Gene Expression Profiles Reveal Distinct Mechanisms Driving Chronic Obstructive Pulmonary Disease Exacerbations
Abstract
1. Introduction
2. Results
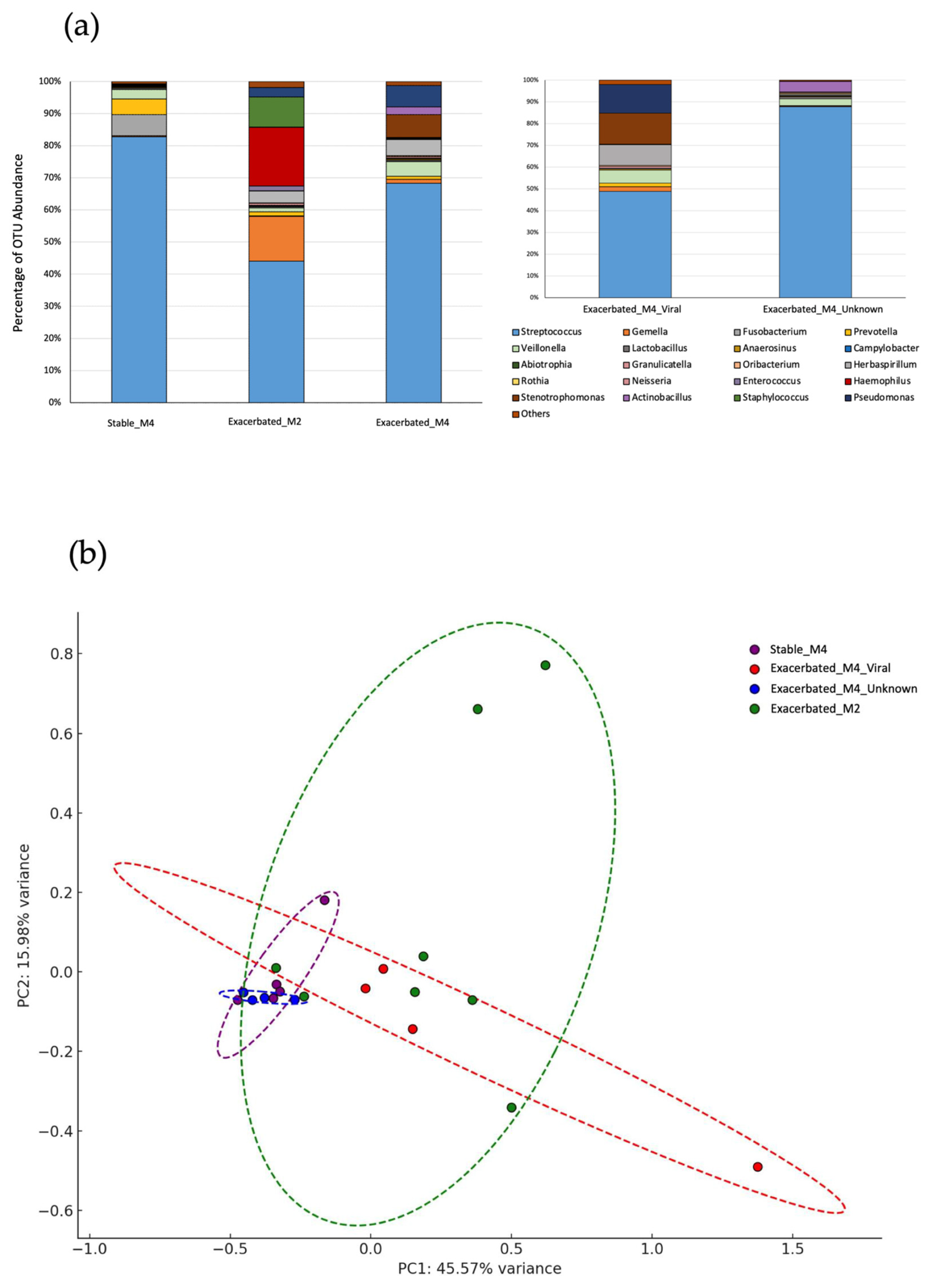
2.1. Sample Classification
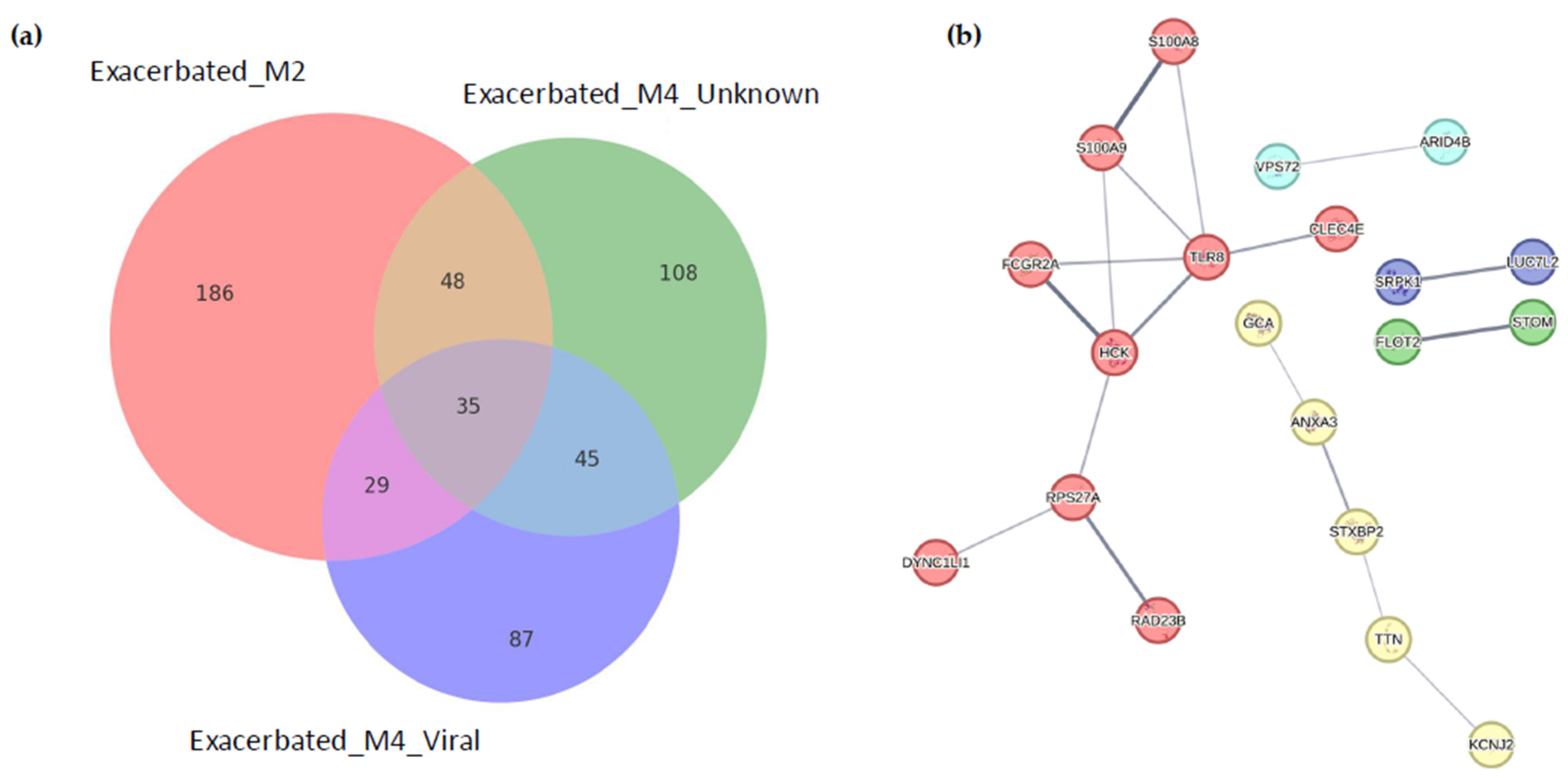
2.2. Identification of Differentially Expressed Genes
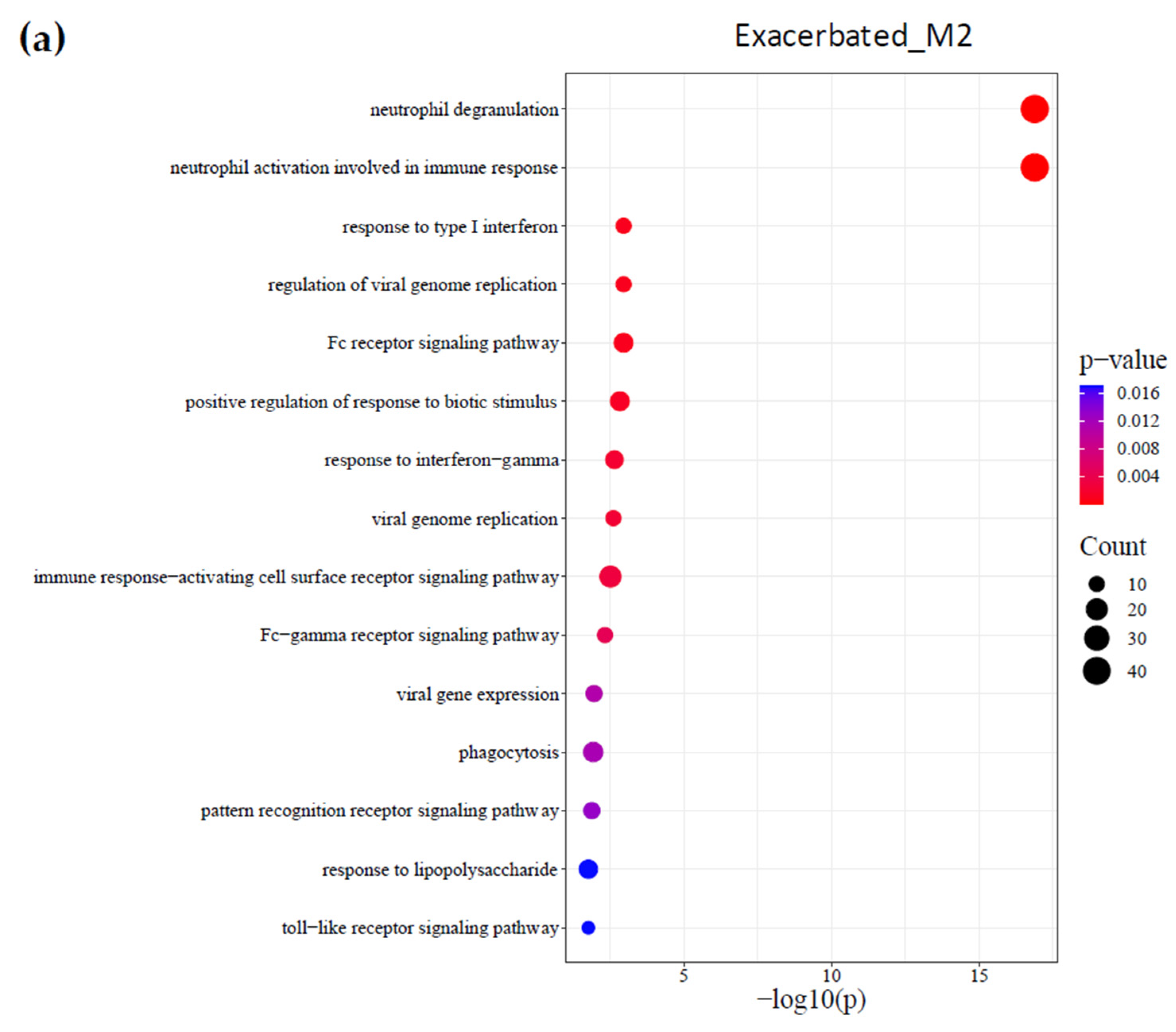
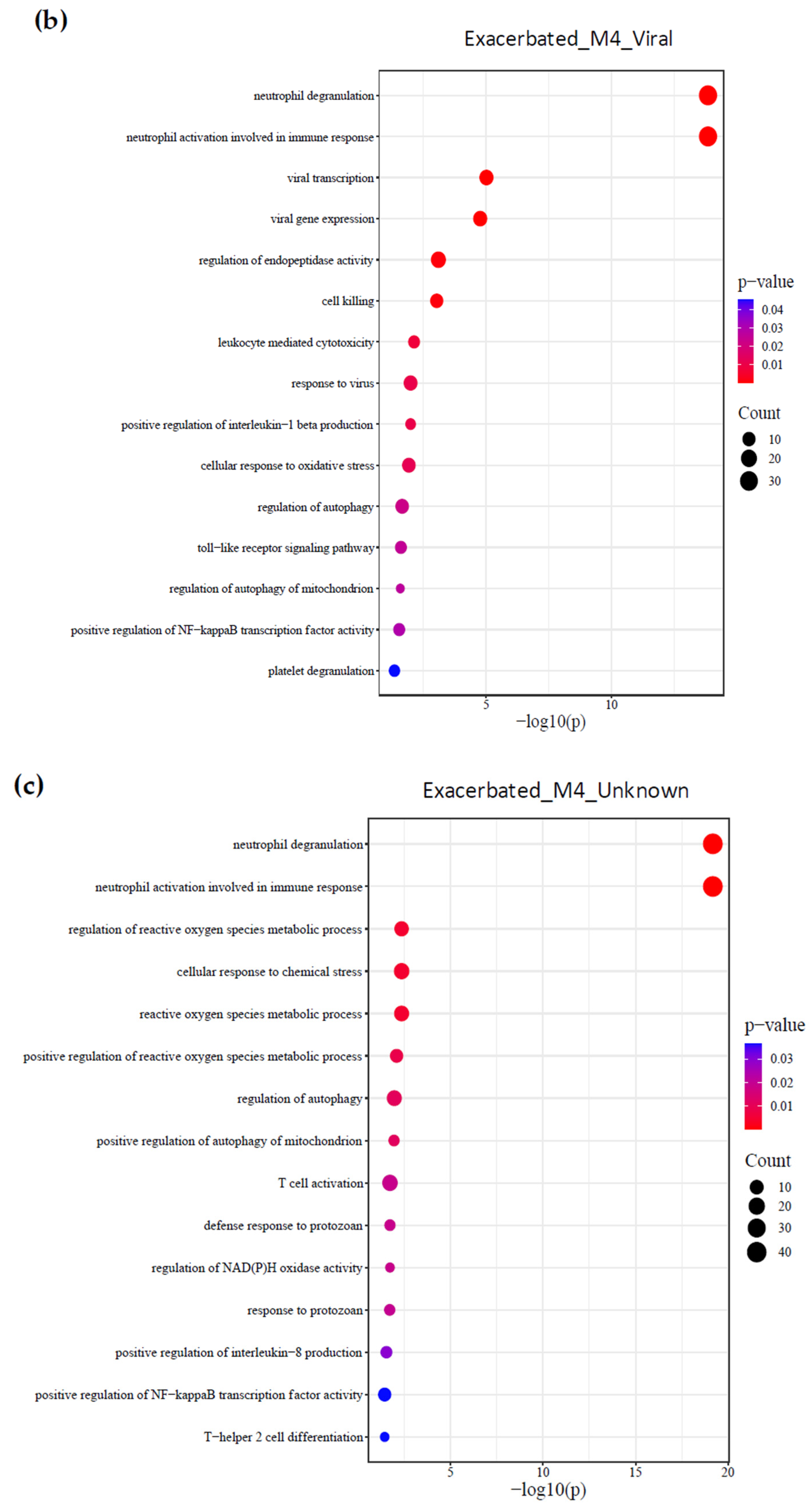
2.3. Identification of Biological Processes
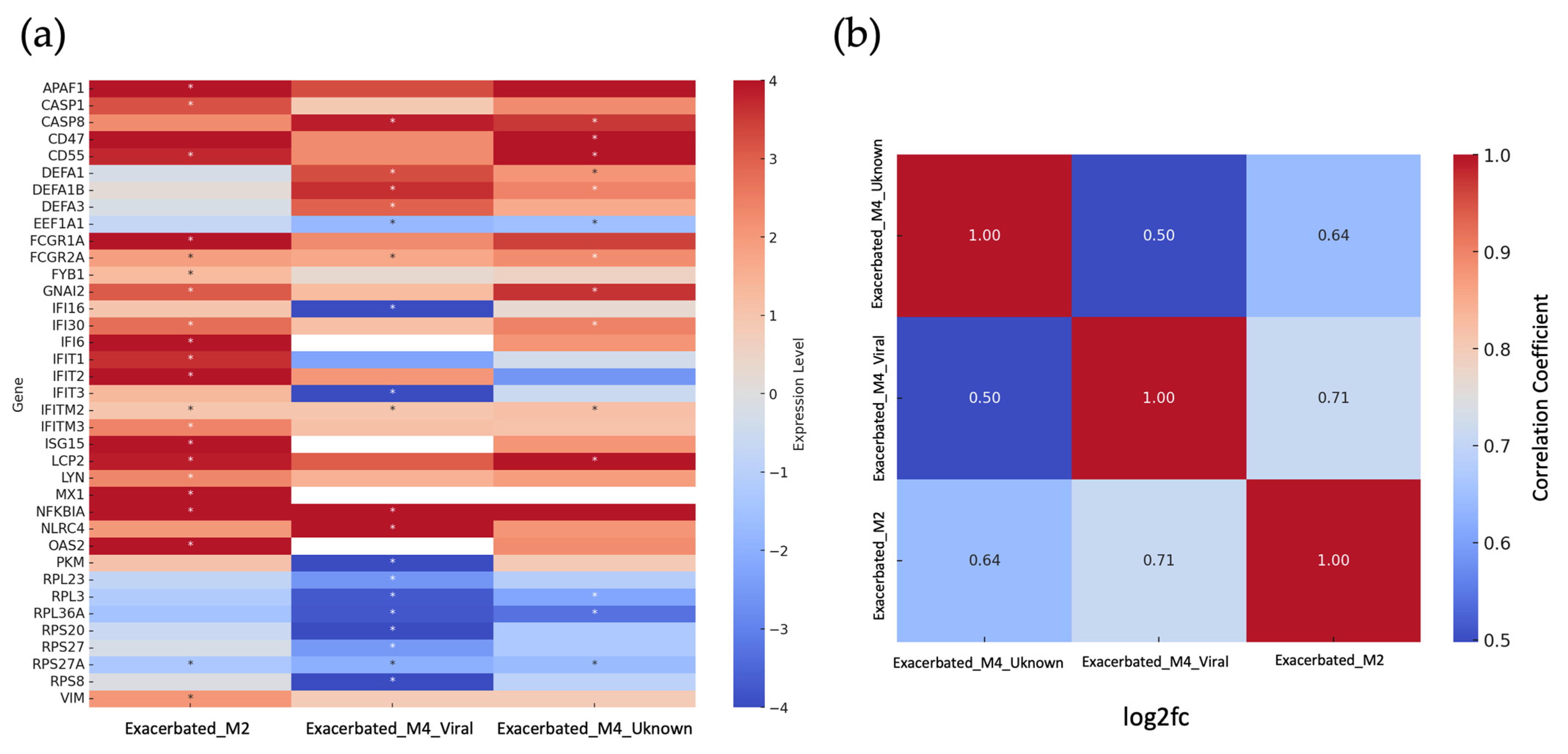
2.4. Identification of Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Blood Transcriptomic Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| rRNA | Ribosomal Ribonucleic Acid |
| PCR | Polymerase Chain Reaction |
| IFITM3 | Interferon-Induced Transmembrane Protein 3 |
| ISG15 | Interferon-Stimulated Gene 15 |
| DEFA3 | Defensin Alpha 3 |
| CD47 | Cluster of Differentiation 47 |
| ROS | Reactive oxygen species |
| NAD(P)H | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen |
| ACOS | Asthma–COPD overlap syndrome |
| NLR | Neutrophil to lymphocyte ratio |
| IFN | Interferon |
| NET | Neutrophil extracellular trap |
| BNC | Blood neutrophil count |
| BEO | Blood eosinophil count |
| IQR | Interquartile range |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| FDR | False discovery rate |
| cDNA | Complementary DNA |
| MinION | Oxford Nanopore MinION Sequencer |
References
