Abstract
Biocontrol microbes are environment friendly and safe for humans and animals. To seek biocontrol microbes effective in suppressing Fusarium oxysporum is important for tomato production. F. oxysporum is a soil-borne pathogen capable of causing wilt in numerous plant species. Therefore, we found a biocontrol bacterium with an excellent control effect from the rhizosphere soil of plant roots. In this work, we focus on two parts of work. The first part is the identification and genomic analysis of the biocontrol bacterium Y-4; the second part is the control efficiency of strain Y-4 on F. oxysporum. For this study, we identified strain Y-4 as Bacillus velezensis. It is an aerobic Gram-positive bacterium that can secrete a variety of extracellular enzymes and siderophores. Strain Y-4 also contains a large number of disease-resistant genes and a gene cluster that forms antibacterial substances. In addition, we found that it significantly inhibited the reproduction of F. oxysporum in a culture dish. In the indoor control effect test, after treatment with strain Y-4 suspension, the disease index of tomato plants decreased significantly. Furthermore, the control efficiency of the plants was 71.88%. At the same time, Y-4 bacterial suspension induced an increase in POD and SOD enzyme activities in tomato leaves, resulting in increased plant resistance. Taken together, strain Y-4 proves to be an effective means of controlling F. oxysporum in tomatoes.
1. Introduction
The tomato (Solanum lycopersicum) of the Solanaceae family is cultivated worldwide [1]. In recent years, tomato has become the leading crop in the world, providing farmers with an important economic resource. However, tomato production is seriously restricted by soil-borne pathogens such as F. oxysporum, which is known to infect different types of hosts and cause serious damage to crops such as cotton, tomatoes, and bananas [2]. F. oxysporum enters the plant through the root and propagates within the vascular bundle, disrupting the ability of the root tissue to transport nutrients to the vascular bundle, resulting in the host’s eventual death, seriously reducing fruit yield and quality. Moreover, the frequent and excessive use of chemical fungicides produces serious environmental and food safety problems [3,4]. Therefore, a study on several topics related to the control of F. oxysporum is necessary.
Biological control is a safe and environmentally friendly option for preventing and treating tomato plant wilt disease. It offers a new alternative that is beneficial for humans and the environment [5]. Biological control has been studied for over 100 years and is considered a viable alternative method to chemical control [6]. It is one of the best potential strategies for the control of soil-borne diseases [7]. Bacteria as the primary source of biological antibacterial agents have the advantages of rapid reproduction and easy preparation of fermentation solutions and the most noticeable control effect [8]. Li et al. found that the A57 strain of Bacillus subtilis could considerably suppress cotton standing blight and yellow wilt, inhibiting the germination of conidia and causing spore deformation to achieve a control effect [9]. Other studies showed that Bacillus megaterium BM1 and B. velezensis RC218 were both capable of considerably reducing the occurrence of wheat blast disease [10,11]. Lu et al. reported that a new species of streptomyces, Streptomyces rimosus M527, not only displayed broad-spectrum antifungal activity but also showed the strongest antagonistic activity against the spore germination of F. oxysporum f.sp. cucumerinum [12]. In addition, biological control has not only focused on the control effects of strains. Studies showed that Bacillus secretes antagonistic compounds. Wang et al. identified three fungal lipopeptides (iturin, surfactin, and bacillomycin D) secreted by Bacillus amylolyticus W19 [13].
In recent years, chemical residues on fruits that are difficult to clean and harmful to consumers and the massive use of chemical agents to control fungal diseases has caused a decline in the structure of soil aggregate and the destruction of microbial communities [14]. Although there are many reports on the biocontrol of B. velezensis against plant fungal disease, there are very few B. velezensis agents now used in agricultural practices. Therefore, it is necessary to screen out more effective B. velezensis biocontrol strains and understand the influences on plants and pathogens.
In this work, we isolated and identified a biocontrol bacterial strain, Y-4, and used it as a research object. The research was mainly carried out in the following ways: (a) an analysis of a potential antibacterial mechanism through whole genome sequencing and metabolic gene clusters, (b) determining the antagonism activity of strain Y-4 on F. oxysporum through a culture dish, (c) evaluating the control efficiency of the Y-4 strain on wild tomatoes indoors, and (d) determining the induction of antioxidant enzymes in tomato leaves by strain Y-4. This study aimed to provide the resources and methods for the control of F. oxysporum, using strain Y-4 as a biological agent.
2. Results
2.1. Isolation of Biocontrol Bacterium Y-4
A total of six biocontrol strains that could inhibit the reproduction of F. oxysporum were selected from the rhizosphere soil. Thus, we took strain Y-4 as the main research object (Table 1).

Table 1.
Preliminary screening of the width of the fungistatic zone.
2.2. Identification of Biocontrol Bacterium Y-4
Y-4 colonies were creamy white, oily, paste-like, smooth at the edges, and slightly folded at the center and smooth and opaque on the surface (Figure 1A). After microscopic observation and after Gram staining, strain Y-4 was Gram-positive with a purple body and rod-shaped bacteria (Figure 1A).
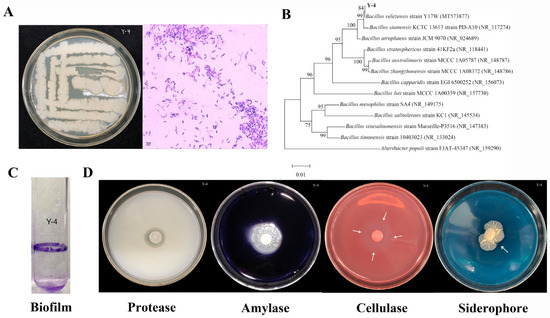
Figure 1.
