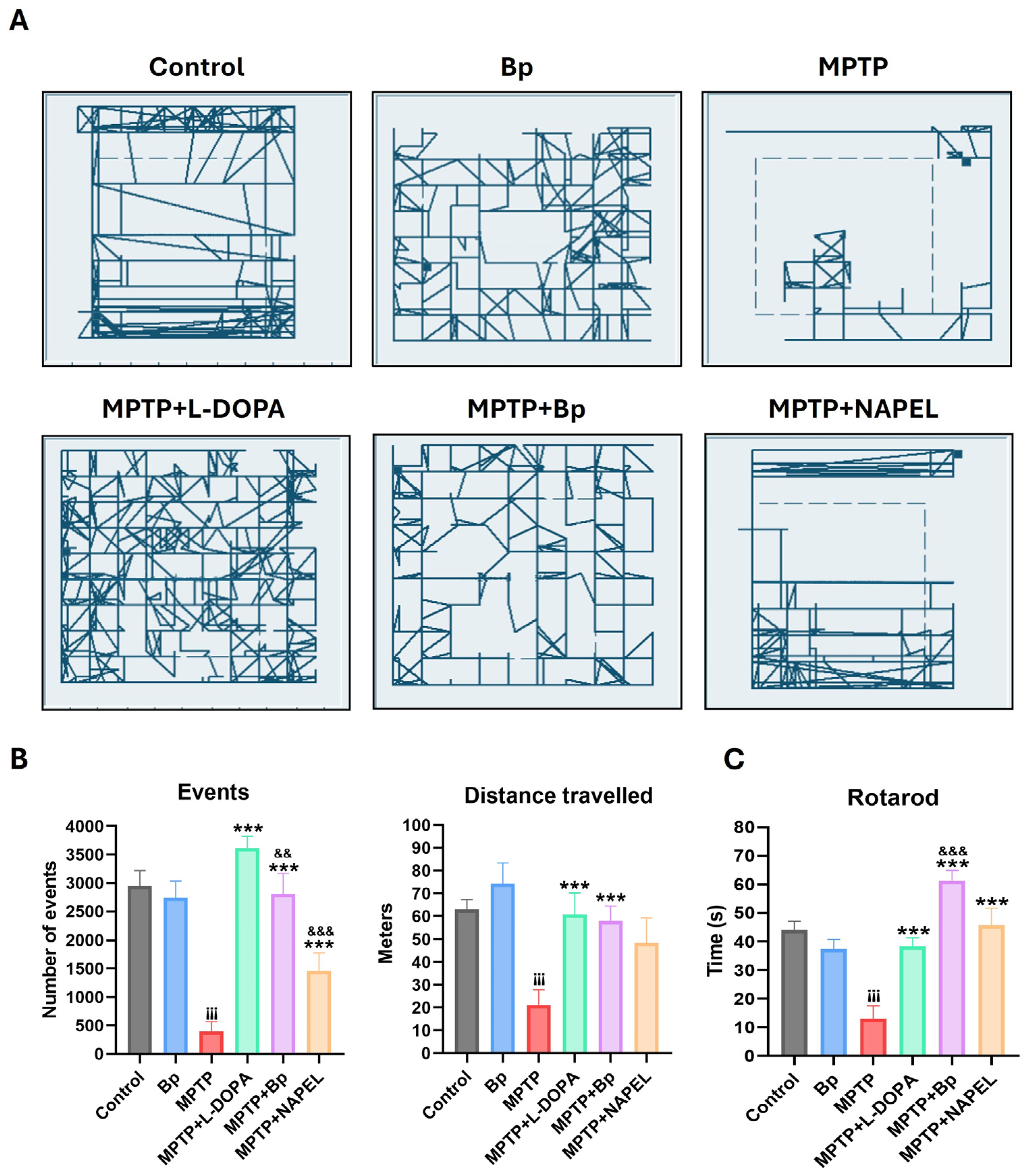
2.1. Motor Function Tests
2.2. Histological Evaluation of the Substantia Nigra by Luxol Fast Blue Staining
2.3. Modulation of the Endogenous Antioxidant System
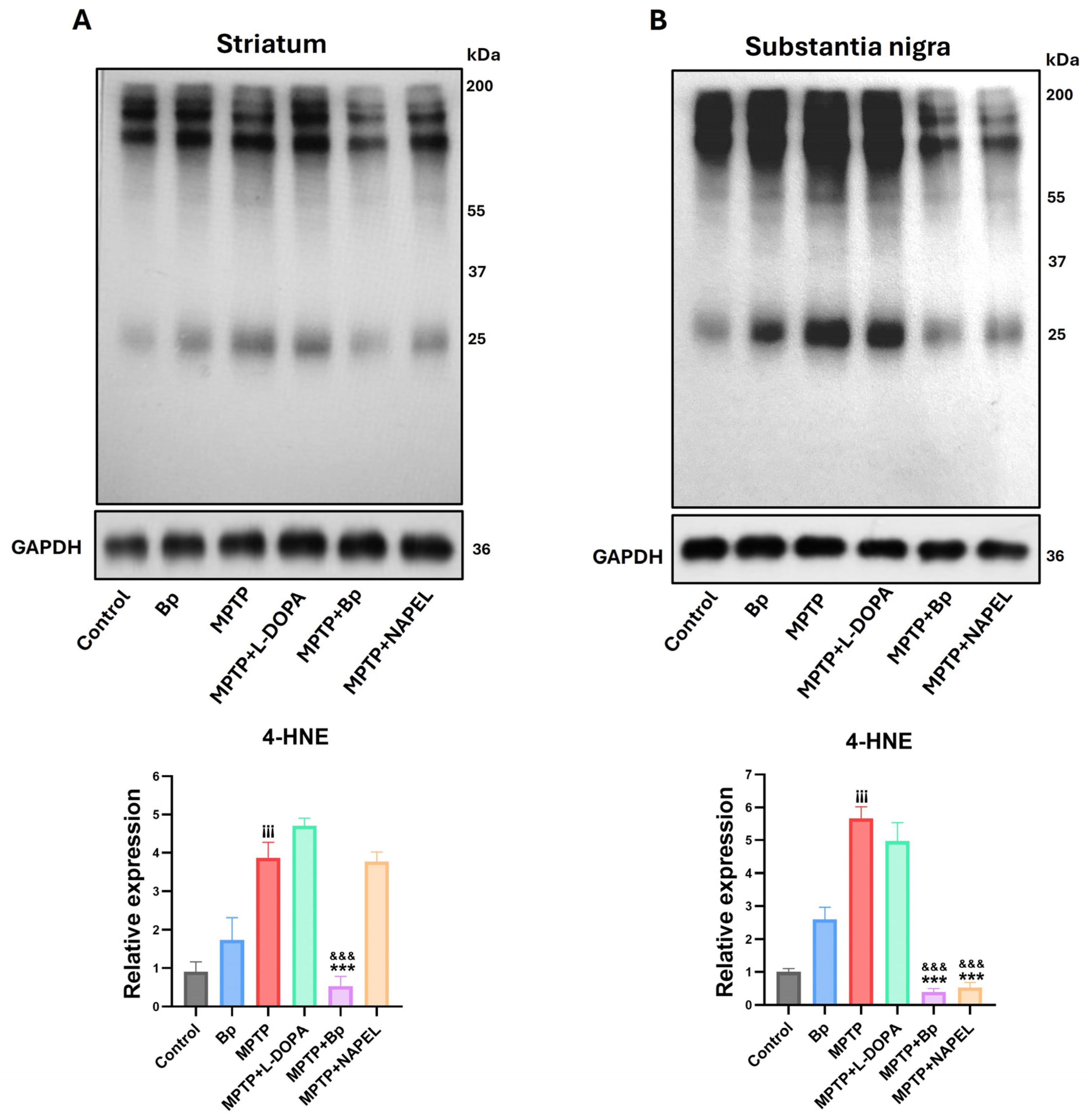
2.4. Neuroprotection Against Lipid Peroxidation
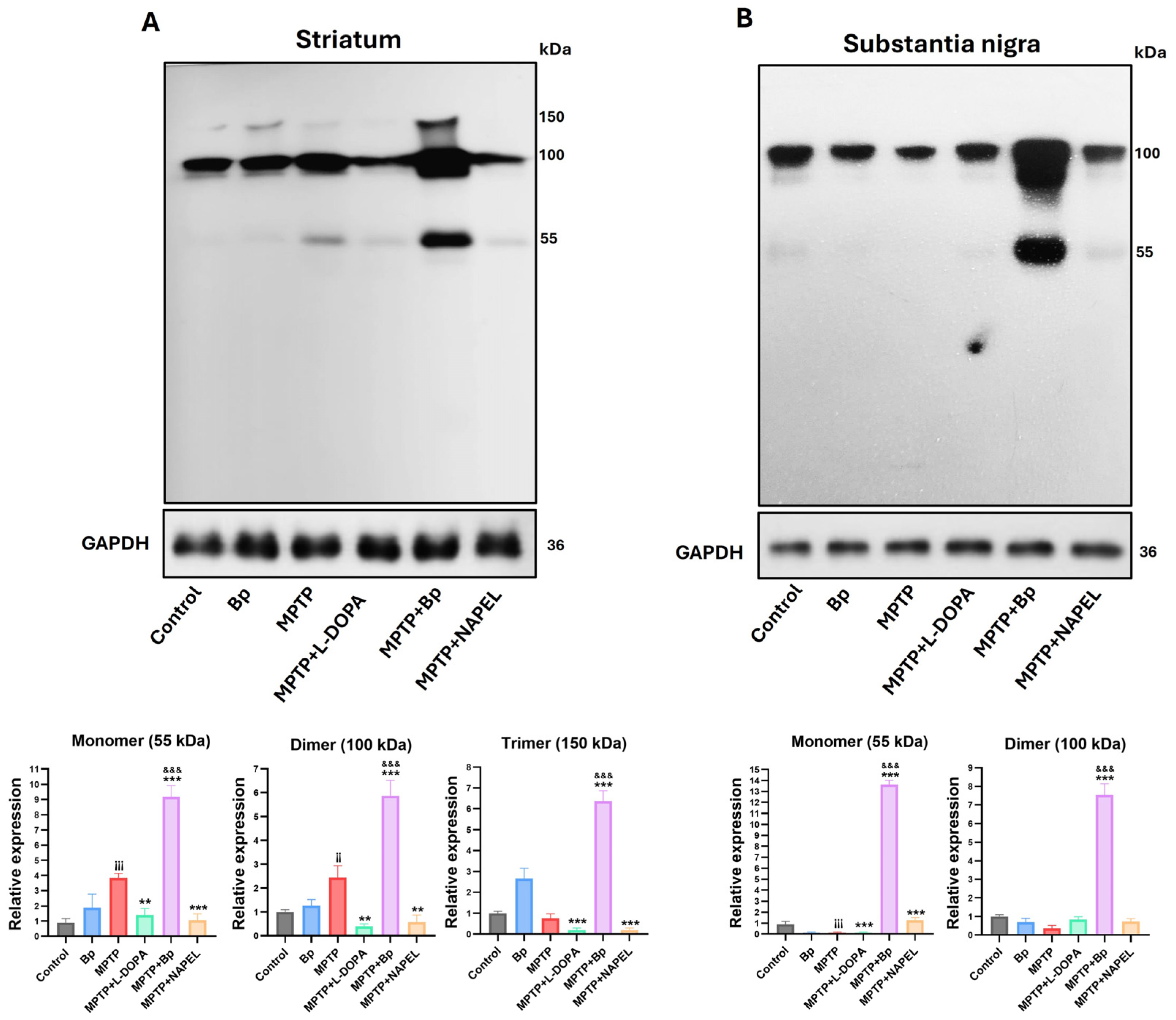
2.5. Expression of Proteins Associated with Cell Signaling and Stress Adaptation
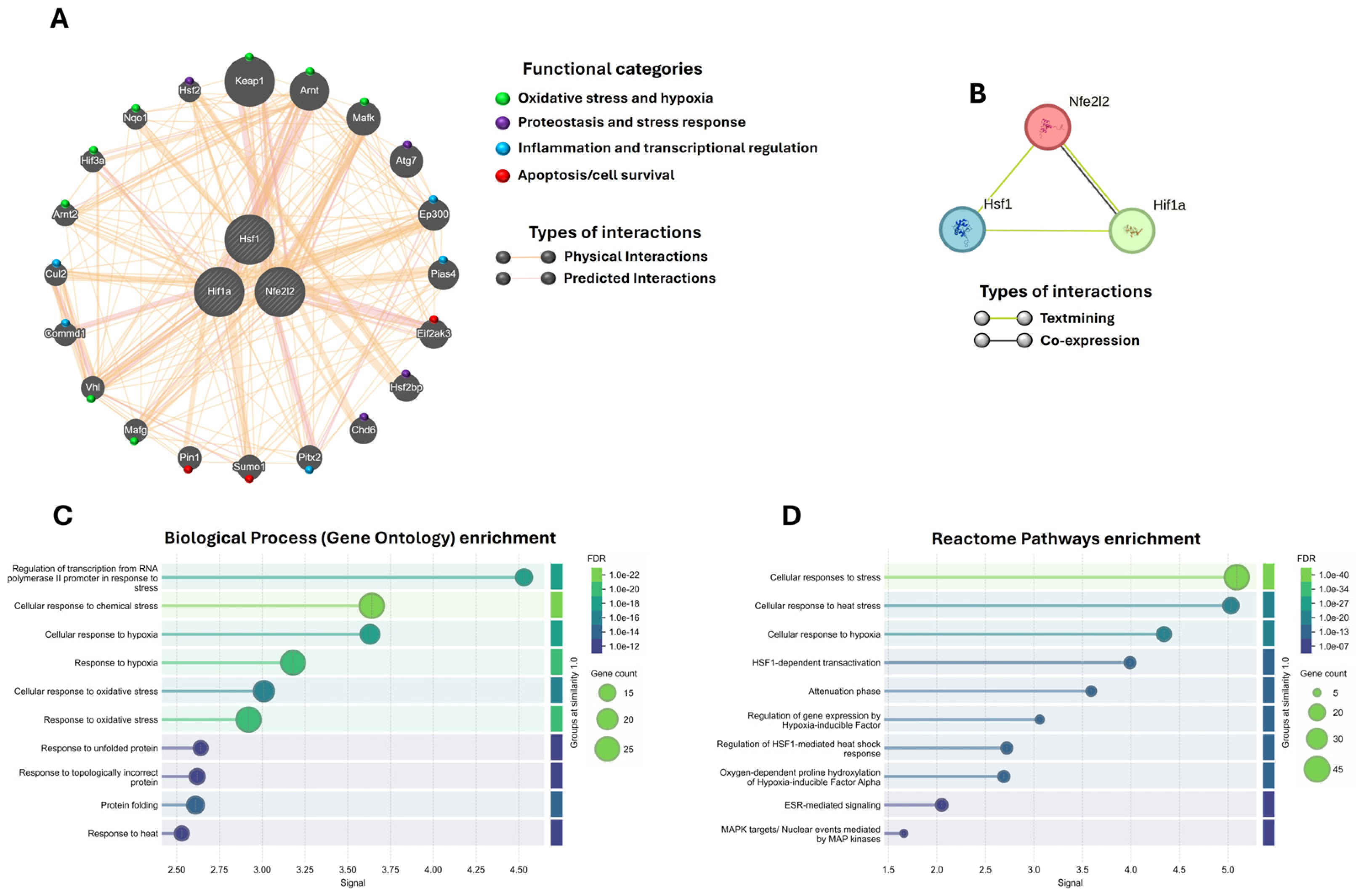
2.6. Nrf2, HSF1, and HIF-1α as Central Nodes of Neuroprotection
3. Discussion
Limitations and Perspectives
4. Materials and Methods
4.1. Procurement of Bioactive Compounds
4.2. Experimental Animals
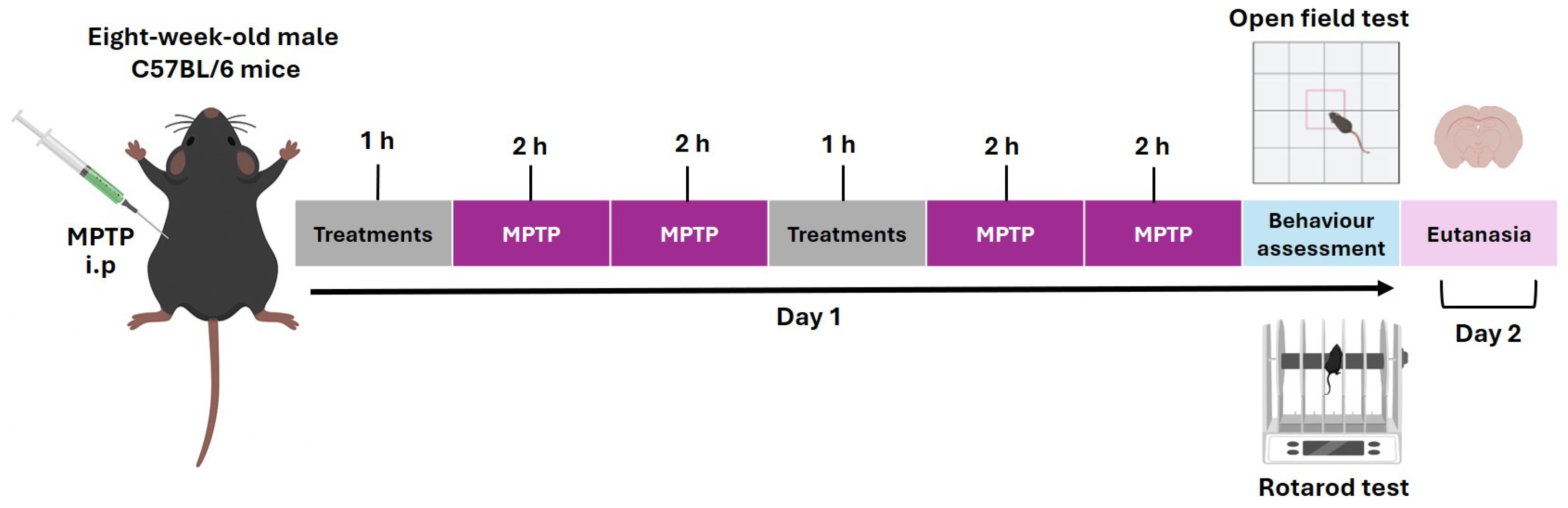
4.3. Experimental Model of MPTP-Induced Parkinson’s Disease
- Healthy control: treated only with saline solution.
