Toxicology Effects of Cadmium in Pomacea canaliculate: Accumulation, Oxidative Stress, Microbial Community, and Transcriptome Analysis
Abstract
1. Introduction
2. Results
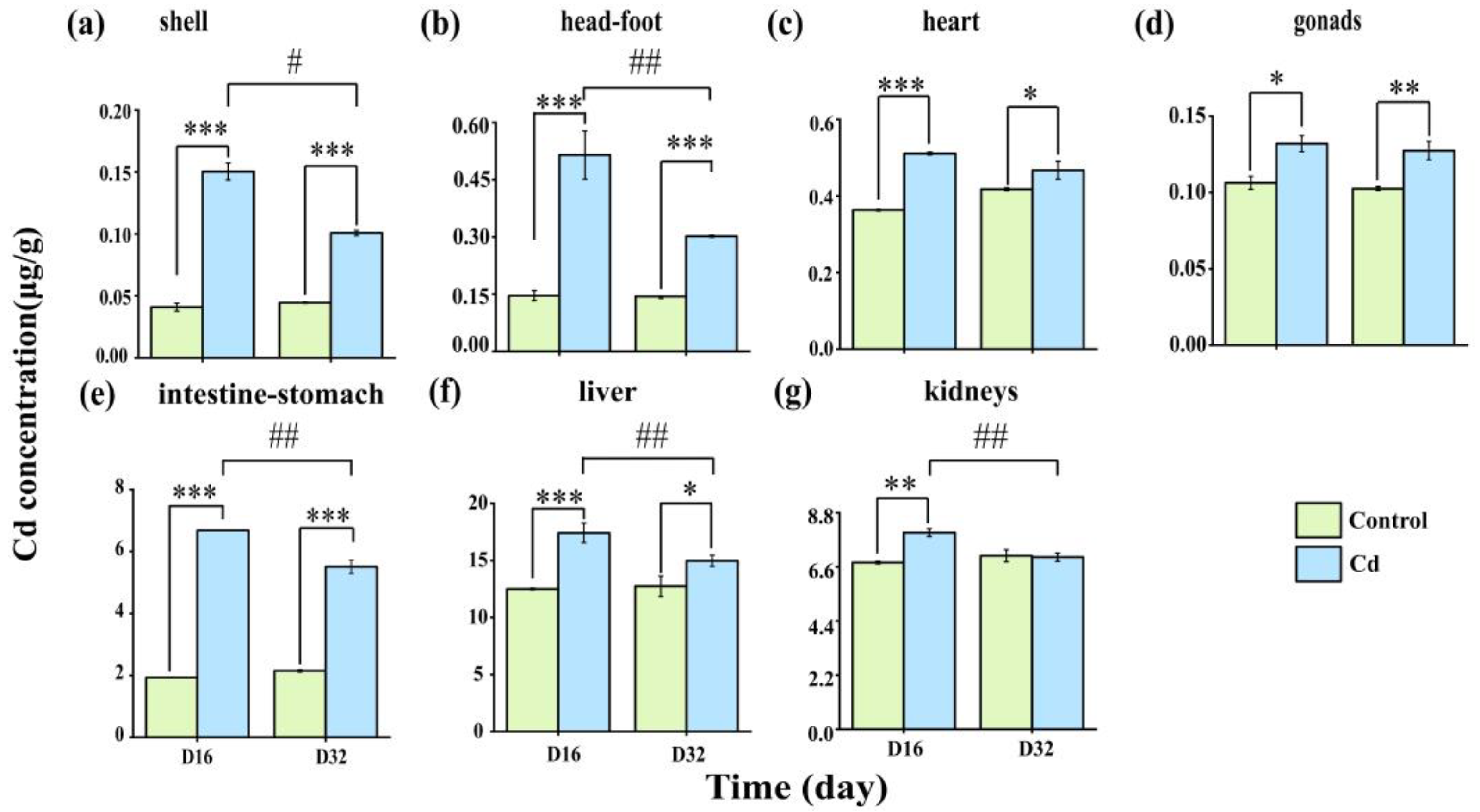
2.1. Cd Accumulation and Elimination in the Tissues of P. canaliculata
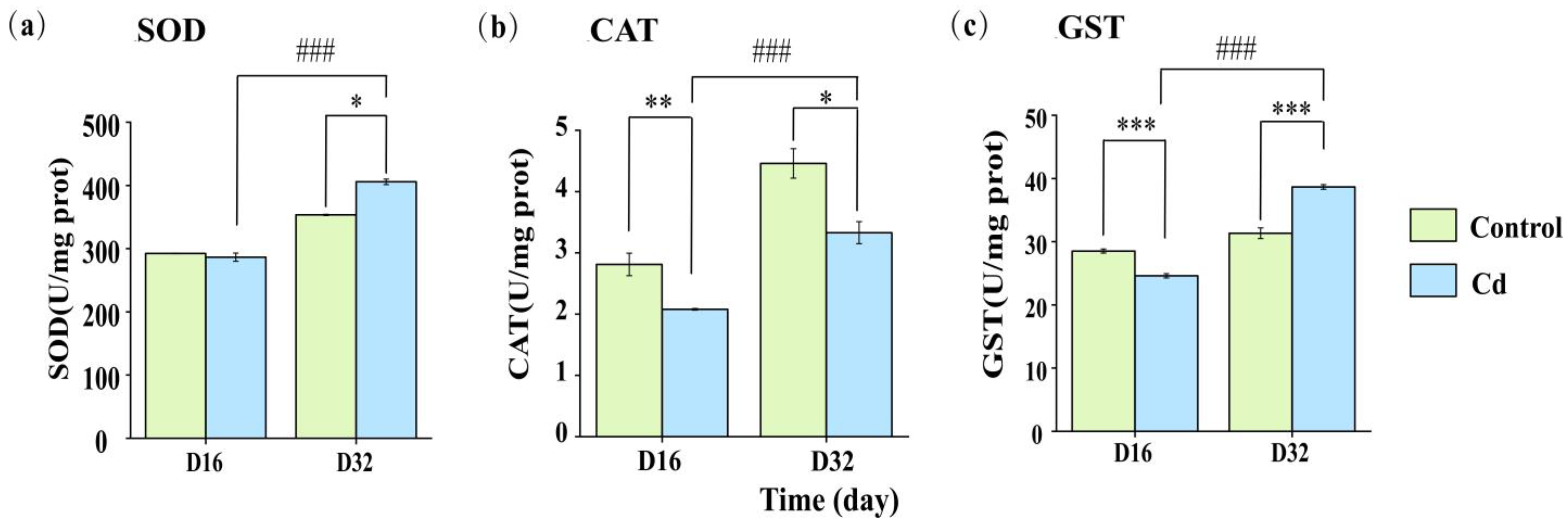
2.2. Enzyme Activity Response of Snails Under Cd Stress
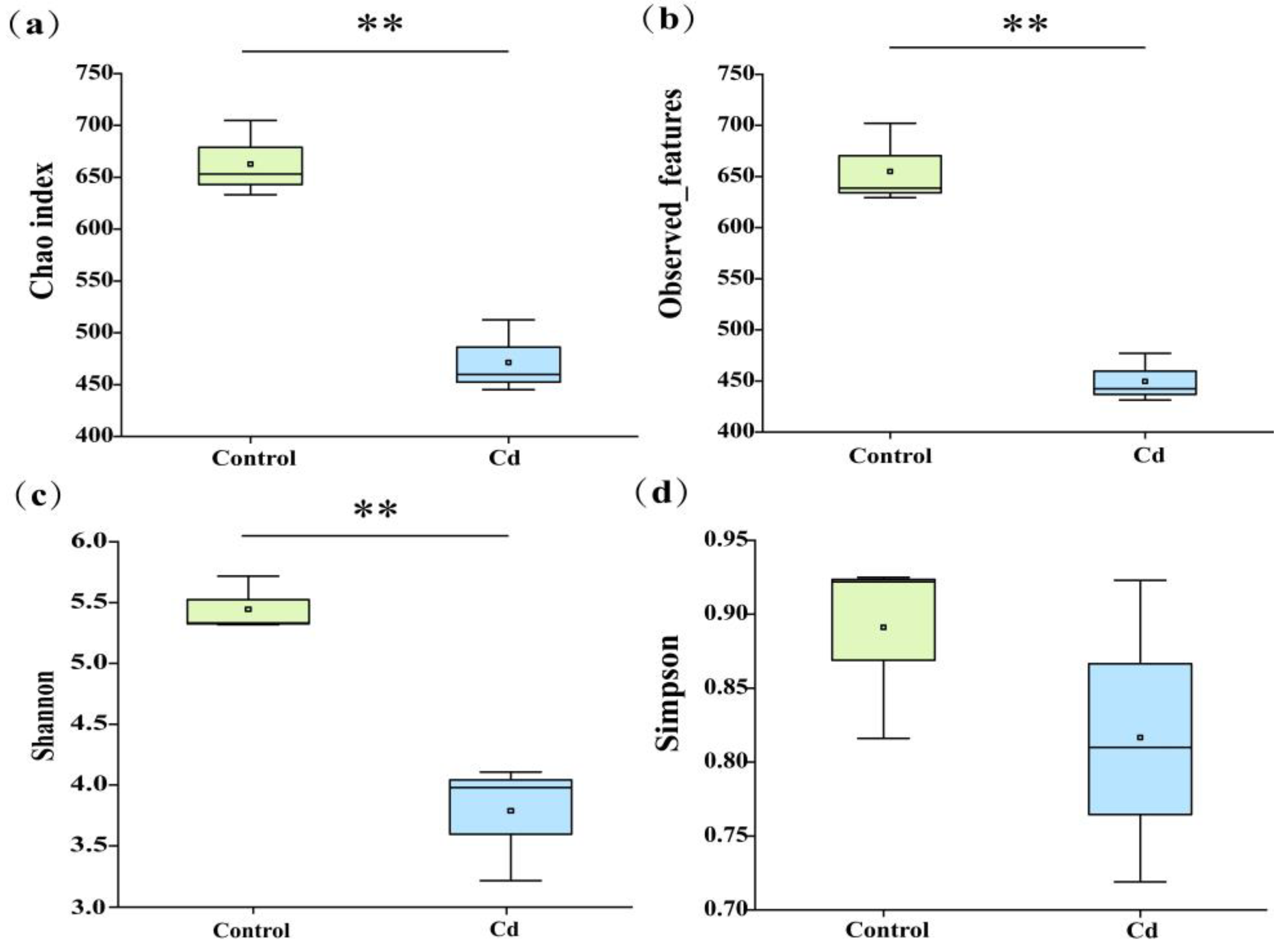
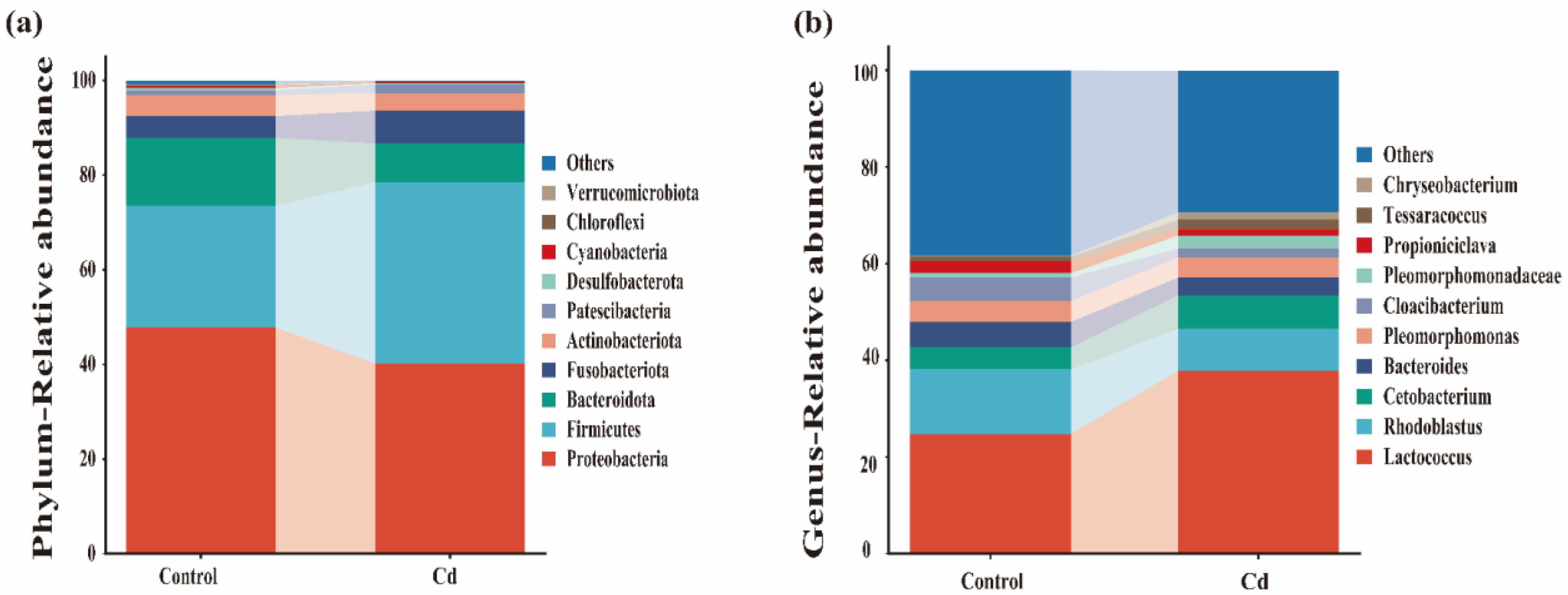
2.3. Characterization of the Gut Microbiota
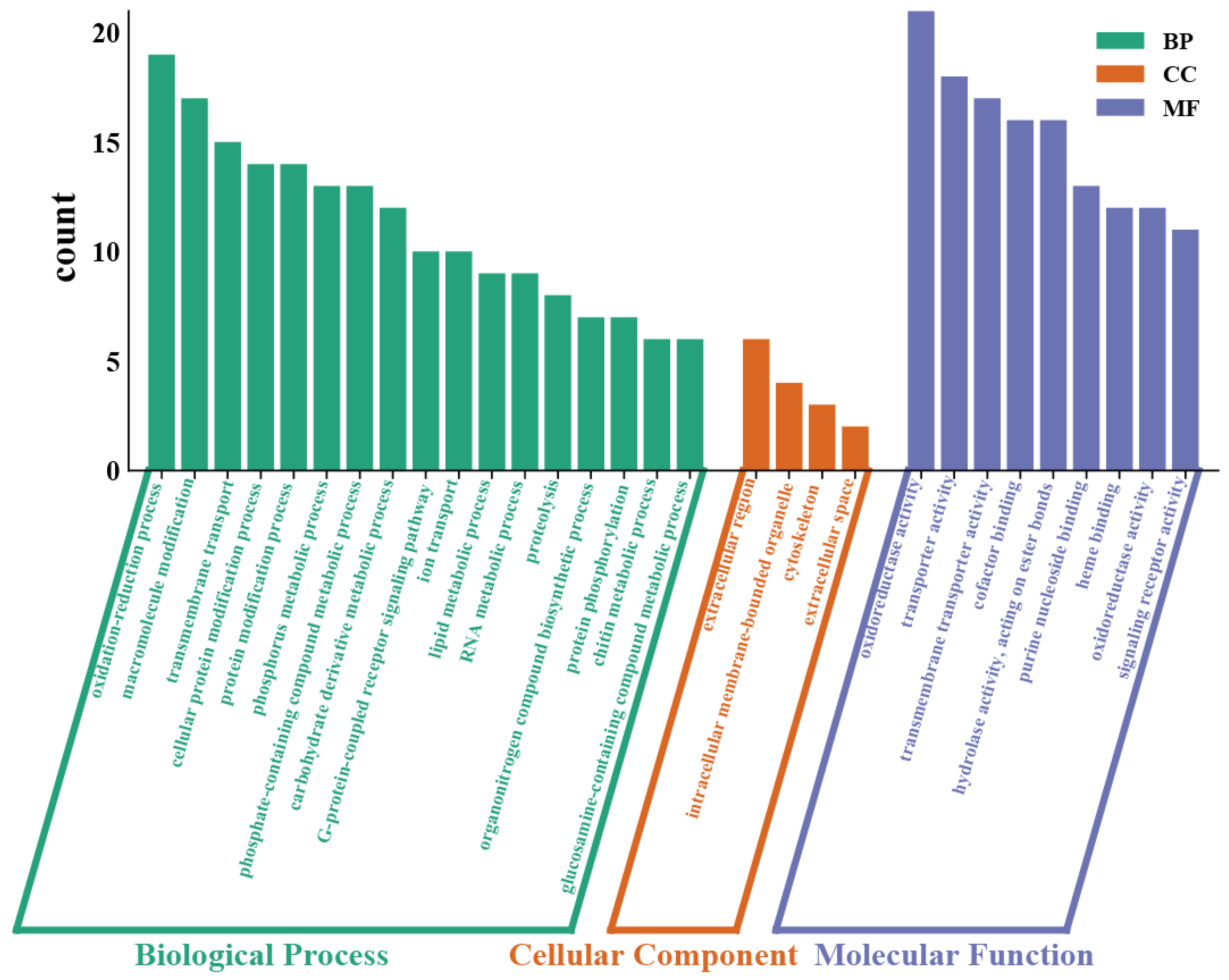
2.4. Transcriptomic Analysis
3. Discussion
3.1. Cd Accumulation and Elimination
3.2. Effect of Cd Stress on the Enzyme Activity
3.3. Taxonomic Composition Changes of Gut Microbiota
3.4. Effects of Cd Exposure on Gene Regulation in the Transcriptome
4. Materials and Methods
4.1. Animals and Experimental Conditions
4.2. Experimental Design
4.3. Enzyme Activity Assay
4.4. Cd Concentration Determination
4.5. Analysis of Gut Microbiota
4.6. RNA Extraction and Transcriptome Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharyya, K.; Sen, D.; Banik, A.K.; Ganguly, S. Adsorptive removal of cadmium from aqueous medium–A critical review. Phys. Chem. Earth 2024, 134, 10353. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Mitigating cadmium accumulation and toxicity in plants: The promising role of nanoparticles. Sci. Total. Environ. 2024, 912, 168826. [Google Scholar] [CrossRef]
- Motta, C.M.; Rosati, L.; Cretì, P.; Montinari, M.R.; Denre, P.; Simoniello, P.; Fogliano, C.; Scudiero, R.; Avallone, B. Histopathological effects of long-term exposure to realistic concentrations of cadmium in the hepatopancreas of Sparus aurata juveniles. Aquat. Toxicol. 2024, 268, 106858. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, F.; Fu, L.; Li, Y.; Li, M.; Yu, Z. Responses of Asian clams (Corbicula fluminea) to low concentration cadmium stress: Whether the depuration phase restores physiological characteristics. Environ. Pollut. 2021, 284, 117182. [Google Scholar] [CrossRef]