Identification and physiological and biochemical characteristics of strainY-4. (A) Observation of the apparent morphology and Gram staining of strain Y-4. (B) Strain Y-4 phylogenetic tree construction. (C) Strain Y-4 biofilm determination map. (D) From left to right, protease, amylase, cellulase, and siderophore secretions (The arrow represents the indicator circle of cellulase and siderophore secreted by the strain).
2.2.1. Molecular Identification of Y-4
The 16S rDNA sequence of strain Y-4 was submitted to NCBI’s GenBank database for homology comparison with reported sequences. The strain with more similar homology to strain Y-4 is B. velezensis Y17W strain (MT573877) (Figure 1B).
To further determine the taxonomic information of strain Y-4, we compared each sequence of strain Y-4 with the NT database. The results are shown in Table 2. According to the identity and score comparison results, the complete genomes of B. velezensis strain BA-26 chromosome and B. velezensis strain CGMCC 11640 chromosome are more similar to that of the Y-4 genome (Table 2). Combined with the comparison results of the 16S rRNA and NT databases, strain Y-4 was determined to be a strain of B. velezensis.

Table 2.
Results of NT library comparison of assembled genomes.
2.2.2. Physiological and Biochemical Identification of Y-4
The results showed that strain Y-4 was an aerobic and alkali-producing type of bacteria. The catalase test was positive. Methyl red was negative (Table 3). Strain Y-4 can form biofilms that protect plants from infections (Figure 1C). Furthermore, it can secrete protease, amylase, cellulase, and siderophores (Table 3 and Figure 1D).

Table 3.
Physiological and biochemical identification.
2.3. Genome-Wide Information of the Strain
From the assembly results, we learned that the subread length of strain Y-4 was 3,984,866 bp, G + C content was 46.45%, and the proportion of the A, T, G, and C base content was 26.75%, 26.80%, 23.25%, and 23.20%, respectively (Figure 2 and Supplemental Table S1). We submitted the sequence data to the NCBI database and obtained the accession number CP139053.
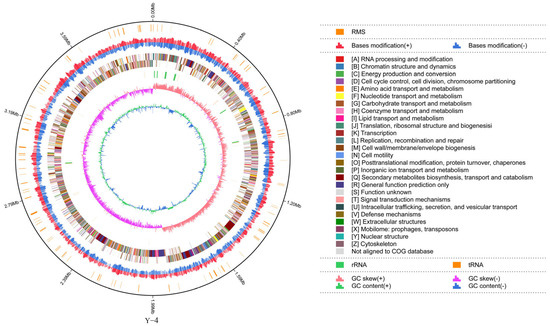
Figure 2.
Genome circle map can display the genome information of the strain Y-4 sample in all directions.
2.4. Genome Structure Annotation Information of the Strain
In total, 4022 coding genes were predicted, with a 3,555,231 bp length for all of the projected coding genes. The predicted number of tRNA was 87, with a total length of 6694 bp. There were 3 types of rRNA, 27 were predicted, and the total length was 41,230 bp (Table 4).

Table 4.
Statistical table for the prediction of basic genetic elements.
2.5. Gene Function Annotation of the Strain
2.5.1. Annotations on Basic Functions of Strain
To study Y-4’s genome function, its sequence was annotated in five databases: NR, SwissProt, COG/KOG, KEGG, and GO. There were 3937 genes annotated in the NR database, 3500 in SwissProt, 3115 in COG/KOG, 2226 in KEGG, and 3055 in GO (Figure 3A).
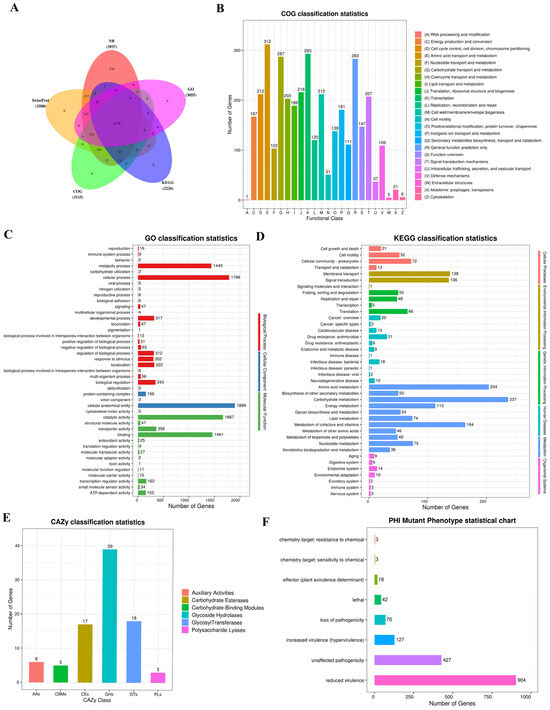
Figure 3.
Basic function database and special function database annotation results illustrated in a diagram. (A) Statistical charts of basic database data. (B) COG function classification diagram. (C) GO Level 2 classification level annotation distribution map. (D) Statistical chart of the Level 2 KEGG annotation result classification. (E) CAZy annotated classification statistical chart. (F) Phenotypic statistics of the PHI mutation.
The COG database is a system annotated by NCBI based on the distance of the gene linear homology. Through sequence similarity alignment, a protein sequence is assigned to a COG cluster. The Y-4 biocontrol bacterium had 3115 (77.84%) annotated genes that were classified into 24 categories (Figure 3B). Among all categories, the number of genes involved in amino acid transport and metabolism was the largest, involving 312 genes (Figure 3B).
A term is the fundamental unit of GO. In the GO database, 3055 genes (76.34%) of the Y-4 genes were annotated (Figure 3C). Cellular anatomical entity had the highest number of genes (1899) in the secondary classification. Then, cellular and metabolic process annotated 1788 and 1440 genes. Catalytic and binding activities annotated 1667 and 1461 genes, respectively (Figure 3C).
