Ultrastructural Study and Immunohistochemical Characteristics of Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta) Brain After Acute Traumatic Injury
Abstract
1. Introduction
2. Results
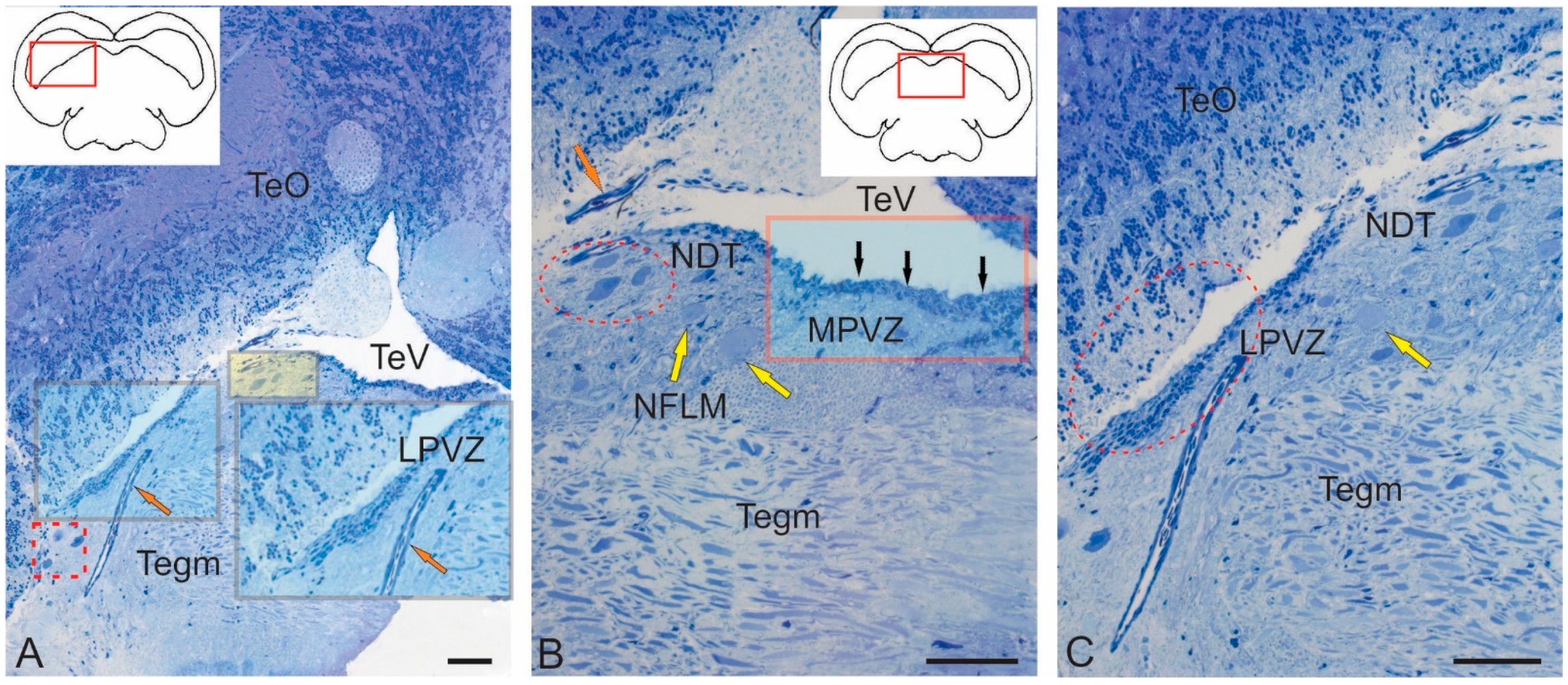
2.1. Ultrastructural Characteristics of Tegmentum Nuclei in Juvenile O. keta
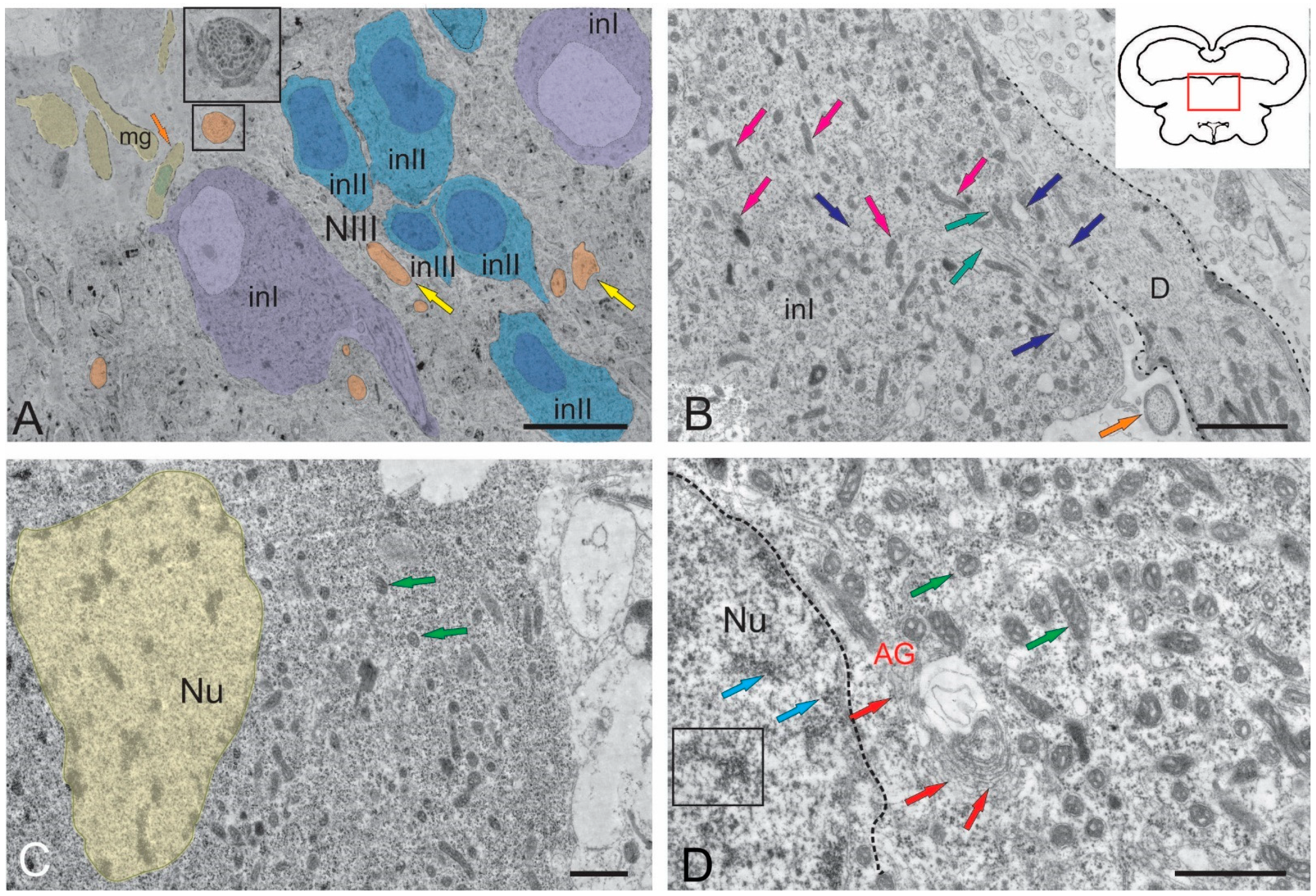
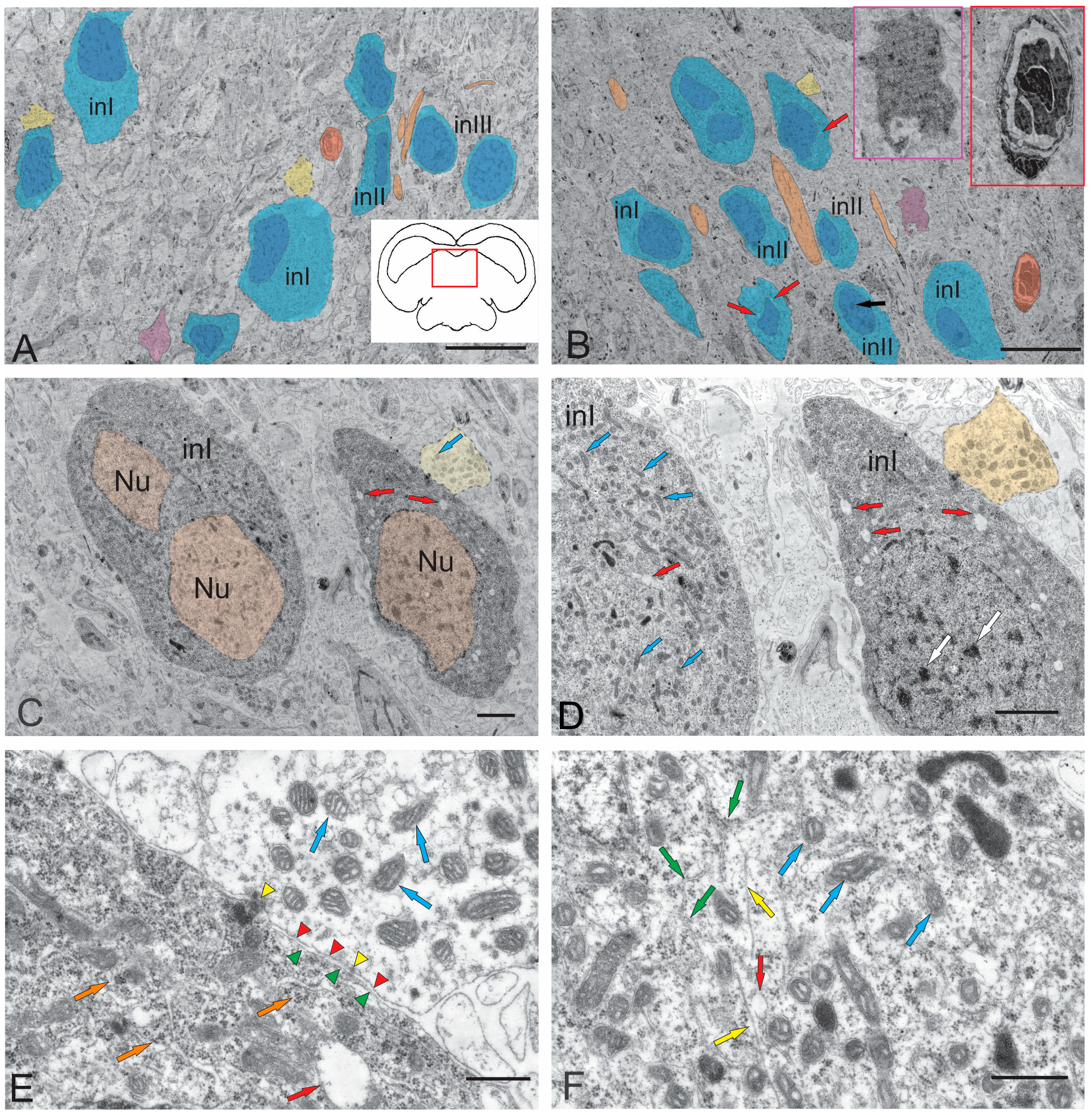
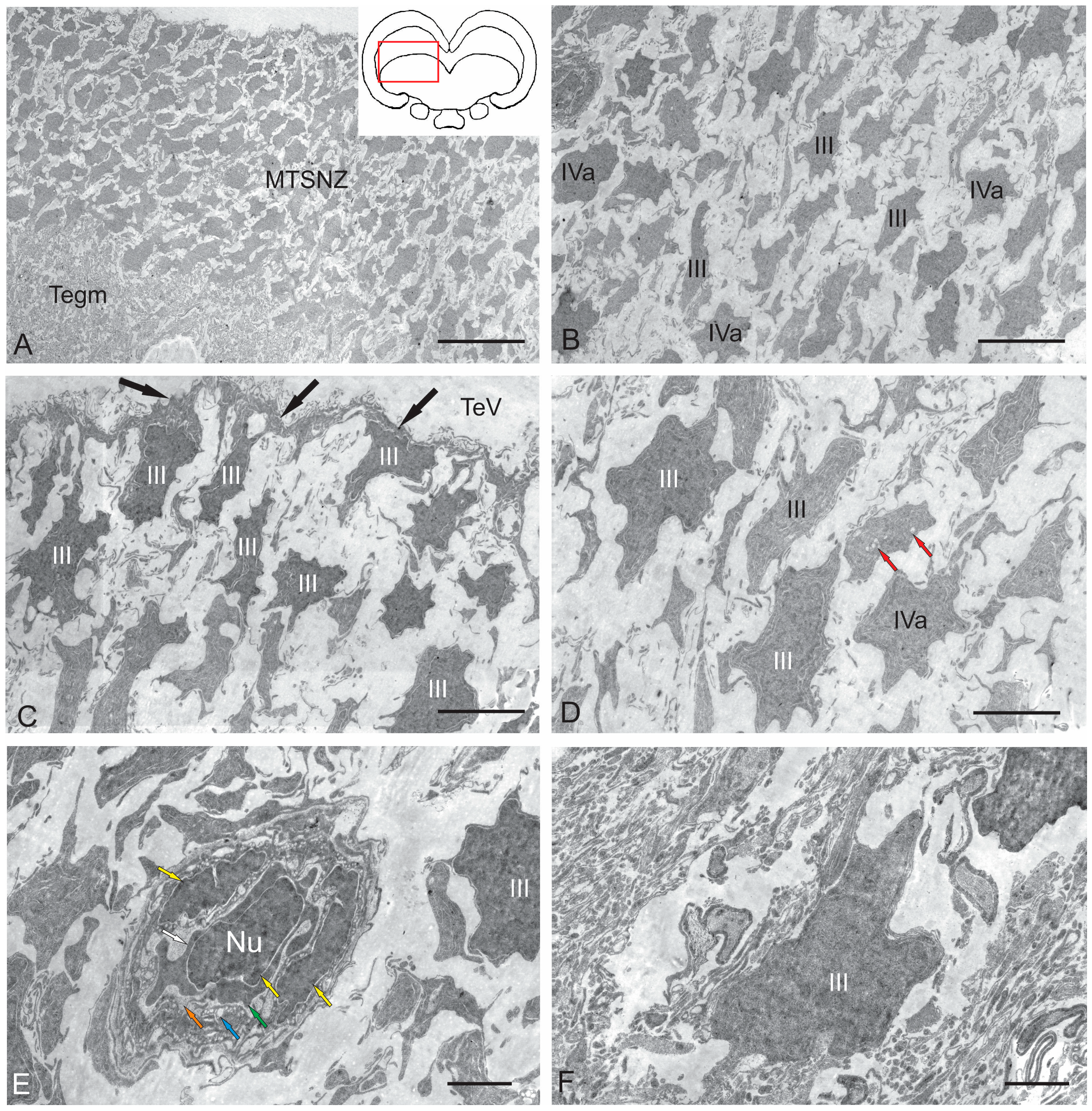
2.1.1. Nucleus of the III Nerve
2.1.2. Nucleus of Fasciculus Longitudinalis Medialis (NFLM)
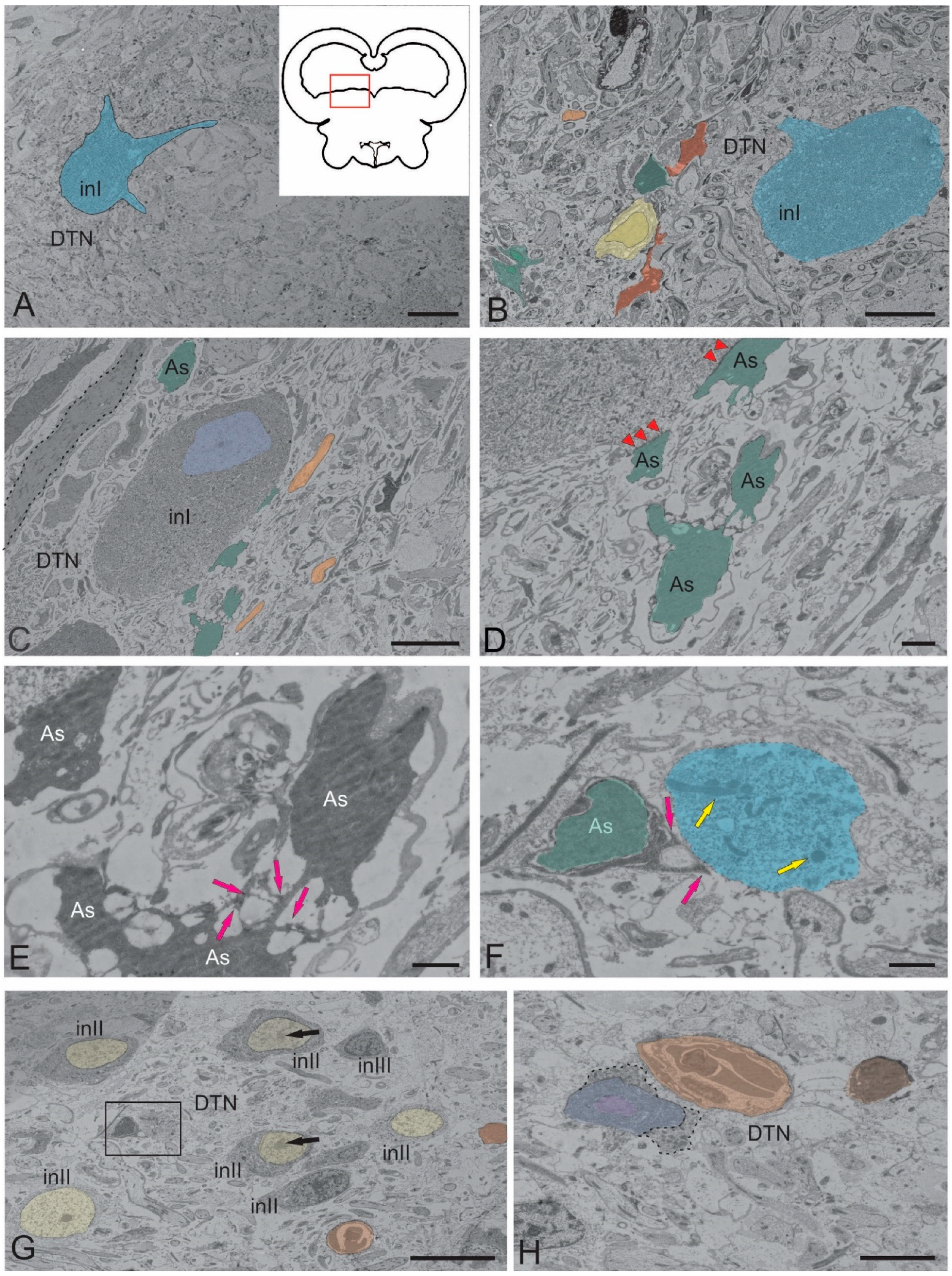
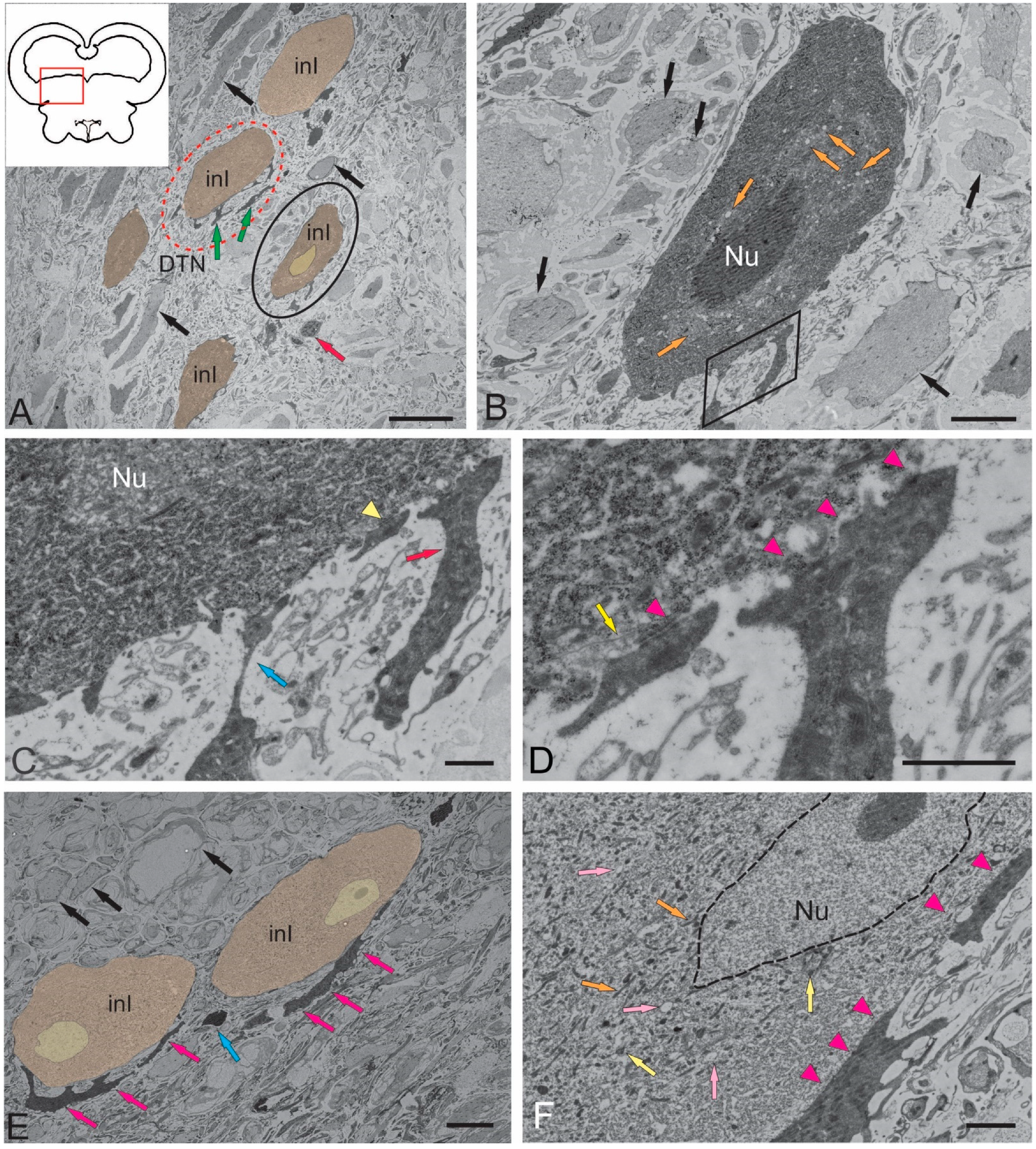
2.1.3. Dorsal Tegmental Nuclei (DTN)
2.2. Ultrastructural Characterization of Neurogenic Zones of the Tegmentum of Juvenile Chum Salmon, O. keta
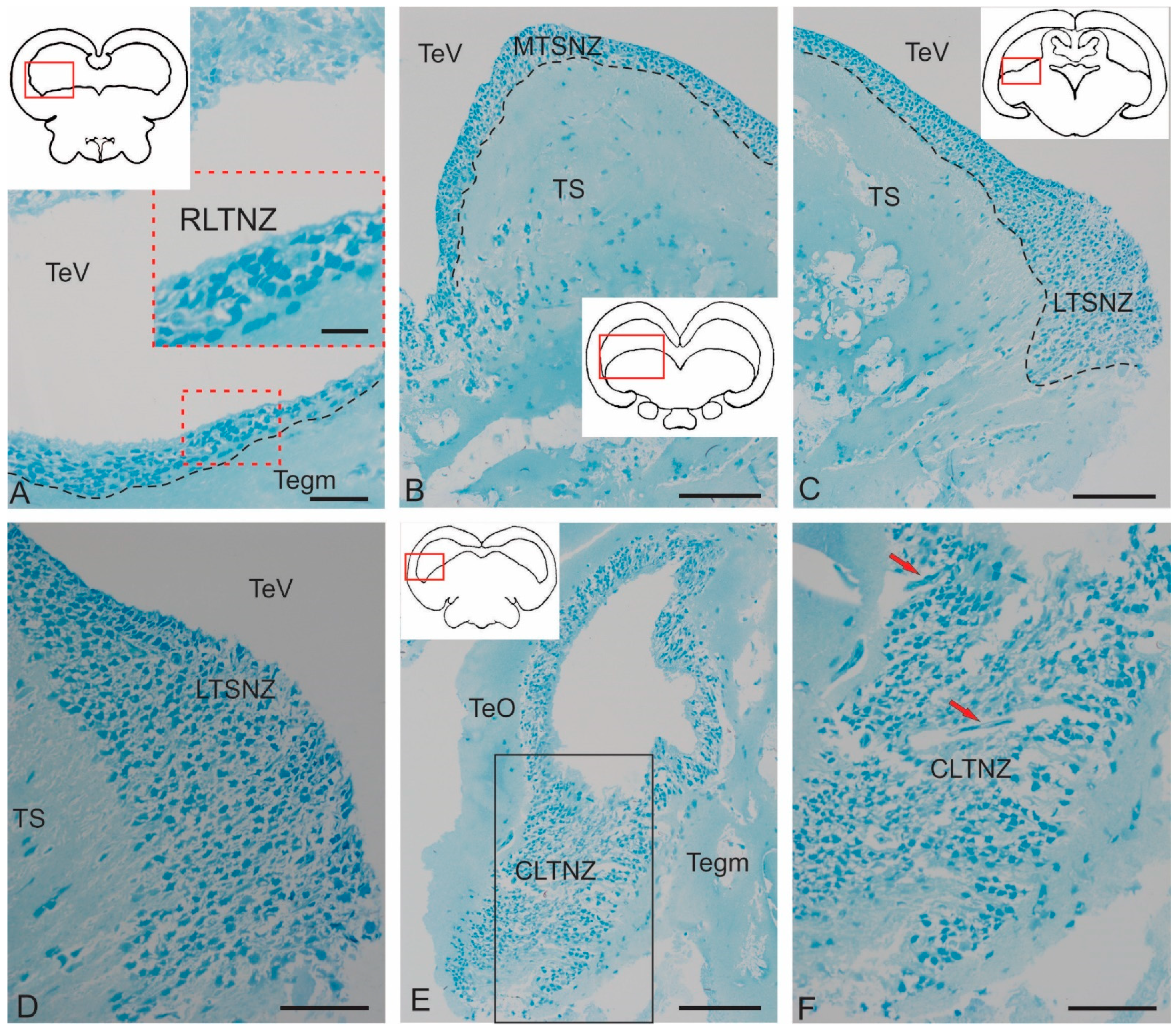
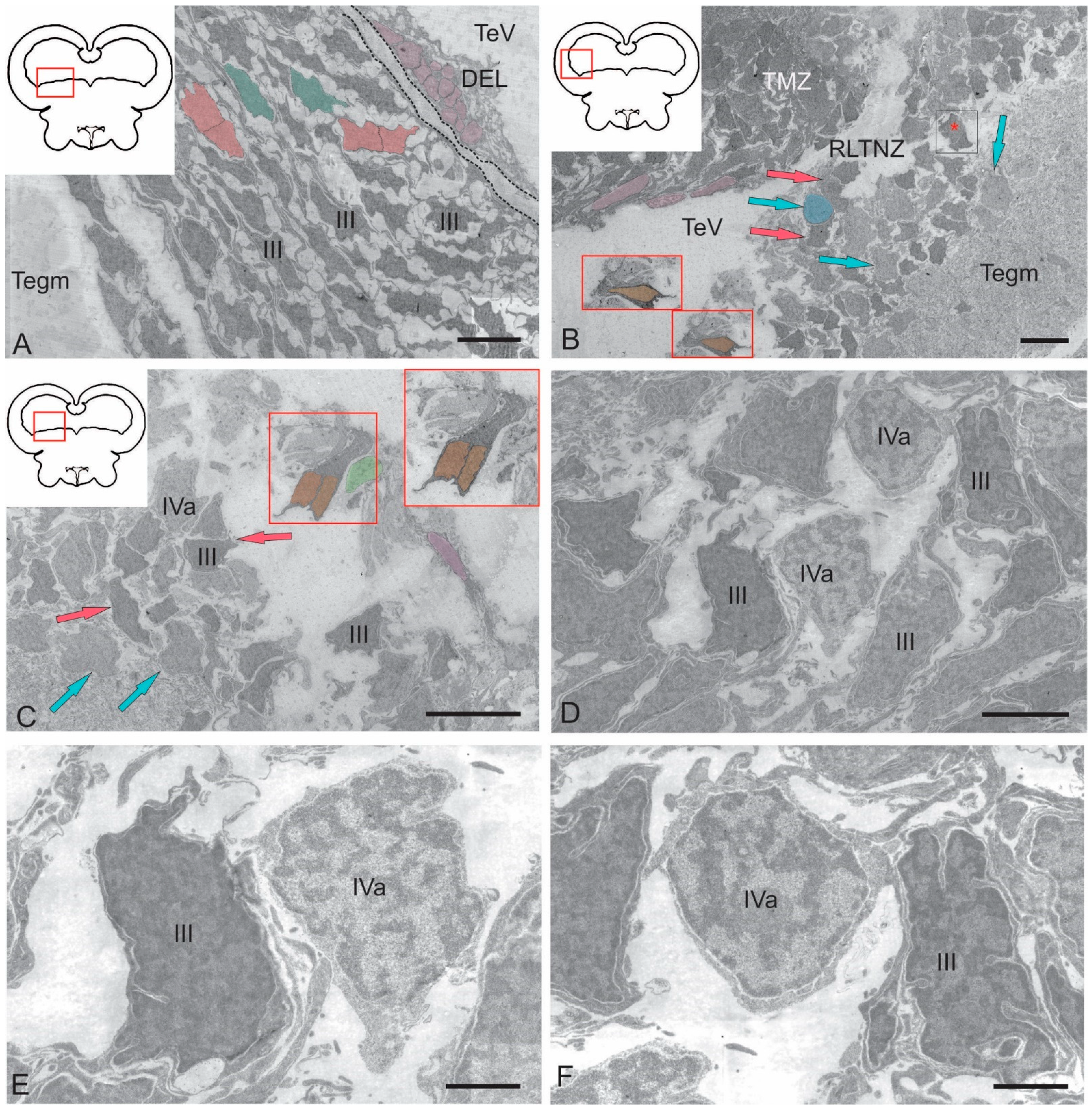
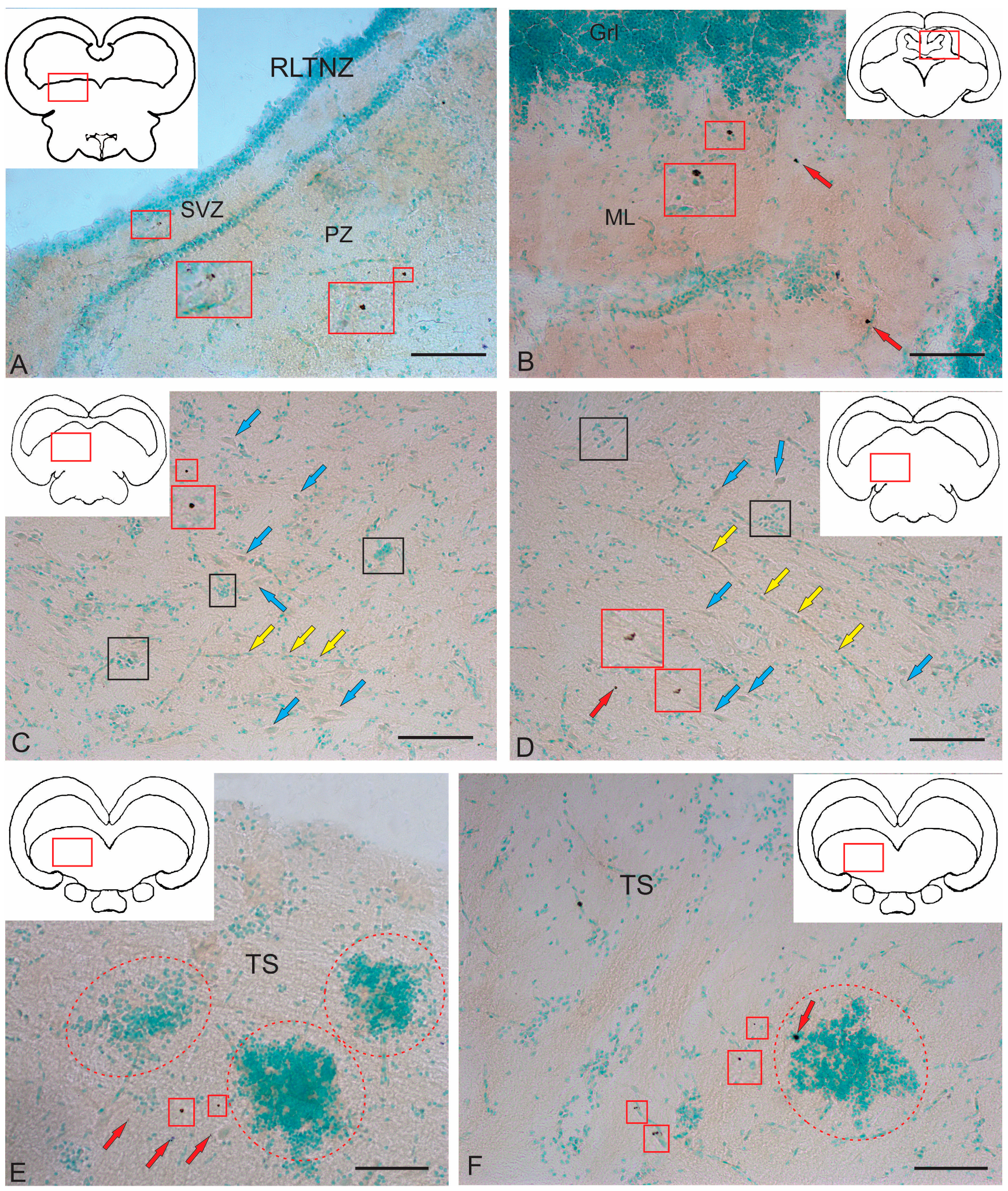
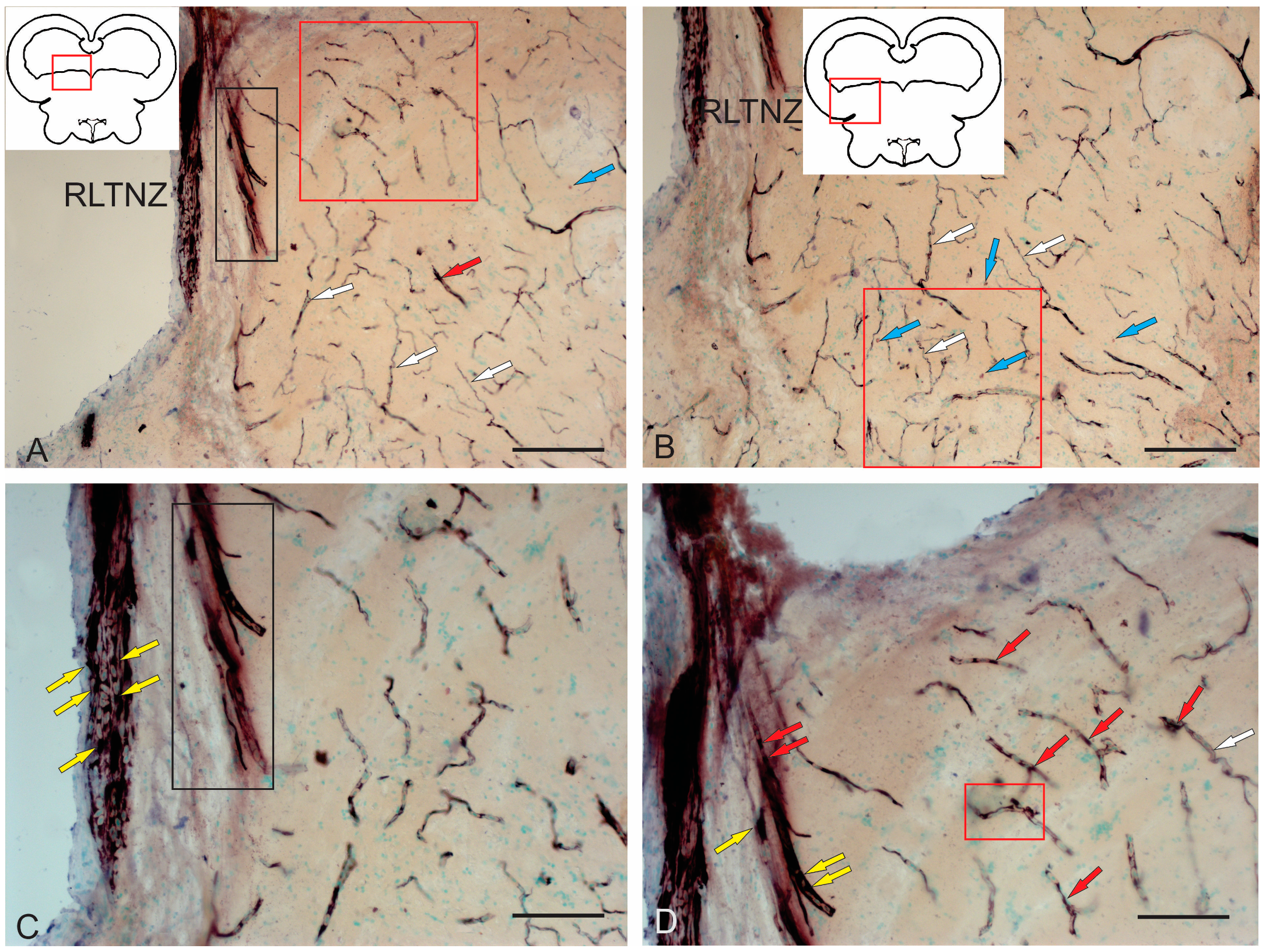
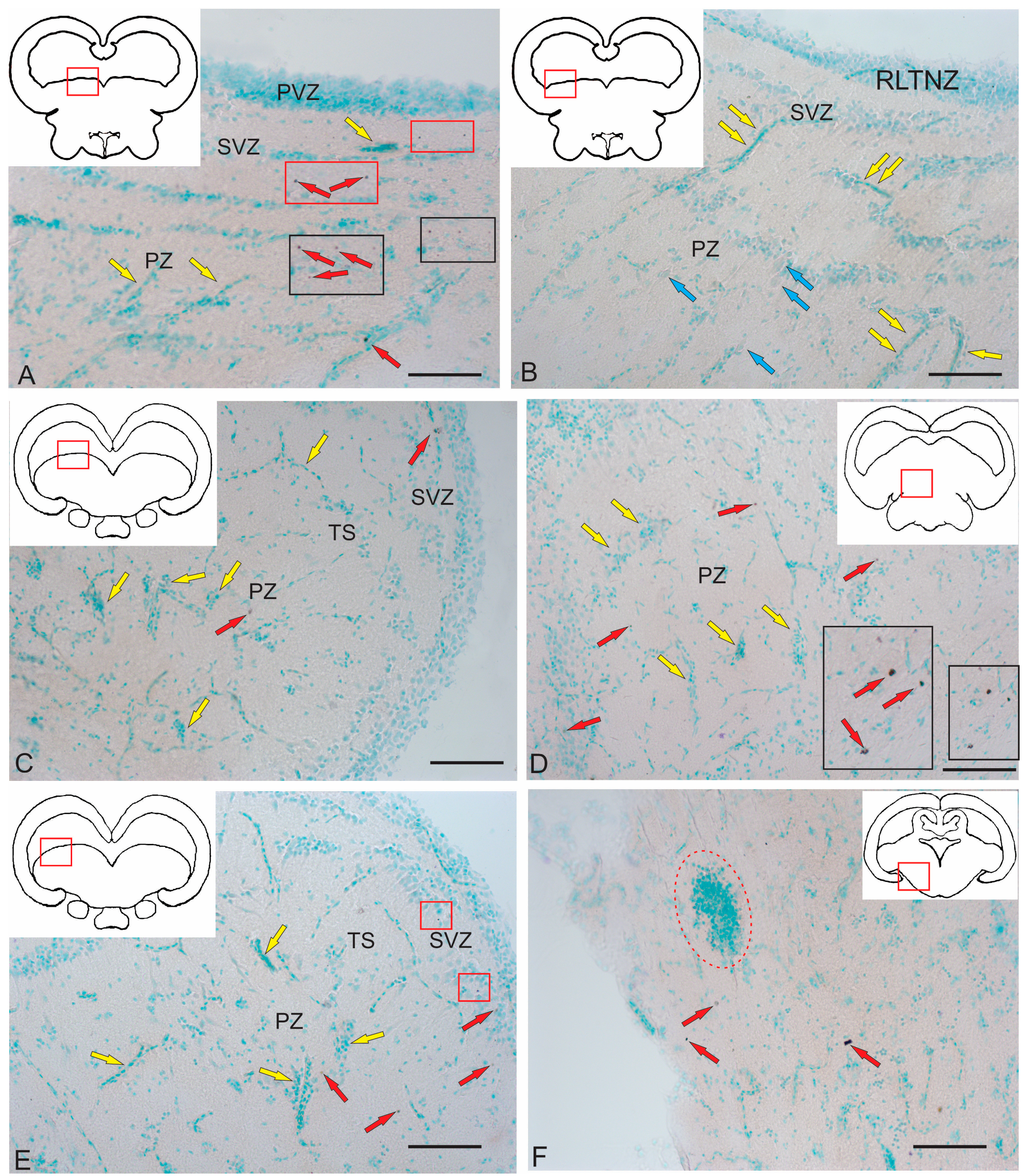
2.2.1. Rostral Lateral Tegmental Neurogenic Zone (RLTNZ)
2.2.2. Torus Semicircularis Neurogenic Zone (TSNZ)
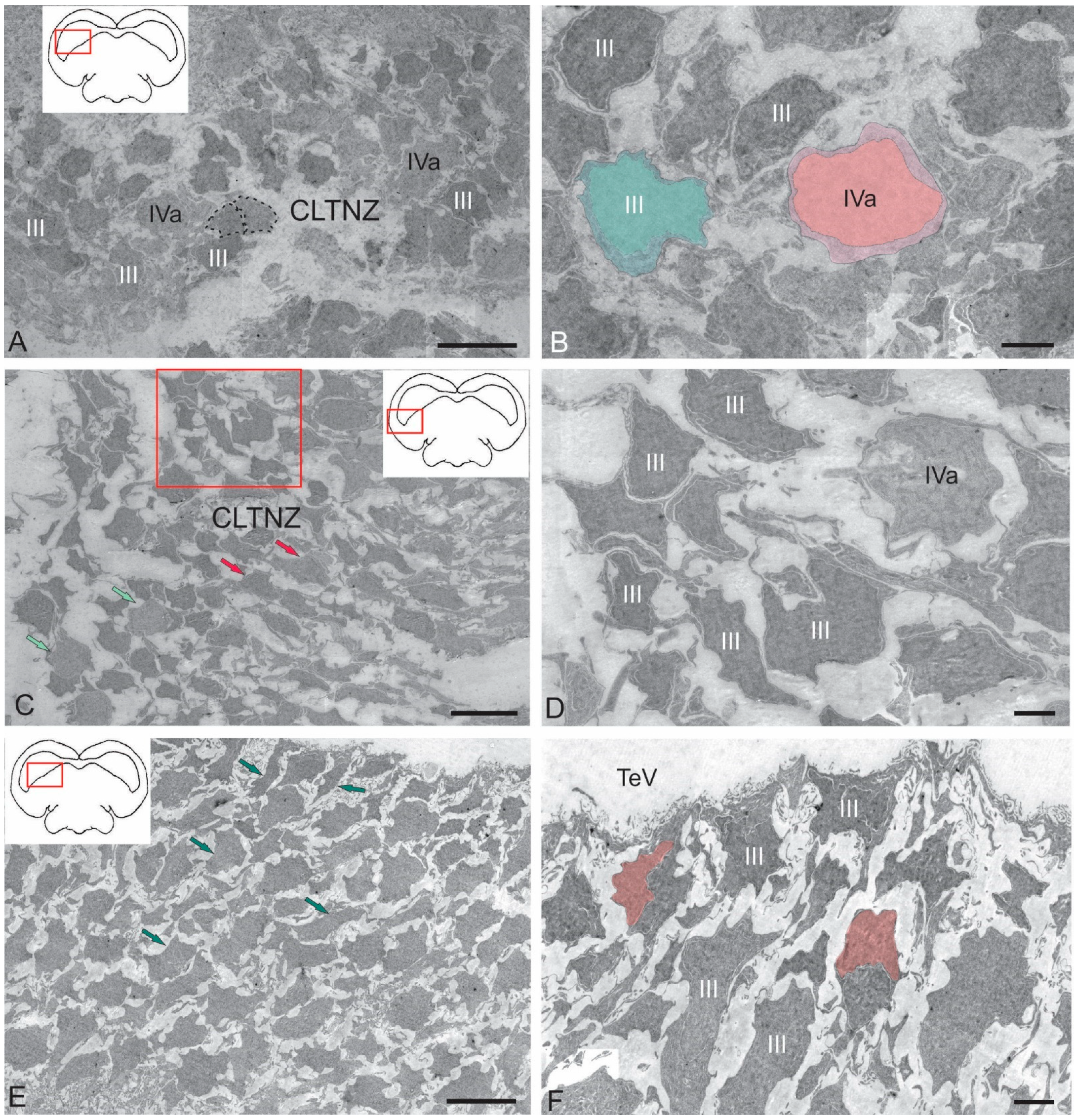
2.2.3. Caudo-Lateral Tegmental Neurogenic Zone (CLTNZ)
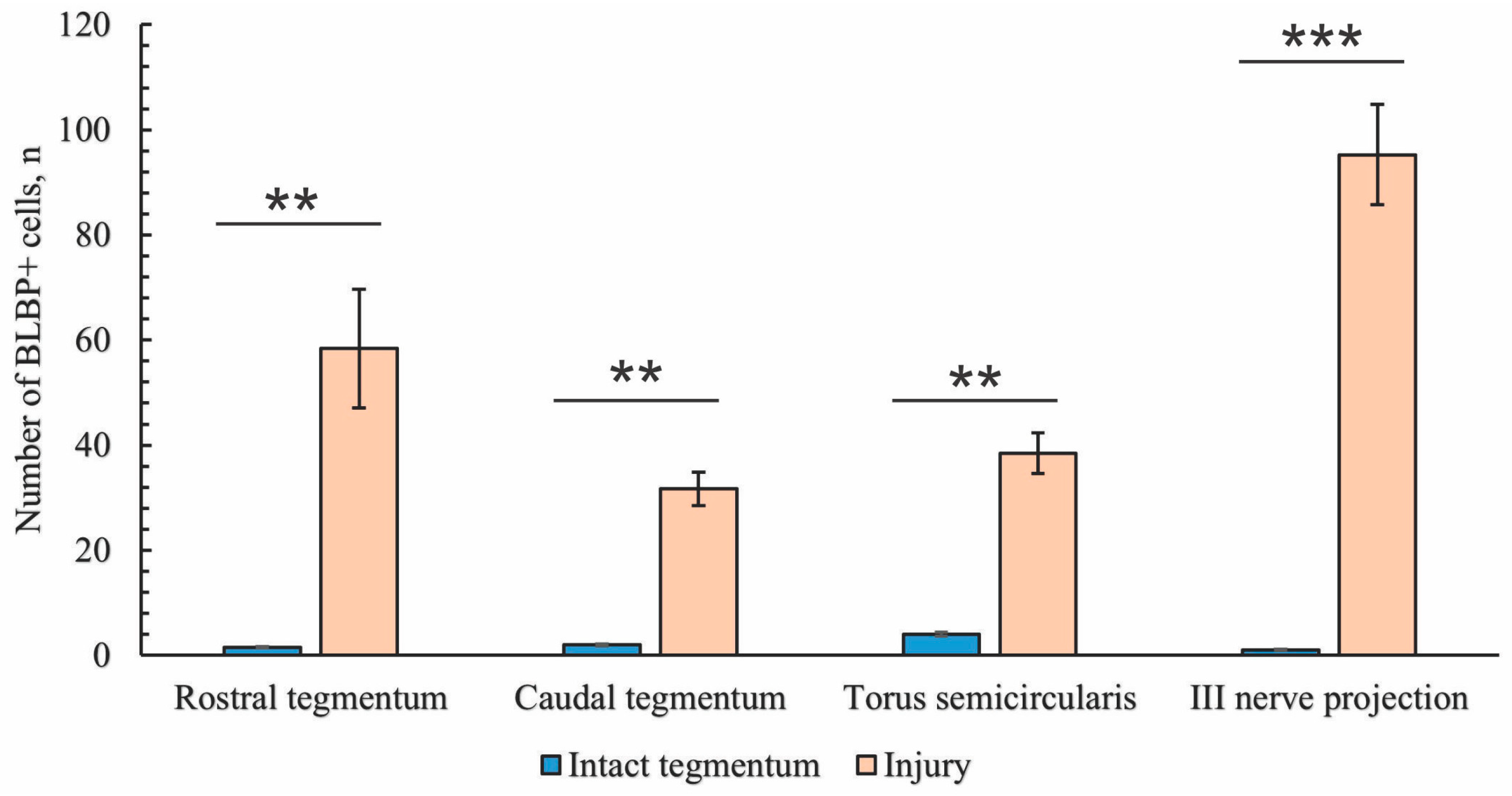
2.3. Immunohistochemical Labeling of BLBP
2.3.1. Immunohistochemical Labeling of BLBP in the Tegmentum of Intact Fish
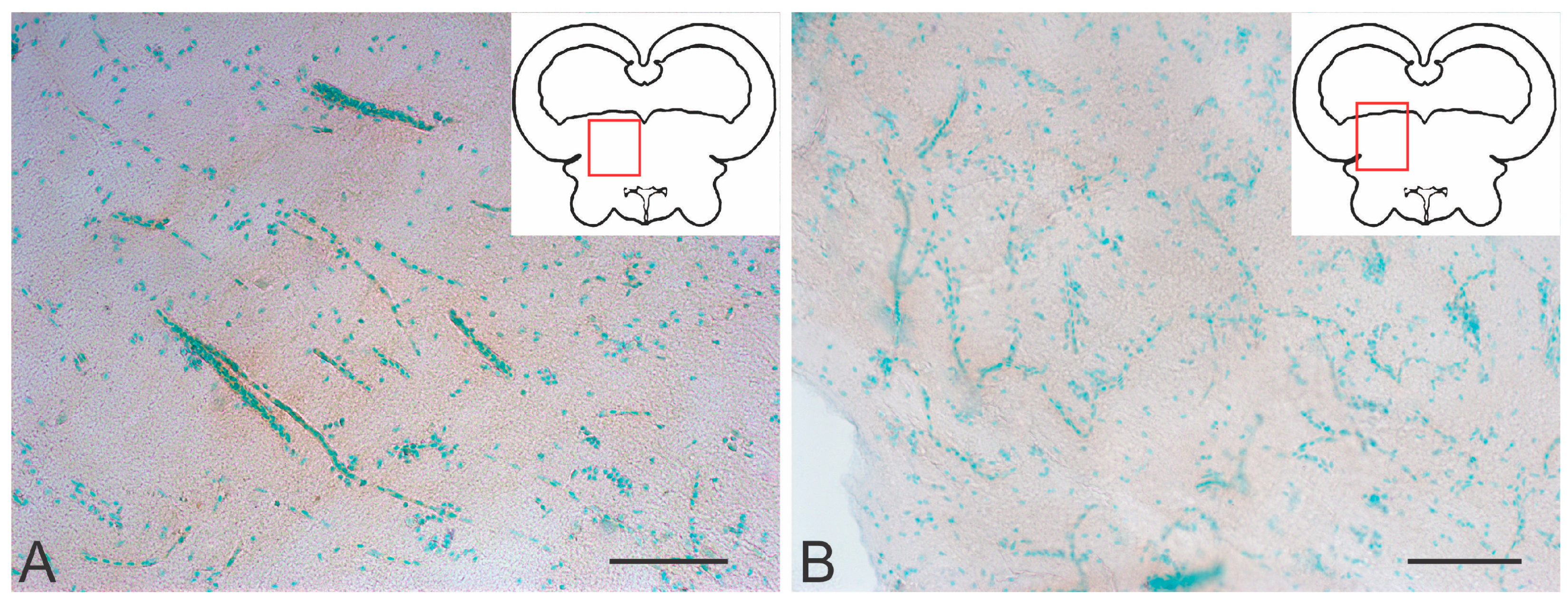
2.3.2. Immunohistochemical Labeling of BLBP in the Tegmentum After Traumatic Injury
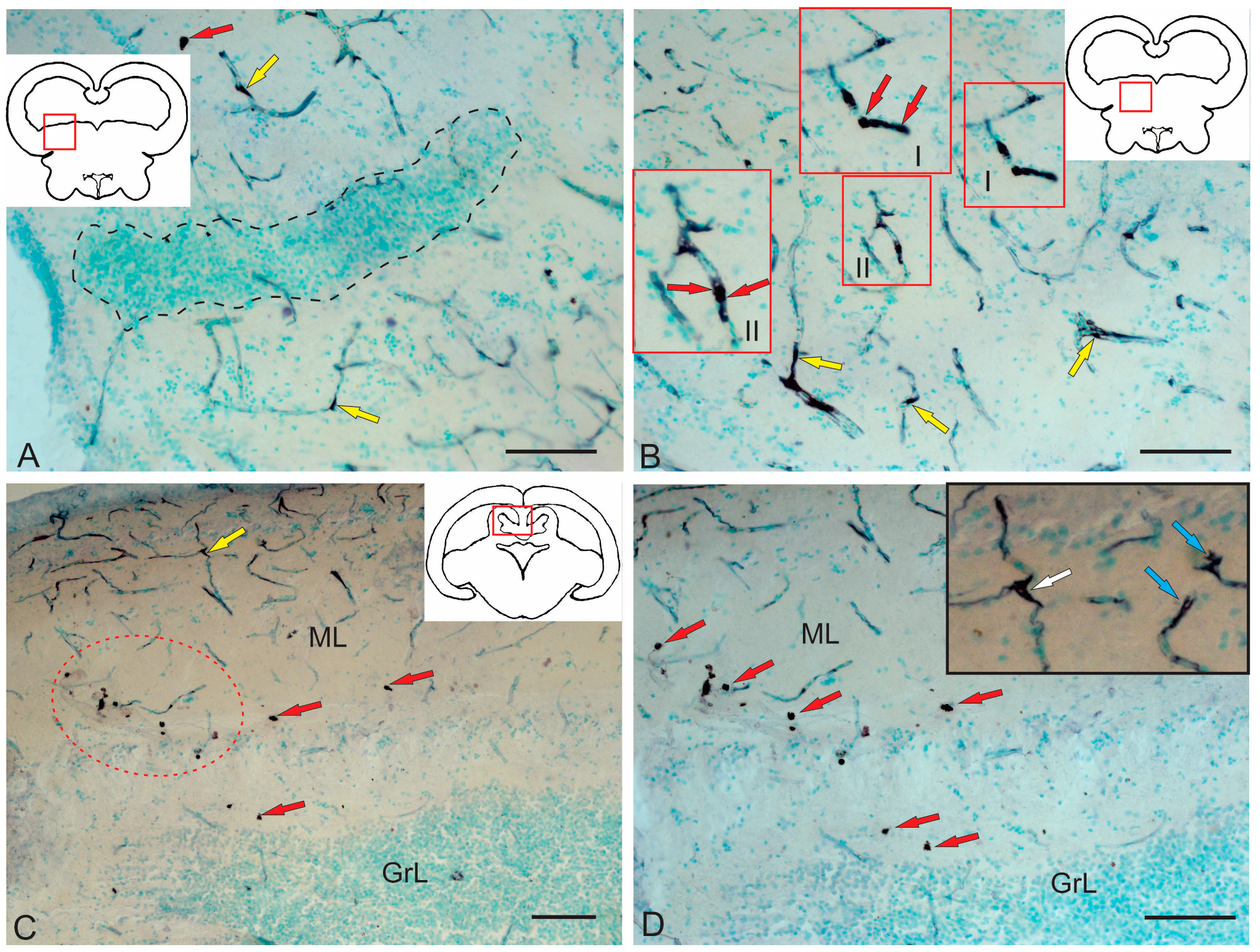
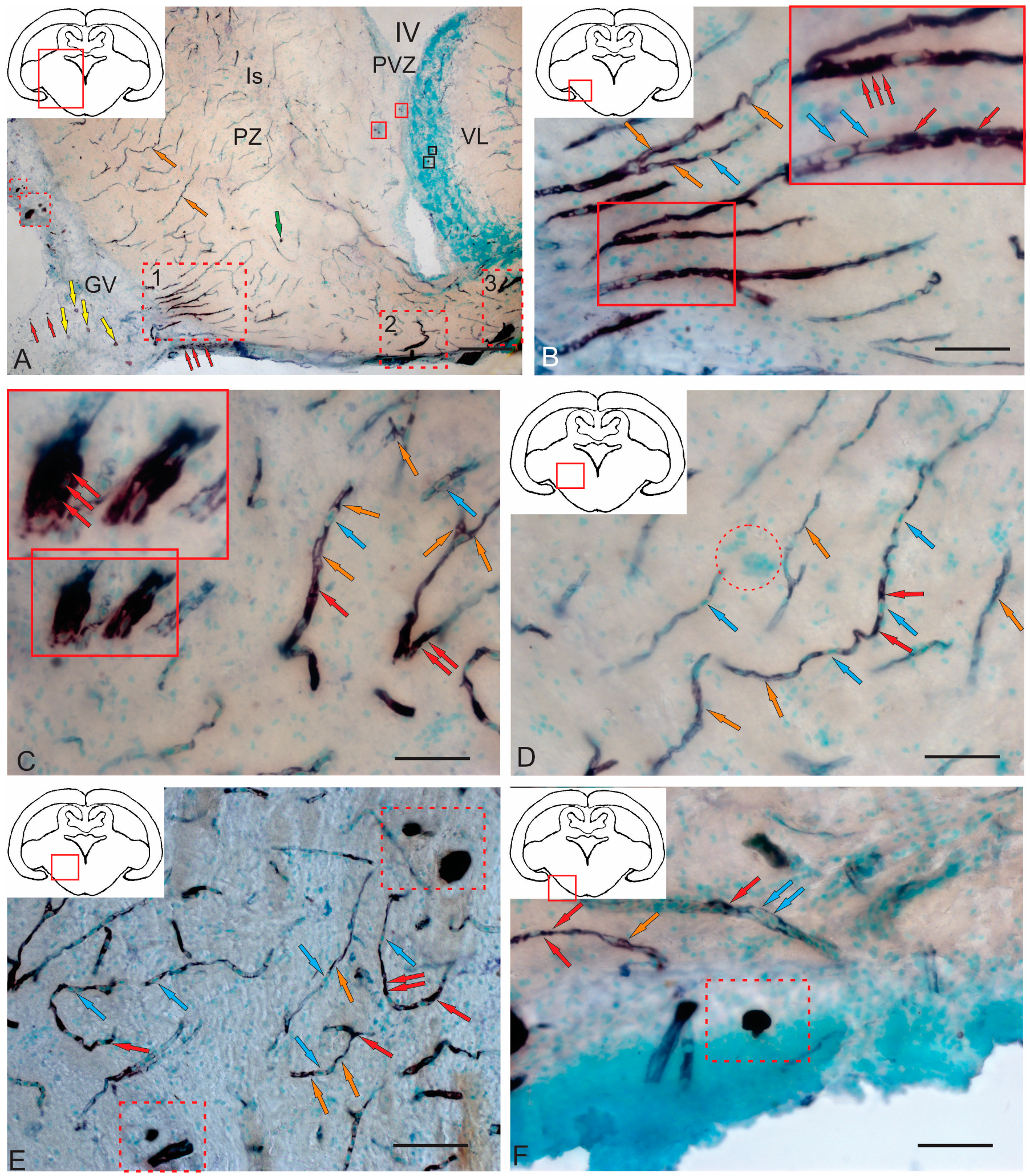
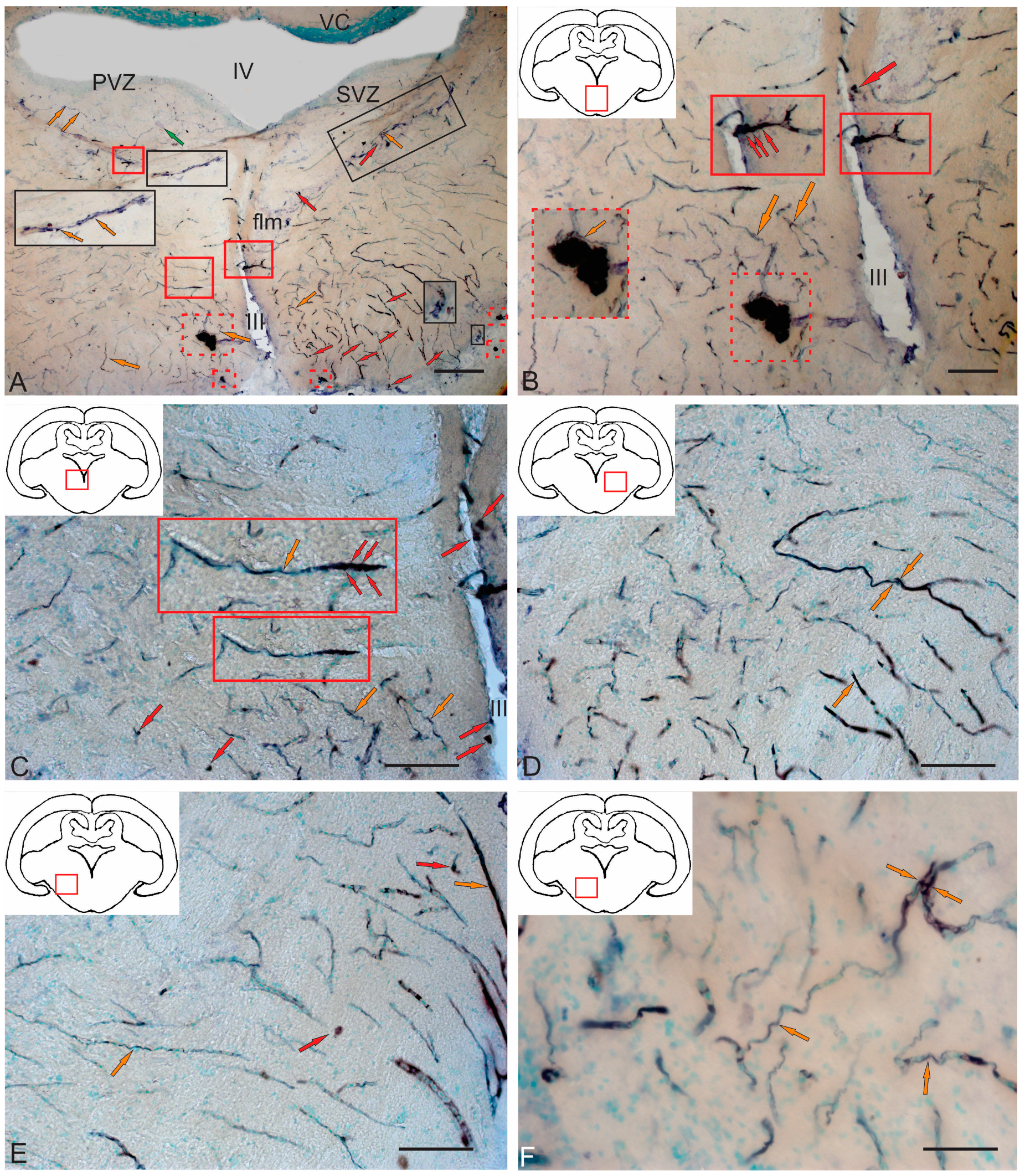
2.3.3. BLBP Expression in RG of the Tegmentum Post-Injury
2.3.4. BLBP Expression in Neurogenic Zones of the Tegmentum After Acute Traumatic Injury
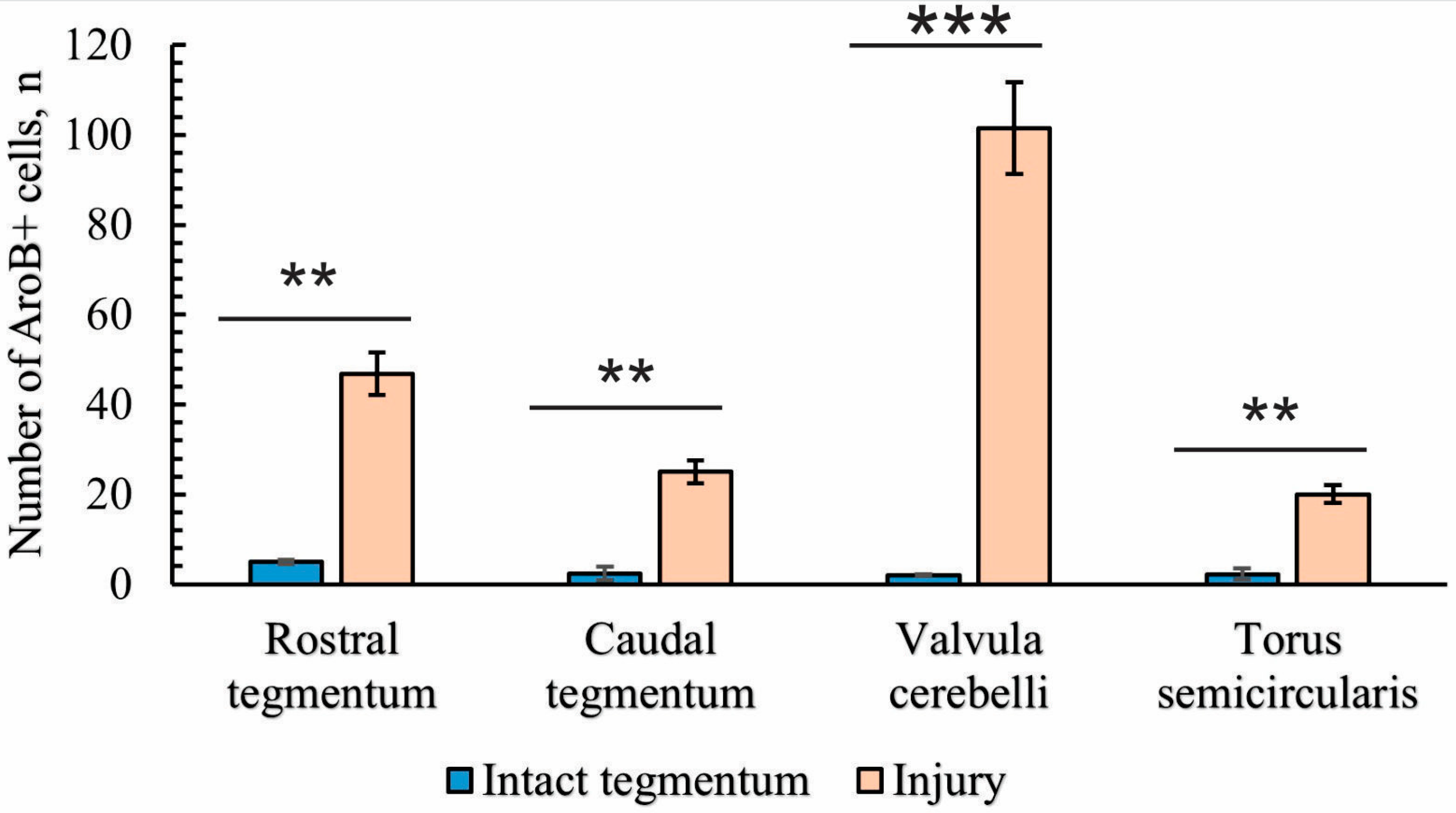
2.4. Immunohistochemical Labeling of Aromatase B (AroB)
2.4.1. Immunohistochemical Labeling of AroB in the Tegmentum of Intact Fish
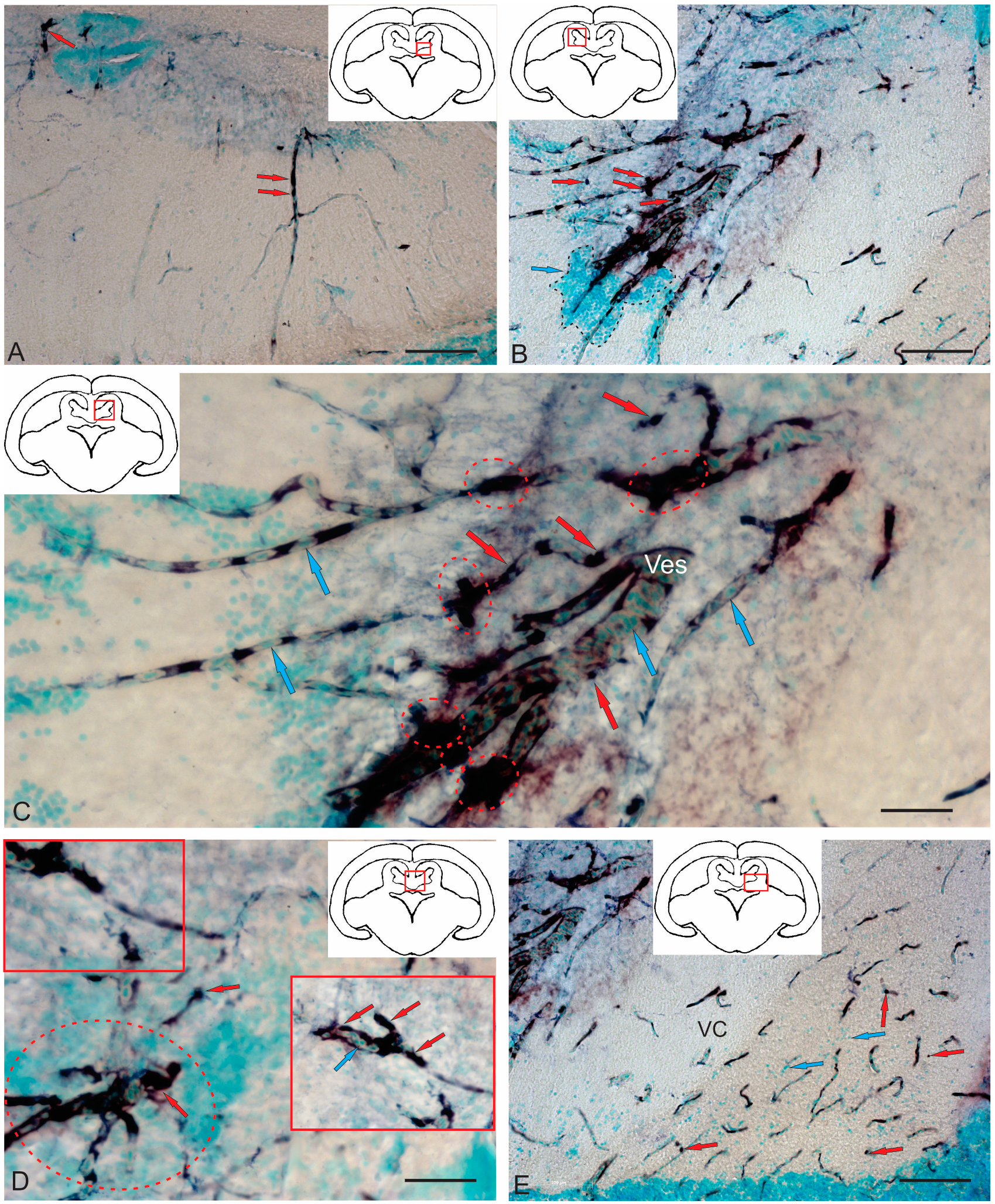
2.4.2. Immunohistochemical Labeling of AroB in the Tegmentum After Traumatic Injury
2.4.3. Immunohistochemical Labeling of AroB in the Rostral Isthmus
2.4.4. Immunohistochemical Labeling of AroB in the Caudal Part of the Isthmus
2.4.5. Immunohistochemical Labeling of AroB in the Valvula Cerebelli
3. Discussion
3.1. Features of the Ultrastructural Organization of the Tegmentum Nuclei in Juvenile Chum Salmon
3.2. Apoptosis in the Tegmentum of Juvenile Chum Salmon and Other Fish
3.3. Neuroglial Interactions in the Tegmentum of Juvenile Chum Salmon
3.4. Neurogenic Zones of the Tegmentum in Juvenile Chum Salmon and Their Involvement in Adult Neurogenesis
3.5. BLBP Expression in the Tegmentum of Intact Juvenile Chum Salmon and in the Post-Traumatic Period
3.6. Aromatase Expression in the Tegmentum of Intact Juvenile Chum Salmon and in the Post-Traumatic Period
4. Materials and Methods
4.1. Experimental Animals
4.2. Transmission Electron Microscopy
4.3. Cell Counting and Visualization
4.4. Experimental Design: Acute Traumatic Injury to Medulla Oblongata
4.5. Preparation of Material for Immunohistochemical Studies
4.6. Immunohistochemical Verification of AroB and BLBP
4.7. Microscopy
4.8. Densitometry
4.9. Statistical Analysis
5. Conclusions
6. Limitations and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase; |
| ACTH | adrenocorticotropic hormone; |
| aNSCs | adult neural stem cells; |
| aNSPCs | adult-type neural stem progenitor cells; |
| AroB | aromatase B; |
| ATP | adenosine triphosphate; |
| Bax | BCL-2-associated X protein; |
| Bcl-2 | B-cell lymphoma 2 (Bcl-2) protein family; |
| Bid | abundant proapoptotic protein of the Bcl-2 family; |
| BLBP | brain lipid-binding protein; |
| BrdU | 2′-deoxy-5-bromodeoxyuridine; |
| Cas | clustered regularly interspaced short palindromic repeats associated proteins; |
| CF | central part of the valve; |
| ChAT | choline acetyltransferase; |
| CLTNZ | caudo-lateral tegmental neurogenic zone; |
| CNS | central nervous system; |
| DEL | dorsal ependymal lamina; |
| DHA | omega-3 polyunsaturated docosahexaenoic acid; |
| DTN | dorsal tegmental nuclei; |
| ERK | extracellular signal-regulated kinases; |
| ERs | estrogen receptors; |
| FLM | fasciculus longitudinalis medialis; |
| GABA | gamma-aminobutyric acid; |
| GFAP | glial fibrillary acidic protein; |
| GS | glutamine synthetase; |
| GV | trigeminal ganglion; |
| Her5 | Hairy/Enhancer of Split [H/E(Spl)]transcription factors; |
| HSP | heat shock proteins; |
| HuC/D | pan-neuronal nuclear label; |
| IHC | immunohistochemical labeling; |
| III | hypothalamic ventricle; |
| IL | interleukin; |
| INI | large neurons; |
| INII | medium-sized neurons; |
| INIII | smaller neurons; |
| JNK | c-Jun N-terminal kinase; |
| LEL | lectins from Lycopersicon esculentum; |
| LPVZ | lateral periventricular zone; |
| LT | lateral torus; |
| LTSNZ | lateral neurogenic zone of torus semicircularis; |
| ML | molecular layer; |
| MOMP | mitochondrial outer membrane permeabilizer; |
| MPVZ | medial periventricular zones; |
| mRNA | messenger ribonucleic acid; |
| MS222 | tricaine methanesulfonate; |
| MT | mitochondria; |
| MTSNZ | medial part of the semilunar torus neurogenic zone; |
| NE | neuroepithelial; |
| NEC | neuroepithelial-like cells; |
| NFLM | nucleus of fasciculus longitudinalis medialis; |
| NIII | nucleus of the oculomotor nerve; |
| NO | nitric oxide; |
| NSCs | neuronal stem cells; |
| NSPCs | neural stem progenitor cells; |
| OsO4 | osmium tetroxide; |
| PCNA | proliferating cell nuclear antigen; |
| PGZ | visual neurogenic niche; |
| PPAR | peroxisome proliferator-activated receptor; |
| PTP | mitochondrial permeability transition pore; |
| PVZ | periventricular zone; |
| RER | rough endoplasmic reticulum; |
| RG | radial glia; |
| RLTNZ | rostral lateral tegmental neurogenic zone; |
| SN | substantia nigra; |
| SVZ | subventricular zone; |
| T cells | T lymphocytes; |
| TEM | transmission electron microscopy; |
| TeO | tectum opticum; |
| TeV | tectal ventricle; |
| TMZ | tectal marginal zone; |