- Parums, D.V. Editorial: Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 Guidelines for COPD, Including COVID-19, Climate Change, and Air Pollution. Med. Sci. Monit. 2023, 29, e942672. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute Exacerbations of Chronic Obstructive Pulmonary Disease: Identification of Biologic Clusters and Their Biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.; Harper-Owen, R.; Bhowmik, A.; Moric, I.; Sanderson, G.; Message, S.; Maccallum, P.; Meade, T.W.; Jeffries, D.J.; Johnston, S.L.; et al. Respiratory Viruses, Symptoms, and Inflammatory Markers in Acute Exacerbations and Stable Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M. Exacerbations of Chronic Obstructive Pulmonary Disease: When Are Bacteria Important? Eur. Respir. J. Suppl. 2002, 36, 9s–19s. [Google Scholar] [CrossRef]
- Wang, H.; Anthony, D.; Selemidis, S.; Vlahos, R.; Bozinovski, S. Resolving Viral-Induced Secondary Bacterial Infection in COPD: A Concise Review. Front. Immunol. 2018, 9, 2345. [Google Scholar] [CrossRef]
- Woerden, H.; Gregory, C.; Martinez-Giron, R.; Burr, M.; Lansdown, A.; Matthews, L.P. Case Series Demonstrating the Presence of Protozoa in the Sputum of a Proportion of Respiratory Patients. Internet J. Lab. Med. 2009, 4, 1. [Google Scholar]
- Sapey, E.; Stockley, R.A. COPD Exacerbations · 2: Aetiology. Thorax 2006, 61, 250–258. [Google Scholar] [CrossRef]
- Chen, Y.-W.R.; Leung, J.M.; Sin, D.D. A Systematic Review of Diagnostic Biomarkers of COPD Exacerbation. PLoS ONE 2016, 11, e0158843. [Google Scholar] [CrossRef]
