Identifying a Biocontrol Bacterium with Disease-Prevention Potential and Employing It as a Powerful Biocontrol Agent Against Fusarium oxysporum
Abstract
1. Introduction
2. Results
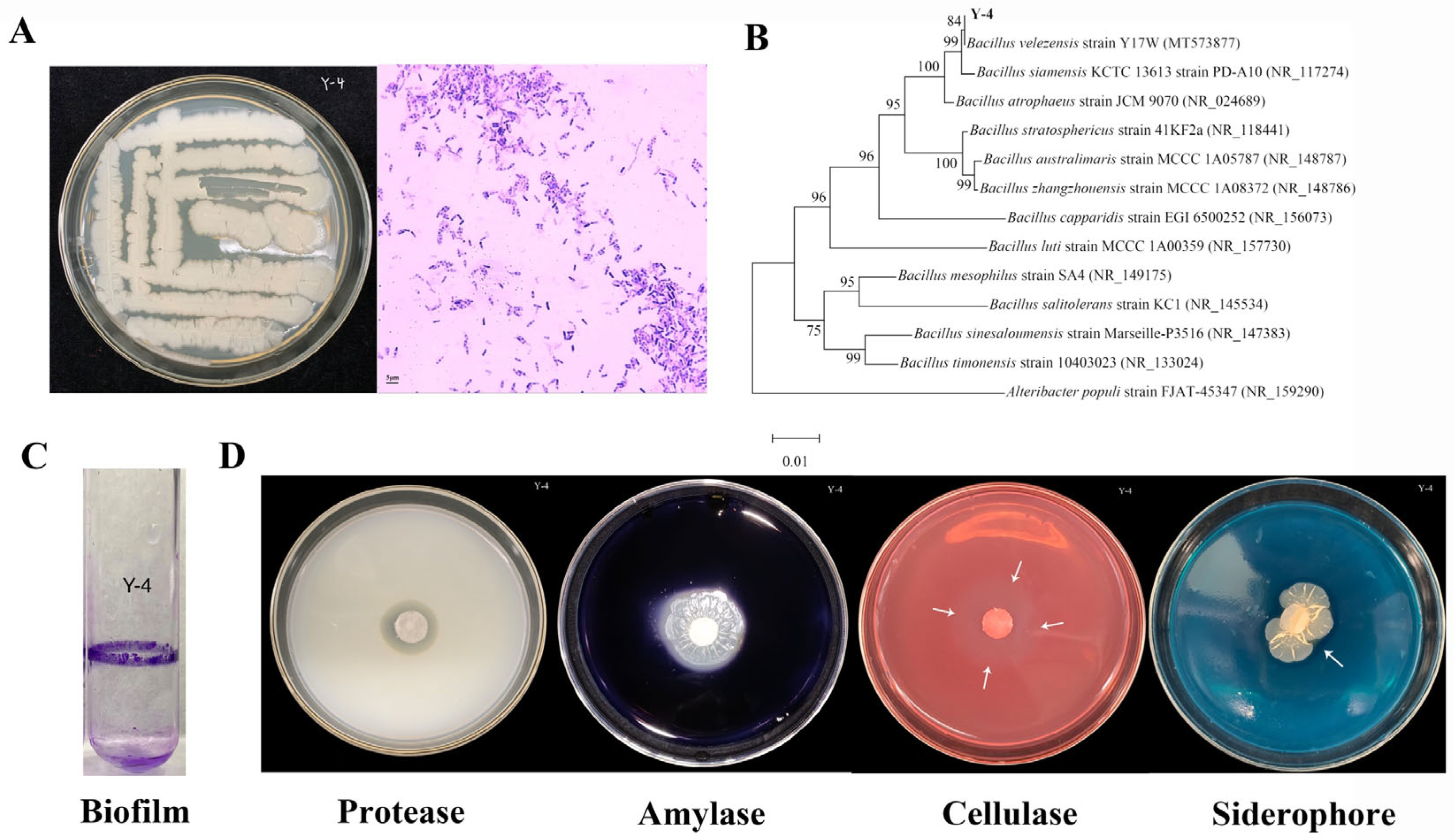
2.1. Isolation of Biocontrol Bacterium Y-4
2.2. Identification of Biocontrol Bacterium Y-4
2.2.1. Molecular Identification of Y-4
2.2.2. Physiological and Biochemical Identification of Y-4
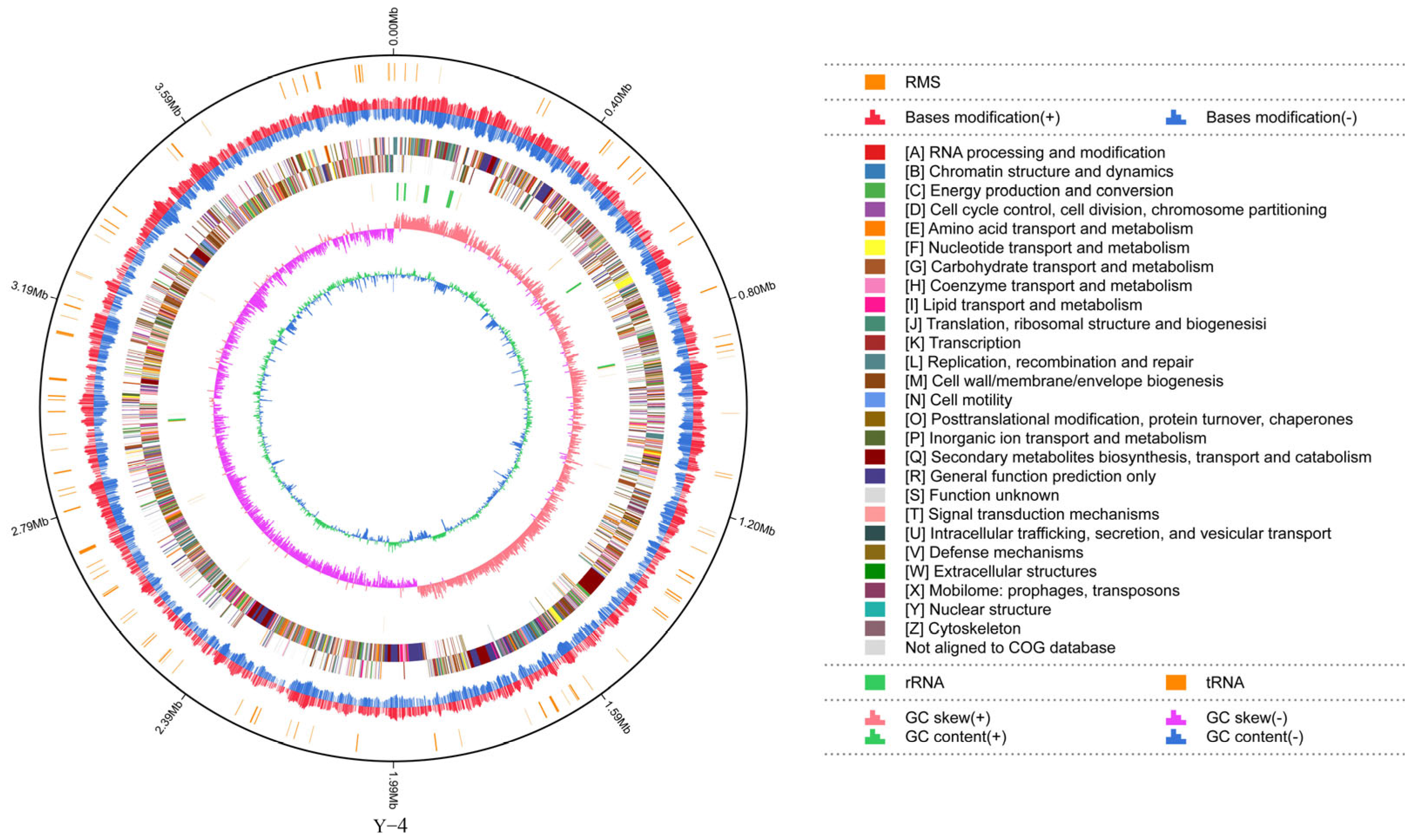
2.3. Genome-Wide Information of the Strain
2.4. Genome Structure Annotation Information of the Strain
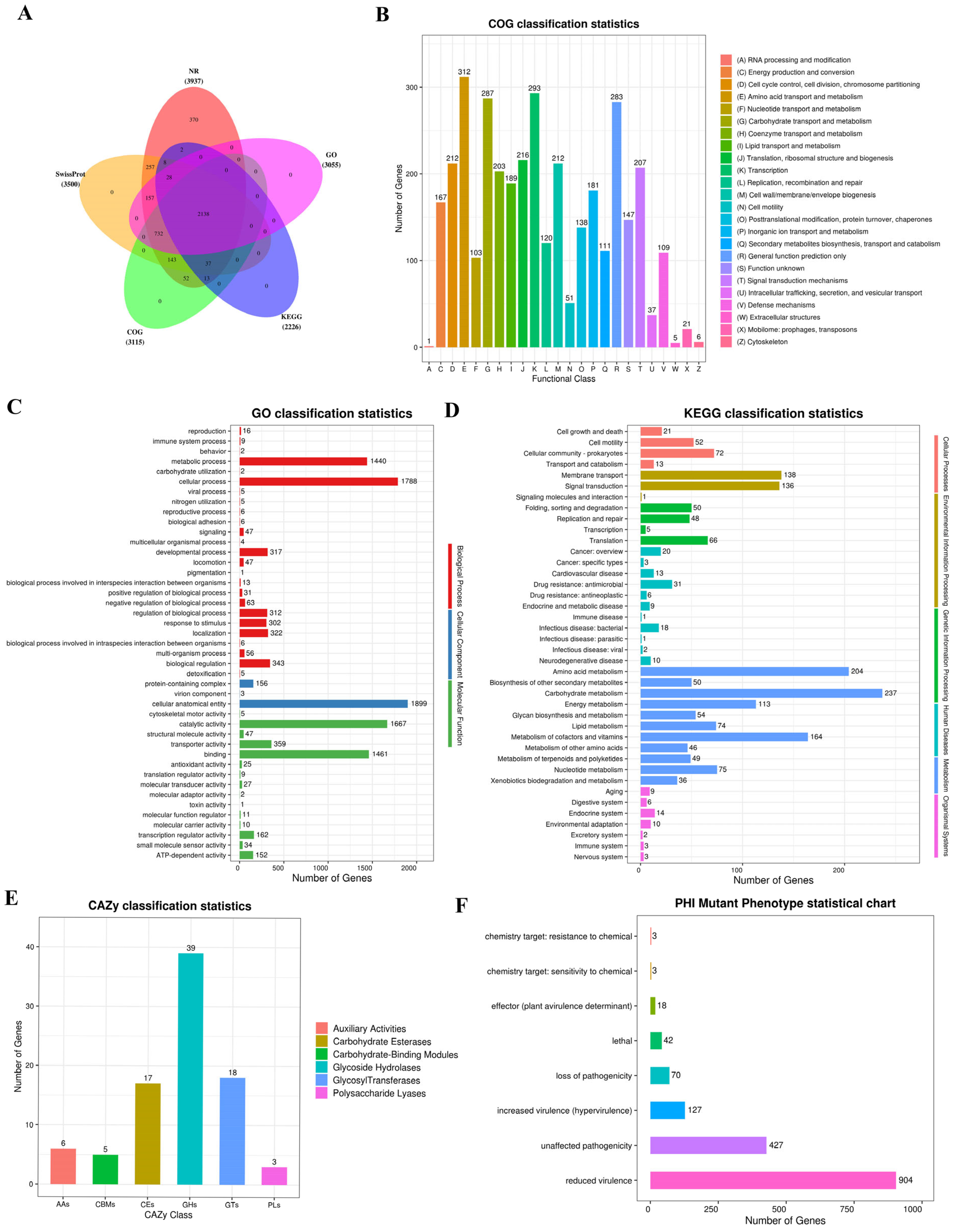
2.5. Gene Function Annotation of the Strain
2.5.1. Annotations on Basic Functions of Strain
2.5.2. Annotation on Special Functions of Strain
2.6. Gene Cluster Analysis of Secondary Metabolites of Strain
2.7. Antifungal Activities of Biocontrol Bacterium Y-4
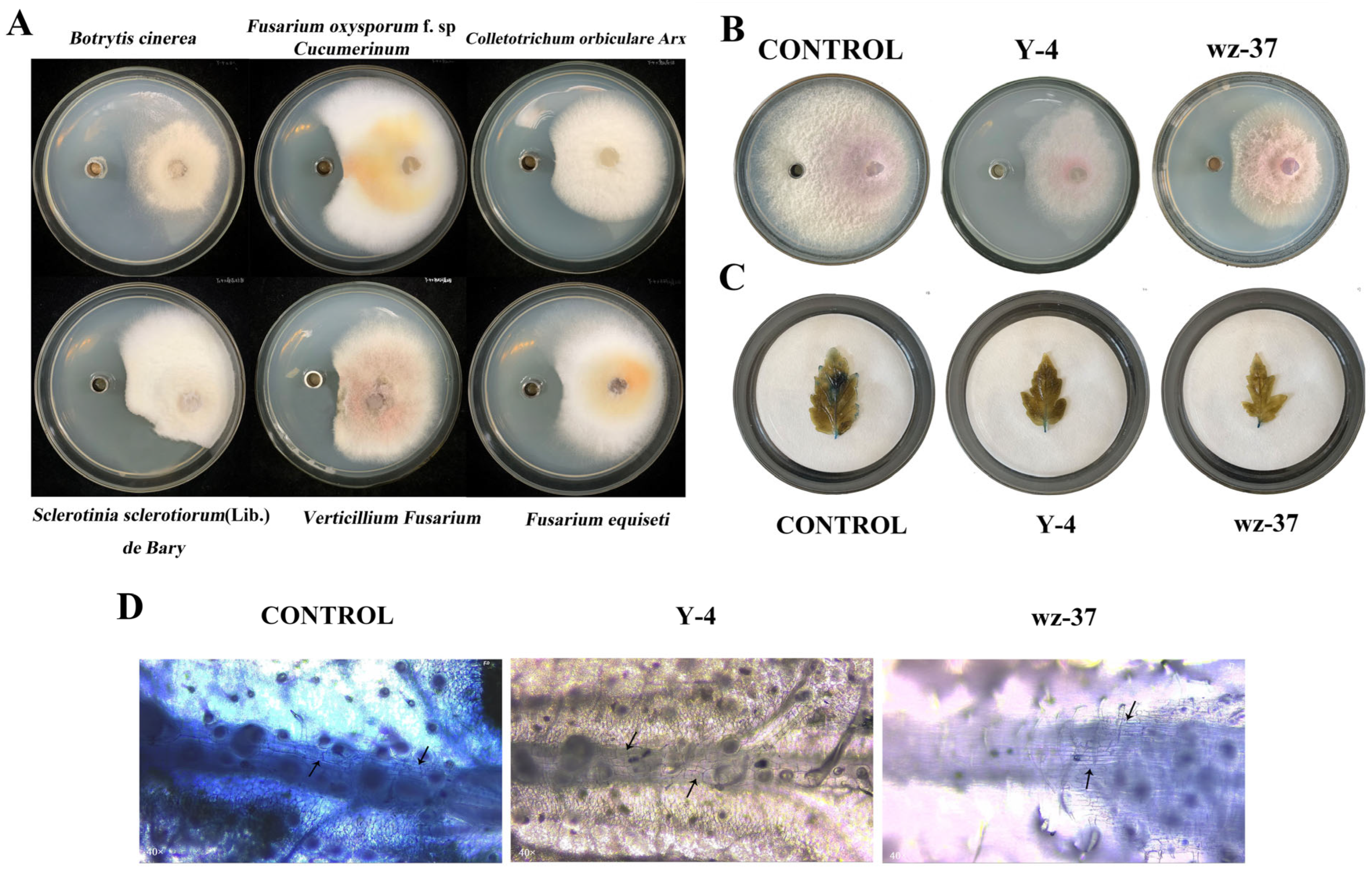
2.7.1. Determination of Broad-Spectrum Bacterial Inhibition of Biocontrol Bacterium Y-4
2.7.2. Determination of the Inhibition Effect of F. oxysporum
2.7.3. Trypan Blue Dyeing Observation of Y-4
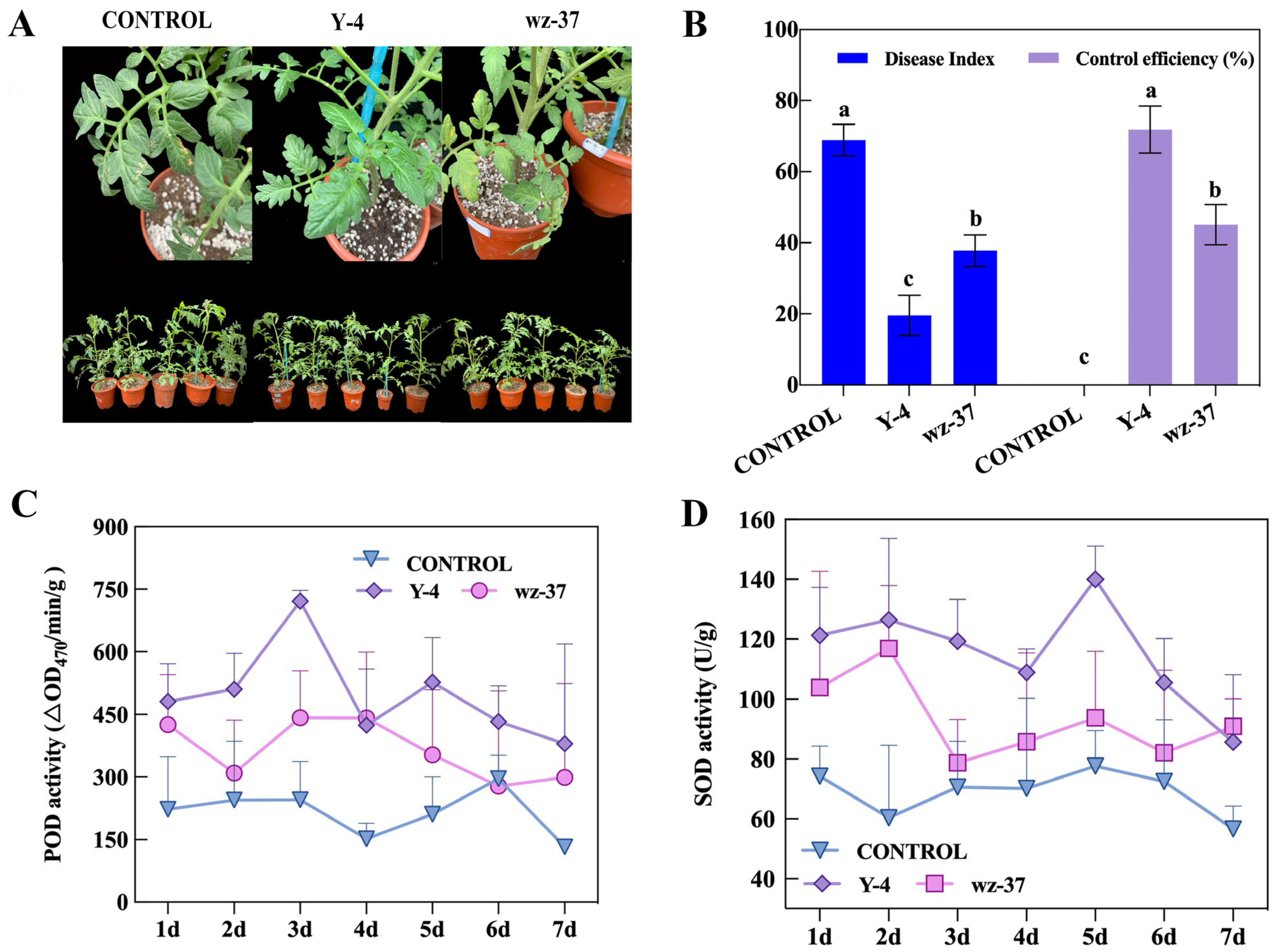
2.8. Determination of Indoor Control Efficiency of Biocontrol Bacterium Y-4
2.9. Induction of Antioxidant Enzymes in Tomato Leaves by Biocontrol Bacterium Y-4
3. Discussion
4. Materials and Methods
4.1. Bacteria, Pathogen, and Culture Conditions
4.2. Tomato Seeds
4.3. Isolation of Biocontrol Bacterium
4.4. Identification of Biocontrol Bacterium
4.4.1. Molecular Identification
4.4.2. Physiological and Biochemical Identification
4.5. Annotation of the Structure and Function of Bacterial Genomes
4.5.1. Genome Structure Annotation
4.5.2. Annotation on the Database of Basic Gene Functions
4.5.3. Annotation on the Database of Special Gene Functions
4.6. Annotation of the Secondary Metabolite Gene Cluster
4.7. Antifungal Activities of Biocontrol Bacterium