- B. procumbens (Bp) control: treated with the aqueous extract of B. procumbens (two doses of 150 mg/kg).
- MPTP: neurological damage induced by MPTP without additional treatment.
- MPTP+L-DOPA: neurological damage induced by MPTP and treated with levodopa.
- MPTP+Bp: neurological damage induced by MPTP and treated with extract of B. procumbens.
- MPTP+NAPEL: neurological damage induced by MPTP and treated with formulation NAPEL.
4.4. Motor Function Tests: Open Field and Rotarod
4.5. Luxol Fast Blue Staining
4.6. Protein Extraction
4.7. Western Blotting
4.8. Interactome and Functional Enrichment Analysis of Nrf2, HSF1, and HIF-1α
4.9. Statistical Analysis and Graphical Representation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Name |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| Nrf2-pS40 | Phosphorylated Nrf2 at serine 40 |
| HO-1 | Heme oxygenase-1 |
| SOD1 | Superoxide dismutase 1 |
| CAT | Catalase |
| GSR | Glutathione-disulfide reductase |
| HSF1 | Heat shock factor 1 |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| AKT | Protein kinase B (PKB/AKT) |
| AKT-pS473 | Phosphorylated AKT at serine 473 |
| β-catenin | Beta-catenin |
| 4-HNE | 4-Hydroxy-2-nonenal |
| DAT | Dopamine transporter |
| MAO-B | Monoamine oxidase B |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| Mafk | Transcription factor MafK |
| Arnt | Aryl hydrocarbon receptor nuclear translocator |
| Vhl | Von Hippel–Lindau tumor suppressor |
| Hsf2bp | Heat shock factor 2 binding protein |
| Atg7 | Autophagy related 7 |
| Chd6 | Chromodomain-helicase-DNA-binding protein 6 |
| Ep300 | E1A binding protein p300 |
| Pias4 | Protein inhibitor of activated STAT 4 |
| Commd1 | COMM domain-containing protein 1 |
| Cul2 | Cullin-2 |
| Pitx2 | Paired-like homeodomain transcription factor 2 |
| Pin1 | Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
| Sumo1 | Small ubiquitin-like modifier 1 |
| Eif2ak3 | Eukaryotic translation initiation factor 2-alpha kinase 3 (also known as PERK) |
| Hmox1 | Heme oxygenase 1 (same as HO-1) |
| Egln1–3 | Prolyl hydroxylase domain-containing proteins 1–3 (EGLN1, EGLN2, EGLN3) |
| Hif1an | Hypoxia-inducible factor 1-alpha inhibitor |
| Hspa1b | Heat shock protein family A member 1B (HSP70 family) |
| Bag3 | BCL2-associated athanogene 3 |
| Xbp1 | X-box binding protein 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
References
- Estadísticas|Parkinson’s Foundation. Available online: https://www.parkinson.org/espanol/entendiendo-parkinson/estadisticas (accessed on 29 March 2025).