In the KEGG database, 2226 (55.62%) of the Y-4 genes were annotated. The results revealed that the metabolism portion had the most annotated genes, with 1102 genes, followed by the environmental information processing section. In the secondary categorization, 237 genes were annotated to carbohydrate metabolism and 204 genes to amino acid metabolism. The number of genes in these two categories is relatively higher than in the others (Figure 3D).
2.5.2. Annotation on Special Functions of Strain
In the CAZy database, strain Y-4 annotated to 85 genes, with glycoside hydrolases having the most genes annotated (39 genes), followed by glycosyl transferases (18 genes) and carbohydrate esterases (17 genes) (Figure 3E). Polysaccharide lyases had the fewest genes annotated, with only three (Figure 3E).
In the PHI mutant phenotype statistics, we observed the most prominent number of reduced virulence genes in the Y-4 sample with a gene count of 904 (Figure 3F). This is substantially greater than the phenotypes of other mutations. It should be noted that reduced virulence is regarded as a key mutation trait for reducing the pathogenicity of pathogenic bacteria (Figure 3F).
2.6. Gene Cluster Analysis of Secondary Metabolites of Strain
According to the anticipated results, the strain Y-4 genome had 13 metabolite synthesis gene clusters. Surfactin (lipopeptide), butirosin A/butirosin B (aminoglycoside), macrolactin H (polyketone), bacillaene (polyketone), fengycin (lipopeptide), difficidin (polyketone), bacillibactin (siderophore), and bacilysin (dipeptide) were identified as fungistatic substances (Table 5). Six compounds (macrolactin H, bacillaene, fengycin, difficidin, bacillibactin, and bacilysin) were 100% similar to known gene clusters, and one compound (surfactin) had 91% similarity. Moreover, the four gene clusters responsible for secondary metabolite synthesis showed no resemblance to the known clusters, and they biosynthesized two terpenes, one T3PKS and one NRPS, respectively (Table 5).

Table 5.
Prediction of secondary metabolite gene clusters of strain Y-4.
2.7. Antifungal Activities of Biocontrol Bacterium Y-4
2.7.1. Determination of Broad-Spectrum Bacterial Inhibition of Biocontrol Bacterium Y-4
In this experiment, the Y-4 bacterial suspension showed different degrees of bactericidal effects against six pathogenic bacteria (B. cinerea, F. oxysporum f.sp. cucumerinum, Colletotrichum orbiculare Arx, Sclerotinia sclerotiorum (Lib.) de Bary, Verticillium fusarium and Fusarium equiseti). These results showed that Y-4 has broad-spectrum bacterial inhibition (Table 6 and Figure 4A).

Table 6.
Broad-spectrum fungistatic band distance of Y-4 (cm).
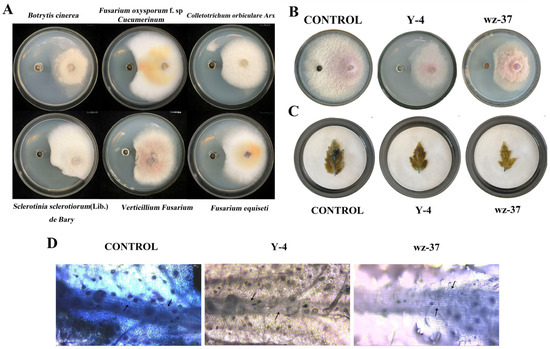
Figure 4.
Inhibitory effect of Y-4 on F. oxysporum. (A) Y-4 broad-spectrum fungistatic map. (B) The effect of culture dish confrontation between Y-4 and F. oxysporum. (C) Observation of the apparent morphology of Trypan Blue staining. (D) Microscopic morphology of leaf veins stained with Trypan Blue. Arrows indicate staining of vein cells.
2.7.2. Determination of the Inhibition Effect of F. oxysporum
Through the culture dish confrontation results, strain Y-4 had the strongest inhibitory effect on F. oxysporum, which was significantly different from CONTROL. In addition, strain wz-37, which was used as a positive control, inhibited the F. oxysporum to a lesser extent than strain Y-4 (Figure 4B and Table 7).

Table 7.
Fungistatic distance of Y-4 to F. oxysporum (cm).
2.7.3. Trypan Blue Dyeing Observation of Y-4
The blue area of the tomato leaves treated with the bacterial suspension was much smaller compared to the control group, among which the stained area of leaves treated with the strain Y-4 bacterial suspension was the smallest, indicating that the leaves had the largest number of viable cells. In contrast, the leaves in the CONTROL were completely stained and the leaf cells were completely inactive. After treatment with strain wz-37, the leaf cells were noticeably stained. However, the prevention and control effect of strain Y-4 was found to be higher than that of strain wz-37 (Figure 4C,D).
2.8. Determination of Indoor Control Efficiency of Biocontrol Bacterium Y-4
In greenhouse prevention studies, the disease index of the plants treated with strain Y-4 was the lowest and the control efficiency was the best, which were 19.56 and 71.88%, respectively (Figure 5A,B). All the plants with the addition of the biological control bacteria had a lower disease index than the CONTROL group. The control efficiency of wz-37 was considerably lower than that of Y-4 (Figure 5B).
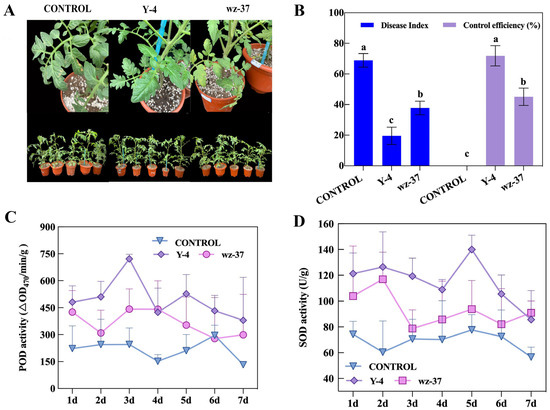
Figure 5.