4.7.1. Determination of Broad-Spectrum Antifungal Effect of Biocontrol Bacterium
4.7.2. Determination of the Inhibition Effect of F. oxysporum
4.7.3. Trypan Blue Dyeing Observation
4.8. Determination of Indoor Control Efficiency of Biocontrol Bacterium
- (1)
- CONTROL+ F. oxysporum: we sprayed the surface of tomato plants with sterile water first, followed by the F. oxysporum suspension.
- (2)
- Y-4 + F. oxysporum: we sprayed the surface of tomato plants with Y-4 suspension first, followed by the F. oxysporum suspension.
- (3)
- wz-37 + F. oxysporum: we sprayed the surface of tomato plants with wz-37 suspension first, followed by the F. oxysporum suspension.
4.9. Induction of Antioxidant Enzymes in Tomato Leaves by Biocontrol Bacterium
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; Park, H.B.; Kim, J.Y.; Choi, S.M.; Lee, D.S.; Kim, D.H.; Lee, Y.H.; Park, C.J.; Jeun, Y.C.; Hong, J.K. Menadione Sodium Bisulfite-Protected Tomato Leaves against Grey Mould via Antifungal Activity and Enhanced Plant Immunity. Plant Pathol. J. 2020, 36, 335, Erratum in Plant Pathol. J. 2021, 37, 204. [Google Scholar] [CrossRef]
- Li, S.P.; Xiao, Q.L.; Yang, H.J.; Huang, J.G.; Li, Y. Characterization of a new Bacillus velezensis as a powerful biocontrol agent against tomato gray mold. Pest. Biochem. Physiol. 2022, 187, 10. [Google Scholar] [CrossRef]
- Pessarakli, M.; Heydari, A. A Review on Biological Control of Fungal Plant Pathogens Using Microbial Antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar]
- Jiang, C.H.; Wu, F.; Yu, Z.Y.; Xie, P.; Ke, H.J.; Li, H.W.; Yu, Y.Y.; Guo, J.H. Study on screening and antagonistic mechanisms of Bacillus amyloliquefaciens 54 against bacterial fruit blotch (BFB) caused by Acidovorax avenae subsp citrulli. Microbiol. Res. 2015, 170, 95–104. [Google Scholar] [CrossRef]