- López Caro, J.C.; Santibáñez, M.; García Rivero, J.L.; Villanueva, M.; Sainz, J.; González Astorqui, P.; Hierro, M.; Rodríguez Porres, M.; Paras Bravo, P.; Mira, A.; et al. Sputum Microbiome Dynamics in Chronic Obstructive Pulmonary Disease Patients during an Exacerbation Event and Post-Stabilization. Respiration 2019, 98, 447–454. [Google Scholar] [CrossRef]
- Lonergan, M.; Dicker, A.J.; Crichton, M.L.; Keir, H.R.; Van Dyke, M.K.; Mullerova, H.; Miller, B.E.; Tal-Singer, R.; Chalmers, J.D. Blood Neutrophil Counts Are Associated with Exacerbation Frequency and Mortality in COPD. Respir. Res. 2020, 21, 166. [Google Scholar] [CrossRef]
- Taylan, M.; Demir, M.; Kaya, H.; Selimoglu Sen, H.; Abakay, O.; Carkanat, A.İ.; Abakay, A.; Tanrikulu, A.C.; Sezgi, C. Alterations of the Neutrophil-Lymphocyte Ratio during the Period of Stable and Acute Exacerbation of Chronic Obstructive Pulmonary Disease Patients. Clin. Respir. J. 2017, 11, 311–317. [Google Scholar] [CrossRef] [PubMed]
- İn, E.; Kuluöztürk, M.; Öner, Ö.; Deveci, F. The Importance of Neutrophil-to-Lymphocyte Ratio in Chronic Obstructive Pulmonary Disease. Turk. Thorac. J. 2016, 17, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, O.; Toussaint, M.; Jackson, M.M.; Mallia, P.; Footitt, J.; Mincham, K.T.; Meyer, G.F.M.; Kebadze, T.; Gilmour, A.; Long, M.; et al. Neutrophil Extracellular Traps Promote Immunopathogenesis of Virus-Induced COPD Exacerbations. Nat. Commun. 2024, 15, 5766. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, Y.; Li, N.; Yu, M.; Zhu, L.; Wang, L.; Chen, F.; Xu, Y.; Liu, J.; Huang, H. Transcriptomic Analysis and Validation Reveal the Pathogenesis and a Novel Biomarker of Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Respir. Res. 2022, 23, 27. [Google Scholar] [CrossRef]
