Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Branching Angle Formation in Tea Plants (Camellia sinensis)
Abstract
1. Introduction
2. Results
2.1. Phenotypic Branching Angles of Different Tea Plant Cultivars
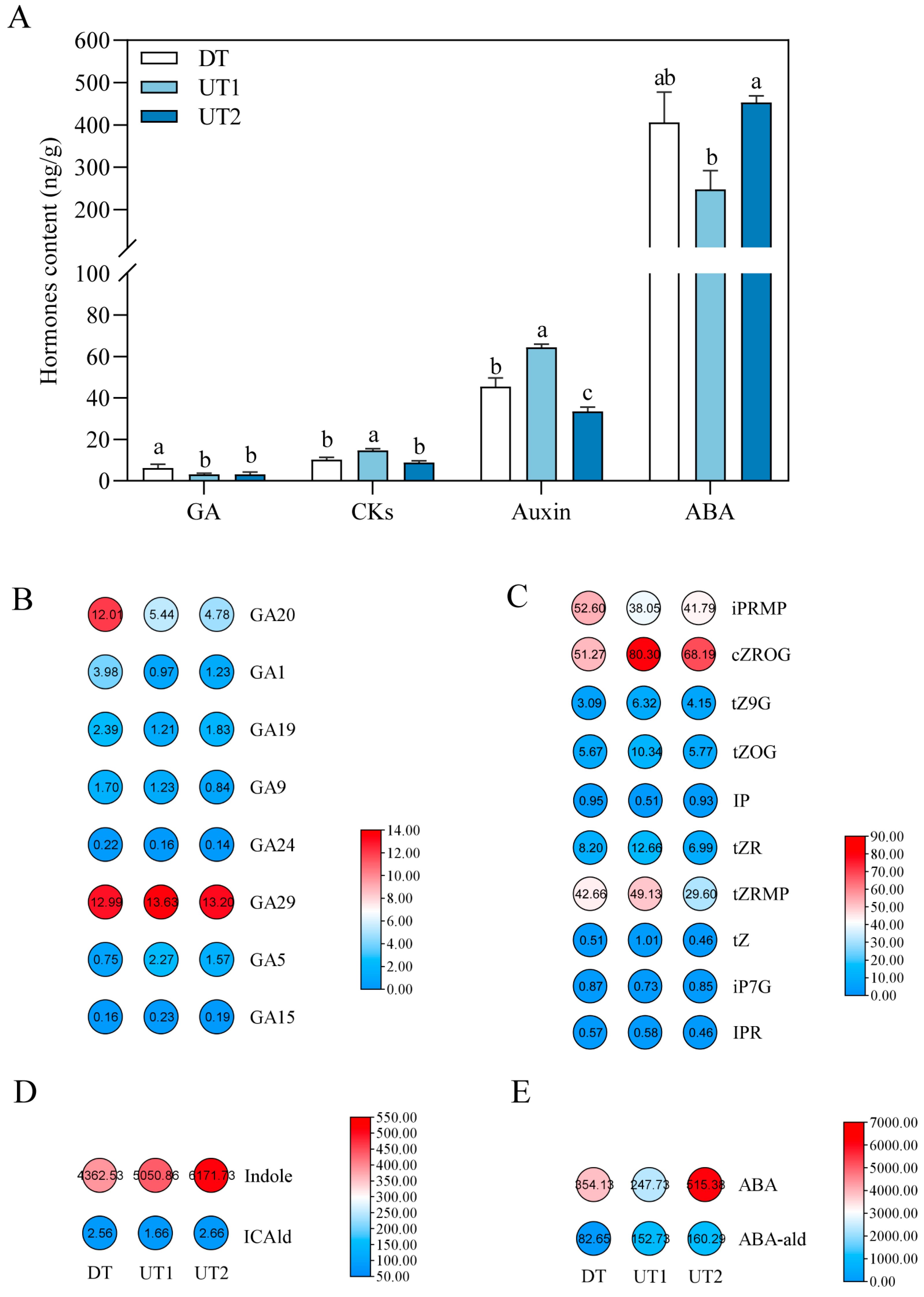
2.2. Relationship Between Endogenous Hormones and the Branching Angle of Tea Plants
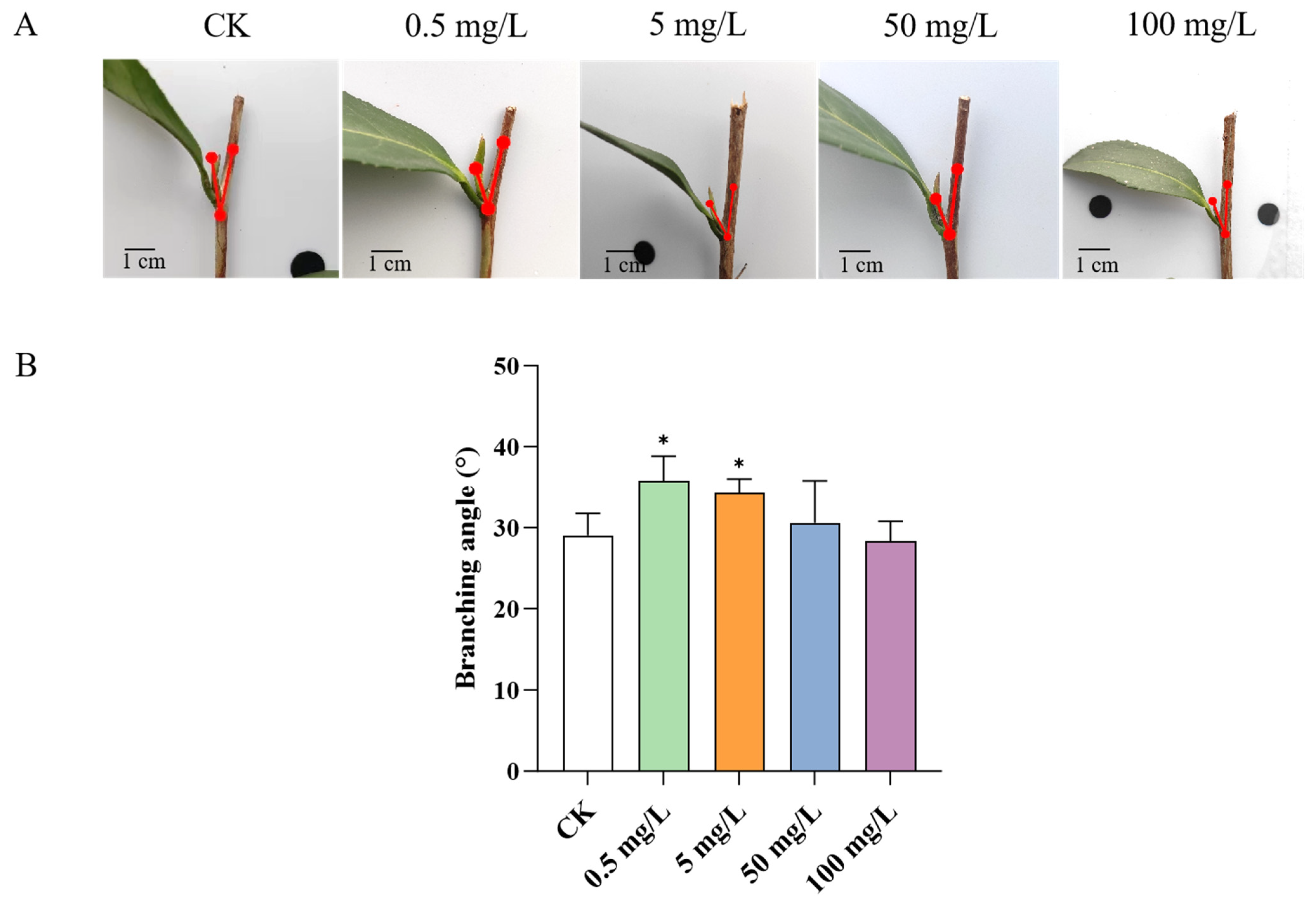
2.3. GA3 Treatment Expands the Branching Angle in Tea Plant Shoots
2.4. Comparative Transcriptome Analysis
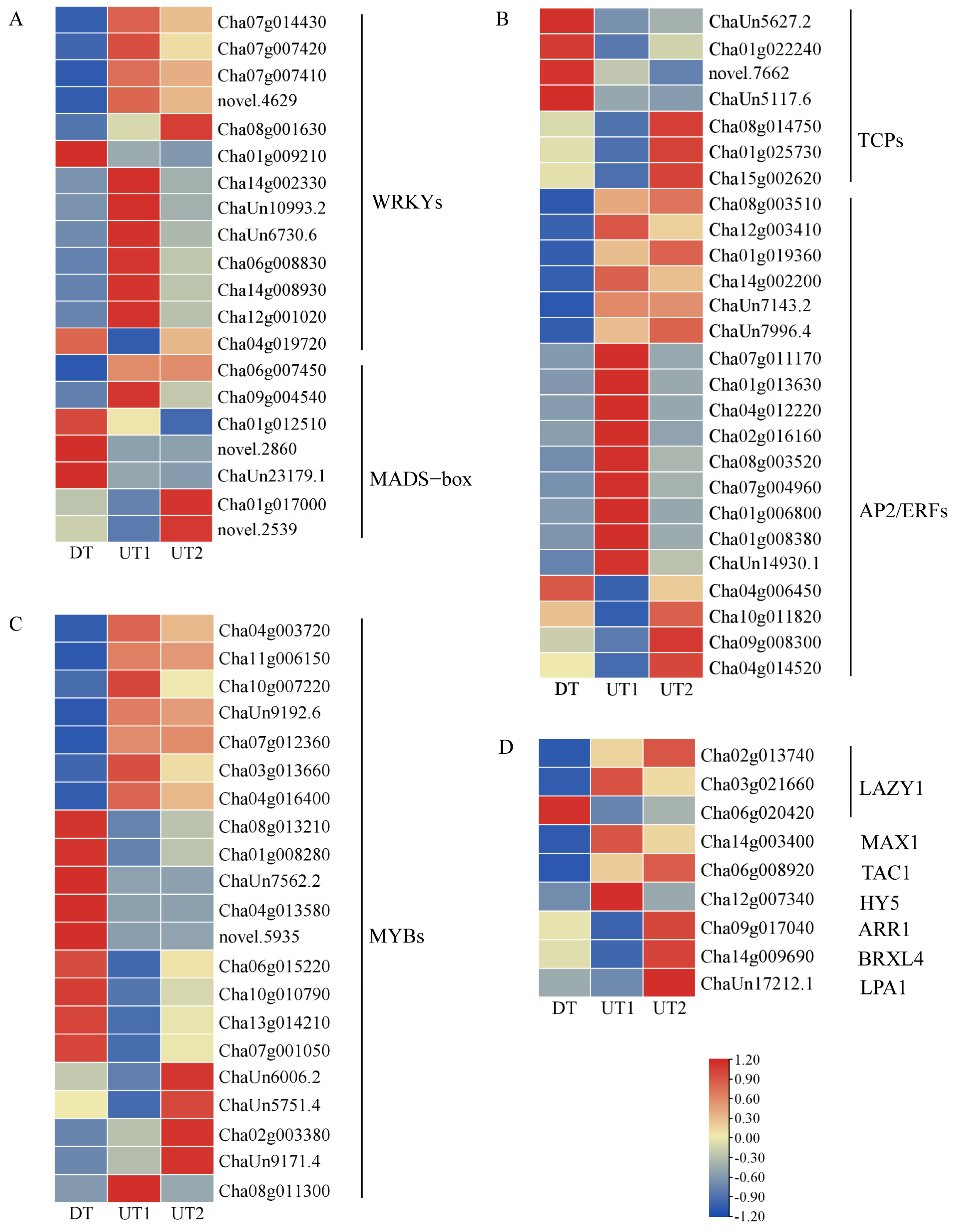
2.5. Transcription Factors Associated with Branching Development in Tea Plants

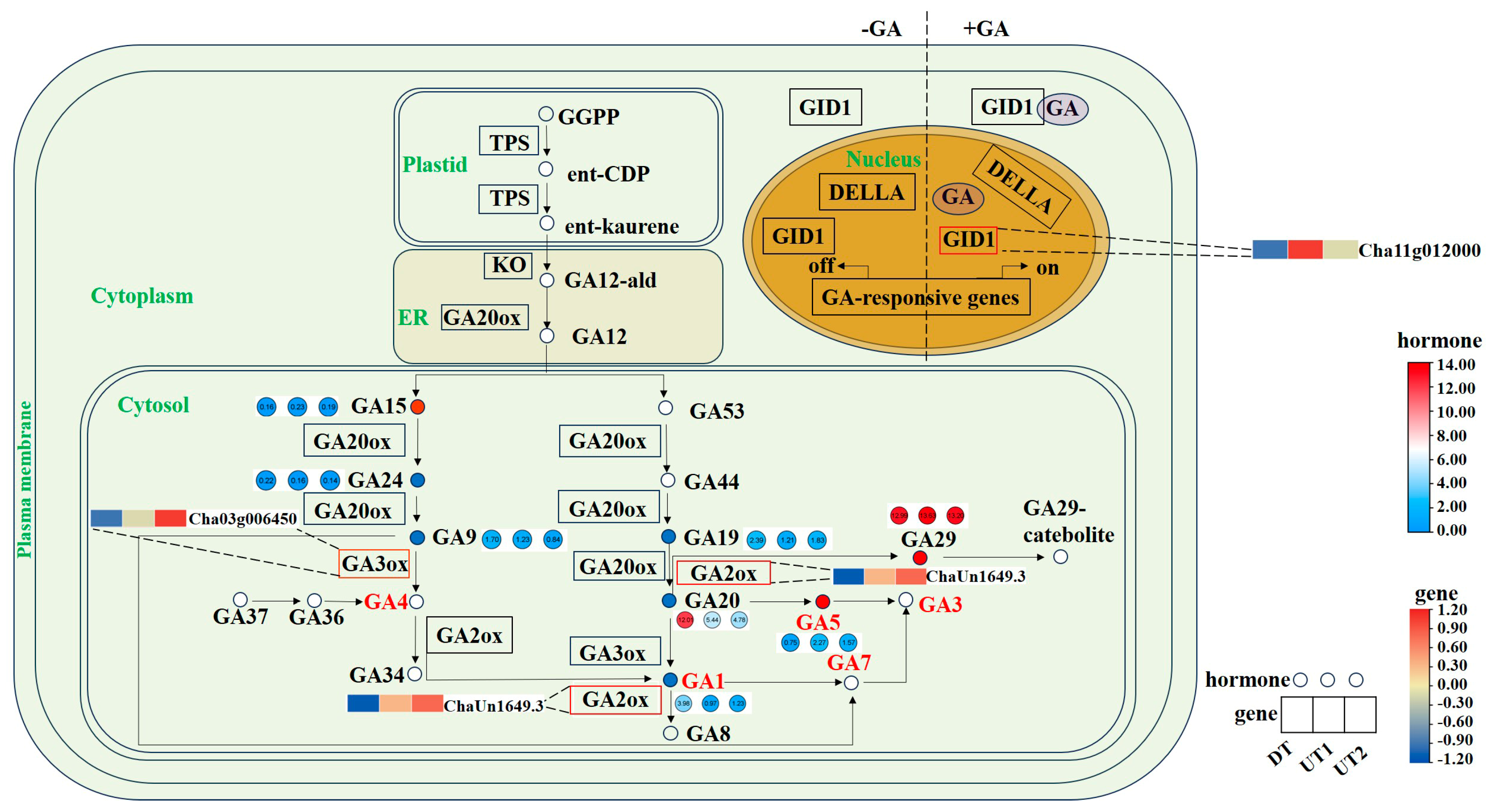
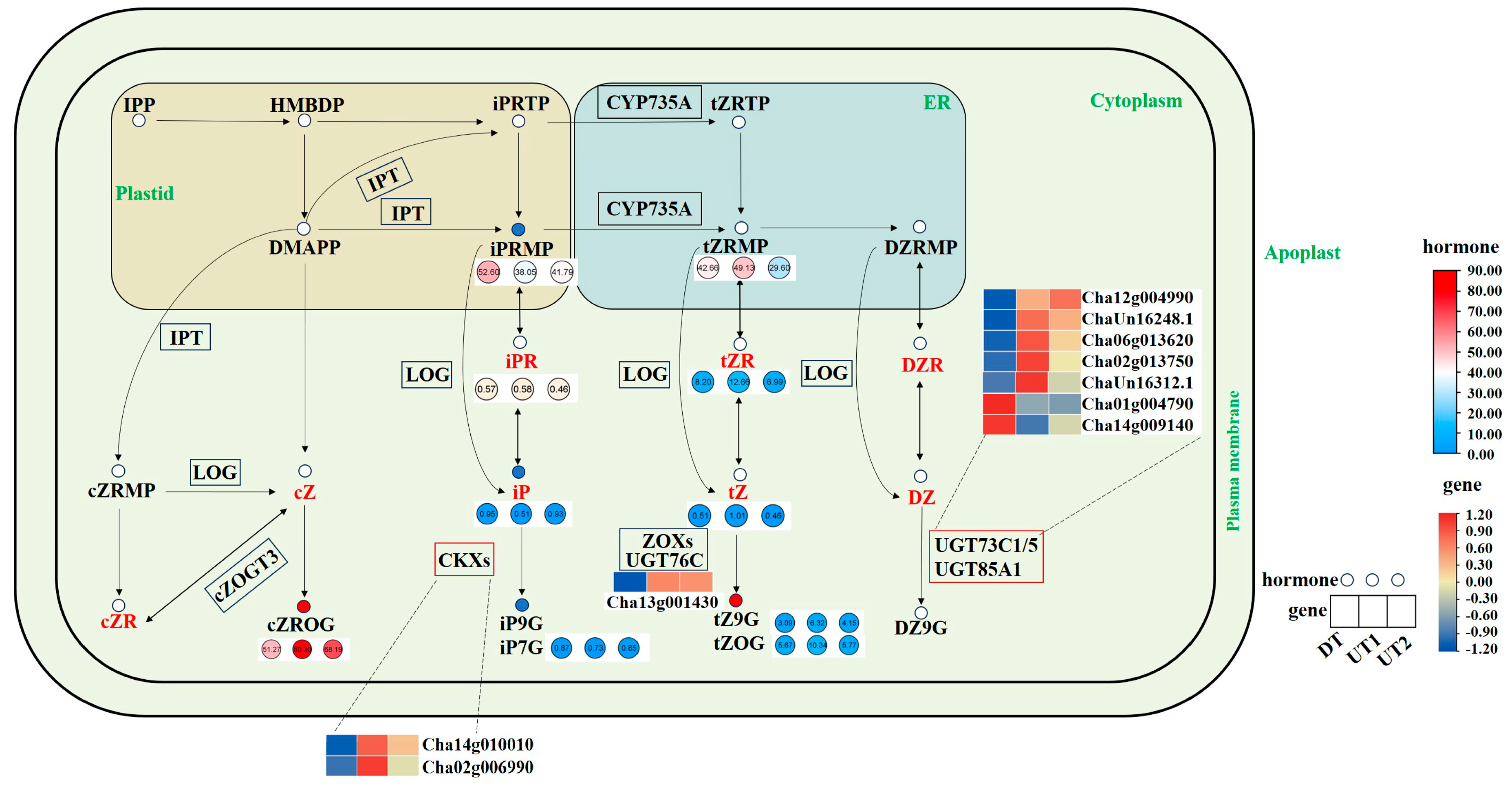
2.6. Combined Analysis of Endogenous Phytohormones and DEGs Related to Plant Hormone Biosynthesis, Metabolism, and Signal Transduction Pathways in the Tea Plant Lateral Bud
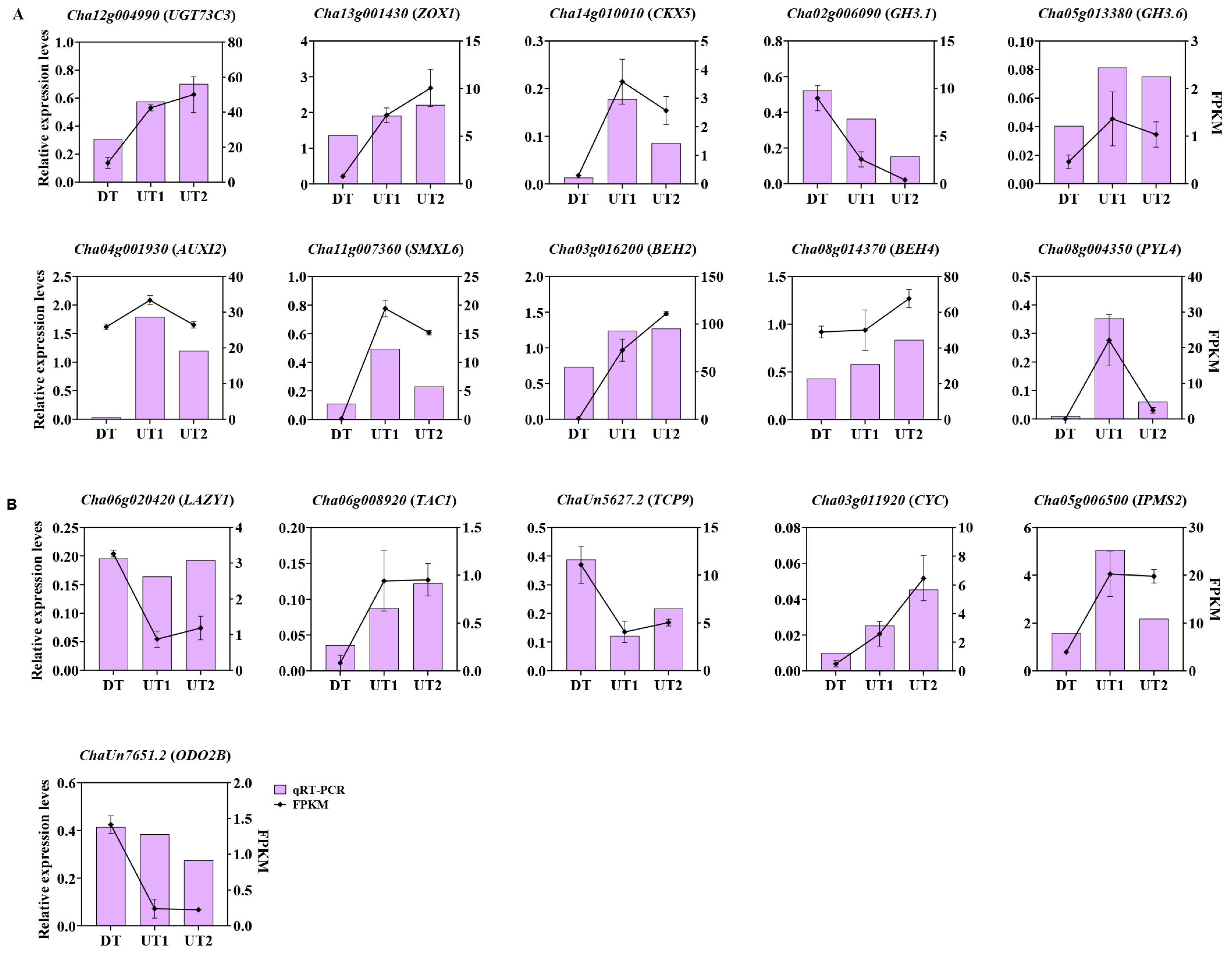
2.7. Transcriptome Validation Using qRT-PCR
3. Discussion
3.1. Plant Hormones Are the Key Factors Affecting the Formation of the Tea Plant Branching Angles
3.2. Transcription Factors Regulate the Formation of the Tea Plant Branching Angle
4. Materials and Methods
4.1. Plant Material and Sample Collection
4.2. RNA Extraction, Library Construction, and Sequencing
4.3. Transcriptomic Data Analysis
4.4. Verification of RNA-Seq Results Using qRT-PCR
4.5. Plant Endogenous Hormone Measurement in the Lateral Bud
4.6. Exogenous Hormone Treatment
4.7. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Smith, S.M.; Li, J. Genetic Regulation of Shoot Architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hu, Y.; Tong, A.; Yan, B.; Lv, Y.; Wang, S.; Ma, W.; Cui, Z.; Wang, X. LAZY1 Controls Tiller Angle and Shoot Gravitropism by Regulating the Expression of Auxin Transporters and Signaling Factors in Rice. Plant Cell Physiol. 