- Elslahi, R.H.; Osman, A.G.; Sherif, A.M.; Elhussein, A.A. Comparative study of the fungicide Benomyl toxicity on some plant growth promoting bacteria and some fungi in pure cultures. Interdiscip. Toxicol. 2014, 7, 12–16. [Google Scholar] [CrossRef]
- Chen, L.; Miao, W.G.; Liu, H.Y.; Nu, E.Z.Y. Antagonistic Mechanism of Brevibacillus brevs A57 against Cotton Mainly Pathogenic Fungus. Acat Agric. Boreali-Occident. Sin. 2008, 17, 149–155. [Google Scholar]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137–143. [Google Scholar] [CrossRef]
- Palazzini, J.; Reynoso, A.; Yerkovich, N.; Zachetti, V.; Ramirez, M.; Chulze, S. Combination of Bacillus velezensis RC218 and Chitosan to Control Fusarium Head Blight on Bread and Durum Wheat under Greenhouse and Field Conditions. Toxins 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.D.; Ma, Z.; Xu, X.H.; Yu, X.P. Isolation and identification of biocontrol agent Streptomyces rimosus M527 against Fusarium oxysporum f. sp cucumerinum. J. Basic Microbiol. 2016, 56, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Yuan, J.; Zhang, J.; Shen, Z.Z.; Zhang, M.X.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Baiome, B.A.; Ye, X.F.; Yuan, Z.Y.; Gaafar, Y.Z.A.; Melak, S.; Cao, H. Identification of Volatile Organic Compounds Produced by Xenorhabdus indica Strain AB and Investigation of Their Antifungal Activities. Appl. Environ. Microbiol. 2022, 88, 17. [Google Scholar] [CrossRef]
- Alam, S.S.; Sakamoto, K.; Inubushi, K. Biocontrol efficiency of Fusarium wilt diseases by a root-colonizing fungus Penicillium sp. Soil Sci. Plant Nutr. 2011, 57, 204–212. [Google Scholar] [CrossRef]
- Wang, W.; Jin, H.; Cong, B.; Zhou, L.; Wei, Z.; Wang, S. Biocontrol effect of composite microbial agent on tomato bacterial wilt. J. Nanjing Agric. Univ. 2022, 45, 1174–1182. [Google Scholar]
- Zhang, D.; Gao, Y.; Wang, Y.; Liu, C.; Shi, C. Advances in taxonomy, antagonistic function and application of Bacillus velezensis. Microbiol. China 2020, 47, 3634–3649. [Google Scholar]