- Obeidat, M.; Nie, Y.; Chen, V.; Shannon, C.P.; Andiappan, A.K.; Lee, B.; Rotzschke, O.; Castaldi, P.J.; Hersh, C.P.; Fishbane, N.; et al. Network-Based Analysis Reveals Novel Gene Signatures in Peripheral Blood of Patients with Chronic Obstructive Pulmonary Disease. Respir. Res. 2017, 18, 72. [Google Scholar] [CrossRef]
- Mycroft, K.; Proboszcz, M.; Paplińska-Goryca, M.; Krenke, R.; Górska, K. Transcriptional Profiles of Peripheral Eosinophils in Chronic Obstructive Pulmonary Disease and Asthma-An Exploratory Study. J. Cell Mol. Med. 2024, 28, e70110. [Google Scholar] [CrossRef]
- Meijer, M.; Rijkers, G.T.; van Overveld, F.J. Neutrophils and Emerging Targets for Treatment in Chronic Obstructive Pulmonary Disease. Expert. Rev. Clin. Immunol. 2013, 9, 1055–1068. [Google Scholar] [CrossRef]
- Horn, M.; Bertling, A.; Brodde, M.F.; Müller, A.; Roth, J.; Van Aken, H.; Jurk, K.; Heilmann, C.; Peters, G.; Kehrel, B.E. Human Neutrophil Alpha-Defensins Induce Formation of Fibrinogen and Thrombospondin-1 Amyloid-like Structures and Activate Platelets via Glycoprotein IIb/IIIa. J. Thromb. Haemost. 2012, 10, 647–661. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Gkikas, I.; Palikaras, K.; Tavernarakis, N. The Role of Mitophagy in Innate Immunity. Front. Immunol. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Bongers, Q.; Comellas, A.P. Impact of Occupational Exposures in Patients with Chronic Obstructive Pulmonary Disease: Current Understanding and Knowledge Gaps. Curr. Opin. Pulm. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.D.; Reddel, H.K.; van Zyl-Smit, R.N.; Agusti, A. The Asthma-COPD Overlap Syndrome: Towards a Revised Taxonomy of Chronic Airways Diseases? Lancet Respir. Med. 2015, 3, 719–728. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, H.C.; Ratier-Cruz, A.; Aleshinloye, O.B.; Martinez-Giron, R.; Gregory, C.; Matthews, I.P. Association between Protozoa in Sputum and Asthma: A Case-Control Study. Respir. Med. 2011, 105, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Al-Husseiny, I.A.; Al-Mayah, Q.S.; Hafedh, A.A. The Role of Intestinal Protozoa in Chronic Obstructive Pulmonary Disease Exacerbation. Ann. Parasitol. 2022, 68, 39–45. [Google Scholar] [CrossRef]
- Meinderts, S.M.; Oldenborg, P.-A.; Beuger, B.M.; Klei, T.R.L.; Johansson, J.; Kuijpers, T.W.; Matozaki, T.; Huisman, E.J.; de Haas, M.; van den Berg, T.K.; et al. Human and Murine Splenic Neutrophils Are Potent Phagocytes of IgG-Opsonized Red Blood Cells. Blood Adv. 2017, 1, 875–886. [Google Scholar] [CrossRef]
- Veerati, P.C.; Troy, N.M.; Reid, A.T.; Li, N.F.; Nichol, K.S.; Kaur, P.; Maltby, S.; Wark, P.A.B.; Knight, D.A.; Bosco, A.; et al. Airway Epithelial Cell Immunity Is Delayed During Rhinovirus Infection in Asthma and COPD. Front. Immunol. 2020, 11, 974. [Google Scholar] [CrossRef]
- Herr, C.; Shaykhiev, R.; Bals, R. The Role of Cathelicidin and Defensins in Pulmonary Inflammatory Diseases. Expert Opin. Biol. Ther. 2007, 7, 1449–1461. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, W. Defensins in Innate Immunity. Curr. Opin. Hematol. 2014, 21, 37–42. [Google Scholar] [CrossRef]