| TS | torus semicircularis; |
| TSNZ | torus semicircularis neurogenic zone; |
| UOD | units of optical density; |
| VI | abducens nerve; |
| VIIL | facial nerve lobes; |
| XL | vagus nerve lobes. |
References
- Lindsey, B.W.; Darabie, A.; Tropepe, V. The cellular composition of neurogenic periventricular zones in the adult zebrafish forebrain. J. Comp. Neurol. 2012, 520, 2275–2316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.K.; Jesuthasan, S.J.; Penney, T.B. Zebrafish forebrain and temporal conditioning. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120462. [Google Scholar] [CrossRef] [PubMed]
- Babchenko, V.Y.; Belova, A.S.; Bashirzade, A.A.; Peskov, A.V.; Giniatullina, A.N.; Shchegolev, B.F. Models of Traumatic Brain Injury in Zebrafish (Danio rerio). Ross. Fiziol. Zhurnal Im. IM Sechenova 2021, 107, 1059–1076. [Google Scholar] [CrossRef]
- Kaslin, J.; Ganz, J.; Brand, M. Stem cells in the adult zebrafish cerebellum: Initiation and maintenance of a novel stem cell niche. J. Neurosci. 2009, 29, 6142–6153. [Google Scholar] [CrossRef]
- Carletti, B.; Rossi, F. Neurogenesis in the cerebellum. Neuroscientist 2008, 14, 91–100. [Google Scholar] [CrossRef]
- Zupanc, G.K.; Sîrbulescu, R.F. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Eur. J. Neurosci. 2011, 34, 917–929. [Google Scholar] [CrossRef]
- Ito, Y.; Tanaka, H.; Okamoto, H.; Ohshima, T. Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev. Biol. 2010, 342, 26–38. [Google Scholar] [CrossRef]
- Joly, J.S.; Recher, G.; Brombin, A.; Ngo, K.; Hartenstein, V. A conserved developmental mechanism builds complex visual systems in insects and vertebrates. Curr. Biol. 2016, 26, R1001–R1009. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Hall, Z.; Heuzé, A.; Joly, J.S.; Tropepe, V.; Kaslin, J. The role of neuro-epithelial-like and radial-glial stem and progenitor cells in development, plasticity, and repair. Prog. Neurobiol. 2018, 170, 99–114. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Aitken, G.E.; Tang, J.K.; Khabooshan, M.; Douek, A.M.; Vandestadt, C.; Kaslin, J. Midbrain tectal stem cells display diverse regenerative capacities in zebrafish. Sci. Rep. 2019, 9, 4420. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Kapustyanov, I.A.; Kluka, G.G. Adult neurogenesis of teleost fish determines high neuronal plasticity and regeneration. Int. J. Mol. Sci. 2024, 25, 3658. [Google Scholar] [CrossRef] [PubMed]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Obukhov, D.K.; Varaksin, A.A. Neurochemical markers of cells of the periventricular brain area in the masu salmon (Oncorhynchus masou, Salmonidae). Ontogenez 2012, 43, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Kapustyanov, I.A.; Varaksin, A.A. Proliferation and Neuro- and Gliogenesis in Normal and Mechanically Damaged Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta). Russ. J. Dev. Biol. 2019, 50, 59–76. [Google Scholar] [CrossRef]
- Lange, C.; Rost, F.; Machate, A.; Reinhardt, S.; Lesche, M.; Weber, A.; Kuscha, V.; Dahl, A.; Brand, M. Single cell sequencing of radial glia progeny reveals the diversity of newborn neurons in the adult zebrafish brain. Development 2020, 147, dev185595. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K. Molecular markers of adult neurogenesis in the telencephalon and tectum of rainbow trout. Int. J. Mol. Sci. 2022, 23, 1188. [Google Scholar] [CrossRef]
- Than-Trong, E.; Bally-Cuif, L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 2015, 63, 1406–1428. [Google Scholar] [CrossRef]
- Diotel, N.; Lillesaar, C.; Strähle, U.; Barbacci, D.; D’Aniello, S.; Gajewski, M.; Rink, E.; Ahn, S.; Mallo, M.; Saha, K.; et al. Common and distinct features of adult neurogenesis and regeneration in the telencephalon of zebrafish and mammals. Front. Neurosci. 2020, 14, 568930. [Google Scholar] [CrossRef]
- Stukaneva, M.E.; Pushchina, E.V. Constitutive neurogenesis in the brain of different vertebrate groups. Neurophysiology 2020, 52, 456–470. [Google Scholar] [CrossRef]
- Dambroise, E.; Simion, M.; Bourquard, T.; Bouffard, S.; Rizzi, B.; Jaszczyszyn, Y.; Bourge, M.; Affaticati, P.; Heuzé, A.; Jouralet, J.; et al. Postembryonic fish brain proliferation zones exhibit neuroepithelial-type gene expression profile. Stem Cells 2017, 35, 1505–1518. [Google Scholar] [CrossRef]
- Obukhov, D.K.; Andreeva, N.G. Evolutionary Morphology of the Nervous System in Vertebrates, 3rd ed.; Textbook; Revised and Expanded; Higher Education: Moscow, Russia, 2024. [Google Scholar]
- Pushchina, E.V. Structure, Chemoarchitecture, and Postembryonic Histogenesis of the CNS in Fish. Ph.D. Thesis, Institute of Marine Biology, Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia, 2012. (In Russian). [Google Scholar]
- Lindsey, B.W.; Tropepe, V. Changes in the social environment induce neurogenic plasticity predominantly in niches residing in sensory structures of the zebrafish brain independently of cortisol levels. Dev. Neurobiol. 2014, 74, 1053–1077. [Google Scholar] [CrossRef] [PubMed]
- Alunni, A.; Cayre, M.; Bénard, S.; Debré, P.; Sernagor, E.; Strähle, U.; Bally-Cuif, L. Evidence for neural stem cells in the medaka optic tectum proliferation zones. Dev. Neurobiol. 2010, 70, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Kaslin, J.; Ganz, J.; Brand, M. Development and specification of cerebellar stem and progenitor cells in zebrafish: From embryo to adult. Neural Dev. 2013, 8, 9. [Google Scholar] [CrossRef]
- Adolf, B.; Bock, E.; Wullimann, M.F.; Brand, M. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 2006, 295, 278–293. [Google Scholar] [CrossRef]
- Grandel, H.; Brand, M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. 2013, 223, 131–147. [Google Scholar] [CrossRef]
- Maruska, K.P.; Carpenter, R.E.; Fernald, R.D. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. J. Comp. Neurol. 2012, 520, 3471–3491. [Google Scholar] [CrossRef]
- Gelinas, D.; Callard, G.V. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. Gen. Comp. Endocrinol. 1997, 106, 155–168. [Google Scholar] [CrossRef]