The control effect of Y-4 on F. oxysporum in an indoor control efficiency test and the change in defense enzyme activity within 7 days. (A) Phenotypic observation of the indoor control efficiency test. (B) Changes in disease index and control index of biocontrol bacterium against F. oxysporum. Different letters indicate significant differences at p ≤ 0.05. (C) POD enzyme activity. (D) SOD enzyme activity.
2.9. Induction of Antioxidant Enzymes in Tomato Leaves by Biocontrol Bacterium Y-4
POD enzyme activity in tomato leaves treated with bacterial suspension of strains Y-4 and wz-37, both reaching a maximum on day 3, with 721.00 min/g for Y-4 and 442.33 min/g for wz-37. The highest POD activity was observed in the strain Y-4 treatment (Figure 5C). Therefore, spraying the leaves with the Y-4 biocontrol suspension could considerably increase the POD enzyme activity and further increase the plant’s resistance to pathogenic bacteria.
The trend of SOD and POD activities in tomato leaves was similar. Strain wz-37 treatments reached the highest value on the second day, which was 116.89 U/g. The difference was that the treatment of strain Y-4 reached its highest value on the fifth day, which was 136.58 U/g. Moreover, strain Y-4 had the highest SOD activity among all groups (Figure 5D). In general, Y-4 bacterial suspensions can boost POD and SOD activities in leaves.
3. Discussion
Among vegetable crops, the tomato stands out as one of the most widely consumed and cultivated worldwide. Tomato fruit production is significant globally, with an annual production of 182 million tons according to the FAO (Food and Agriculture Organization of the United Nations). Tomatoes are easily infected by diseases during growth, resulting in a decline in fruit quality. F. oxysporum is a key pathogen that causes Fusarium wilt in tomato plants [15]. To reduce the threat of this pathogen, an efficient and ecofriendly control agent for F. oxysporum is required. Furthermore, we were discovered that Y-4 can be used to prevent a range of fungal illnesses and had potent broad-spectrum antibacterial action (Figure 4A).
Bacillus is a widely employed biological resource for crop protection due to its antagonistic and growth-promoting properties. Bacillus is a commonly used biological resource for protecting crops [16]. B. velezensis is a branch of Bacillus and is a relatively lately discovered type. The prospects for development are strong, and it has many ways it can be used [17,18]. It was previously found that B. velezensis can prevent and control various kinds of plant-disease-causing pathogens, such as Verticillium dahliae Kleb, Alternaria brassicae, Pyricularia grisea (Cooke) Sacc, and Lettuce Root Rot (Pythium) [19,20,21]. In this work, we isolated and identified a strain of B. velezensis Y-4 from soil that showed strong antifungal activity. In this test, strain Y-4 could effectively inhibit F. oxysporum infections in plants, thereby reducing the disease index of tomato plants. Through indoor prevention experiments, we found that strain Y-4 was successful in preventing disease caused by F. oxysporum, with it controlling up to 71.88%, considerably higher than that of the CONTROL, and decreasing the disease index (Figure 5B). Previous studies showed that the control effect of B. velezensis D18 bacterial suspension on banana blight was 67.9%, which could effectively control the occurrence of fungal diseases [22]. In this study, the control efficiency of the Y-4 bacterial suspension was better than that of the D18 bacterial suspension. Wang et al. isolated a bacterial strain (SF18-3) and identified it as B. velezensis. Its fermentation liquor effectively controlled Xanthomonas campestris pv.vesicatoria (Doidge) Dye (Bacterial Spot Disease of Pepper), with a control efficiency of 70.17% [23].
POD and SOD are two defense enzymes that exist widely in plants. When plants are biologically stressed, they frequently enhance the activity of defensive enzymes to resist fungal infection. In our study, POD and SOD activities increased at first and then decreased gradually (Figure 5C,D). POD activity was consistent with the research results of Wang et al. [24]. Our results showed that, after spraying foliar with a biocontrol agent, the strains first propagated and occupied a favorable living space to inhibit the infection of F. oxysporum. When F. oxysporum invades plants, it will cause a large amount of ROS to accumulate. ROS will destroy biofilms and cause membrane peroxidation. The SOD enzyme plays an important role in clearing ROS and alleviating the damage caused by ROS to plants.
More kinds of bioactive metabolites are produced by B. velezensis than by other bacterial strains [25]. We analyzed 13 gene clusters in strain Y-4, of which 9 gene clusters can synthesize known antibiotics, including locillomycin/locillomycin B/locillomycin C, surfactin, butirosin A/butirosin B, macrolactin H, bacillaene, fengycin, and others (Table 5). Fengycin, bacillibactin, and surfactin are classic lipopeptide antibacterial substances. Thurlow et al. reported that a strain of B. velezensis AP193 can produce the polyketide compound difficidin [26]. B. velezensis FZB4 can produce related surfactin and fengycin substances, which achieve antibacterial effects by changing the cell wall of the mycelium or conidia of specific fungi [27]. Fengycin has been reported to be the main antibacterial substance produced by B. velezensis. It can significantly inhibit the growth of Ralstonia solanacearum and F. oxysporum [28] and inhibit the growth of Fusarium solani by interfering with the cell membrane [29]. Bacilysin is a non-ribosomal formed dipeptide antibiotic composed of L-alanine and L-anticapsin, produced by Bacillus [30]. Metabolites produced by microorganisms can regulate the exchange of substances and competition and interaction between organisms [31]; they are also used as a starter to induce resistance in plants. Therefore, B. velezensis has broad application prospects in biopesticides.
