Soloxolone N-3-(Dimethylamino)propylamide Suppresses Tumor Growth and Mitigates Doxorubicin-Induced Hepatotoxicity in RLS40 Lymphosarcoma-Bearing Mice
Abstract
1. Introduction
2. Results
2.1. Sol-DMAP Potentiates DOX and Exhibits Direct Antitumor Activity in Murine RLS40 Lymphosarcoma In Vivo
2.1.1. Sol-DMAP Enhances the Antitumor Efficacy of DOX by Increasing Its Intratumoral Concentration
2.1.2. Sol-DMAP Is Well-Tolerated in RLS40 Lymphosarcoma-Bearing Mice
2.1.3. Sol-DMAP Decreases Necrotic Area and Mitotic Activity in RLS40 Lymphosarcoma Tissue
2.1.4. Sol-DMAP Induces ROS-Independent Cell Death via Apoptosis and G1-Arrest in RLS40 Cells In Vitro
2.2. Sol-DMAP Demonstrates a Hepatoprotective Effect in DOX-Treated Mice
2.2.1. Sol-DMAP Attenuates DOX-Induced Liver Damage in RLS40 Tumor-Bearing Mice
2.2.2. Sol-DMAP Induced Antioxidant Response in Hepatocyte-Like Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. In Vivo Experiments
4.1.1. Mice
4.1.2. Tumor Transplantation and Design of Animal Experiment
4.1.3. Toxicity Assessment
4.1.4. Histology, Morphometry, and Immunohistochemistry
4.2. Identification of Compound Concentrations in Murine Blood and Tissues Using HPLS-MS/MS
4.2.1. Blood Pharmacokinetics of Sol-DMAP
4.2.2. Preparation of Calibrators and Experimental Samples for Quantification of Sol-DMAP in Mice Blood
4.2.3. Preparation of Stock Solutions of Sol-DMAP and DOX and Internal Standard Working Solutions for HPLC-MS/MS Analysis of Tumors
4.2.4. Tumor Homogenization Protocol
4.2.5. Preparation of Calibrators and Experimental Samples for Quantification of Sol-DMAP and DOX in Tumors
4.2.6. Instrumentation and LC-MS/MS Conditions
4.3. In Vitro Experiments
4.3.1. Cell Cultures and Evaluated Compound
4.3.2. Cell Viability Assay
4.3.3. Apoptosis Assay
4.3.4. Caspase-3/-7 Activity Assay
4.3.5. ROS Accumulation Assay
4.3.6. Cell Cycle Analysis
4.3.7. Total RNA Isolation
4.3.8. Quantitative Real-Time PCR (RT-qPCR)
4.4. In Silico Prediction
4.4.1. Molecular Docking
4.4.2. Structure Similarity Analysis
4.4.3. Biological Activity Prediction
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, M.; Chen, X.-Y.; Huang, P.; Fleishman, J.S.; Yang, D.-H.; Wu, Z.-X.; Ke, Z.-F.; Chen, Z.-S. Understanding and Overcoming Multidrug Resistance in Cancer. Nat. Rev. Clin. Oncol. 2025, 22, 760–780. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Tian, Q.; Teng, Q.-X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.-S. Understanding and Targeting Resistance Mechanisms in Cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Pastan, I.H. The Role of Multidrug Resistance Efflux Pumps in Cancer: Revisiting a JNCI Publication Exploring Expression of the MDR1 (P-Glycoprotein) Gene. J. Natl. Cancer Inst. 2015, 107, djv222. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, P.; Ali, V.; Verma, M. Role of Membrane-Embedded Drug Efflux ABC Transporters in the Cancer Chemotherapy. Oncol. Rev. 2020, 14, 448. [Google Scholar] [CrossRef]