- Forlano, P.M.; Marchaterre, M.A.; Deitcher, D.L.; Bass, A.H. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: Aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001, 21, 8943–8955. [Google Scholar] [CrossRef]
- Pellegrini, E.; Mouriec, K.; Anglade, I.; Menuet, A.; Le Page, Y.; Gueguen, M.M.; Brion, F.; Kah, O. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 2007, 501, 150–167. [Google Scholar] [CrossRef]
- Mouriec, K.; Lareyre, J.J.; Tong, S.K.; Le Page, Y.; Vaillant, C.; Pellegrini, E.; Pakdel, F.; Chung, B.C.; Kah, O.; Anglade, I. Early regulation of brain aromatase (cyp19a1b) by estrogen receptors during zebrafish development. Dev. Dyn. 2009, 238, 2641–2651. [Google Scholar] [CrossRef]
- Strobl-Mazzulla, P.H.; Nuñez, A.; Pellegrini, E.; Gueguen, M.M.; Kah, O.; Somoza, G.M. Progenitor radial cells and neurogenesis in pejerrey fish forebrain. Brain Behav. Evol. 2010, 76, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Coumailleau, P.; Kah, O. Cyp19a1 (aromatase) expression in the Xenopus brain at different developmental stages. J. Neuroendocrinol. 2014, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Maurice, P.; Pichon, C.; Montagnac, G.; Le Maire, M.; de Kerdrel, T.; Dupré, L.; Gorvel, J.P.; Chabert, C.; Marguet, D.; Boge, G. A generic approach for the purification of signaling complexes that specifically interact with the carboxyl-terminal domain of G protein-coupled receptors. Mol. Cell. Proteom. 2008, 7, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Bykova, M.E.; Varaksin, A.A. Post-traumatic expressions of aromatase B, glutamine synthetase, and cystathionine-beta-synthase in the cerebellum of juvenile chum salmon. Int. J. Mol. Sci. 2024, 25, 3299. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Noctor, S.C.; Kriegstein, A.R. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex 2006, 16 (Suppl. S1), i152–i161. [Google Scholar] [CrossRef]
- Brocca, M.E.; García-Segura, L.M. Non-reproductive functions of aromatase in the central nervous system under physiological and pathological conditions. Cell. Mol. Neurobiol. 2019, 39, 473–481. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Wright, C.L.; McCarthy, M.M. A critical period in Purkinje cell development is mediated by local estradiol synthesis, disrupted by inflammation, and has enduring consequences only for males. J. Neurosci. 2016, 36, 10039–10049. [Google Scholar] [CrossRef]
- Cambiasso, M.J.; Maggi, R.; Eguiguren, M.; Acosta, M.; Roldan, P.; Panzica, G.C. Interaction of sex chromosome complement, gonadal hormones, and neuronal steroid synthesis on the sexual differentiation of mammalian neurons. J. Neurogenet. 2017, 31, 300–306. [Google Scholar] [CrossRef]
- Diotel, N.; Rodriguez Viales, R.; Armant, O.; März, M.; Ferg, M.; Rastegar, S.; Strähle, U. Comprehensive expression map of transcription regulators in the adult zebrafish telencephalon reveals distinct neurogenic niches. J. Comp. Neurol. 2015, 523, 1202–1221. [Google Scholar] [CrossRef]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; de Girolamo, P. BDNF, brain and regeneration: Insights from zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef]
- Krelin, Y.; Gelfand, M.; Ibragimov, A.; Engel, S.; Berdichevsky, Y.; Zreik, F.; Voronov, E.; Benharroch, D.; Kaneti, J.; Hanna, S.; et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007, 67, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Sánchez, R.; Sali, A.; Heintz, N. Ligand specificity of brain lipid-binding protein. J. Biol. Chem. 1996, 271, 24711–24719. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Ball, J.; Simopoulos, A.P. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 5–13. [Google Scholar] [CrossRef]
- Gronert, K. Lipid autacoids in inflammation and injury responses: A matter of privilege. Mol. Interv. 2008, 8, 28–35. [Google Scholar] [CrossRef]
- Diotel, N.; Chao, J.; Tardy, M.; Vaillant, C. Aromatase in the brain of teleost fish: Expression, regulation and putative functions. Front. Neuroendocrinol. 2010, 31, 172–192. [Google Scholar] [CrossRef]
- Diotel, N.; Vaillant, C.; Kah, O.; Pellegrini, E. Mapping of brain lipid binding protein (Blbp) in the brain of adult zebrafish, co-expression with aromatase B and links with proliferation. Gene Expr. Patterns 2016, 20, 42–54. [Google Scholar] [CrossRef]
- Tong, S.K.; Mouriec, K.; Kuo, M.W.; Pellegrini, E.; Gueguen, M.M.; Brion, F.; Kah, O.; Chung, B.C. A cyp19a1b-gfp (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells. Genesis 2009, 47, 67–73. [Google Scholar] [CrossRef]
- März, M.; Chapouton, P.; Diotel, N.; Vaillant, C.; Hesl, B.; Takamiya, M.; Lam, C.S.; Kah, O.; Bally-Cuif, L.; Strähle, U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010, 58, 870–888. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A. Neurolin expression in the optic nerve and immunoreactivity of Pax6-positive niches in the brain of rainbow trout. Neural Regen. Res. 2019, 14, 156–171. [Google Scholar] [CrossRef]
- Boneva, N.B.; Kaplamadzhiev, D.B.; Sahara, S.; Kikuchi, H.; Pyko, I.V.; Kikuchi, M.; Tonchev, A.B.; Yamashima, T. Expression of fatty acid-binding proteins in adult hippocampal neurogenic niche of postischemic monkeys. Hippocampus 2011, 21, 162–171. [Google Scholar] [CrossRef]
- Matsumata, M.; Sakayori, N.; Maekawa, M.; Owada, Y.; Yoshikawa, T.; Osumi, N. The effects of Fabp7 and Fabp5 on postnatal hippocampal neurogenesis in the mouse. Stem Cells 2012, 30, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Schnell, A.; Chappuis, S.; Schmutz, I.; Brai, E.; Ripperger, J.A.; Schaad, O.; Welzl, H.; Descombes, P.; Alberi, L.; Albrecht, U. The nuclear receptor REV-ERBα regulates Fabp7 and modulates adult hippocampal neurogenesis. PLoS ONE 2014, 9, e99883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Jeng, S.R.; Yueh, W.S.; Pen, Y.T.; Gueguen, M.M.; Pasquier, J.; Dufour, S.; Chang, C.F.; Kah, O. Expression of aromatase in radial glial cells in the brain of the Japanese eel provides insight into the evolution of the cyp19a1 gene in Actinopterygians. PLoS ONE 2012, 7, e44750. [Google Scholar] [CrossRef] [PubMed]