- Xu, Y.; Gui, Y.; Zhi, D.; Pi, J.; Liu, X.; Xiang, J.; Li, D.; Li, J. Protective effects of calcium against cadmium-induced toxicity in juvenile grass carp (Ctenopharyngodon idellus). Ecotoxicol. Environ. Saf. 2023, 258, 114972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wong, J.; Wang, L.; Sun, F.; Lee, M.; Yue, G.H. Unveiling the underwater threat: Exploring cadmium’s adverse effects on tilapia. Sci. Total. Environ. 2024, 912, 169104. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Z.; Zhang, Y.; Zhang, P.; Liu, J.; Cheng, Y.; Zhang, L.; Li, Y. Effect on intestinal microbiota, bioaccumulation, and oxidative stress of Carassius auratus gibelio under waterborne cadmium exposure. Fish Physiol. Biochem. 2020, 46, 2299–2309. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Hao, Z.; Liu, Y.; Qiu, M.; Kang, L.; Teng, X.; Tang, Y. Cadmium induced time-dependent kidney injury in common carp via mitochondrial pathway: Impaired mitochondrial energy metabolism and mitochondrion-dependent apoptosis. Aquat. Toxicol. 2023, 261, 106570. [Google Scholar] [CrossRef]
- Li, H.; Liang, Z.; Wu, L.; Ke, Y.; Que, H.; Shi, B. The role of Zip1 and Zip3 in cadmium accumulation in Fujian oyster (Crassostrea angulata). Front. Mar. Sci. 2024, 11, 1412127. [Google Scholar] [CrossRef]
- Ya, J.; Li, X.; Wang, L.; Kou, H.; Wang, H.; Zhao, H. The effects of chronic cadmium exposure on the gut of Bufo gargarizans larvae at metamorphic climax: Histopathological impairments, microbiota changes and intestinal remodeling disruption. Ecotoxicol. Environ. Saf. 2020, 195, 110523. [Google Scholar] [CrossRef] [PubMed]
- Motta-Romero, H.A.; Perez-Donado, C.E.; Auchtung, J.M.; Rose, D.J. Toxicity of cadmium on dynamic human gut microbiome cultures and the protective effect of cadmium-tolerant bacteria autochthonous to the gut. Chemosphere 2023, 338, 139581. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; Ju, Z.; Wang, H.; Zhao, H. Exposure to cadmium induced gut histopathological damages and microbiota alterations of Chinese toad (Bufo gargarizans) larvae. Ecotoxicol. Environ. Saf. 2019, 180, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zink, L.; Simonis, C.; Wiseman, S.; Pyle, G.G. In vitro characterization of cadmium transport across the gastro-intestinal membrane of the fathead minnow (Pimephales promelas) in the presence and absence of microplastics. Environ. Toxicol. Pharmacol. 2023, 101, 104195. [Google Scholar] [CrossRef]