- García-Valero, J.; Olloquequi, J.; Montes, J.F.; Rodríguez, E.; Martín-Satué, M.; Texidó, L.; Ferrer Sancho, J. Deficient Pulmonary IFN-β Expression in COPD Patients. PLoS ONE 2019, 14, e0217803. [Google Scholar] [CrossRef]
- Monk, P.D.; Brookes, J.L.; Tear, V.J.; Batten, T.N.; Newall, C.; Mankowski, M.; Crooks, M.G.; Singh, D.; Chaudhuri, R.; Leaker, B.; et al. Nebulised Interferon Beta-1a (SNG001) in the Treatment of Viral Exacerbations of COPD. Respir. Res. 2024, 25, 228. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, P.L.; Mallia, P.; Cox, M.J.; Footitt, J.; Willis-Owen, S.A.G.; Homola, D.; Trujillo-Torralbo, M.-B.; Elkin, S.; Kon, O.M.; Cookson, W.O.C.; et al. Outgrowth of the Bacterial Airway Microbiome after Rhinovirus Exacerbation of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Stables (n = 5) | Exacerbated (n = 16) | p-Value |
|---|---|---|---|
| Age (years, median, IQR) | 76.0 (67.0–79.0) | 74.0 (67.75–79.25) | 0.736 |
| Sex at birth | |||
| Male | 5 (100.00%) | 13 (81.25%) | 0.754 |
| Female | 0 (0%) | 3 (18.75%) | |
| GOLD | |||
| 1E | 0 (0%) | 1 (6.25%) | 0.758 |
| 2E | 0 (0%) | 2 (12.5%) | |
| 3E | 3 (60.00%) | 7 (43.75%) | |
| 4E | 2 (40.00%) | 5 (31.25%) | |
| Microbiome | |||
| Stable or unknown exacerbated (M4) | 5 (100%) | 4 (25.0%) | 0.016 |
| Viral exacerbated (M4 + respiratory virus) | 4 (25.0%) | ||
| Bacterial exacerbated (M2) | 8 (50.0%) | ||
| Cell count (cells/µL, median, IQR) | |||