2021, 61, 2111–2125. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Liang, Y.; Li, J.; Wang, Y. Molecular Basis Underlying Rice Tiller Angle: Current Progress and Future Perspectives. Mol. Plant 2022, 15, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Liu, J.; Zhang, P.; Kudoyarova, G.; Liu, C.J.; Zhang, K. Spatially Distributed Cytokinins: Metabolism, Signaling, and Transport. Plant Commun. 2024, 5, 100936. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Le Moigne, M.A.; Bertheloot, J.; Crespel, L.; Perez-Garcia, M.D.; Ogé, L.; Demotes-Mainard, S.; Hamama, L.; Davière, J.M.; Sakr, S. BRANCHED1: A Key Hub of Shoot Branching. Front. Plant Sci. 2019, 10, 76. [Google Scholar] [CrossRef]
- Liu, D.; Yan, G.; Wang, S.; Yu, L.; Lin, W.; Lu, S.; Guo, L.; Yang, Q.Y.; Dai, C. Comparative Transcriptome Profiling Reveals the Multiple Levels of Crosstalk in Phytohormone Networks in Brassica napus. Plant Biotechnol. J. 2023, 21, 1611–1627. [Google Scholar] [CrossRef]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pěnčík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins Are Initial Targets of Light in the Control of Bud Outgrowth. Plant Physiol. 2016, 172, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhao, J.; Hong, J.; Zhu, B.; Xia, S.; Zhu, E.; Han, P.; Zhang, K. Cytokinins Regulate Rice Lamina Joint Development and Leaf Angle. Plant Physiol. 2023, 191, 56–69. [Google Scholar] [CrossRef]
- Seale, M.; Bennett, T.; Leyser, O. BRC1 Expression Regulates Bud Activation Potential but Is Not Necessary or Sufficient for Bud Growth Inhibition in Arabidopsis. Development 2017, 144, 1661–1673. [Google Scholar] [PubMed]