- Bao, X.; Wang, W.; Chen, X.; Feng, Y.; Xu, X.; Sun, G.; Li, B.; Liu, X.; Li, Z.; Yang, J. Exploration of immune response mechanisms in cadmium and copper co-exposed juvenile golden cuttlefish (Sepia esculenta) based on transcriptome profiling. Front. Immunol. 2022, 13, 963931. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kang, X.; Shang, D.; Ning, J.; Ding, H.; Zhai, Y.; Sheng, X. Hyperaccumulation of cadmium by scallop Chlamys farreri revealed by comparative transcriptome analysis. BioMetals 2020, 33, 397–413. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Park, S.Y.; Choi, J. Expression of catalase and glutathione S-transferase genes in Chironomus riparius on exposure to cadmium and nonylphenol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 399–408. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Li, P.; Liu, L.; Li, Z.-H. Transcriptomic and proteomic analysis of Chinese rare minnow (Gobiocypris rarus) larvae in response to acute waterborne cadmium or mercury stress. Aquat. Toxicol. 2022, 246, 106134. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, J.; Saveanu, L.; Martín, P.R.; Shi, Z.; Zhang, J. Invasiveness of Pomacea canaliculata: The Differences in Life History Traits of Snail Populations from Invaded and Native Areas. Agronomy 2023, 13, 1259. [Google Scholar] [CrossRef]
- Dummee, V.; Tanhan, P.; Kruatrachue, M.; Damrongphol, P.; Pokethitiyook, P. Histopathological changes in snail, Pomacea canaliculata, exposed to sub-lethal copper sulfate concentrations. Ecotoxicol. Environ. Saf. 2015, 122, 290–295. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. GigaScience 2018, 7, giy101. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Peng, Z.; Wu, H.; Zhang, X.; Ji, C.; Peng, X. Stress responses in expressions of microRNAs in mussel Mytilus galloprovincialis exposed to cadmium. Ecotoxicol. Environ. Saf. 2021, 212, 111927. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Kang, X.; Ding, H.; Sheng, X.; Tan, Z. Bioaccessibility and transformation of cadmium in different tissues of Zhikong scallops (Chlamys farreri) during in vitro gastrointestinal digestion. Food Chem. 2023, 402, 134285. [Google Scholar] [CrossRef]