| Leukocytes | 8825 (7973–9443) | ||
| Neutrophils | 6245 (4695–6918) | ||
| Lymphocytes | 1275 (758–1965) | ||
| Eosinophils | 135 (65–258) |
| Gene Name | Description |
|---|---|
| AC010970.1 | Long intergenic non-protein coding RNA |
| ANXA3 | Annexin A3 |
| ARID4B | AT-rich interaction domain 4B |
| CLEC4E | C-type lectin domain family 4 member E |
| DYNC1LI1 | Dynein cytoplasmic 1 light intermediate chain 1 |
| ELP3 | Elongator acetyltransferase complex subunit 3 |
| FCGR2A | Fc fragment of IgG receptor IIa |
| FKBP5 | FK506 binding protein 5 |
| FLOT2 | Flotillin 2 |
| GCA | Grancalcin |
| HCK | Hematopoietic cell kinase |
| HDLBP | High-density lipoprotein binding protein |
| IFITM2 | Interferon-induced transmembrane protein 2 |
| JPT1 | Jupiter microtubule-associated homolog 1 |
| KCNJ2 | Potassium inwardly rectifying channel |
| LUC7L2 | LUC7-like protein 2 |
| MAPKAP1 | Mitogen-activated protein kinase associated protein 1 |
| MATR3 | Matrin 3 |
| MED23 | Mediator complex subunit 23 |
| PRR13 | Proline-rich protein 13 |
| RAD23B | RAD23 homolog B |
| RASGRP4 | RAS guanyl-releasing protein 4 |
| RBM47 | RNA-binding motif protein 47 |
| RCBTB2 | Regulator of chromosome condensation and BTB domain containing 2 |
| RPS27A | Ribosomal protein S27a |
| S100A8 | S100 calcium-binding protein A8 |
| S100A9 | S100 calcium-binding protein A9 |
| SH3GLB1 | SH3 domain-containing protein L-binding protein 1 |
| SRPK1 | Serine/arginine-rich protein kinase 1 |
| STOM | Stomatin |
| STXBP2 | Syntaxin binding protein 2 |