- García-Segura, L.M.; He, F.; Watanabe, M. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neuroscience 1999, 89, 567–578. [Google Scholar] [CrossRef]
- Pérez, S.E.; Gallego, V.; Korf, H.W. Distribution of choline acetyltransferase (ChAT) immunoreactivity in the brain of the adult trout and tract-tracing observations on the connections of the nuclei of the isthmus. J. Comp. Neurol. 2000, 428, 450–474. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Karpenko, A.A. Distribution of cholineacetyltransferase histochemistry in isthmus and medulla of Oncorhynchus masu. Tract-tracing observation on the ascending meso-pontine cholinergic system. Tsitologiia 2007, 49, 581–593. (In Russian) [Google Scholar]
- Clemente, D.; Porteros, A.; Weruaga, E.; Alonso, J.R.; Arenzana, F.J.; Aijón, J.; Arévalo, R. Cholinergic elements in the zebrafish central nervous system: Histochemical and immunohistochemical analysis. J. Comp. Neurol. 2004, 474, 75–107. [Google Scholar] [CrossRef]
- Muller, T.; Vernier, P.; Wullimann, M.F. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004, 1011, 156–169. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.X.; Wang, F.W.; Zhang, Q.; Du, Z.X.; Zhan, J.M.; Yuan, Q.H.; Ling, E.A.; Hao, A.J. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H2S pathway. Neuroscience 2013, 237, 106–117. [Google Scholar] [CrossRef]
- Wullimann, M.F. The central nervous system. In The Physiology of Fishes, 2nd ed.; Evans, D.H., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 295–320. [Google Scholar]
- Pushchina, E.V.; Obukhov, D.K.; Varaksin, A.A. Features of adult neurogenesis and neurochemical signaling in the Cherry salmon Oncorhynchus masou brain. Neural Regen. Res. 2013, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- AnvariFar, H.; Adineh, H.; Khamisabadi, S.; Pahlavan, M. Apoptosis in fish: Environmental factors and programmed cell death. Cell Tissue Res. 2017, 368, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.H.; Clint, S.C.; Takimoto, N.; Hughes, A.T.L.; Wellbrock, U.M.; Meissner, D. Spatio-temporal distribution of microglia/macrophages during regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: A quantitative analysis. Brain Behav. Evol. 2003, 62, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Israels, L.G.; Israels, E.D. Apoptosis. Oncology 1999, 4, 332–339. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis: Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K.; Prudnikov, I.M. GFAP expression in the optic nerve and increased H2S generation in the integration centers of the rainbow trout (Oncorhynchus mykiss) brain after unilateral eye injury. Neural Regen. Res. 2020, 15, 1867–1886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colavincenzo, J.; Levine, R.L. Myelin debris clearance during Wallerian degeneration in the goldfish visual system. J. Neurosci. Res. 2000, 59, 47–62. [Google Scholar] [CrossRef]
- Ghali, R.P.; Leclerc, L.; Levine, R.L. Mononuclear cell proliferation and hyperplasia during Wallerian degeneration in the visual system of the goldfish in the presence or absence of regenerating optic axons. Brain Res. 2000, 854, 178–188. [Google Scholar] [CrossRef]
- Levine, R.L.; Evans, M.D. The source of reactive cells during central Wallerian degeneration in the goldfish: A differential irradiation protocol. Exp. Neurol. 2002, 173, 136–144. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J. Comp. Neurol. 2001, 433, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Scapigliati, G.; Buonocore, F.; Mazzini, M. Biological activity of cytokines: An evolutionary perspective. Curr. Pharm. Des. 2006, 12, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
- Huising, M.O.; van der Meulen, T.; Flik, G.; Verburg-van Kemenade, B.M. Three novel carp CXC chemokines are expressed early in ontogeny and at nonimmune sites. Eur. J. Biochem. 2004, 271, 4094–4106. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.H.; Kompass, K.S.; Horschke, I.; Ott, R.; Schwarz, H. Apoptosis after injuries in the cerebellum of adult teleost fish. Exp. Neurol. 1998, 152, 221–230. [Google Scholar] [CrossRef]
- Berthelet, J.; Dubrez, L. Regulation of apoptosis by inhibitors of apoptosis (IAPs). Cells 2013, 2, 163–187. [Google Scholar] [CrossRef]
- Metzstein, M.M.; Stanfield, G.M.; Horvitz, H.R. Genetics of programmed cell death in C. elegans: Past, present and future. Trends Genet. 1998, 14, 410–416. [Google Scholar] [CrossRef]
- Rajendran, R.S.; Wellbrock, U.M.; Zupanc, G.K. Apoptotic cell death, long-term persistence, and neuronal differentiation of aneuploid cells generated in the adult brain of teleost fish. Dev. Neurobiol. 2008, 68, 1257–1268. [Google Scholar] [CrossRef]
- Sakata, S.; Yan, Y.; Satou, Y.; Momoi, A.; Ngo-Hazelett, P.; Nozaki, M.; Furutani-Seiki, M.; Postlethwait, J.H.; Yonehara, S.; Sakamaki, K. Conserved function of caspase-8 in apoptosis during bony fish evolution. Gene 2007, 396, 134–148. [Google Scholar] [CrossRef]
- Laing, K.J.; Holland, J.; Bonilla, S.; Cunningham, C.; Secombes, C.J. Cloning and sequencing of caspase 6 in rainbow trout, Oncorhynchus mykiss, and analysis of its expression under conditions known to induce apoptosis. Dev. Comp. Immunol. 2001, 25, 303–312. [Google Scholar] [CrossRef]
- Zeiss, C.J. The apoptosis-necrosis continuum: Insights from genetically altered mice. Vet. Pathol. 2003, 40, 481–495. [Google Scholar] [CrossRef]
- Kulms, D.; Schwarz, T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar] [PubMed]
- Leist, M.; Single, B.; Naumann, H.; Fava, E.; Simon, B.; Kühnle, S.; Nicotera, P. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp. Cell Res. 1999, 249, 396–403. [Google Scholar] [CrossRef]