- Li, R.; Hao, Y.; Shen, Y.; Gui, L.; Lv, W.; Yuan, L.; Du, B.; Xie, L.; Li, J.; Xu, X. Impact of cadmium and diclofenac exposure on biochemical responses, transcriptome, gut microflora, and growth performance in grass carp (Ctenopharyngodon idella). Chemosphere 2024, 360, 142428. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, V.; Haase, H.; Maares, M. Interaction and competition for intestinal absorption by zinc, iron, copper, and manganese at the intestinal mucus layer. J. Trace Elements Med. Biol. 2024, 84, 127459. [Google Scholar] [CrossRef]
- Souid, G.; Souayed, N.; Yaktiti, F.; Maaroufi, K. Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol. Environ. Saf. 2013, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Jebali, J.; Chouba, L.; Banni, M.; Boussetta, H. Comparative study of the bioaccumulation and elimination of trace metals (Cd, Pb, Zn, Mn and Fe) in the digestive gland, gills and muscle of bivalve Pinna nobilis during a field transplant experiment. J. Trace Elements Med. Biol. 2014, 28, 212–217. [Google Scholar] [CrossRef]
- Jing, W.; Lang, L.; Lin, Z.; Liu, N.; Wang, L. Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere 2019, 219, 321–327. [Google Scholar] [CrossRef]
- Cinier, C.d.C.; Petit-Ramel, M.; Faure, R.; Garin, D.; Bouvet, Y. Kinetics of cadmium accumulation and elimination in carp Cyprinus carpio tissues. Comp. Biochem. Physiol. 1999, 122, 345–352. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, J.; Xie, Y.; Chang, X.; Li, J.; Ren, C.; Zhu, J.; Ren, L.; Qi, K.; Bai, Z.; et al. Dual role of cadmium in rat liver: Inducing liver injury and inhibiting the progression of early liver cancer. Toxicol. Lett. 2022, 355, 62–81. [Google Scholar] [CrossRef]
- Thangal, S.H.; Priya, R.N.; Vasuki, C.; Gayathri, V.; Anandhan, K.; Yogeshwaran, A.; Muralisankar, T.; Ramesh, M.; Rajaram, R.; Santhanam, P.; et al. The impact of ocean acidification and cadmium toxicity in the marine crab Scylla serrata: Biological indices and oxidative stress responses. Chemosphere 2023, 345, 140447. [Google Scholar] [CrossRef]
- Lee, J.-W.; Jo, A.-H.; Lee, D.-C.; Choi, C.Y.; Kang, J.-C.; Kim, J.-H. Review of cadmium toxicity effects on fish: Oxidative stress and immune responses. Environ. Res. 2023, 236, 116600. [Google Scholar] [CrossRef]