According to earlier research, B. velezensis has functional genes that prevent harmful bacteria. For example, a strain of B. velezensis isolated from rice rhizosphere can induce resistance to disease in Arabidopsis thaliana and an overproduction of the PAD4 gene in plants [32]. In the CAZy database, strain Y-4 involves 39 genes annotated in glycoside hydrolases (Figure 3E). The majority of glycoside hydrolases are linked to cell wall metabolism, defense mechanisms, secondary metabolism, glycolipid metabolism, and signal transduction [33]. In the PHI database, we found that up to 904 genes in strain Y-4 were annotated into reduced virulence (Figure 3F). Therefore, we speculated that the antagonistic effect of strain Y-4 was due to the existence of a disease-resistant gene. The analysis results of the PHI data showed us the direction for further in-depth research.
4. Materials and Methods
4.1. Bacteria, Pathogen, and Culture Conditions
B. velezensis wz-37 was previously isolated in the laboratory from rhizosphere soil samples of tomatoes, cucumbers, corn, and wheat. The wz-37 was deposited in the China General Microbiological Culture Collection Centre (CGMCC No. 15766) (Beijing, China). The bacterial isolates were grown on LB agar media [34] at 28 °C in the dark (the same as below unless stated otherwise) for 3 days before being used.
F. oxysporum, B. cinerea, F. oxysporum f.sp. cucumerinum, Colletotrichum orbiculare Arx, Verticillium fusarium, Fusarium equiseti, and Sclerotinia sclerotiorum (Lib.) de Bary were preserved in the Horticultural Biotechnology Laboratory of Northeast Agricultural University, China (Harbin, China). The pathogen was grown on potato dextrose agar (PDA) [35] at 28 °C in the dark (the same as below unless stated otherwise) for 7 d before being used.
Bacterial suspension: On a clean bench, the purified cultures Y-4 and wz-37 (Quantity: 1%) were inoculated into liquid LB agar media and placed in a shaker at 200 rpm, and the bacterial suspension was obtained by shaking the culture for 48 h. We used sterile water to adjust the bacterial suspension concentration to 1 × 109 CFU/mL.
F. oxysporum suspension: F. oxysporum was incubated in culture dishes for 7 days, and the mycelium was allowed to grow all over the Petri dish, with germinating spores attached to the surface. The mycelium was rinsed with sterile water to disperse the spores in the solution and filtered, and the spore solution was adjusted using a spectrophotometer. We used sterile water to adjust the F. oxysporum suspension concentration to 1 × 106 CFU/mL.
4.2. Tomato Seeds
The tomato variety used in the experiment was “DRK0568” (also known as the Provence tomato). The seedlings were cultured in the Horticultural Biotechnology Laboratory of Northeast Agricultural University, China (Harbin, China).
4.3. Isolation of Biocontrol Bacterium
Soil samples were collected from the rhizosphere soil of healthy Schefflera heptaphylla plants in Xishuangbanna Autonomous Prefecture, Yunnan Province, China. The surface soil was removed, and soil samples were collected at a depth of 5–10 cm. Sampling was performed using the multipoint sampling method, and samples were kept in self-sealing bags and numbered.
Soil samples were used to isolate the biocontrol bacterium. The isolation method described for biocontrol strains followed the procedure of Li et al. [36]. After the colony grew out on the culture dish, it was randomly selected for purification. Using F. oxysporum as an indicator pathogen, the bacteria with a good antagonistic effect were screened using the culture dish antagonistic method.
4.4. Identification of Biocontrol Bacterium
The biocontrol bacterium was cultured on an LB agar media and observed for morphology (the underlining method is shown in Supplemental Figure S1).
4.4.1. Molecular Identification
The DNA extraction and identification of the strain via 16S rRNA were conducted by Beijing LiuheHuada Gene Technology Co., China (Beijing, China). The DNA extraction method was according to the instructions of the Bacterial Genome Kit (Tiangen Biochemical Technology Co., Ltd., Beijing, China). The 16S rRNA sequences of strain Y-4 were submitted to the GenBank database of the NCBI and subjected to BLAST comparison and homology with the reported sequences. The phylogenetic tree of Y-4 evaluated in 1000 replicates was constructed using the neighbor-joining method in the MEGA 10.2.6 software.
The whole genome sequencing analysis of strain Y-4 was operated by the Wuhan Frasergen company, China (Wuhan, China). For the bacterial genome assembly part, pb_assembly_hifi_microbial (from smrtlink11.0.0) software was used for assembly, and the second-generation data pair reads-based assembly correction was used to obtain the final assembly results using pilon-1.24 software [37]. Each sequence was compared with the NT database using the BLASTn tool of the ncbi-blast-2.11.0+ software [38] with the comparison options “-task megablast -outfmt 5 -evalue 1e-5 -max_target_seqs 3”. The remaining settings were set to default values. The strain identification was determined by combining the results of the NT database comparison and 16S rRNA gene sequencing.
4.4.2. Physiological and Biochemical Identification
Aerobic and anaerobic, catalase, glucose oxidative fermentation, and methyl red tests all refer to Berger’s Manual for the identification of bacteria [39]. Determination of the biofilm-forming ability followed the method of Sun et al. [40]. We referred to Tagele et al. for the determination of protease [41] and Slama et al. for the determination of amylase [42]. In the determination of cellulase, the cellulase detection culture dish was configured. A 6 mm diameter sterile filter paper was placed at the center of the test culture dish and 2 μL of Y-4 bacteria suspension cultured for 48 h was dripped onto the filter paper. The detection culture dish was placed in a constant-temperature incubator at 28 °C to culture for 3–7 days. If there was a transparent halo around the filter paper, it indicated that the strain could produce cellulase. The determination of siderophores followed the procedure of Bernhard Schwyn and J.B. Neilands (1987) [43]. The presence of an orange halo around the colony indicated siderophore production. All experiments in this part were repeated three times.