- Del Rosario Cárdenas-Aquino, M.; Sarria-Guzmán, Y.; Martínez-Antonio, A. Review: Isoprenoid and Aromatic Cytokinins in Shoot Branching. Plant Sci. 2022, 319, 111240. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose Is an Early Modulator of the Key Hormonal Mechanisms Controlling Bud Outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones Regulate Rice Tiller Angle by Attenuating Shoot Gravitropism through Inhibiting Auxin Biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; van De Sande, K.; Leyser, H.M. MAX1 and MAX2 Control Shoot Lateral Branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, F.; Liao, H.; Pan, W.; Zhang, W.; Xu, B.; Yang, X. Transcriptome and Metabolite Analysis Related to Branch Development in Two Genotypes of Eucalyptus urophylla. Mol. Genet. Genom. 2021, 296, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- González-Grandío, E.; Pajoro, A.; Franco-Zorrilla, J.M.; Tarancón, C.; Immink, R.G.; Cubas, P. Abscisic Acid Signaling Is Controlled by a BRANCHED1/HD-ZIP I Cascade in Arabidopsis Axillary Buds. Proc. Natl. Acad. Sci. USA 2017, 114, E245–E254. [Google Scholar] [CrossRef]
- Spencer, V.M.R.; Bentall, L.; Harrison, C.J. Diverse Branching Forms Regulated by a Core Auxin Transport Mechanism in Plants. Development 2023, 150, dev201209. [Google Scholar] [CrossRef] [PubMed]
- Wolbang, C.M.; Davies, N.W.; Taylor, S.A.; Ross, J.J. Gravistimulation Leads to Asymmetry of Both Auxin and Gibberellin Levels in Barley pulvini. Physiol. Plant 2007, 131, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, D.; Yan, X.; Cui, L.; Wang, Z.; Yuan, H. The Role of Gibberellins in Improving the Resistance of Tebuconazole-Coated Maize Seeds to Chilling Stress by Microencapsulation. Sci. Rep. 2016, 6, 35447. [Google Scholar] [CrossRef]