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Luo, K.; Liu, Y.; Zhou, M.; Yan, S.; Shi, H.; Cai, Y. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem. Toxicol. 2013, 58, 61–67. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Wang, G.; Guan, T.; Zhu, C.; Li, J.; Wang, H. Effects of cadmium on antioxidant and non-specific immunity of Macrobrachium nipponense. Ecotoxicol. Environ. Saf. 2021, 224, 112651. [Google Scholar] [CrossRef]
- Hernández-Cruz, E.Y.; Amador-Martínez, I.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Chaverri, J.P. Renal damage induced by cadmium and its possible therapy by mitochondrial transplantation. Chem. Biol. Interactions 2022, 361, 109961. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidneys Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cao, L.; Liu, J.; Ren, Z.; Zhao, B.; Dou, S. Effects of seawater acidification and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total Environ. 2020, 718, 137234. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, H.M.; Williams, C.R.; Gallagher, E.P. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat. Toxicol. 2012, 110–111, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cheng, J.; Bao, L.; Zhu, X.; Li, H.; Chen, X.; Zhang, Y.; Zhang, J.; Chu, W.; Pan, Y.; et al. Exposure to waterborne cadmium induce oxidative stress, autophagy and mitochondrial dysfunction in the liver of Procypris merus. Ecotoxicol. Environ. Saf. 2020, 204, 111051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Feng, Y.; Ren, N.; Sun, K. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci. Total Environ. 2019, 666, 944–955. [Google Scholar] [CrossRef]
- Wang, H.; Han, Q.; Chen, Y.; Hu, G.; Xing, H. Novel insights into cytochrome P450 enzyme and solute carrier families in cadmium-induced liver injury of pigs. Ecotoxicol. Environ. Saf. 2021, 211, 111910. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-C.; Choi, Y.J.; Kim, J.-H. Toxic effects of waterborne cadmium exposure on hematological parameters, oxidative stress, neurotoxicity, and heat shock protein 70 in juvenile olive flounder, Paralichthys olivaceus. Fish Shellfish. Immunol. 2022, 122, 476–483. [Google Scholar] [CrossRef]