4.5. Annotation of the Structure and Function of Bacterial Genomes
4.5.1. Genome Structure Annotation
We used Glimmer (v3.02) [44] to find the coding genes of microbial DNA (particularly bacteria, archaea, and viruses), tRNAscan-SE (2.0.9) [45] to predict the tRNA of the microbial genome, and RNAmmer (v1.2) [46] to predict rRNA.
4.5.2. Annotation on the Database of Basic Gene Functions
Regarding the basic function annotation, we compared the predicted gene protein sequences to the Cluster of Orthologous Groups of Proteins (COG), KEGG, and GO databases.
We used diamond (v2.0.9.147) software to compare the protein sequence of the predicted gene to the COG2020 database using the BLASTp command. The E value 1e-5, and the hit with the highest score is selected as the final annotation result. For KEGG, we used the BLASTp command of the diamond (v2.0.9.147) software to align the protein sequence of the predicted gene into the entire library of KOBAS-v3.0 [47] and then used KOBAS-v3.0 software to analyze the comparison results to map the gene ID to KO. Finally, the hierarchical relationship table in the KEGG BRITE library (last updated: 11 October 2021) was used to annotate each level. For GO, we mapped GO term annotations through ID mapping (20210616) using SwissProt annotation results and then annotated at various levels using go-basic.obo (v2021-09-01).
4.5.3. Annotation on the Database of Special Gene Functions
To annotate CAZy [48], we employed dbCAN2 (an automatic web annotation tool for carbohydrate-related enzymes, annotation software HMMER-v3.3.2, parameter E Value <= 1e-5, coverage >= 0.35). In addition, we utilized diamond (v2.0.9.147) software’s BLASTp command to compare the predicted gene’s protein sequence to the PHI database.
4.6. Annotation of the Secondary Metabolite Gene Cluster
In this study, the gene clusters produced by the secondary metabolites of the strain were analyzed using anti-SMASH (v5.1.1).
4.7. Antifungal Activities of Biocontrol Bacterium
4.7.1. Determination of Broad-Spectrum Antifungal Effect of Biocontrol Bacterium
The abovementioned pathogens were made into 6 mm fungal chunks (we punched holes vertically on the surface of the fungal culture medium and used a sterile inoculation needle to pick it out to obtain a fungal chunk). The fungal chunk was inoculated on the right side of the sterile PDA agar media center, and the sterile Oxford cup was placed on the left. Using a pipette, 80–100 μL of Y-4 and wz-37 bacterial suspensions were injected into the Oxford cups. On the right side of the control group was an F. oxysporum chunk, and 80–100 μL of sterile water was added to the Oxford cup on the left side. We used wz-37 as a positive control group. The culture dish was sealed and incubated in a constant-temperature oven at 28 °C for 7 days. The width of the inhibition zone was measured. The test was repeated three times.
4.7.2. Determination of the Inhibition Effect of F. oxysporum
First, we made F. oxysporum chunks (the production method of F. oxysporum chunks is the same as in Section 4.7.1). The culture dishes were filled with PDA agar media and dried. We placed the F. oxysporum chunks on the right side of the Petri dish and placed the sterile Oxford cup on the left side. Using a pipette, 80–100 μL of Y-4 and wz-37 bacterial suspensions were injected into Oxford cups, and CONTROL added an equal amount of sterile water to them. The culture dish was sealed and incubated in a constant-temperature oven at 28 °C for 7 days. The width of the inhibition zone was measured. The test was repeated three times.
4.7.3. Trypan Blue Dyeing Observation
The pretreatment for the leaves was as follows: The leaves were immersed in 75% ethanol for 30 s. After rinsing, the leaves were immersed in 3% sodium hypochlorite for 3 min and then rinsed three times with sterile water. The petioles were wrapped in sterile, moist, skimmed cotton wool. We applied sterile water (CONTROL), Y-4 bacterial suspension, and wz-37 bacterial suspension (1 × 109 CFU/mL) to the leaves. A suspension of F. oxysporum spores (1 × 106 CFU/mL) was evenly sprayed on dried leaves. A staining test was performed after 3 days. The dyeing process followed the method described by Yin et al. [49]. The stained leaves were observed for cell color under an electron microscope. Deep cell color indicated leaf cell death, and light color indicated leaf cells were active.
4.8. Determination of Indoor Control Efficiency of Biocontrol Bacterium
Biocontrol suspension and F. oxysporum suspension (preparation method followed Section 4.1) were placed into two spray bottles, respectively. First, we sprayed biocontrol suspension evenly on the surface of the plant. After 24 h, we sprayed F. oxysporum suspension.
After 15 days of treatment, plant disease indices and prevention effects were recorded. The test was repeated five times. The disease index and disease control effect were calculated using the following formulas [50]. The treatment was as follows:
- (1)
- CONTROL+ F. oxysporum: we sprayed the surface of tomato plants with sterile water first, followed by the F. oxysporum suspension.
- (2)
- Y-4 + F. oxysporum: we sprayed the surface of tomato plants with Y-4 suspension first, followed by the F. oxysporum suspension.
- (3)
- wz-37 + F. oxysporum: we sprayed the surface of tomato plants with wz-37 suspension first, followed by the F. oxysporum suspension.
Disease index = [∑(The number of diseased plants in this grade × Disease grade)/(Total number of plants investigated × the highest disease grade)] × 100.
Control efficiency (%) = [(Disease index of control − Disease index of treated group)/Disease index of control] × 100.
4.9. Induction of Antioxidant Enzymes in Tomato Leaves by Biocontrol Bacterium
Leaf samples were collected after 7 days of treatment of the plants, as in Section 4.8. The collection period lasted 7 days. For the sampling standard, leaves with the same growth position and similar leaf size were selected and liquid nitrogen was used for quick freezing, followed by storage in −80 °C refrigerators. Peroxidase (POD) and superoxide (SOD) kits (Suzhou Grace Biotechnology Co., Ltd., Suzhou, China) were used to determine the activity of defense enzymes.