- Zheng, L.; Wen, J.; Liu, J.; Meng, X.; Liu, P.; Cao, N.; Dong, J.; Wang, T. From Model to Alfalfa: Gene Editing to Obtain Semidwarf and Prostrate Growth Habits. Crop J. 2022, 10, 932–941. [Google Scholar] [CrossRef]
- Hollender, C.A.; Hill, J.L., Jr.; Waite, J.; Dardick, C. Opposing Influences of TAC1 and LAZY1 on Lateral Shoot Orientation in Arabidopsis. Sci. Rep. 2020, 10, 6051. [Google Scholar] [CrossRef]
- Kim, J.I.; Baek, D.; Park, H.C.; Chun, H.J.; Oh, D.H.; Lee, M.K.; Cha, J.Y.; Kim, W.Y.; Kim, M.C.; Chung, W.S.; et al. Overexpression of Arabidopsis YUCCA6 in Potato Results in High-Auxin Developmental Phenotypes and Enhanced Resistance to Water Deficit. Mol. Plant 2013, 6, 337–349. [Google Scholar] [CrossRef]
- Che, X.; Splitt, B.L.; Eckholm, M.T.; Miller, N.D.; Spalding, E.P. BRXL4-LAZY1 Interaction at the Plasma Membrane Controls Arabidopsis Branch Angle and Gravitropism. Plant J. 2023, 113, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Peng, H.; Yue, C.N.; Li, W.J.; Tong, Z.F.; Yang, P.X. Selection of Core Evaluation Indices and Construction of a Comprehensive Evaluation Method for Machine-Harvested Tea Plant Cultivars. Euphytica 2021, 218, 162. [Google Scholar] [CrossRef]
- Xia, X.; Mi, X.; Jin, L.; Guo, R.; Zhu, J.; Xie, H.; Liu, L.; An, Y.; Zhang, C.; Wei, C.; et al. CsLAZY1 Mediates Shoot Gravitropism and Branch Angle in Tea Plants (Camellia sinensis). BMC Plant Biol. 2021, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, C.; Wei, J.; Han, W. Effects of Exogenous 6-Benzyladenine on Dwarfing, Shoot Branching, and Yield of Tea Plant (Camellia sinensis). HortScience Horts 2018, 53, 651–655. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of Plant Hormone Response Pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Van Wees, S.C.M. Plant Hormone Functions and Interactions in Biological Systems. Plant J. 2021, 105, 287–289. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Wenkel, S.; Chandler, J.W.; Werr, W.; Jiao, Y. Spatiotemporal Control of Axillary Meristem Formation by Interacting Transcriptional Regulators. Development 2018, 145, dev158352. [Google Scholar] [CrossRef]
- Goda, H.; Sawa, S.; Asami, T.; Fujioka, S.; Shimada, Y.; Yoshida, S. Comprehensive Comparison of Auxin-Regulated and Brassinosteroid-Regulated Genes in Arabidopsis. Plant Physiol. 2004, 134, 1555–1573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Wang, J.G.; Wang, L.Y.; Wang, J.F.; Wang, Q.; Yu, P.; Bai, M.Y.; Fan, M. Gibberellin Repression of Axillary Bud Formation in Arabidopsis by Modulation of DELLA-SPL9 Complex Activity. J. Integr. Plant Biol. 2020, 62, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, J.; Jiang, Y.; Zhang, J.; Ni, Y.; Zhang, P.; Yao, Z.; Jiao, Z.; Li, H.; Li, L.; et al. Wheat Gibberellin Oxidase Genes and Their Functions in Regulating Tillering. PeerJ 2023, 11, e15924. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wang, L.Y.; Wu, L.Y.; Zhang, C.C.; Li, H.L.; Tan, L.Q.; Cao, H.L.; Cheng, H. Transcriptome Analysis of Indole-3-Butyric Acid-Induced Adventitious Root Formation in Nodal Cuttings of Camellia sinensis (L.). PLoS ONE 2014, 9, e107201. [Google Scholar] [CrossRef] [PubMed]