- Tian, Y.; Lei, Y.; Wang, Y.; Lai, J.; Wang, J.; Xia, F. Mechanism of Multidrug Resistance to Chemotherapy Mediated by P-glycoprotein (Review). Int. J. Oncol. 2023, 63, 119. [Google Scholar] [CrossRef]
- Volm, M.; Efferth, T. Role of P-Glycoprotein for Resistance of Tumors to Anticancer Drugs: From Bench to Bedside. In Resistance to Targeted ABC Transporters in Cancer; Efferth, T., Ed.; Springer: Cham, Swizerland, 2015; pp. 1–26. [Google Scholar]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three Decades of P-Gp Inhibitors: Skimming Through Several Generations and Scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal Chemistry Strategies to Discover P-Glycoprotein Inhibitors: An Update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef] [PubMed]
- Rybalkina, E.Y.; Moiseeva, N.I.; Karamysheva, A.F.; Eroshenko, D.V.; Konysheva, A.V.; Nazarov, A.V.; Grishko, V.V. Triterpenoids with Modified A-Ring as Modulators of P-Gp-Dependent Drug-Resistance in Cancer Cells. Chem. Biol. Interact. 2021, 348, 109645. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.Y.F.; Chang, P.M.H. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef] [PubMed]
- Cascorbi, I. P-Glycoprotein: Tissue Distribution, Substrates, and Functional Consequences of Genetic Variations BT—Drug Transporters. In Drug Transporters. Handbook of Experimental Pharmacology; Fromm, M.F., Kim, R.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 261–283. ISBN 978-3-642-14541-4. [Google Scholar]
- Stupp, R.; Bauer, J.; Pagani, O.; Gerard, B.; Cerny, T.; Sessa, C.; Bastian, G.; Sarkany, M.; Schläpfer, J.; Giroux, B.; et al. Ventricular Arrhythmia and Torsade de Pointe: Dose Limiting Toxicities of the MDR-Modulator S9788 in a Phase I Trial. Ann. Oncol. 1998, 9, 1233–1242. [Google Scholar] [CrossRef]
- Ferry, D.R.; Traunecker, H.; Kerr, D.J. Clinical Trials of P-Glycoprotein Reversal in Solid Tumours. Eur. J. Cancer 1996, 32A, 1070–1081. [Google Scholar] [CrossRef]
- Wan, M.; Xiao, J.; Liu, J.; Yang, D.; Wang, Y.; Liu, J.; Huang, L.; Liu, F.; Xiong, G.; Liao, X.; et al. Cyclosporine A Induces Hepatotoxicity in Zebrafish Larvae via Upregulating Oxidative Stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 266, 109560. [Google Scholar] [CrossRef]
- Mudd, T.W.; Guddati, A.K. Management of Hepatotoxicity of Chemotherapy and Targeted Agents. Am. J. Cancer Res. 2021, 11, 3461–3474. [Google Scholar]
- Radeva, L.; Yoncheva, K. Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress. Molecules 2025, 30, 3311. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Mercês, É.A.B.; Portela, F.S.; Malheiro, L.F.L.; Silva, H.B.L.; De Benedictis, L.M.; De Benedictis, J.M.; d’Ávilla e Silva, C.C.; Santos, A.C.L.; Rosa, D.P.; et al. An Integrated View of Cisplatin-Induced Nephrotoxicity, Hepatotoxicity, and Cardiotoxicity: Characteristics, Common Molecular Mechanisms, and Current Clinical Management. Clin. Exp. Nephrol. 2024, 28, 711–727. [Google Scholar] [CrossRef]