4.10. Statistical Analysis
Excel: numerical values were the mean ± standard deviation (SD) of triplicates (Version 16.77.1). Significance analysis was performed using the one-way ANOVA test (p ≤ 0.05); multiple group comparisons were performed using Tukey after ANOVA in SPSS (Version 26.0). The error bars indicate the SD of the data. The column and line chart were drawn using GraphPad Prism 9 (Version 9.0.0).
5. Conclusions
In conclusion, strain Y-4 as a biocontrol bacterium has been widely used in plant disease management owing to its high efficiency, safety, and environmental friendliness. Strain Y-4 is an aerobic bacterium, which can produce biofilm and many extracellular enzymes. Strain Y-4 plays a crucial role in reducing F. oxysporum-induced blight, safeguarding tomato plants against harmful bacteria and ensuring high-quality and high-yield tomato fruits. In summary, strain Y-4 is a newly discovered and highly effective agent for pathogen control.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26020700/s1.
Author Contributions
Conceptualization, Q.W., Z.S., T.L., X.C. and J.L.; data curation, Q.W.; Formal analysis, Q.W.; investigation, Q.W., Z.S., Z.Z. and T.F.; methodology, Q.W., T.L. and X.C.; resources, X.C. and A.W.; software, Q.W.; Supervision, X.C. and A.W.; validation, Q.W.; visualization, Q.W.; writing—original draft preparation, Q.W.; writing—review and editing, Q.W. and X.C. Funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Program of Shandong (2024LZGCQY015) and National Natural Science Foundation of China (32072588). In addition, we are grateful for the support provided by the Taishan Industry Leading Talent Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; Park, H.B.; Kim, J.Y.; Choi, S.M.; Lee, D.S.; Kim, D.H.; Lee, Y.H.; Park, C.J.; Jeun, Y.C.; Hong, J.K. Menadione Sodium Bisulfite-Protected Tomato Leaves against Grey Mould via Antifungal Activity and Enhanced Plant Immunity. Plant Pathol. J. 2020, 36, 335, Erratum in Plant Pathol. J. 2021, 37, 204. [Google Scholar] [CrossRef]
- Li, S.P.; Xiao, Q.L.; Yang, H.J.; Huang, J.G.; Li, Y. Characterization of a new Bacillus velezensis as a powerful biocontrol agent against tomato gray mold. Pest. Biochem. Physiol. 2022, 187, 10. [Google Scholar] [CrossRef]
- Pessarakli, M.; Heydari, A. A Review on Biological Control of Fungal Plant Pathogens Using Microbial Antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar]
- Jiang, C.H.; Wu, F.; Yu, Z.Y.; Xie, P.; Ke, H.J.; Li, H.W.; Yu, Y.Y.; Guo, J.H. Study on screening and antagonistic mechanisms of Bacillus amyloliquefaciens 54 against bacterial fruit blotch (BFB) caused by Acidovorax avenae subsp citrulli. Microbiol. Res. 2015, 170, 95–104. [Google Scholar] [CrossRef]
- Elslahi, R.H.; Osman, A.G.; Sherif, A.M.; Elhussein, A.A. Comparative study of the fungicide Benomyl toxicity on some plant growth promoting bacteria and some fungi in pure cultures. Interdiscip. Toxicol. 2014, 7, 12–16. [Google Scholar] [CrossRef]
- Chen, L.; Miao, W.G.; Liu, H.Y.; Nu, E.Z.Y. Antagonistic Mechanism of Brevibacillus brevs A57 against Cotton Mainly Pathogenic Fungus. Acat Agric. Boreali-Occident. Sin. 2008, 17, 149–155. [Google Scholar]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137–143. [Google Scholar] [CrossRef]
- Palazzini, J.; Reynoso, A.; Yerkovich, N.; Zachetti, V.; Ramirez, M.; Chulze, S. Combination of Bacillus velezensis RC218 and Chitosan to Control Fusarium Head Blight on Bread and Durum Wheat under Greenhouse and Field Conditions. Toxins 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.D.; Ma, Z.; Xu, X.H.; Yu, X.P. Isolation and identification of biocontrol agent Streptomyces rimosus M527 against Fusarium oxysporum f. sp cucumerinum. J. Basic Microbiol. 2016, 56, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Yuan, J.; Zhang, J.; Shen, Z.Z.; Zhang, M.X.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Baiome, B.A.; Ye, X.F.; Yuan, Z.Y.; Gaafar, Y.Z.A.; Melak, S.; Cao, H. Identification of Volatile Organic Compounds Produced by Xenorhabdus indica Strain AB and Investigation of Their Antifungal Activities. Appl. Environ. Microbiol. 2022, 88, 17. [Google Scholar] [CrossRef]
- Alam, S.S.; Sakamoto, K.; Inubushi, K. Biocontrol efficiency of Fusarium wilt diseases by a root-colonizing fungus Penicillium sp. Soil Sci. Plant Nutr. 2011, 57, 204–212. [Google Scholar] [CrossRef]
- Wang, W.; Jin, H.; Cong, B.; Zhou, L.; Wei, Z.; Wang, S. Biocontrol effect of composite microbial agent on tomato bacterial wilt. J. Nanjing Agric. Univ. 2022, 45, 1174–1182. [Google Scholar]
- Zhang, D.; Gao, Y.; Wang, Y.; Liu, C.; Shi, C. Advances in taxonomy, antagonistic function and application of Bacillus velezensis. Microbiol. China 2020, 47, 3634–3649. [Google Scholar]