- Waldie, T.; Leyser, O. Cytokinin Targets Auxin Transport to Promote Shoot Branching. Plant Physiol. 2018, 177, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A Pin1 Family Gene, Ospin1, Involved in Auxin-Dependent Adventitious Root Emergence and Tillering in Rice. Plant Cell Physiol. 2005, 46, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Young, N.F.; Ferguson, B.J.; Antoniadi, I.; Bennett, M.H.; Beveridge, C.A.; Turnbull, C.G. Conditional Auxin Response and Differential Cytokinin Profiles in Shoot Branching Mutants. Plant Physiol. 2014, 165, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Y.; Huang, C.; Li, C.; Feng, Y.; Wang, H.; Ding, C.; Li, N.; Wang, L.; Zeng, J.; et al. Uncovering the Complex Regulatory Network of Spring Bud Sprouting in Tea Plants: Insights from Metabolic, Hormonal, and Oxidative Stress Pathways. Front. Plant Sci. 2023, 14, 1263606. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Petrásek, J.; Zádníková, P.; Hoyerová, K.; Pesek, B.; Raz, V.; Swarup, R.; Bennett, M.; Zazímalová, E.; Benková, E.; et al. The Auxin Influx Carriers AUX1 and LAX3 Are Involved in Auxin-Ethylene Interactions During Apical Hook Development in Arabidopsis thaliana Seedlings. Development 2010, 137, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.J.; Catalá, C. Evaluating Auxin Distribution in Tomato (Solanum Lycopersicum) through an Analysis of the PIN and AUX/LAX Gene Families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated Role of ABA in Seed Maturation, Dormancy, and Germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Finlayson, S.A. Abscisic Acid Is a General Negative Regulator of Arabidopsis Axillary Bud Growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [PubMed]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C.; et al. Arabidopsis Class I and Class Ⅱ TCP Transcription Factors Regulate Jasmonic Acid Metabolism and Leaf Development Antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar]