- Xie, S.S.; Yu, H.G.; Li, E.Z.; Wang, Y.; Liu, J.; Jiang, H.Y. Identification of miRNAs Involved in Bacillus velezensis FZB42-Activated Induced Systemic Resistance in Maize. Int. J. Mol. Sci. 2019, 20, 14. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Béjar, V.; Martínez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Errington, J. Bacillus subtilis sporulation: Regulation of gene expression and control of morphogenesis. Microbiol. Rev. 1993, 57, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Bao, X.; Li, W.; An, T.; Sun, H. Solation, Purification and Identification of a Bacillus velezensis B18 and its Control Effect on Banana Wilt. Genom. Appl. Biol. 2021, 40, 3209–3215. [Google Scholar]
- Wang, H.; Liu, L.; Yu, S.; Guan, T.; Zou, C.; Li, B.; Zheng, L. Identification and Evaluation of Bacillus subtilis SF18-3 Against Bacterial Spot Disease of Pepper. J. Shenyang Agric. Univ. 2024, 55, 417–425. [Google Scholar]
- Wang, X.Y.; Zhou, X.N.; Cai, Z.B.; Guo, L.; Chen, X.L.; Chen, X.; Liu, J.Y.; Feng, M.F.; Qiu, Y.W.; Zhang, Y.; et al. A Biocontrol Strain of Pseudomonas aeruginosa CQ-40 Promote Growth and Control Botrytis cinerea in Tomato. Pathogens 2021, 10, 17. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.-H. Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Thurlow, C.M.; Williams, M.A.; Carrias, A.; Ran, C.; Newman, M.; Tweedie, J.; Allison, E.; Jescovitch, L.N.; Wilson, A.E.; Terhune, J.S.; et al. Bacillus velezensis AP193 exerts probiotic effects in channel catfish (Ictalurus punctatus) and reduces aquaculture pond eutrophication. Aquaculture 2019, 503, 347–356. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, B.; Zhu, Y.; Xiao, R.; Yang, W.; Ge, C.; Chen, Z. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 2020, 20, 160. [Google Scholar] [CrossRef]