- Hogan, R.J.; Taylor, W.R.; Cuchens, M.A.; Naftel, J.P.; Clem, L.W.; Miller, N.W.; Chinchar, V.G. Induction of target cell apoptosis by channel catfish cytotoxic cells. Cell Immunol. 1999, 195, 110–118. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Krumschnabel, G.; Podrabsky, J.E. Fish as model systems for the study of vertebrate apoptosis. Apoptosis 2009, 14, 1–21. [Google Scholar] [CrossRef]
- Whitley, D.; Goldberg, S.P.; Jordan, W.D. Heat shock proteins: A review of the molecular chaperones. J. Vasc. Surg. 1999, 29, 748–751. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Simonsen, L.O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol. Rev. 1989, 69, 315–382. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Celi, M.; Peñafiel, L.G.; Berra, J.A.; de Lima, M.F.D.C. Elevated cortisol modulates Hsp70 and Hsp90 gene expression and protein in sea bass head kidney and isolated leukocytes. Gen. Comp. Endocrinol. 2012, 175, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M. “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 2004, 117, 2641–2651. [Google Scholar] [CrossRef]
- van Ham, T.J.; Kokel, D.; Peterson, R.T. Apoptotic cells are cleared by directional migration and elmo1-dependent macrophage engulfment. Curr. Biol. 2012, 22, 830–836. [Google Scholar] [CrossRef]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef]
- Furlan, A.; Manousopoulou, M.B.; Franchini, C.M.O.S.P.; Ferreira, L.P.D. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, 6346. [Google Scholar] [CrossRef]
- Chapouton, P.; Jagasia, R.; Bally-Cuif, L. Adult neurogenesis in non-mammalian vertebrates. BioEssays 2007, 29, 745–757. [Google Scholar] [CrossRef]
- Chapouton, P.; Godinho, L. Neurogenesis. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 100, pp. 73–126. [Google Scholar] [CrossRef] [PubMed]
- Haydon, P.G. GLIA: Listening and talking to the synapse. Nat. Rev. Neurosci. 2001, 2, 185–193. [Google Scholar] [CrossRef]
- Goenaga, J.; Zamorín, L.H.; García-Sáez, M.G.; Gracia-Navarro, A.S. Calcium signaling in astrocytes and gliotransmitter release. Front. Synaptic Neurosci. 2023, 15, 1138577. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, Y.; Choi, J.H.; Park, S.S.; Lee, H.K.; Kim, J.H. Astrocytes promote ethanol-induced enhancement of intracellular Ca2+ signals through intercellular communication with neurons. iScience 2021, 24, 102436. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.; Sîrbulescu, R.F. Teleost fish as a model system to study successful regeneration of the central nervous system. Curr. Top. Microbiol. Immunol. 2013, 367, 193–233. [Google Scholar] [PubMed]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A. Mechanical brain injury increases cells’ production of cystathionine β-synthase and glutamine synthetase, but reduces Pax2 expression in the telencephalon of juvenile chum salmon. Int. J. Mol. Sci. 2021, 22, 1279. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Jackson, W.T.; Ransom, C.B.; Amara, S.G.; Kato, S. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 2008, 28, 6659–6663. [Google Scholar] [CrossRef]
- Halassa, M.M.; Florian, C.; Tzeng, R.C.; Vauclair, F.; Ahsan, S.; Haydon, P.G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007, 27, 6473–6477. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.R.; Kim, A.E.; Packer, J.G.; Kim, D.H.; Ryu, J.H.; Wu, Y.; Kwon, H. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, W.; Zhang, J.; Huang, S.; Xu, Q.; Xu, G. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J. Neurosci. 2020, 40, 7355–7374. [Google Scholar] [CrossRef]
- Fuente-Martin, E.; Lopez, M.; Mendez, P.; Mallo, F.; Gallego, R.; Dieguez, C.; Nogueiras, R. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev. Endocr. Metab. Disord. 2013, 14, 331–338. [Google Scholar] [CrossRef]
- Porter, J.T.; McCarthy, K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997, 51, 439–455. [Google Scholar] [CrossRef]
- Benveniste, E.N.; Qin, H. Type I interferons as anti-inflammatory mediators. Sci. STKE 2007, 2007, pe70. [Google Scholar] [CrossRef]
- Crotti, A.; Ransohoff, R.M. Microglial physiology and pathophysiology: Insights from genome-wide transcriptional profiling. Immunity 2016, 44, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Battisti, W.P.; Cuoghi, B.; Turrini, M.; Fornari, R.; Mancini, D.; Spagnoli, M.; Cacciabue, D.; Passamonti, M. Macrophages, microglia, and astrocytes are rapidly activated after crush injury of the goldfish optic nerve: A light and electron microscopic analysis. J. Comp. Neurol. 1995, 354, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.D.; Wilson, B.R. Light microscopic study of degenerating cobalt-filled optic axons in goldfish: Role of microglia and radial glia in debris removal. J. Comp. Neurol. 1989, 282, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, L.; Pederson, K.; Levine, R.L. Specific uptake of intracranial horseradish peroxidase (HRP) by microglial cells in the goldfish. Neurosci. Lett. 1996, 208, 13–16. [Google Scholar] [CrossRef]
- Cuoghi, B.; Mola, L. Microglia of teleosts: Facing a challenge in neurobiology. Eur. J. Histochem. 2007, 51, 231–240. [Google Scholar] [PubMed]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Cuoghi, B.; Blasiol, L.; Sabatini, M.A. ACTH occurrence in teleosts supramedullary neuron clusters: A neuron-glial common language? Gen. Comp. Endocrinol. 2003, 132, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, I.; Pica, A.; Grimaldi, M.C. Immunohistochemical detection of ACTH and MSH cells in the hypophysis of the hermaphroditic teleost, Diplodus sargus. Eur. J. Histochem. 2000, 44, 397–406. [Google Scholar]