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s Disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef]
- Stocchi, F.; Bravi, D.; Emmi, A.; Antonini, A. Parkinson Disease Therapy: Current Strategies and Future Research Priorities. Nat. Rev. Neurol. 2024, 20, 695–707. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Beheshti, I. Exploring Risk and Protective Factors in Parkinson’s Disease. Cells 2025, 14, 710. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Bisaglia, M. Oxidative Stress and Neuroinflammation in Parkinson’s Disease: The Role of Dopamine Oxidation Products. Antioxidants 2023, 12, 955. [Google Scholar] [CrossRef]
- Mustapha, M.; Taib, C.N.M. MPTP-Induced Mouse Model of Parkinson’s Disease: A Promising Direction for Therapeutic Strategies. Bosn. J. Basic Med. Sci. 2021, 21, 422–433. [Google Scholar] [CrossRef]
- Mondal, S.; Firdous, S.M. Unrevealing the Molecular Mechanisms of MPTP-Induced Parkinson’s in Experimental Animals. Med. Chem. Res. 2025, 34, 2398–2413. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; García-Morales, V.; Suleiman-Martos, S.; Rivas-Domínguez, A.; Mohamed-Mohamed, H.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L.; González-Acedo, A. Current Treatments and New, Tentative Therapies for Parkinson’s Disease. Pharmaceutics 2023, 15, 770. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Paniagua, M.; Vázquez-Álvarez, A.M.; Valverde-Aguilar, G.; Vergara-Aragón, P. Current Treatments in Parkinson’s Including the Proposal of an Innovative Dopamine Microimplant. Rev. Médica Hosp. Gen. México 2016, 79, 79–87. [Google Scholar] [CrossRef]
- Kaur, T.; Sidana, P.; Kaur, N.; Choubey, V.; Kaasik, A. Unraveling Neuroprotection in Parkinson’s Disease: Nrf2–Keap1 Pathway’s Vital Role amidst Pathogenic Pathways. Inflammopharmacology 2024, 32, 2801–2820. [Google Scholar] [CrossRef]
- Huenchuguala, S.; Segura-Aguilar, J. Natural Compounds That Activate the KEAP1/Nrf2 Signaling Pathway as Potential New Drugs in the Treatment of Idiopathic Parkinson’s Disease. Antioxidants 2024, 13, 1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pi, J.; Zhang, Q. Signal Amplification in the KEAP1-NRF2-ARE Antioxidant Response Pathway. Redox Biol. 2022, 54, 102389. [Google Scholar] [CrossRef]
- Bhurtel, S.; Bok, E.; Katila, N.; Kim, J.; Choi, D.-Y. Activation of Nrf2 by Methylene Blue Is Associated with the Neuroprotection against MPP+ Induced Toxicity via Ameliorating Oxidative Stress and Mitochondrial Dysfunction. Biochem. Pharmacol. 2021, 192, 114719. [Google Scholar] [CrossRef]
- Chen, H.; Ma, D.; Yue, F.; Qi, Y.; Dou, M.; Cui, L.; Xing, Y. The Potential Role of Hypoxia-Inducible Factor-1 in the Progression and Therapy of Central Nervous System Diseases. Curr. Neuropharmacol. 2022, 20, 1651–1666. [Google Scholar] [CrossRef]
- Qu, Z.; Titus, A.S.C.L.S.; Xuan, Z.; D’Mello, S.R. Neuroprotection by Heat Shock Factor-1 (HSF1) and Trimerization-Deficient Mutant Identifies Novel Alterations in Gene Expression. Sci. Rep. 2018, 8, 17255. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Kostov, R.V.; Dinkova-Kostova, A.T. Transcription Factors Hsf1 and Nrf2 Engage in Crosstalk for Cytoprotection. Trends Pharmacol. Sci. 2015, 36, 6–14. [Google Scholar] [CrossRef]
- Acuña-Pilarte, K.; Koh, M.Y. The HIF Axes in Cancer: Angiogenesis, Metabolism, and Immune-Modulation. Trends Biochem. Sci. 2025, 50, 677–694. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and Comprehensive Insights into HIF-1 Stabilization under Normoxic Conditions: Implications for Cellular Adaptation and Therapeutic Strategies in Cancer. Cell. Mol. Biol. Lett. 2025, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-Inducible Factor (HIF-1)α: Its Protein Stability and Biological Functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova, S.A.; Kokoreva, N.E.; Guzhova, I.V.; Alhasan, B.A.; Margulis, B.A.; Nikotina, A.D. Unveiling the HSF1 Interaction Network: Key Regulators of Its Function in Cancer. Cancers 2024, 16, 4030. [Google Scholar] [CrossRef]
- Pérez-Mora, S.; Pérez-Ishiwara, D.G.; Salgado-Hernández, S.V.; Medel-Flores, M.O.; Reyes-López, C.A.; Rodríguez, M.A.; Sánchez-Monroy, V.; Gómez-García, M.d.C. Entamoeba Histolytica: In Silico and In Vitro Oligomerization of EhHSTF5 Enhances Its Binding to the HSE of the EhPgp5 Gene Promoter. Int. J. Mol. Sci. 2024, 25, 4218. [Google Scholar] [CrossRef]