- Wang, C.C.; Ye, X.J.; Ng, T.B.; Zhang, W.J. Study on the Biocontrol Potential of Antifungal Peptides Produced by Bacillus velezensis against Fusarium solani That Infects the Passion Fruit Passiflora edulis. J. Agric. Food Chem. 2021, 69, 2051–2061. [Google Scholar] [CrossRef]
- Li, Q.; Liao, S.; Wei, J.; Xing, D.; Xiao, Y.; Yang, Q. Isolation of Bacillus subtilis strain SEM-2 from silkworm excrement and characterisation of its antagonistic effect against Fusarium spp. Can. J. Microbiol. 2020, 66, 401–412. [Google Scholar] [CrossRef]
- Charlop-Powers, Z.; Owen, J.G.; Reddy, B.V.B.; Ternei, M.A.; Brady, S.F. Chemical-biogeographic survey of secondary metabolism in soil. Proc. Natl. Acad. Sci. USA. 2014, 111, 3757–3762. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Zhao, X.; Li, Y.; Wang, R.; Huang, Y.; Qi, G. Engineering Bacillus velezensis with high production of acetoin primes strong induced systemic resistance in Arabidopsis thaliana. Microbiol. Res. 2019, 227, 126297. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 2008, 227, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, Y.; Wang, M.; Liu, W.; Lu, F.; Li, Y. Effects of Different Integration Sites on the Expression of Exogenous Alkaline Protease in Bacillus amyloliquefaciens. Biotechnol. Bull. 2022, 38, 253–260. [Google Scholar]
- Afsharmanesh, H.; Ahmadzadeh, M.; Javan-Nikkhah, M.; Behboudi, K. Improvement in biocontrol activity of Bacillus subtilis UTB1 against Aspergillus flavus using gamma-irradiation. Crop. Prot. 2014, 60, 83–92. [Google Scholar] [CrossRef]
- Li, B.; Xu, L.H.; Lou, M.M.; Li, F.; Zhang, Y.D.; Xie, G.L. Isolation and characterization of antagonistic bacteria against bacterial leaf spot of Euphorbia pulcherrima. Lett. Appl. Microbiol. 2008, 46, 450–455. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.D.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Whitman, W.B.; Goodfellow, M.; Kämpfer, P. Bergey’s Manual of Systematic Bacteriology. Vol. 5 The Actinobacteria; Springer: New York, NY, USA, 2012; p. 2012. [Google Scholar]