- Xie, S.S.; Yu, H.G.; Li, E.Z.; Wang, Y.; Liu, J.; Jiang, H.Y. Identification of miRNAs Involved in Bacillus velezensis FZB42-Activated Induced Systemic Resistance in Maize. Int. J. Mol. Sci. 2019, 20, 14. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Béjar, V.; Martínez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Errington, J. Bacillus subtilis sporulation: Regulation of gene expression and control of morphogenesis. Microbiol. Rev. 1993, 57, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Bao, X.; Li, W.; An, T.; Sun, H. Solation, Purification and Identification of a Bacillus velezensis B18 and its Control Effect on Banana Wilt. Genom. Appl. Biol. 2021, 40, 3209–3215. [Google Scholar]
- Wang, H.; Liu, L.; Yu, S.; Guan, T.; Zou, C.; Li, B.; Zheng, L. Identification and Evaluation of Bacillus subtilis SF18-3 Against Bacterial Spot Disease of Pepper. J. Shenyang Agric. Univ. 2024, 55, 417–425. [Google Scholar]
- Wang, X.Y.; Zhou, X.N.; Cai, Z.B.; Guo, L.; Chen, X.L.; Chen, X.; Liu, J.Y.; Feng, M.F.; Qiu, Y.W.; Zhang, Y.; et al. A Biocontrol Strain of Pseudomonas aeruginosa CQ-40 Promote Growth and Control Botrytis cinerea in Tomato. Pathogens 2021, 10, 17. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.-H. Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Thurlow, C.M.; Williams, M.A.; Carrias, A.; Ran, C.; Newman, M.; Tweedie, J.; Allison, E.; Jescovitch, L.N.; Wilson, A.E.; Terhune, J.S.; et al. Bacillus velezensis AP193 exerts probiotic effects in channel catfish (Ictalurus punctatus) and reduces aquaculture pond eutrophication. Aquaculture 2019, 503, 347–356. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, B.; Zhu, Y.; Xiao, R.; Yang, W.; Ge, C.; Chen, Z. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 2020, 20, 160. [Google Scholar] [CrossRef]
- Wang, C.C.; Ye, X.J.; Ng, T.B.; Zhang, W.J. Study on the Biocontrol Potential of Antifungal Peptides Produced by Bacillus velezensis against Fusarium solani That Infects the Passion Fruit Passiflora edulis. J. Agric. Food Chem. 2021, 69, 2051–2061. [Google Scholar] [CrossRef]
- Li, Q.; Liao, S.; Wei, J.; Xing, D.; Xiao, Y.; Yang, Q. Isolation of Bacillus subtilis strain SEM-2 from silkworm excrement and characterisation of its antagonistic effect against Fusarium spp. Can. J. Microbiol. 2020, 66, 401–412. [Google Scholar] [CrossRef]
- Charlop-Powers, Z.; Owen, J.G.; Reddy, B.V.B.; Ternei, M.A.; Brady, S.F. Chemical-biogeographic survey of secondary metabolism in soil. Proc. Natl. Acad. Sci. USA. 2014, 111, 3757–3762. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Zhao, X.; Li, Y.; Wang, R.; Huang, Y.; Qi, G. Engineering Bacillus velezensis with high production of acetoin primes strong induced systemic resistance in Arabidopsis thaliana. Microbiol. Res. 2019, 227, 126297. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 2008, 227, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, Y.; Wang, M.; Liu, W.; Lu, F.; Li, Y. Effects of Different Integration Sites on the Expression of Exogenous Alkaline Protease in Bacillus amyloliquefaciens. Biotechnol. Bull. 2022, 38, 253–260. [Google Scholar]
- Afsharmanesh, H.; Ahmadzadeh, M.; Javan-Nikkhah, M.; Behboudi, K. Improvement in biocontrol activity of Bacillus subtilis UTB1 against Aspergillus flavus using gamma-irradiation. Crop. Prot. 2014, 60, 83–92. [Google Scholar] [CrossRef]
- Li, B.; Xu, L.H.; Lou, M.M.; Li, F.; Zhang, Y.D.; Xie, G.L. Isolation and characterization of antagonistic bacteria against bacterial leaf spot of Euphorbia pulcherrima. Lett. Appl. Microbiol. 2008, 46, 450–455. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.D.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Whitman, W.B.; Goodfellow, M.; Kämpfer, P. Bergey’s Manual of Systematic Bacteriology. Vol. 5 The Actinobacteria; Springer: New York, NY, USA, 2012; p. 2012. [Google Scholar]
- Sun, D.; Zhuo, T.; Hu, X.; Fan, X.; Zou, H. Identification of a Pseudomonas putida as biocontrol agent for tomato bacterial wilt disease. Biol. Control 2017, 114, 45–50. [Google Scholar] [CrossRef]
- Tagele, S.B.; Kim, S.W.; Lee, H.G.; Kim, H.S.; Lee, Y.S. Effectiveness of multi-trait Burkholderia contaminans KNU17BI1 in growth promotion and management of banded leaf and sheath blight in maize seedling. Microbiol. Res. 2018, 214, 8–18. [Google Scholar] [CrossRef]
- Slama, H.B.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium Antagonistic Bacteria From Contrasting Niches Designated the Endophyte Bacillus halotolerans as Plant Warden Against Fusarium. Front. Microbiol. 2018, 9, 3236. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rodland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. 2), W316–W322. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2008, 37 (Suppl. 1), D233–D238. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, J.; Zeng, L.; Goh, M.; Leung, H.; Khush, G.S.; Wang, G.L. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol. Plant-Microbe Interact. MPMI 2000, 13, 869–876. [Google Scholar] [CrossRef]
- Sato, I.; Yoshida, S.; Iwamoto, Y.; Aino, M.; Hyakumachi, M.; Shimizu, M.; Takahashi, H.; Ando, S.; Tsushima, S. Suppressive Potential of Paenibacillus Strains Isolated from the Tomato Phyllosphere against Fusarium Crown and Root Rot of Tomato. Microbes Environ. 2014, 29, 168–177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).