- Dorantes-Palma, D.; Pérez-Mora, S.; Azuara-Liceaga, E.; Pérez-Rueda, E.; Pérez-Ishiwara, D.G.; Coca-González, M.; Medel-Flores, M.O.; Gómez-García, C. Screening and Structural Characterization of Heat Shock Response Elements (HSEs) in Entamoeba Histolytica Promoters. Int. J. Mol. Sci. 2024, 25, 1319. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Hardy, A.; Schofield, C.J. The Human Oxygen Sensing Machinery and Its Manipulation. Chem. Soc. Rev. 2008, 37, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Priya, S. Expanding Role of Molecular Chaperones in Regulating α-Synuclein Misfolding; Implications in Parkinson’s Disease. Cell. Mol. Life Sci. CMLS 2016, 74, 617–629. [Google Scholar] [CrossRef]
- Tanaka, K.; Galduróz, R.F.S.-; Gobbi, L.T.B.; Galduróz, J.C.F. Ginkgo Biloba Extract in an Animal Model of Parkinson’s Disease: A Systematic Review. Curr. Neuropharmacol. 2013, 11, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Olang, C.A.; Lewis, G.; Mandalaneni, K.; Anand, N.; Gorantla, V.R. An Overview of Parkinson’s Disease: Curcumin as a Possible Alternative Treatment. Cureus 2022, 14, e25032. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Yang, X.; Yv, Q.; Ye, F.; Chen, S.; Cui, Y.; Gu, L.; Zhu, M.; Li, W. Baicalin Exerts Neuroprotective Actions by Regulating the Nrf2-NLRP3 Axis in Toxin-Induced Models of Parkinson’s Disease. Chem. Biol. Interact. 2024, 387, 110820. [Google Scholar] [CrossRef]
- Kosmopoulou, D.; Lafara, M.-P.; Adamantidi, T.; Ofrydopoulou, A.; Grabrucker, A.M.; Tsoupras, A. Neuroprotective Benefits of Rosmarinus Officinalis and Its Bioactives against Alzheimer’s and Parkinson’s Diseases. Appl. Sci. 2024, 14, 6417. [Google Scholar] [CrossRef]
- Martínez-Cuazitl, A.; Gómez-García, M.d.C.; Hidalgo-Alegria, O.; Flores, O.M.; Núñez-Gastélum, J.A.; Martínez, E.S.M.; Ríos-Cortés, A.M.; Garcia-Solis, M.; Pérez-Ishiwara, D.G. Characterization of Polyphenolic Compounds from Bacopa Procumbens and Their Effects on Wound-Healing Process. Molecules 2022, 27, 6521. [Google Scholar] [CrossRef]
- Martínez-Cuazitl, A.; Gómez-García, M.D.C.; Pérez-Mora, S.; Rojas-López, M.; Delgado-Macuil, R.J.; Ocampo-López, J.; Vázquez-Zapién, G.J.; Mata-Miranda, M.M.; Pérez-Ishiwara, D.G. Polyphenolic Compounds Nanostructurated with Gold Nanoparticles Enhance Wound Repair. Int. J. Mol. Sci. 2023, 24, 17138. [Google Scholar] [CrossRef]
- Pérez-Mora, S.; Ocampo-López, J.; Gómez-García, M.D.C.; Pérez-Ishiwara, D.G. BFNB Enhances Hair Growth in C57BL/6 Mice through the Induction of EGF and FGF7 Factors and the PI3K-AKT-β-Catenin Pathway. Int. J. Mol. Sci. 2023, 24, 12110. [Google Scholar] [CrossRef]
- Pérez-Mora, S.; Ocampo-López, J.; Gómez-García, M.d.C.; Salgado-Hernández, S.V.; Flores-Martinez, Y.M.; Pérez-Ishiwara, D.G. Polyphenols from Bacopa Procumbens Nanostructured with Gold Nanoparticles Stimulate Hair Growth Through Apoptosis Modulation in C57BL/6 Mice. Pharmaceutics 2025, 17, 222. [Google Scholar] [CrossRef]
- Nagarajan, S.; Chellappan, D.R.; Chinnaswamy, P.; Thulasingam, S. Ferulic Acid Pretreatment Mitigates MPTP-Induced Motor Impairment and Histopathological Alterations in C57BL/6 Mice. Pharm. Biol. 2015, 53, 1591–1601. [Google Scholar] [CrossRef]
- Kawagoe, S.; Matsusaki, M.; Mabuchi, T.; Ogasawara, Y.; Watanabe, K.; Ishimori, K.; Saio, T. Mechanistic Insights into Oxidative Response of Heat Shock Factor 1 Condensates. JACS Au 2025, 5, 606–617. [Google Scholar] [CrossRef]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef]
- Wada, M.; Ang, M.J.; Weerasinghe-Mudiyanselage, P.D.E.; Kim, S.-H.; Kim, J.-C.; Shin, T.; Moon, C. Behavioral Characterization in MPTP/p Mouse Model of Parkinson’s Disease. J. Integr. Neurosci. 2021, 20, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Yang, W.; Fu, Y. Levodopa Improves Behavioral Deficits of Mice with Parkinson’s Disease Symptoms via Curbing NLRP3 Inflammasome Activation and Enhancing Tyrosine Hydroxylase Levels in the Striatum and Substantia Nigra. J. Integr. Neurosci. 2024, 23, 2. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Negida, A.; Ahmed, H.; Abdel-Daim, M.M. Neuroprotective Mechanisms of Plant Extracts against MPTP Induced Neurotoxicity: Future Applications in Parkinson’s Disease. Biomed. Pharmacother. 2017, 85, 635–645. [Google Scholar] [CrossRef]
- Xu, N.; Xing, S.; Li, J.; Pang, B.; Liu, M.; Fan, M.; Zhao, Y. Water Extract of Ginseng Alleviates Parkinsonism in MPTP-Induced Parkinson’s Disease Mice. PLoS ONE 2024, 19, e0296424. [Google Scholar] [CrossRef]
- Chang, H.-C.; Liu, K.-F.; Teng, C.-J.; Lai, S.-C.; Yang, S.-E.; Ching, H.; Wu, C.-R. Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress. Nutrients 2019, 11, 252. [Google Scholar] [CrossRef]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective Effect of Naringenin against MPTP-Induced Oxidative Stress. Int. J. Neurosci. 2019, 129, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Yarim, G.F.; Kazak, F.; Yarim, M.; Sozmen, M.; Genc, B.; Ertekin, A.; Gokceoglu, A. Apigenin Alleviates Neuroinflammation in a Mouse Model of Parkinson’s Disease. Int. J. Neurosci. 2025, 135, 505–514. [Google Scholar] [CrossRef]