- Kurdziel, K.A.; Kiesewetter, D.O.; Carson, R.E.; Eckelman, W.C.; Herscovitch, P. Biodistribution, Radiation Dose Estimates, and in Vivo Pgp Modulation Studies of 18F-Paclitaxel in Nonhuman Primates. J. Nucl. Med. 2003, 44, 1330–1339. [Google Scholar]
- Hubensack, M.; Müller, C.; Höcherl, P.; Fellner, S.; Spruss, T.; Bernhardt, G.; Buschauer, A. Effect of the ABCB1 Modulators Elacridar and Tariquidar on the Distribution of Paclitaxel in Nude Mice. J. Cancer Res. Clin. Oncol. 2008, 134, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, S.; Mizuno, T.; Fukudo, M.; Katsura, T. Effect of P-Glycoprotein and Breast Cancer Resistance Protein Inhibition on the Pharmacokinetics of Sunitinib in Rats. Drug Metab. Dispos. 2013, 41, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-L.; Zhuang, X.-M.; Yang, H.-Y.; Yuan, M.; Xu, L.; Li, H. Inhibition of P-Glycoprotein Gene Expression and Function Enhances Triptolide-Induced Hepatotoxicity in Mice. Sci. Rep. 2015, 5, 11747. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.H.; Franke, N.E.; Tolboom, R.; Buckle, T.; Rosing, H.; Lopez-Lazaro, L.; Schellens, J.H.M.; Beijnen, J.H.; van Tellingen, O. Disposition and Toxicity of Trabectedin (ET-743) in Wild-Type and Mdr1 Gene (P-Gp) Knock-out Mice. Investig. New Drugs 2010, 28, 145–155. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Dou, J.; Gong, L. Research Progress on the Protective Effects of Licorice-Derived 18β-Glycyrrhetinic Acid against Liver Injury. Acta Pharmacol. Sin. 2021, 42, 18–26. [Google Scholar] [CrossRef]
- Prikhodko, V.A.; Matuzok, T.M.; Karev, V.E.; Karavaeva, A.V.; Spasenkova, O.M.; Kirillova, N.V.; Ivkin, D.Y.; Okovityi, S.V. Glycyrrhizinic Acid and Phosphatidylcholine Combination as a Preventive Therapy for Experimental Murine Non-Alcoholic Steatohepatitis. Livers 2024, 4, 63–83. [Google Scholar] [CrossRef]
- Moralev, A.D.; Zenkova, M.A.; Markov, A.V. Complex Inhibitory Activity of Pentacyclic Triterpenoids against Cutaneous Melanoma In Vitro and In Vivo: A Literature Review and Reconstruction of Their Melanoma-Related Protein Interactome. ACS Pharmacol. Transl. Sci. 2024, 7, 3358–3384. [Google Scholar] [CrossRef]
- Baltina, L.; Karimova, E.; Nugumanov, T.; Petrova, S.; Gabdrakhmanova, S.; Khisamutdinova, R. Synthesis, Modification and Biological Activity of 2,3-Indoles of Glycyrrhetinic Acid. Nat. Prod. Res. 2025, 39, 3991–3996. [Google Scholar] [CrossRef] [PubMed]
- Moralev, A.D.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Markov, A.V. Soloxolone N-3-(Dimethylamino)Propylamide Restores Drug Sensitivity of Tumor Cells with Multidrug-Resistant Phenotype via Inhibition of P-Glycoprotein Efflux Function. Molecules 2024, 29, 4939. [Google Scholar] [CrossRef] [PubMed]
- Mironova, N.; Shklyaeva, O.; Andreeva, E.; Popova, N.; Kaledin, V.; Nikolin, V.; Vlassov, V.; Zenkova, M. Animal Model of Drug-Resistant Tumor Progression. Ann. N. Y. Acad. Sci. 2006, 1091, 490–500. [Google Scholar] [CrossRef]