- Li, S.; Qian, Z.; Yang, J.; Lin, Y.; Li, H.; Chen, L. Seasonal variation in structure and function of gut microbiota in Pomacea canaliculata. Ecol. Evol. 2022, 12, e9162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, H.; Li, D.; Zeng, W.; Huang, J.; Wu, Z. Comparison of gut microbiome in the Chinese mud snail (Cipangopaludina chinensis) and the invasive golden apple snail (Pomacea canaliculata). PeerJ 2022, 10, e13245. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Y.; Hou, J.-L.; Li, J.; Feng, X.-L.; Liu, X.-H.; Li, D.-L.; Xiang, J.-G.; Li, J.-H. Comparative profiling of the skin and gut microbiota during metamorphosis in Chinese spiny frogs (Quasipaa spinosa) highlights microbial communities involved in disease pathogenesis. Aquaculture 2024, 585, 740726. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Ma, H.-L.; Liu, G.-X.; Fan, S.-G.; Deng, Y.-Q.; Jiang, J.-J.; Feng, J.; Guo, Z.-X. Toxic effects of cadmium exposure on intestinal histology, oxidative stress, microbial community, and transcriptome change in the mud crab (Scylla paramamosain). Chemosphere 2023, 326, 138464. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Si, J.; Li, P.; Gao, H.; Li, W.; Chen, Y. What happens to gut microorganisms and potential repair mechanisms when meet heavy metal(loid)s. Environ. Pollut. 2023, 317, 120780. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Chen, C.; Feng, Y.; Ren, N.; Sun, K. Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere 2020, 242, 125105. [Google Scholar] [CrossRef]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef] [PubMed]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Da, Y.M.; Li, S.S.; Li, Y.Q.; Deng, L.Y.; Li, M.J.; Huang, T.; Sun, Q.Y.; Shirin, J.; Zhou, G.W. Effects of cadmium on the intestinal health of the snail Bradybaena ravida Benson. Ecotoxicology 2024, 33, 849–858. [Google Scholar] [CrossRef]
- Xie, M.; Hao, Q.; Olsen, R.E.; Ringø, E.; Yang, Y.; Zhang, Z.; Ran, C.; Zhou, Z. Growth performance, hepatic enzymes, and gut health status of common carp (Cyprinus carpio) in response to dietary Cetobacterium somerae fermentation product. Aquac. Rep. 2022, 23, 101049. [Google Scholar] [CrossRef]