- Sun, D.; Zhuo, T.; Hu, X.; Fan, X.; Zou, H. Identification of a Pseudomonas putida as biocontrol agent for tomato bacterial wilt disease. Biol. Control 2017, 114, 45–50. [Google Scholar] [CrossRef]
- Tagele, S.B.; Kim, S.W.; Lee, H.G.; Kim, H.S.; Lee, Y.S. Effectiveness of multi-trait Burkholderia contaminans KNU17BI1 in growth promotion and management of banded leaf and sheath blight in maize seedling. Microbiol. Res. 2018, 214, 8–18. [Google Scholar] [CrossRef]
- Slama, H.B.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium Antagonistic Bacteria From Contrasting Niches Designated the Endophyte Bacillus halotolerans as Plant Warden Against Fusarium. Front. Microbiol. 2018, 9, 3236. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rodland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. 2), W316–W322. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2008, 37 (Suppl. 1), D233–D238. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, J.; Zeng, L.; Goh, M.; Leung, H.; Khush, G.S.; Wang, G.L. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol. Plant-Microbe Interact. MPMI 2000, 13, 869–876. [Google Scholar] [CrossRef]
- Sato, I.; Yoshida, S.; Iwamoto, Y.; Aino, M.; Hyakumachi, M.; Shimizu, M.; Takahashi, H.; Ando, S.; Tsushima, S. Suppressive Potential of Paenibacillus Strains Isolated from the Tomato Phyllosphere against Fusarium Crown and Root Rot of Tomato. Microbes Environ. 2014, 29, 168–177. [Google Scholar] [CrossRef]
| Strain Number | Inhibition Zone/cm |
|---|---|
| Y-1 | 0.41 ± 0.05 c |
| Y-2 | 0.47 ± 0.03 bc |
| Y-3 | 0.73 ± 0.10 ab |
| Y-4 | 0.79 ± 0.09 a |
| Y-5 | 0.57 ± 0.14 abc |
| Y-9 | 0.49 ± 0.01 bc |
| Query_id | Y_4_chr | Y_4_chr | Y_4_chr |
|---|---|---|---|
| Query_length | 3,984,866 | 3,984,866 | 3,984,866 |
| Subject_id | CP046918.1 | CP026610.1 | CP071970.1 |
| Subject_length | 4,035,062 | 4,322,979 | 4,160,003 |