- Sen’kova, A.V.; Mironova, N.L.; Patutina, O.A.; Ageeva, T.A.; Zenkova, M.A. The Toxic Effects of Polychemotherapy onto the Liver Are Accelerated by the Upregulated MDR of Lymphosarcoma. ISRN Oncol. 2012, 2012, 721612. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to Different Experimental Organ Systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Odarenko, K.V.; Sen’kova, A.V.; Salomatina, O.V.; Markov, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Markov, A.V. Soloxolone Para-Methylanilide Effectively Suppresses Aggressive Phenotype of Glioblastoma Cells Including TGF-Β1-Induced Glial-Mesenchymal Transition in Vitro and Inhibits Growth of U87 Glioblastoma Xenografts in Mice. Front. Pharmacol. 2024, 15, 1428924. [Google Scholar] [CrossRef]
- Markov, A.V.; Ilyina, A.A.; Salomatina, O.V.; Sen’kova, A.V.; Okhina, A.A.; Rogachev, A.D.; Salakhutdinov, N.F.; Zenkova, M.A. Novel Soloxolone Amides as Potent Anti-Glioblastoma Candidates: Design, Synthesis, In Silico Analysis and Biological Activities In Vitro and In Vivo. Pharmaceuticals 2022, 15, 603. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.V.; Sen’kova, A.V.; Babich, V.O.; Odarenko, K.V.; Talyshev, V.A.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Logashenko, E.B. Dual Effect of Soloxolone Methyl on LPS-Induced Inflammation in Vitro and in Vivo. Int. J. Mol. Sci. 2020, 21, 7876. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.V.; Kel, A.E.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Logashenko, E.B. Deep Insights into the Response of Human Cervical Carcinoma Cells to a New Cyano Enone-Bearing Triterpenoid Soloxolone Methyl: A Transcriptome Analysis. Oncotarget 2019, 10, 5267–5297. [Google Scholar] [CrossRef] [PubMed]
- Gatbonton-Schwager, T.; Yagishita, Y.; Joshi, T.; Wakabayashi, N.; Srinivasan, H.; Suzuki, T.; Yamamoto, M.; Kensler, T.W. A Point Mutation at C151 of Keap1 of Mice Abrogates NRF2 Signaling, Cytoprotection in Vitro, and Hepatoprotection in Vivo by Bardoxolone Methyl (CDDO-Me). Mol. Pharmacol. 2023, 104, 51–61. [Google Scholar] [CrossRef]
- Reisman, S.A.; Buckley, D.B.; Tanaka, Y.; Klaassen, C.D. CDDO-Im Protects from Acetaminophen Hepatotoxicity through Induction of Nrf2-Dependent Genes. Toxicol. Appl. Pharmacol. 2009, 236, 109–114. [Google Scholar] [CrossRef]
- Delgado-Montemayor, C.; Cordero-Pérez, P.; Salazar-Aranda, R.; Waksman-Minsky, N. Models of Hepatoprotective Activity Assessment. Med. Univ. 2015, 17, 222–228. [Google Scholar] [CrossRef]
- Singh, R.; Czaja, M.J. Regulation of Hepatocyte Apoptosis by Oxidative Stress. J. Gastroenterol. Hepatol. 2007, 22, S45–S48. [Google Scholar] [CrossRef]
- Meng, X.; Waddington, J.C.; Tailor, A.; Lister, A.; Hamlett, J.; Berry, N.; Park, B.K.; Sporn, M.B. CDDO-Imidazolide Targets Multiple Amino Acid Residues on the Nrf2 Adaptor, Keap1. J. Med. Chem. 2020, 63, 9965–9976. [Google Scholar] [CrossRef]