- Gu, J.; Kong, A.; Guo, C.; Liu, J.; Li, K.; Ren, Z.; Zhou, Y.; Tang, M.; Shi, H. Cadmium perturbed lipid profile and induced liver dysfunction in mice through phosphatidylcholine remodeling and promoting arachidonic acid synthesis and metabolism. Ecotoxicol. Environ. Saf. 2022, 247, 114254. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.-Y.; Zhou, X.-P.; Liu, Z.-P.; Song, X.-B.; Wang, Z.-Y.; Wang, L. Cadmium disrupts autophagic flux by inhibiting cytosolic Ca2+ -dependent autophagosome-lysosome fusion in primary rat proximal tubular cells. Toxicology 2017, 383, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, T.; Yuan, J.; Sun, J.; Yuan, Y.; Gu, J.; Liu, X.; Bian, J.; Liu, Z. Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes. Toxicol. Lett. 2020, 321, 32–43. [Google Scholar] [CrossRef]
- Cong, Y.; Chi, Q.; Teng, X.; Li, S. The Protection of Selenium Against Cadmium-Induced Mitochondrial Damage via the Cytochrome P450 in the Livers of Chicken. Biol. Trace Element Res. 2019, 190, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, B.; Liu, X.; Liu, D.; Zhou, Z.; Wang, P. Influence of co-exposure to sulfamethazine on the toxicity and bioaccumulation kinetics of chlorpyrifos in zebrafish (Danio rerio). Chemosphere 2022, 308, 136317. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, L.; Wang, K.; Huang, R.; Yu, W.; Yan, B.; Wang, H.; Zhang, C.; Yang, Z.; Liu, Z. Role of endoplasmic reticulum stress in cadmium-induced hepatocyte apoptosis and the protective effect of quercetin. Ecotoxicol. Environ. Saf. 2022, 241, 113772. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, H.; Bai, L.; Wang, L.; Zou, X. Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult. Sci. 2020, 99, 3215–3228. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Liu, Y. Effects of cadmium on the actin cytoskeleton in renal mesangial cells. Can. J. Physiol. Pharmacol. 2013, 91, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Q.-G.; Huang, L.; Ye, Z.; Wang, X.; Xiao, T.; Wu, Y.; Zhang, W.; Yan, B. Intestinal uptake and low transformation increase the bioaccumulation of inorganic arsenic in freshwater zebrafish. J. Hazard. Mater. 2022, 434, 128904. [Google Scholar] [CrossRef]
- Hu, F.X.; Yin, L.; Dong, F.L.; Zheng, M.Y.; Zhao, Y.X.; Fu, S.R.; Zhang, W.N.; Chen, X.H. Effects of long-term cadmium exposure on growth, antioxidant defense and DNA methylation in juvenile Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2021, 241, 106014. [Google Scholar] [CrossRef]