- Delgado, R.; Mena, J.; Hsieh, C.-Y.; Ganea, D. Melanocortin peptides inhibit production of proinflammatory cytokines and nitric oxide by activated microglia. J. Leukoc. Biol. 1998, 63, 740–745. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Dowding, A.J.; Maggs, A.; Scholes, J. Diversity amongst the microglia in growing and regenerating fish CNS: Immunohistochemical characterization using FL.1, an anti-macrophage monoclonal antibody. Glia 1991, 4, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Cuoghi, B.; Marini, M. Ultrastructural and cytochemical features of the supramedullary neurons of the pufferfish Diodon holacanthus (L.) (Osteichthyes). Tissue Cell 2001, 33, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Aijón, J.; Lara, J.M.; Muñoz, A.; Leal, E.; Gutiérrez, J.C.; Varela, C.; González, J. Enzyme histochemical identification of microglial cells in the retina of a fish (Tinca tinca). Neurosci. Lett. 1999, 263, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Acarin, L.; Castro, M.C.; Vela, J.M.; González, B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J. Histochem. Cytochem. 1994, 42, 1033–1041. [Google Scholar] [CrossRef]
- Nakanishi, M.; Aoyama, Y.; Shimizu, H.; Matsuda, T.; Saito, Y.; Yamamoto, Y.; Watanabe, K. Microglia-derived interleukin-6 and leukemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur. J. Neurosci. 2007, 25, 649–658. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Zupanc, G.K.H. Neurogenesis and neuronal regeneration in the adult fish brain. J. Comp. Physiol. A 2006, 192, 649–670. [Google Scholar] [CrossRef]
- Pinteaux, E.; Rothwell, N.J.; Luheshi, G.N. Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J. Neurochem. 2002, 83, 754–763. [Google Scholar] [CrossRef]
- Sarkar, R.; Kuo, J.; Hsu, K.; Huang, C. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ. Res. 1996, 78, 225–230. [Google Scholar] [CrossRef]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration, and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Tropepe, V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog. Neurobiol. 2006, 80, 281–307. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.H.; Hinsch, K.; Gage, F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005, 488, 290–319. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Hsiao, C.; Sweeney, L.; Aztiria, L.; Moens, C.; Nguyen, A. Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia 2010, 58, 1345–1363. [Google Scholar] [CrossRef]
- Seeley, E.S.; Nachury, M.V. The perennial organelle: Assembly and disassembly of the primary cilium. J. Cell Sci. 2010, 123 Pt 4, 511–518. [Google Scholar] [CrossRef]
- Götz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef]
- Willardsen, M.I.; Link, B.A. Cell biological regulation of division fate in vertebrate neuroepithelial cells. Dev. Dyn. 2011, 240, 1865–1879. [Google Scholar] [CrossRef][Green Version]
- Galant, S.; McGowan, D.; DeMarco, K.; Wullimann, M.F. Embryonic origin and lineage hierarchies of the neural progenitor subtypes building the zebrafish adult midbrain. Dev. Biol. 2016, 420, 120–135. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A. Constitutive neurogenesis and neuronal plasticity in the adult cerebellum and brainstem of rainbow trout Oncorhynchus mykiss. Int. J. Mol. Sci. 2024, 25, 5595. [Google Scholar] [CrossRef]
- Merkle, F.T.; Mirzadeh, Z.; Alvarez-Buylla, A. Mosaic organization of neural stem cells in the adult brain. Science 2007, 317, 381–384. [Google Scholar] [CrossRef]
- Lledo, P.M.; Merkle, F.T.; Alvarez-Buylla, A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008, 31, 392–400. [Google Scholar] [CrossRef]
- Feng, L.; Hatten, M.E.; Heintz, N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron 1994, 12, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, J.C.; Hatten, M.E. Glial-guided granule neuron migration in vitro: A high-resolution time-lapse video microscopic study. J. Neurosci. 1987, 7, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Q.; Liu, X.L.; Hatten, M.E. The weaver gene encodes a nonautonomous signal for CNS neuronal differentiation. Cell 1992, 68, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.O.; Heintz, N.; Hatten, M.E. Cerebellar granule cell neurogenesis is regulated by cell-cell interactions in vitro. Neuron 1991, 6, 705–715. [Google Scholar] [CrossRef]
- Hatten, M.E.; Liem, R.K.; Mason, C.A. Two forms of cerebellar glial cells interact differently with neurons in vitro. J. Cell Biol. 1984, 98, 193–204. [Google Scholar] [CrossRef]
- Menuet, A.; Pellegrini, E.; Brion, F.; Gueguen, M.M.; Anglade, I.; Pakdel, F.; Kah, O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005, 485, 304–320. [Google Scholar] [CrossRef]
- Okubo, K.; Watanabe, T.; Iida, H.; Hiramatsu, N.; Matsuda, K.; Hayashi, Y.; Yoshimura, T. Sex differences in aromatase gene expression in the medaka brain. J. Neuroendocrinol. 2011, 23, 412–423. [Google Scholar] [CrossRef]
- Azcoitia, I.; Luque, G.M.; García-Segura, L.M.; Watanabe, M. Aromatase expression by reactive astroglia is neuroprotective. Ann. N. Y. Acad. Sci. 2003, 1007, 298–305. [Google Scholar] [CrossRef]
- Carswell, H.V.O.; Gordon, K.; Farr, T.; MacKenzie, A.; Sweeney, J. Brain aromatase overexpression does not reduce ischaemic damage nor improve long-term functional outcome after experimental stroke: A magnetic resonance imaging and behavioural study. J. Cereb. Blood Flow Metab. 2005, 25 (Suppl. S1), S293. [Google Scholar] [CrossRef]
- Peterson, R.S.; Sutherland, M.; Niblock, M.M.; Zhang, H.; Nowakowski, R.S.; Rybak, C.E.; Sisk, C.L. Radial glia express aromatase in the injured zebra finch brain. J. Comp. Neurol. 2004, 475, 261–269. [Google Scholar] [CrossRef]
- Peterson, R.S.; Saldanha, C.J.; Schlinger, B.A. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata). J. Neuroendocrinol. 2001, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.A.; Saldanha, C.J. Neuroinflammation induces glial aromatase expression in the uninjured songbird brain. J. Neuroinflamm. 2011, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Mehos, C.J.; Nelson, L.H.; Saldanha, C.J. A quantification of the injury-induced changes in central aromatase, oestrogenic milieu and steroid receptor expression in the zebra finch. J. Neuroendocrinol. 2016, 28, 12348. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.L.; Brownrout, J.L.; Saldanha, C.J. Neuroinflammation and neurosteroidogenesis: Reciprocal modulation during injury to the adult zebra finch brain. Physiol. Behav. 2018, 187, 51–56. [Google Scholar] [CrossRef]