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; León, R. KEAP1-NRF2 Protein–Protein Interaction Inhibitors: Design, Pharmacological Properties and Therapeutic Potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar] [CrossRef]
- Asano, W.; Hantani, R.; Uhara, T.; Debaene, F.; Nomura, A.; Yamaguchi, K.; Adachi, T.; Otake, K.; Harada, K.; Hantani, Y. Screening Approaches for the Identification of Nrf2-Keap1 Protein-Protein Interaction Inhibitors Targeting Hot Spot Residues. SLAS Discov. 2024, 29, 100125. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Dylenova, E.P.; Goncharova, D.B.; Zhigzhitzhapov, B.V.; Emelyanova, E.A.; Polonova, A.V.; Tykheev, Z.A.; Bazarsadueva, S.V.; Taraskina, A.S.; Pintaeva, E.T.; et al. Functional Activity of the Antioxidant System of Artemisia Genus Plants in the Republic of Buryatia (Russia) and Its Significance in Plant Adaptation. Plants 2024, 13, 2630. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; De Feo, V.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-Gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Imbs, T.I.; Usoltseva, R.V.; Ermakova, S.P. Brown Alga Costaria Costata as a Source of Phlorethol That Inhibits TPA-Induced Neoplastic Cell Transformation and Progression of Human Breast Cancer Cells via AKT/GSK-3β/CDKs Pathway. Algal Res. 2025, 90, 104162. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Kazakova, O.B. Antiviral Potency of Lupane and Oleanane Alkynyl-Derivatives against Human Cytomegalovirus and Papillomavirus. J. Antibiot. 2024, 77, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Chen, P. Effect of Tumor Shape and Size on Drug Delivery to Solid Tumors. J. Biol. Eng. 2012, 6, 4. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, J.; Zhao, L.; Luo, Q.; Jin, X. Expression and Clinical Significance of Multidrug Resistance Proteins in Brain Tumors. J. Exp. Clin. Cancer Res. 2010, 29, 122. [Google Scholar] [CrossRef]
- Patel, K.J.; Tannock, I.F. The Influence of P-Glycoprotein Expression and Its Inhibitors on the Distribution of Doxorubicin in Breast Tumors. BMC Cancer 2009, 9, 356. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, Y.; Wang, Z.-Y.; Li, X.; Zheng, P.; Zhang, T.-C. MRTF-A Can Activate Nrf2 to Increase the Resistance to Doxorubicin. Oncotarget 2017, 8, 8436–8446. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, F.; Sun, Z.; Zhou, W.; Li, Z.; You, Q.; Guo, Q.; Hu, R. Drug Resistance Associates with Activation of Nrf2 in MCF-7/DOX Cells, and Wogonin Reverses It by down-Regulating Nrf2-Mediated Cellular Defense Response. Mol. Carcinog. 2013, 52, 824–834. [Google Scholar] [CrossRef]
- Ryoo, I.G.; Kim, G.; Choi, B.H.; Lee, S.H.; Kwak, M.K. Involvement of NRF2 Signaling in Doxorubicin Resistance of Cancer Stem Cell-Enriched Colonospheres. Biomol. Ther. 2016, 24, 482. [Google Scholar] [CrossRef]