| Identity | 1,278,306/1,281,807 (99.73) | 902,961/904,588 (99.82) | 733,597/734,823 (99.83) |
| Score | 2,347,330 | 1,661,360 | 1,350,150 |
| E value | 0 | 0 | 0 |
| Description | B. velezensis strain BA-26 chromosome, complete genome | B. velezensis strain CGMCC 11640 chromosome, complete genome | B. amyloliquefaciens strain XJ5 chromosome, complete genome |
| Physiological and Biochemical Identification | Y-4 |
|---|---|
| Aerobic or anaerobic | Aerobic type |
| Catalase test | + |
| Glucose oxidative fermentation | Alkali-producing type |
| Methyl red | − |
| Biofilm | + |
| Protease | + |
| Amylase | + |
| Cellulase | + |
| Siderophore | + |
| Basic Gene Element Prediction | ||||
|---|---|---|---|---|
| Coding Gene | Genome_Size (bp) | Gene_Num (#) | Gene_TotalLen (bp) | Gene_AverageLen (bp) |
| 3,984,866 | 4002 | 3,555,231 | 888.36 | |
| tRNA | Number | Total_len (bp) | Average_Len (bp) | %of_genome |
| 87 | 6694 | 76.94 | 0.17 | |
| rRNA | Number | Total_len (bp) | Average_Len (bp) | %of_genome |
| 27 | 41,230 | 1527.04 | 1.03 | |
| Region | Type | From | To | Most Similar Known Cluster | Similarity |
|---|---|---|---|---|---|
| Region 1 | NRPS, transAT-PKS | 197,788 | 275,401 | Locillomycin/locillomycin B/locillomycin C | 28% |
| Region 2 | NRPS | 353,992 | 418,853 | Surfactin | 91% |
| Region 3 | PKS-like | 942,114 | 983,358 | butirosin A/butirosin B | 7% |
| Region 4 | Terpene | 1,068,980 | 1,086,136 | - | |
| Region 5 | transAT-PKS | 1,404,718 | 1,491,156 | macrolactin H | 100% |
| Region 6 | transAT-PKS, T3PKS, NRPS | 1,718,562 | 1,818,285 | Bacillaene | 100% |
| Region 7 | NRPS, transAT-PKS, betalactone | 1,898,242 | 2,034,192 | Fengycin | 100% |
| Region 8 | Terpene | 2,085,270 | 2,080,153 | - | |
| Region 9 | T3PKS | 2,145,247 | 2,186,347 | - | |
| Region 10 | transAT-PKS | 2,314,539 | 2,408,331 | Difficidin | 100% |
| Region 11 | NRP-metallophore, NPRS, RiPP-like | 3,031,566 | 3,083,360 | Bacillibactin | 100% |