- Pedersen, A.L.; Saldanha, C.J. Reciprocal interactions between prostaglandin E2- and estradiol-dependent signaling pathways in the injured zebra finch brain. J. Neuroinflamm. 2017, 14, 262. [Google Scholar] [CrossRef]
- Brinton, R.D. Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends Pharmacol. Sci. 2009, 30, 212–222. [Google Scholar] [CrossRef]
- Garcia-Segura, L.M.; McCarthy, M.M. Minireview: Role of glia in neuroendocrine function. Endocrinology 2004, 145, 1082–1086. [Google Scholar] [CrossRef]
- Lephart, E.D. A review of brain aromatase cytochrome P450. Brain Res. Rev. 1996, 22, 1–26. [Google Scholar] [CrossRef]
- McEwen, B. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 2002, 57, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Gerhold, L.M.; Böttner, M.; Rau, S.W.; Dela Cruz, C.; Yang, E.; Zhu, H.; Yu, J.; Cashion, A.B.; Kindy, M.S.; et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 2007, 500, 1064–1075. [Google Scholar] [CrossRef]
- Ubuka, T.; Ukena, K.; Tsutsui, K.; Ebling, F.J.P. Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis. Nat. Commun. 2014, 5, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Chen, W.; Wu, Y.; Ge, W.; Zhang, L.; Zhang, W. Aromatase (Cyp19a1b) in the pituitary is dynamically involved in the upregulation of lhb but not fshb in the vitellogenic female ricefield eel Monopterus albus. Endocrinology 2014, 155, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M.; Azcoitia, I.; DonCarlos, L.L. Aromatase: A neuroprotective enzyme. Prog. Neurobiol. 2003, 71, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palmero, I.; Ortiz-Rodriguez, A.; Melcangi, R.C.; Caruso, D.; Garcia-Segura, L.M.; Rune, G.M.; Arevalo, M.A. Oestradiol synthesized by female neurons generates sex differences in neuritogenesis. Sci. Rep. 2016, 6, 31891. [Google Scholar] [CrossRef]
- Chamniansawat, S.; Chongthammakun, S. A priming role of local estrogen on exogenous estrogen-mediated synaptic plasticity and neuroprotection. Exp. Mol. Med. 2012, 44, 403–411. [Google Scholar] [CrossRef]
- Li, R.; He, P.; Cui, J.; Staufenbiel, M.; Harada, N.; Shen, Y. Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol. Neurobiol. 2013, 47, 857–867. [Google Scholar] [CrossRef]
- Saldanha, C.J.; Burstein, S.R.; Duncan, K.A. Induced synthesis of oestrogens by glia in the songbird brain. J. Neuroendocrinol. 2013, 25, 1032–1038. [Google Scholar] [CrossRef]
- Luchetti, S.; van Eden, C.G.; Schuurman, K.; van Strien, M.E.; Swaab, D.F.; Huitinga, I. Gender differences in multiple sclerosis: Induction of estrogen signaling in male and progesterone signaling in female lesions. J. Neuropathol. Exp. Neurol. 2014, 73, 123–135. [Google Scholar] [CrossRef]
- Spence, R.D.; Zhen, Y.; White, S.; Schlinger, B.A.; Day, L.B. Recovery of motor and cognitive function after cerebellar lesions in a songbird: Role of estrogens. Eur. J. Neurosci. 2009, 29, 1225–1234. [Google Scholar] [CrossRef]
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef]
- Magistretti, P.; Alla9man, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Mathiisen, T.; Lehre, K.; Danbolt, N.; Ottersen, O. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Di Liegro, C.; Schiro, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Mishra, A.; Reynolds, J.; Chen, Y.; Gourine, A.; Rusakov, D.; Attwell, D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016, 19, 1619–1627. [Google Scholar] [CrossRef]

| Type of Cells | Tegmental Neurons | ||
|---|---|---|---|
| Large InI | Medial InII | Small InIII | |
| Sample size | n = 24 | n = 22 | n = 21 |
| Nucleus nervus oculomotoris NIII | |||
| Long axis of cells soma (µm) | 21.15 ± 4.43 | 17.29 ± 2.63 | 9.47 ± 0.39 |
| Short axis of cells soma (µm) | 13.97 ± 0.46 | 8.4 ± 1.01 | 5.33 ± 0.27 |
| Nucleus | 1 | 1 | 1 |
| Contour | ovoid | ovoid; irregular ± invaginations | ovoid; elongated |
| Long axis (µm) | 10.25 ± 0.52 | 8.69 ±0.93 | 5.46 ± 0.63 |
| Short axis (µm) | 6.71 ± 0.83 | 5.45 ± 0.63 | 3.61 ± 0.53 |
| Chromatin | reticulated euchromatin | reticulated; clumped heterochromatin | clumped euchromatin, heterochromatin |
| Color | medium-light | medium | medium |
| Nucleoli | 1 or 2 | 1 or 2, rarely visible | 1 |
| Cytoplasm | |||
| Percentage/color | abundant/light | abundant/light | medium/light |
| Mitochondria | many | few | few |
| Vacuoles | many; small | few | few; small |
| Lipid droplets | yes | yes | yes |
| Dense bodies | yes | yes | no |
| Cell contacts | yes | no | no |
| Nucleus of fasciculus longitudinalis medialis (NFLM) | |||
| Sample size | n = 26 | n = 20 | n = 23 |
| Long axis of cells soma (µm) | 15.72 ± 0.83 | 11.41 ± 1.61 | 9.08 ± 0.29 |
| Short axis of cells soma (µm) | 8.96 ± 1.21 | 6.02 ± 0.91 | 6.13 ± 0.22 |
| Nucleus | 1–2 | 1 | 1 |
| Shape | ovoid; irregular ± invaginations | ovoid; irregular ± invaginations | ovoid; irregular |
| Long axis (µm) | 9.45 ±1.47 | 6.89 ± 0.96 | 7.77 ± 0.49 |
| Short axis (µm) | 5.49 ±1.44 | 3.55 ± 0.52 | 5.69 ± 0.32 |
| Chromatin | some clumped | more clumped | heterochromatin |
| Color | light | light | light |
| Nucleoli | 1 or 2 | 1 or 2 | 1 or more |
| Cytoplasm | |||
| Percentage/color | 70–80%, light gray or gray | 50–60%, denser, gray | 20–30%, light, grainy |
| Mitochondria | many | few | few |
| Vacuoles | few | few | few; small |
| Lipid droplets | many | fewer but larger | single |
| Dense bodies | yes | yes | single |
| Cell contacts | large synaptic terminals | with InII | - |
| Dorsal tegmental nucleus DTN | |||
| Sample size | n = 21 | n = 25 | n = 20 |
| Long axis of cells soma (µm) | 47.95 ± 5.3 | 11.87 ± 1.17 | 5.85 ± 0.25 |
| Short axis of cells soma (µm) | 22.24 ± 2.59 | 5.35 ± 0.87 | 3.33 ± 0.13 |
| Nucleus | 1 or no | 1 | 1 |
| Contour | ovoid | ovoid; irregular ± invaginations | - |
| Long axis (µm) | 12.47 ± 2.45 | 7.62 ± 1.04 | 4.66 ± 0.34 |