- Wang, L.; An, H.; Yu, F.; Yang, J.; Ding, H.; Bao, Y.; Xie, H.; Huang, D. The Neuroprotective Effects of Paeoniflorin against MPP+-Induced Damage to Dopaminergic Neurons via the Akt/Nrf2/GPX4 Pathway. J. Chem. Neuroanat. 2022, 122, 102103. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-Gallate Modulates Peripheral Immunity in the MPTP-Induced Mouse Model of Parkinson’s Disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, A.; Lee, H.J.; Ur Rehman, I.; Khan, I.; Alam, S.I.; Kim, M.O. Lupeol, a Plant-Derived Triterpenoid, Protects Mice Brains against Aβ-Induced Oxidative Stress and Neurodegeneration. Biomedicines 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Williamson, T.P.; Johnson, D.A.; Johnson, J.A. Activation of the Nrf2-ARE Pathway by siRNA Knockdown of Keap1 Reduces Oxidative Stress and Provides Partial Protection from MPTP-Mediated Neurotoxicity. Neurotoxicology 2012, 33, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kaidery, N.A.; Banerjee, R.; Yang, L.; Smirnova, N.A.; Hushpulian, D.M.; Liby, K.T.; Williams, C.R.; Yamamoto, M.; Kensler, T.W.; Ratan, R.R.; et al. Targeting Nrf2-Mediated Gene Transcription by Extremely Potent Synthetic Triterpenoids Attenuate Dopaminergic Neurotoxicity in the MPTP Mouse Model of Parkinson’s Disease. Antioxid. Redox Signal. 2013, 18, 139–157. [Google Scholar] [CrossRef]
- Yang, X.-X.; Yang, R.; Zhang, F. Role of Nrf2 in Parkinson’s Disease: Toward New Perspectives. Front. Pharmacol. 2022, 13, 919233. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Jazwa, A.; Rojo, A.I.; García, C.; Fernández-Ruiz, J.; Grochot-Przeczek, A.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Cuadrado, A. Different Susceptibility to the Parkinson’s Toxin MPTP in Mice Lacking the Redox Master Regulator Nrf2 or Its Target Gene Heme Oxygenase-1. PLoS ONE 2010, 5, e11838. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.H.; Trist, B.G.; Nikseresht, S.; Harwood, R.; Roudeau, S.; Rowlands, B.D.; Kreilaus, F.; Cottam, V.; Mor, D.; Richardson, M.; et al. Parkinson-like Wild-Type Superoxide Dismutase 1 Pathology Induces Nigral Dopamine Neuron Degeneration in a Novel Murine Model. Acta Neuropathol. 2025, 149, 22. [Google Scholar] [CrossRef]
- Hussain, S.; Hass, B.S.; Slikker, W.; Ali, S.F. Reduced Levels of Catalase Activity Potentiate MPP+-Induced Toxicity: Comparison between MN9D Cells and CHO Cells. Toxicol. Lett. 1999, 104, 49–56. [Google Scholar] [CrossRef]
- Liao, Y.; Gu, Y.; Wang, J.; Tian, Y.; Ni, X.; Zhou, L.; Ye, Y.; Xia, G. HSF1 Inhibits Microglia Activation to Attenuate Neuroinflammation via Regulating miR-214-3p and NFATc2 in Parkinson’s Disease. Folia Neuropathol. 2023, 61, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Ekimova, I.V.; Plaksina, D.V.; Pastukhov, Y.F.; Lapshina, K.V.; Lazarev, V.F.; Mikhaylova, E.R.; Polonik, S.G.; Pani, B.; Margulis, B.A.; Guzhova, I.V.; et al. New HSF1 Inducer as a Therapeutic Agent in a Rodent Model of Parkinson’s Disease. Exp. Neurol. 2018, 306, 199–208. [Google Scholar] [CrossRef]
- Fujimaki, A.; Ohuchi, K.; Takizawa, S.; Murakami, T.; Kurita, H.; Hozumi, I.; Wen, X.; Kitamura, Y.; Wu, Z.; Maekawa, Y.; et al. The Neuroprotective Effects of FG-4592, a Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitor, against Oxidative Stress Induced by Alpha-Synuclein in N2a Cells. Sci. Rep. 2023, 13, 15629. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Hatori, Y.; Kubo, T.; Saito, S.; Kitamura, H.; Akagi, R. NRF2 and HSF1 Coordinately Regulate Heme Oxygenase-1 Expression. Biochem. Biophys. Res. Commun. 2018, 506, 7–11. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. HIF1, HSF1, and NRF2: Oxidant-Responsive Trio Raising Cellular Defenses and Engaging Immune System. Chem. Res. Toxicol. 2022, 35, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Mazumder, M.K.; Paul, R.; Choudhury, A.; Choudhury, S.; Borah, A. L-DOPA Treatment in MPTP-Mouse Model of Parkinson’s Disease Potentiates Homocysteine Accumulation in Substantia Nigra. Neurosci. Lett. 2016, 628, 225–229. [Google Scholar] [CrossRef]
- Hassani, S.; Esmaeili, A. The Neuroprotective Effects of Ferulic Acid in Toxin-Induced Models of Parkinson’s Disease: A Review. Ageing Res. Rev. 2024, 97, 102299. [Google Scholar] [CrossRef]
- Geng, X.; Tian, X.; Tu, P.; Pu, X. Neuroprotective Effects of Echinacoside in the Mouse MPTP Model of Parkinson’s Disease. Eur. J. Pharmacol. 2007, 564, 66–74. [Google Scholar] [CrossRef]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef]