| Region 12 | NPRS | 3,376,282 | 3,444,726 | - | |
| Region 13 | Other | 3,649,950 | 3,691,368 | Bacilysin | 100% |
| Name of Pathogen | Inhibition Zone Width/cm |
|---|---|
| B. cinerea | 0.90 ± 0.01 a |
| F. oxysporum f.sp cucumerinum | 0.60 ± 0.02 c |
| C. orbiculare Arx | 0.61 ± 0.18 c |
| V. fusarium | 0.72 ± 0.0025 ab |
| F. equiseti | 0.87 ± 0.07 b |
| S. sclerotiorum (Lib.) de Bary | 0.71 ± 0.005 ab |
| Strain Number | Inhibition Zone Width/cm |
|---|---|
| CONTROL | 0.00 ± 0.00 c |
| Y-4 | 0.69 ± 0.04 a |
| wz-37 | 0.45 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Sun, Z.; Li, T.; Fan, T.; Zhou, Z.; Liu, J.; Chen, X.; Wang, A. Identifying a Biocontrol Bacterium with Disease-Prevention Potential and Employing It as a Powerful Biocontrol Agent Against Fusarium oxysporum. Int. J. Mol. Sci. 2025, 26, 700. https://doi.org/10.3390/ijms26020700
Wang Q, Sun Z, Li T, Fan T, Zhou Z, Liu J, Chen X, Wang A. Identifying a Biocontrol Bacterium with Disease-Prevention Potential and Employing It as a Powerful Biocontrol Agent Against Fusarium oxysporum. International Journal of Molecular Sciences. 2025; 26(2):700. https://doi.org/10.3390/ijms26020700
Chicago/Turabian StyleWang, Qi, Zhenshu Sun, Tiantian Li, Tiantian Fan, Ziqi Zhou, Jiayin Liu, Xiuling Chen, and Aoxue Wang. 2025. "Identifying a Biocontrol Bacterium with Disease-Prevention Potential and Employing It as a Powerful Biocontrol Agent Against Fusarium oxysporum" International Journal of Molecular Sciences 26, no. 2: 700. https://doi.org/10.3390/ijms26020700
APA StyleWang, Q., Sun, Z., Li, T., Fan, T., Zhou, Z., Liu, J., Chen, X., & Wang, A. (2025). Identifying a Biocontrol Bacterium with Disease-Prevention Potential and Employing It as a Powerful Biocontrol Agent Against Fusarium oxysporum. International Journal of Molecular Sciences, 26(2), 700. https://doi.org/10.3390/ijms26020700