| TLR8 | Toll-like receptor 8 |
| TTN | Titin |
| VPS72 | Vacuolar protein sorting 72 |
| ZNF512 | Zinc finger protein 512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello-Perez, M.; García-Pachón, E.; Gonzalo-Jimenez, N.; Ruiz-García, M.; Zamora-Molina, L.; Baeza-Martinez, C.; Galiana, A. Gene Expression Profiles Reveal Distinct Mechanisms Driving Chronic Obstructive Pulmonary Disease Exacerbations. Int. J. Mol. Sci. 2025, 26, 627. https://doi.org/10.3390/ijms26020627
Bello-Perez M, García-Pachón E, Gonzalo-Jimenez N, Ruiz-García M, Zamora-Molina L, Baeza-Martinez C, Galiana A. Gene Expression Profiles Reveal Distinct Mechanisms Driving Chronic Obstructive Pulmonary Disease Exacerbations. International Journal of Molecular Sciences. 2025; 26(2):627. https://doi.org/10.3390/ijms26020627
Chicago/Turabian StyleBello-Perez, Melissa, Eduardo García-Pachón, Nieves Gonzalo-Jimenez, Montserrat Ruiz-García, Lucía Zamora-Molina, Carlos Baeza-Martinez, and Antonio Galiana. 2025. "Gene Expression Profiles Reveal Distinct Mechanisms Driving Chronic Obstructive Pulmonary Disease Exacerbations" International Journal of Molecular Sciences 26, no. 2: 627. https://doi.org/10.3390/ijms26020627
APA StyleBello-Perez, M., García-Pachón, E., Gonzalo-Jimenez, N., Ruiz-García, M., Zamora-Molina, L., Baeza-Martinez, C., & Galiana, A. (2025). Gene Expression Profiles Reveal Distinct Mechanisms Driving Chronic Obstructive Pulmonary Disease Exacerbations. International Journal of Molecular Sciences, 26(2), 627. https://doi.org/10.3390/ijms26020627