- Salomatina, O.V.; Sen’kova, A.V.; Moralev, A.D.; Savin, I.A.; Komarova, N.I.; Salakhutdinov, N.F.; Zenkova, M.A.; Markov, A.V. Novel Epoxides of Soloxolone Methyl: An Effect of the Formation of Oxirane Ring and Stereoisomerism on Cytotoxic Profile, Anti-Metastatic and Anti-Inflammatory Activities In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 6214. [Google Scholar] [CrossRef]
- Markov, A.V.; Sen’kova, A.V.; Popadyuk, I.I.; Salomatina, O.V.; Logashenko, E.B.; Komarova, N.I.; Ilyina, A.A.; Salakhutdinov, N.F.; Zenkova, M.A. Novel 3′-Substituted-1′,2′,4′-Oxadiazole Derivatives of 18βh-Glycyrrhetinic Acid and Their o-Acylated Amidoximes: Synthesis and Evaluation of Antitumor and Anti-Inflammatory Potential in Vitro and in Vivo. Int. J. Mol. Sci. 2020, 21, 3511. [Google Scholar] [CrossRef]
- Satomi, Y.; Nishino, H.; Shibata, S. Glycyrrhetinic Acid and Related Compounds Induce G1 Arrest and Apoptosis in Human Hepatocellular Carcinoma HepG2. Anticancer Res. 2005, 25, 4043–4047. [Google Scholar]
- Liu, J.-J.; Nilsson, A.; Oredsson, S.; Badmaev, V.; Zhao, W.-Z.; Duan, R.-D. Boswellic Acids Trigger Apoptosis via a Pathway Dependent on Caspase-8 Activation but Independent on Fas/Fas Ligand Interaction in Colon Cancer HT-29 Cells. Carcinogenesis 2002, 23, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Cameron, S. Effects of Systemic Chemotherapy on the Liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Ferreira, M.J.U.; Dos Santos, D.J.V.A. Molecular Docking Characterizes Substrate-Binding Sites and Efflux Modulation Mechanisms within P-Glycoprotein. J. Chem. Inf. Model. 2013, 53, 1747–1760. [Google Scholar] [CrossRef]
- Lin, J.H.; Yamazaki, M. Role of P-Glycoprotein in Pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Lund, M.; Petersen, T.S.; Dalhoff, K.P. Clinical Implications of P-Glycoprotein Modulation in Drug–Drug Interactions. Drugs 2017, 77, 859–883. [Google Scholar] [CrossRef]
- Liu, M.; Reddy, N.M.; Higbee, E.M.; Potteti, H.R.; Noel, S.; Racusen, L.; Kensler, T.W.; Sporn, M.B.; Reddy, S.P.; Rabb, H. The Nrf2 Triterpenoid Activator, CDDO-Imidazolide, Protects Kidneys from Ischemia–Reperfusion Injury in Mice. Kidney Int. 2014, 85, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ye, M.; Wang, C.; Wang, Z.; Zhou, W. Protective Effect of CDDO-Imidazolide against Intestinal Ischemia/Reperfusion Injury in Mice. Eur. J. Inflamm. 2018, 16, 2058739218802681. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Moralev, A.D.; Markov, O.V.; Zenkova, M.A.; Markov, A. V Novel Cross-Cancer Hub Genes in Doxorubicin Resistance Identified by Transcriptional Mapping. Biomedicines 2025, 13, 2527. [Google Scholar] [CrossRef]