- Kodzhahinchev, V.; Rachamalla, M.; Dissi, A.A.; Niyogi, S.; Weber, L.P. Examining the subchronic (28-day) effects of aqueous Cd-BaP co-exposure on detoxification capacity and cardiac function in adult zebrafish (Danio rerio). Aquat. Toxicol. 2023, 263, 106672. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Campoy-Diaz, A.D.; Arribére, M.A.; Guevara, S.R.; Vega, I.A. Bioindication of mercury, arsenic and uranium in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Bioconcentration and depuration in tissues and symbiotic corpuscles. Chemosphere 2018, 196, 196–205. [Google Scholar] [CrossRef]
- Juarez, A.; Vega, I.A.; Mayorga, L.S.; Guevara, S.R.; Arribére, M.A. An Arsenic-76 radiotracer to study the routes of assimilation, hemolymph distribution, and tissue inventories in the bioindicator organism Pomacea canaliculata. Sci. Total. Environ. 2022, 815, 152760. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Bi, X.; Liu, Y.; Li, H.; Li, D.; Chen, G. Toxicology Effects of Cadmium in Pomacea canaliculate: Accumulation, Oxidative Stress, Microbial Community, and Transcriptome Analysis. Int. J. Mol. Sci. 2025, 26, 751. https://doi.org/10.3390/ijms26020751
Qiu M, Bi X, Liu Y, Li H, Li D, Chen G. Toxicology Effects of Cadmium in Pomacea canaliculate: Accumulation, Oxidative Stress, Microbial Community, and Transcriptome Analysis. International Journal of Molecular Sciences. 2025; 26(2):751. https://doi.org/10.3390/ijms26020751
Chicago/Turabian StyleQiu, Mingxin, Xiaoyang Bi, Yuanyang Liu, Huashou Li, Dongqin Li, and Guikui Chen. 2025. "Toxicology Effects of Cadmium in Pomacea canaliculate: Accumulation, Oxidative Stress, Microbial Community, and Transcriptome Analysis" International Journal of Molecular Sciences 26, no. 2: 751. https://doi.org/10.3390/ijms26020751
APA StyleQiu, M., Bi, X., Liu, Y., Li, H., Li, D., & Chen, G. (2025). Toxicology Effects of Cadmium in Pomacea canaliculate: Accumulation, Oxidative Stress, Microbial Community, and Transcriptome Analysis. International Journal of Molecular Sciences, 26(2), 751. https://doi.org/10.3390/ijms26020751