- Shang, X.; Han, Z.; Zhang, D.; Wang, Y.; Qin, H.; Zou, Z.; Zhou, L.; Zhu, X.; Fang, W.; Ma, Y. Genome-Wide Analysis of the TCP Gene Family and Their Expression Pattern Analysis in Tea Plant (Camellia sinensis). Front. Plant Sci. 2022, 13, 840350. [Google Scholar]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.; Ali, J.; Li, Z. Overexpression of OsMYB48-1, a Novel MYB-Related Transcription Factor, Enhances Drought and Salinity Tolerance in Rice. PLoS ONE 2014, 9, e92913. [Google Scholar]
- Nie, J.; Wen, C.; Xi, L.; Lv, S.; Zhao, Q.; Kou, Y.; Ma, N.; Zhao, L.; Zhou, X. The AP2/ERF Transcription Factor CmERF053 of Chrysanthemum Positively Regulates Shoot Branching, Lateral Root, and Drought Tolerance. Plant Cell Rep. 2018, 37, 1049–1060. [Google Scholar] [PubMed]
- Fu, Q.; Cao, H.; Wang, L.; Lei, L.; Di, T.; Ye, Y.; Ding, C.; Li, N.; Hao, X.; Zeng, J.; et al. Transcriptome Analysis Reveals That Ascorbic Acid Treatment Enhances the Cold Tolerance of Tea Plants through Cell Wall Remodeling. Int. J. Mol. Sci. 2023, 24, 10059. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, H.; Chang, Y.; Ma, C.; Wang, L.; Hao, X.; Li, A.; Cheng, H.; Wang, L.; Cui, P.; et al. Population Sequencing Enhances Understanding of Tea Plant Evolution. Nat. Commun. 2020, 11, 4447. [Google Scholar]
- Zheng, Q.; Guo, L.; Huang, J.; Hao, X.; Li, X.; Li, N.; Wang, Y.; Zhang, K.; Wang, X.; Wang, L.; et al. Comparative Transcriptomics Provides Novel Insights into the Mechanisms of Selenium Accumulation and Transportation in Tea Cultivars (Camellia sinensis (L.) O. Kuntze). Front. Plant Sci. 2023, 14, 1268537. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. Clusterprofiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and Evaluation of Reliable Reference Genes for Quantitative Real-Time PCR Analysis in Tea Plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, H.; Zhang, X.; Khashi, U.R.M.; Mazzoleni, S.; Du, M.; Wu, F. Plant Extracellular Self-DNA Inhibits Growth and Induces Immunity Via the Jasmonate Signaling Pathway. Plant Physiol. 2023, 192, 2475–2491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, X.; Huang, J.; Wang, L.; Zheng, Q.; Li, H.; Chen, Y.; Tang, J.; Hao, X.; Wang, X.; et al. Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Branching Angle Formation in Tea Plants (Camellia sinensis). Int. J. Mol. Sci. 2025, 26, 604. https://doi.org/10.3390/ijms26020604
Zhu J, Li X, Huang J, Wang L, Zheng Q, Li H, Chen Y, Tang J, Hao X, Wang X, et al. Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Branching Angle Formation in Tea Plants (Camellia sinensis). International Journal of Molecular Sciences. 2025; 26(2):604. https://doi.org/10.3390/ijms26020604
Chicago/Turabian StyleZhu, Jinping, Xiaoman Li, Jianyan Huang, Lu Wang, Qinghua Zheng, Hanjia Li, Yao Chen, Junwei Tang, Xinyuan Hao, Xinchao Wang, and et al. 2025. "Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Branching Angle Formation in Tea Plants (Camellia sinensis)" International Journal of Molecular Sciences 26, no. 2: 604. https://doi.org/10.3390/ijms26020604
APA StyleZhu, J., Li, X., Huang, J., Wang, L., Zheng, Q., Li, H., Chen, Y., Tang, J., Hao, X., Wang, X., Huang, Y., & Zeng, J. (2025). Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Branching Angle Formation in Tea Plants (Camellia sinensis). International Journal of Molecular Sciences, 26(2), 604. https://doi.org/10.3390/ijms26020604