- Markov, A.V.; Savin, I.A.; Zenkova, M.A.; Sen’kova, A. V Identification of Novel Core Genes Involved in Malignant Transformation of Inflamed Colon Tissue Using a Computational Biology Approach and Verification in Murine Models. Int. J. Mol. Sci. 2023, 24, 4311. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.-H.; Vreven, T.; Weng, Z. ZDOCK Server: Interactive Docking Prediction of Protein-Protein Complexes and Symmetric Multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Karatzas, E.; Zamora, J.E.; Athanasiadis, E.; Dellis, D.; Cournia, Z.; Spyrou, G.M. ChemBioServer 2.0: An Advanced Web Server for Filtering, Clustering and Networking of Chemical Compounds Facilitating Both Drug Discovery and Repurposing. Bioinformatics 2020, 36, 2602–2604. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]


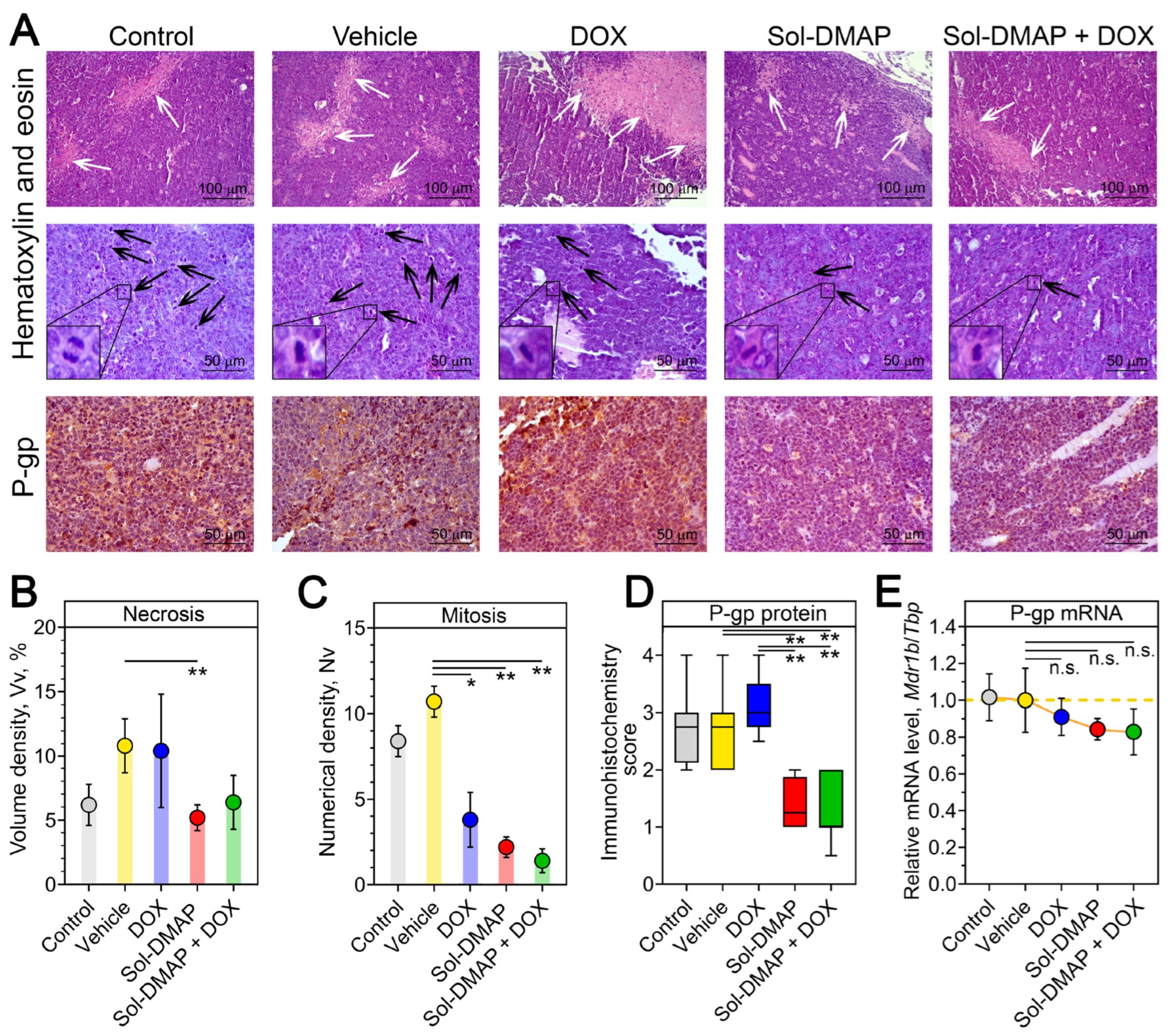


| Healthy | Control | Vehicle | DOX | Sol-DMAP | Sol-DMAP + DOX | |
|---|---|---|---|---|---|---|
| Unchanged liver tissue, Vv, % | 71.2 ± 2.3 | 24.7 ± 1.6 | 33.9 ± 1.4 | 33.4 ± 3.3 * | 42.2 ± 3.3 **# | 56 ± 2.8 **##^^ |
| Dystrophy, Vv, % | 7 ± 0.6 | 37.9 ± 1.9 | 27.5 ± 1.9 | 23.1 ± 2.9 ** | 16.9 ± 2.3 **# | 10.5 ± 1.3 **##^ |
| Necrosis, Vv, % | 7.6 ± 1.5 | 24.8 ± 1.2 | 26.8 ± 1.1 | 20.9 ± 1.7 # | 24 ± 1.1 | 16 ± 3 *# |
| Total destructive changes, Vv, % | 14.6 ± 1.5 | 62.7 ± 1.7 | 54.6 ± 1.7 | 44 ± 3.9 **# | 40.9 ± 3.1 **## | 26.5 ± 4.2 **##^ |
| Blood vessels, Vv, % | 4.3 ± 0.4 | 6.1 ± 0.6 | 5.3 ± 0.5 | 15.5 ± 0.7 **## | 5.8 ± 0.6 ^^ | 5.8 ± 0.4 ^^ |
| Other, Vv, % | 9.9 ± 0.8 | 6.6 ± 0.3 | 6.6 ± 0.6 | 7 ± 0.8 | 11.1 ± 0.9 **##^ | 11.8 ± 1.7 *#^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moralev, A.D.; Sen’kova, A.V.; Firsova, A.A.; Solomina, D.E.; Rogachev, A.D.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Markov, A.V. Soloxolone N-3-(Dimethylamino)propylamide Suppresses Tumor Growth and Mitigates Doxorubicin-Induced Hepatotoxicity in RLS40 Lymphosarcoma-Bearing Mice. Int. J. Mol. Sci. 2025, 26, 11912. https://doi.org/10.3390/ijms262411912
Moralev AD, Sen’kova AV, Firsova AA, Solomina DE, Rogachev AD, Salomatina OV, Salakhutdinov NF, Zenkova MA, Markov AV. Soloxolone N-3-(Dimethylamino)propylamide Suppresses Tumor Growth and Mitigates Doxorubicin-Induced Hepatotoxicity in RLS40 Lymphosarcoma-Bearing Mice. International Journal of Molecular Sciences. 2025; 26(24):11912. https://doi.org/10.3390/ijms262411912
Chicago/Turabian StyleMoralev, Arseny D., Aleksandra V. Sen’kova, Alina A. Firsova, Daria E. Solomina, Artem D. Rogachev, Oksana V. Salomatina, Nariman F. Salakhutdinov, Marina A. Zenkova, and Andrey V. Markov. 2025. "Soloxolone N-3-(Dimethylamino)propylamide Suppresses Tumor Growth and Mitigates Doxorubicin-Induced Hepatotoxicity in RLS40 Lymphosarcoma-Bearing Mice" International Journal of Molecular Sciences 26, no. 24: 11912. https://doi.org/10.3390/ijms262411912
APA StyleMoralev, A. D., Sen’kova, A. V., Firsova, A. A., Solomina, D. E., Rogachev, A. D., Salomatina, O. V., Salakhutdinov, N. F., Zenkova, M. A., & Markov, A. V. (2025). Soloxolone N-3-(Dimethylamino)propylamide Suppresses Tumor Growth and Mitigates Doxorubicin-Induced Hepatotoxicity in RLS40 Lymphosarcoma-Bearing Mice. International Journal of Molecular Sciences, 26(24), 11912. https://doi.org/10.3390/ijms262411912