| Short axis (µm) | 7.26 ± 0.81 | 4.18 ± 0.75 | 2.92 ± 0.29 |
| Chromatin | evenly distributed; non-clumped | heterochromatin | heterochromatin |
| Color | light or dark | light or medium | medium, coarse grain |
| Nucleoli | 1 | 1 | few |
| Cytoplasm | |||
| Percentage/color | 70–80%, dense gray or gray | 60–70%, dense, coarse-grained | 20–30%, gray, fine-grained |
| Mitochondria | many | intermediate | few |
| Vacuoles | few | few | few |
| Lipid droplets | many | few | no |
| Dense bodies | yes | yes | yes |
| Cell contacts | with astrocytes | - | - |
| Type of Cells | Ependyma | III | IVa | Neuroepithelial Cells |
|---|---|---|---|---|
| Sample size | n = 32 | n = 51 | n = 59 | n = 7 |
| Long axis of cells soma (µm) | 1.73 ± 0.42 | 7.22 ± 0.83 | 6.72 ± 0.04 | 11.36 ± 0.39 |
| Short axis of cells soma (µm) | 0.99 ± 0.17 | 3.93 ± 0.69 | 5.66 ± 0.09 | 4.23 ± 0.03 |
| Nucleus | ||||
| Shape | elliptical; elongated ± few invaginations | elongated; irregular ± invaginations | ovoid, irregular ± invaginations | ovoid, elongated ± invaginations |
| Long axis (µm) | 0.78 ± 0.06 | 6.62 ± 0.47 | 5.85 ± 0.19 | 5.28 ± 0.38 |
| Short axis (µm) | 0.52 ± 0.07 | 3.35 ± 0.29 | 4.79 ± 0.21 | 2.52 ± 0.25 |
| Chromatin | evenly distributed; heterochromatin | evenly distributed; non-clumped | reticulated; clumped hetero | reticulated; clumped hetero. |
| Color | light | medium | dark | medium |
| Nucleoli | 1 or 2 | 1 or 2 | 1 or 2, rarely visible | 1 or 2 |
| Cytoplasm | ||||
| Percentage/color | abundant/light | scanty/light | scanty/medium | abundant/medium |
| Mitochondria | many | few | few | few |
| Microvilli | few to many | no | no | few |
| Vacuoles | Many, large | no | few | few |
| Lipid droplets | yes | no or 1 | no | no |
| Dense bodies | yes | yes | no | yes |
| Localization | RLTNZ, TMZ | RLTNZ, TSNZ, CLTNZ | RLTNZ, TSNZ, CLTNZ | RLTNZ |
| Contacts | DEL, capillaries | III, IVa | III, IVa | DEL, ependyma |
| Brain Areas | Type of Cells | Cell Size, μm * | Optical Density **, UOD |
|---|---|---|---|
| Intact fish | |||
| Rostral tegmentum, SVZ | Elongated | 4.02 ± 0.54/2.28 ± 0.4 5.75 ± 0.6/4.4 ± 0.4 | 68 ± 24.04 87 ± 8.8 |
| NIII | Small Elongated | 4.44 ± 0.55/3.32 ± 0.16 8.24 ± 0.7/6.61 ± 0.6 | 54.5 ± 0.71 82 ± 8.6 |
| Torus semicircularis | Small Elongated | 2.76 ± 0.57/2.37 ± 0.64 8.36 + 0.8/5.89 ± 0.6 | 56.75 ± 30.78 96.67 ± 34.85 |
| Valvula cerebelli, ML | Oval Elongated | 6 ± 0.6/3.84 ± 0.4 8.59 ± 0.93/6.65 ± 0.35 | 95.5 ± 23.33 86 ± 8.6 |
| Traumatic injury of medulla oblongata | |||
| Rostral tegmentum | Small Migrating Oval Elongated | 4.65 ± 0.55/2.83 ± 0.81 7.04 ± 1.29/2.95 ± 0.89 6.72 ± 6.72/3.75 ± 3.75 6.38 ± 6.38/4.62 ± 4.62 | 135.8 ± 13.75 142.4 ± 15.44 132.4 ± 20.31 149 ± 8.25 |
| Caudal tegmentum | Small Migrating Oval | 4.76 ± 0.82/3.28 ± 1.11 8.01 ± 1.65/3.28 ± 0.59 6.932 ± 1.03/3.52 ± 0.7 | 132.8 ± 19.4 143.43 ± 10.21 132.8 ± 13.03 |
| NIII | Rounded Small Migrating Oval Elongated | 5.61 ± 0.5/5.53 ± 0.4 5 ± 0.39/2.98 ± 0.49 8.61 ± 1.11/3.24 ± 0.99 6.26 ± 1.04/3.89 ± 0.99 6.54 ± 0.44/4.98 ± 0.5 | 119 ± 45.12 135.2 ± 26.7 153.3 ± 14.22 119.4 ± 42.86 133.8 ± 36.77 |
| Torus semicircularis | Small Migrating | 4.23 ± 0.67/2.31 ± 0.86 7.17 ± 2.05/2.97 ± 0.27 | 151.8 ± 16.38 160 ± 8.09 |
| Brain Areas | Type of Cells | Cell Size, μm * | Optical Density **, UOD |
|---|---|---|---|
| Intact fish | |||
| Rostral tegmentum, SVZ Medio-basal tegmentum | Small, Undifferentiated | 3.79 ± 0.84/2.82 ± 0.4 | 63 ± 16.27 |
| NIII | Oval Rounded | 8.24 ± 1.41/4.88 ± 0.23 5.79 ± 0.6/4.57 ± 0.5 | 106.75 ± 9.74 48.33 ± 10.02 |
| Torus semicircularis, SVZ PZ | Small, Undifferentiated | 4.38 ± 1.47/3.22 ± 1.03 | 56.5 ± 6.36 |
| Traumatic injury of medulla oblongata | |||
| Rostral isthmus | Large Small Migrating Oval Elongated | 19.24 ± 3.25/13.79 ± 3.17 4.11 ± 0.47/2.35 ± 0.63 7.64 ± 1.67/2.84 ± 1.25 6.11 ± 0.68/3.5 ± 0.5 5.94 ± 0.6/4.8 ± 0.4 | 65 ± 30.95 150.75 ± 9.36 95.2 ± 44.91 152 ± 11.8 159.5 ± 2.38 |
| Caudal isthmus | Small Migrating Oval Elongated | 4.7 ± 1.11/2.56 ± 0.63 8.93 ± 0.87/3.15 ± 1.05 6.79 ± 1.2/3.26 ± 0.6 7.65 ± 2.29/5.81 ± 1 | 134 ± 40.48 124.82 ± 26.49 140.8 ± 18.9 128.14 ± 16.19 |
| Valvula cerebelli, Granular layer | Small Migrating Oval Elongated | 4.33 ± 0.85/2.21 ± 0.11 7.42 ± 1.75/2.67 ± 0.54 8.26 ± 2.07/4.94 ± 1.25 0.32 ± 6.52/0.01 ± 5.45 | 149.4 ± 15.06 150.5 ± 9.43 142.29 ± 19.32 148 ± 15.62 |
| No. | Antibodies | Manufacturer | Dilution | Catalog Number | Marker |
|---|---|---|---|---|---|
| 1 | BLBP | Abcam, Cambridge, UK | 1:300 | ab32423 | BLBP |
| 2 | AroB (cyp19a1b) | Abcam, Cambridge, UK | 1:300 | ab106168 | AroB (cyp19a1b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushchina, E.V.; Pimenova, E.A.; Kapustyanov, I.A.; Bykova, M.E. Ultrastructural Study and Immunohistochemical Characteristics of Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta) Brain After Acute Traumatic Injury. Int. J. Mol. Sci. 2025, 26, 644. https://doi.org/10.3390/ijms26020644
Pushchina EV, Pimenova EA, Kapustyanov IA, Bykova ME. Ultrastructural Study and Immunohistochemical Characteristics of Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta) Brain After Acute Traumatic Injury. International Journal of Molecular Sciences. 2025; 26(2):644. https://doi.org/10.3390/ijms26020644
Chicago/Turabian StylePushchina, Evgeniya V., Evgeniya A. Pimenova, Ilya A. Kapustyanov, and Mariya E. Bykova. 2025. "Ultrastructural Study and Immunohistochemical Characteristics of Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta) Brain After Acute Traumatic Injury" International Journal of Molecular Sciences 26, no. 2: 644. https://doi.org/10.3390/ijms26020644
APA StylePushchina, E. V., Pimenova, E. A., Kapustyanov, I. A., & Bykova, M. E. (2025). Ultrastructural Study and Immunohistochemical Characteristics of Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta) Brain After Acute Traumatic Injury. International Journal of Molecular Sciences, 26(2), 644. https://doi.org/10.3390/ijms26020644