- Liu, L.-X.; Chen, W.-F.; Xie, J.-X.; Wong, M.-S. Neuroprotective Effects of Genistein on Dopaminergic Neurons in the Mice Model of Parkinson’s Disease. Neurosci. Res. 2008, 60, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, C.; Fan, Y.; Yan, P.; Shi, D.; Zhang, Y. Neuroprotection by Paeoniflorin in the MPTP Mouse Model of Parkinson’s Disease. Neuropharmacology 2017, 116, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Sigmond, T.; Hotzi, B.; Bohár, B.; Fazekas, D.; Deák, V.; Vellai, T.; Barna, J. HSF1Base: A Comprehensive Database of HSF1 (Heat Shock Factor 1) Target Genes. Int. J. Mol. Sci. 2019, 20, 5815. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Muñoz, L.I.O. NORMA Oficial Mexicana NOM-062-ZOO-1999. In Especificaciones Tecnicas Para la Produccion, Cuidado y Uso de los Animales de Laboratorio; SAGARPA: Mexico City, Mexico, 1999. [Google Scholar]
- Wu, D.C.; Jackson-Lewis, V.; Vila, M.; Tieu, K.; Teismann, P.; Vadseth, C.; Choi, D.-K.; Ischiropoulos, H.; Przedborski, S. Blockade of Microglial Activation Is Neuroprotective in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model of Parkinson Disease. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 1763–1771. [Google Scholar] [CrossRef]
- Lane, E.; Dunnett, S. Animal Models of Parkinson’s Disease and L-Dopa Induced Dyskinesia: How Close Are We to the Clinic? Psychopharmacology 2008, 199, 303–312. [Google Scholar] [CrossRef]
- Farfán-García, E.D.; Abad-García, A.; Alatorre, A.; Pérez-Capistran, T.; Querejeta, E.; Soriano-Ursúa, M.A. Olive Oil Limited Motor Disruption and Neuronal Damage in Parkinsonism Induced by MPTP Administration. Toxicol. Res. Appl. 2020, 4, 2397847320922939. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Humana Press/Springer Nature: Totowa, NJ, USA, 2009; pp. 1–20. ISBN 978-1-60761-302-2. [Google Scholar]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A Rotarod Test for Evaluation of Motor Skill Learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef]
- Karl, T.; Pabst, R.; von Hörsten, S. Behavioral Phenotyping of Mice in Pharmacological and Toxicological Research. Exp. Toxicol. Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA Prediction Server: Biological Network Integration for Gene Prioritization and Predicting Gene Function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Hernández, S.V.; Martínez-Retamoza, L.; Ocadiz-Delgado, R.; Pérez-Mora, S.; Cedeño-Arboleda, G.E.; Gómez-García, M.d.C.; Gariglio, P.; Pérez-Ishiwara, D.G. miRNAs Dysregulated in Human Papillomavirus-Associated Benign Prostatic Lesions and Prostate Cancer. Cancers 2025, 17, 26. [Google Scholar] [CrossRef] [PubMed]




| Metabolite | Administered Dose (mg/kg) | Solvent | References |
|---|---|---|---|
| Naringenin | 10 | DMSO 10% | Sugumar et al. [42] |
| Apigenin | 10 | DMSO 20% | Yarim et al. [43] |
| Paeoniflorin | 10 | Water | Wang et al. [44] |
| (−)-Epicatechin | 3 | DMSO 1% | Zhou et al. [45] |
| Lupeol | 6 | DMSO 20% | Ahmad et al. [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rodríguez, M.; Pérez-Mora, S.; Soriano-Ursúa, M.A.; Gómez-García, M.d.C.; Flores-Martinez, Y.M.; Ocampo-López, J.; Zamorano-Carrillo, A.; Viveros-Bartolomé, J.M.; Pérez-Ishiwara, D.G. Aqueous Extract of Bacopa procumbens and the NAPEL Formulation Mitigate MPTP-Induced Neurotoxicity via Nrf2/HSF1/HIF-1α Signaling in a Parkinson’s Disease Model. Int. J. Mol. Sci. 2025, 26, 11914. https://doi.org/10.3390/ijms262411914
Pérez-Rodríguez M, Pérez-Mora S, Soriano-Ursúa MA, Gómez-García MdC, Flores-Martinez YM, Ocampo-López J, Zamorano-Carrillo A, Viveros-Bartolomé JM, Pérez-Ishiwara DG. Aqueous Extract of Bacopa procumbens and the NAPEL Formulation Mitigate MPTP-Induced Neurotoxicity via Nrf2/HSF1/HIF-1α Signaling in a Parkinson’s Disease Model. International Journal of Molecular Sciences. 2025; 26(24):11914. https://doi.org/10.3390/ijms262411914
Chicago/Turabian StylePérez-Rodríguez, Maribel, Salvador Pérez-Mora, Marvin A. Soriano-Ursúa, María del Consuelo Gómez-García, Yazmin Montserrat Flores-Martinez, Juan Ocampo-López, Absalom Zamorano-Carrillo, José Manuel Viveros-Bartolomé, and David Guillermo Pérez-Ishiwara. 2025. "Aqueous Extract of Bacopa procumbens and the NAPEL Formulation Mitigate MPTP-Induced Neurotoxicity via Nrf2/HSF1/HIF-1α Signaling in a Parkinson’s Disease Model" International Journal of Molecular Sciences 26, no. 24: 11914. https://doi.org/10.3390/ijms262411914
APA StylePérez-Rodríguez, M., Pérez-Mora, S., Soriano-Ursúa, M. A., Gómez-García, M. d. C., Flores-Martinez, Y. M., Ocampo-López, J., Zamorano-Carrillo, A., Viveros-Bartolomé, J. M., & Pérez-Ishiwara, D. G. (2025). Aqueous Extract of Bacopa procumbens and the NAPEL Formulation Mitigate MPTP-Induced Neurotoxicity via Nrf2/HSF1/HIF-1α Signaling in a Parkinson’s Disease Model. International Journal of Molecular Sciences, 26(24), 11914. https://doi.org/10.3390/ijms262411914







